Abstract
Background:
Clopidogrel low response (CLR) is an independent risk factor of adverse outcomes in patients undergoing percutaneous coronary intervention (PCI), and intensified antiplatelet treatments (IAT) guided by platelet function assays might overcome laboratory CLR. However, whether IAT improves clinical outcomes is controversial.
Methods:
Relevant trials were identified in PubMed, the Cochrane Library, and the Chinese Medical Journal Network databases from their establishment to September 9, 2014. Trials were screened using predefined inclusion criteria. Conventional meta-analysis and cumulative meta-analysis were performed using the Review Manager 5.0 and STATA 12.0 software programs.
Results:
Thirteen randomized controlled trials involving 5111 patients with CLR were recruited. During a follow-up period of 1–12 months, the incidences of cardiovascular (CV) death, nonfatal myocardial infarction (MI), and stent thrombosis were significantly lower in the IAT arm than in the conventional antiplatelet treatment arm (relative risk [RR] = 0.45, 95% confidence interval [CI]: 0.36–0.57, P < 0.000,01), whereas bleeding was similar between the two arms (RR = 1.05, 95% CI: 0.86–1.27, P = 0.65).
Conclusions:
IAT guided by platelet function assays reduces the risk of CV death, nonfatal MI, and stent thrombosis (ST) without an increased risk of bleeding in patients undergoing PCI and with CLR.
Keywords: Coronary Artery Disease, Individualized Medicine, Platelet Aggregation Inhibitor, Platelet Function Test
INTRODUCTION
Conventional antiplatelet treatment (CAT) with clopidogrel in addition to aspirin has been recommended for patients undergoing percutaneous coronary intervention (PCI) since 2002.[1] However, patients have significantly varied responses to clopidogrel, and clopidogrel low response (CLR) is an independent risk factor of adverse clinical outcomes in patients undergoing PCI.[2,3,4,5,6]
A few randomized controlled trials (RCTs) investigated the efficacy of intensified antiplatelet treatment (IAT) guided by platelet function assays, but the results were controversial. The Gauging Responsiveness with a VerifyNow Assay-Impact on Thrombosis and Safety (GRAVITAS)[7] and Testing Platelet Reactivity in Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy with Prasugrel (TRIGGER-PCI)[8] trials showed that patients with CLR did not benefit from IAT guided by the VerifyNow Assay. However, several other randomized trials reported that IAT significantly reduced the incidence of stent thrombosis (ST) or composite endpoints compared to CAT for patients with CLR undergoing stent implantation.[9,10] Accordingly, we conducted this meta-analysis to elucidate whether patients with CLR can benefit from IAT guided by platelet function assays.
METHODS
Search strategy
The literature was scanned using computerized searches of PubMed, the Cochrane Library, and the Chinese Medical Journal Network databases from their establishment to September 9, 2014. The search terms included three parts, CLR, clinical events, and method of platelet function test. The search terms for CLR included clopidogrel, low response or no response or high on-treatment platelet reactivity (HOPR) or high residual platelet reactivity or high platelet reactivity or resistance. The search terms for clinical events included death or myocardial infarction (MI) or ST or bleeding. The search terms for method of platelet function test included VerifyNow or light transmission aggregation (LTA) or LTA or aggregometer or aggregometry or multiple electrode aggregometry (MEA) or multiplate analyzer or thrombelastography (TEG) or vasodilator-stimulated phosphoprotein (VASP) or LTA or TEG or VASP or MEA or platelet function analyser-100. The article type was limited to RCTs. No language restriction was enforced.
Selection of studies and data extraction
Studies were selected according to the following criteria: (1) studies that recruited patients with coronary artery disease (CAD) undergoing PCI and initially treated with aspirin plus clopidogrel; (2) studies that identified CLR by platelet function assays; (3) studies that randomly assigned the CLR patients into CAT or IAT arms; and (4) studies that reported clinical adverse events, including cardiovascular (CV) death, nonfatal MI, ST, or bleeding.
A total of 397 papers were initially screened, and 132 papers remained after limiting the article type to clinical trials. Finally, 11 papers that met the study criteria were identified. An additional two papers were included after reviewing the citations of the screened papers.[11,12] The details of the paper selection are described in Figure 1. Two experienced authors (Xu and Zhang) independently extracted the following data from the selected studies: author name and year of publication, study design, clinical diagnosis of participants, type of stent implanted, method of platelet function assay, cut-off line of HOPR, number of patients randomized, the strategy of treatment adjustment, duration of follow-up, and clinical outcomes. Disagreements were resolved by discussion or consultation with a third reviewer (Li).
Figure 1.
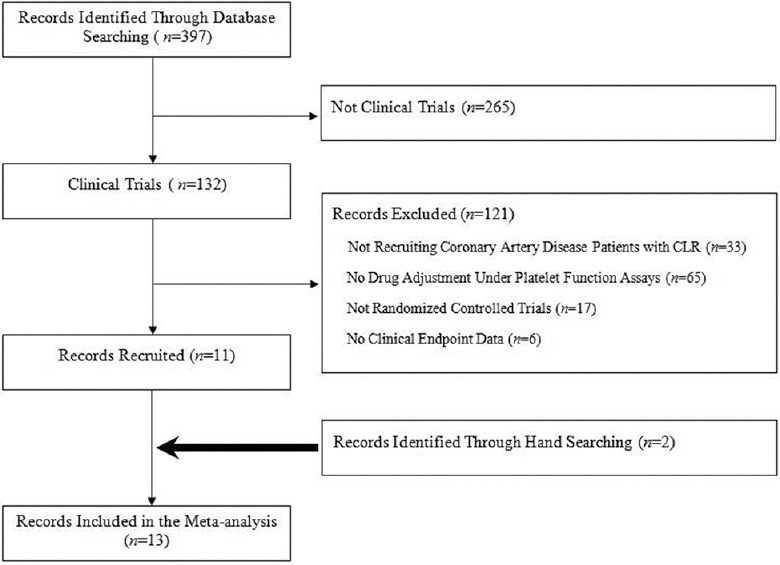
Flow diagram of literature search of this meta-analysis. CLR: Clopidogrel low response.
Quality assessment
The quality of the recruited studies was assessed using the modified Jadad scale,[13] which is based on methods of randomization, allocation concealment, blinding, and loss to follow-up. The total Jadad score was 7, which was based on a score of 2 for randomization, a score of 2 for allocation concealment, a score of 2 for blinding, and a score of 1 for the description of withdrawals and dropouts. We defined low-quality studies as those with a score of 1–3 and high-quality studies as those with a score of 4–7.[14,15]
Statistical analysis
A conventional meta-analysis was performed using Review Manager 5.0.0 (The Cochrane Collaboration, Oxford, England), and a cumulative meta-analysis was performed using STATA 12.0 (Lakeway Drive, College Station, Texas, USA). A subgroup meta-analysis was performed for different platelet function assays. The results are presented as the incidence of outcomes, relative risk (RR), 95% confidence intervals (CIs), and P value. The Cochran Q-test and the I2 test (with 95% CIs) were used to assess heterogeneity. A fixed-effects model was used if I2 < 50%, and a random-effects model was used if I2 ≥ 50%. Publication bias was analyzed using funnel plots and was quantified by Egger's test. A value of P < 0.05 was considered statistically significant.
RESULTS
Characteristics of the included studies
Thirteen RCTs with 5111 CLR patients were finally recruited, and the characteristics of these studies are summarized in Table 1. Assessment of the methodological quality using the modified Jadad scale demonstrated that all 13 studies were ranked as high quality [Table 2]. The sample sizes of the studies ranged from 44 to 2214, and the participants were aged from 45 to 80 years. Platelet function assays, including the VerifyNow P2Y12, MEA, LTA, TEG, and VASP were used in the recruited studies. In total, 2695 patients were randomly allocated to the IAT arm and 2416 patients to the CAT arm. The follow-up duration ranged from 1 to 12 months, with a mean follow-up period of 5.69 ± 4.60 months.
Table 1.
Characteristics of included studies
| Authors | Design | Participant diagnosis | Platelet function assay | Cutoff line for HOPR | Number of patients with HOPR | Number of patients in IAT group | Number of patients in CAT group | Intensified antiplatelet treatment | Follow-up duration (months) |
|---|---|---|---|---|---|---|---|---|---|
| Aradi et al. 2011[16] | RCT | Stable angina | LTA | LTA ≥34% | 74 | 36 | 38 | Clopidogrel 150 mg | 12 |
| Ari et al. 2012[17] | RCT | CAD | VerifyNow | Inhibition value <40% | 94 | 47 | 47 | Clopidogrel 150 mg | 6 |
| Bonello et al. 2008[18] | RCT | Refractory angina pectoris, silent ischemia, or NSTEMI | VASP | PRI >50% | 162 | 78 | 84 | Clopidogrel 600–2400 mg | 1 |
| Bonello et al. 2009[9] | RCT | Refractory angina pectoris, silent ischemia, or NSTEMI | VASP | PRI ≥50% | 429 | 215 | 214 | Clopidogrel 600–2400 mg | 1 |
| Cuisset et al. 2008[11] | RCT | Sable angina or with a positive coronary function test | LTA | LTA >70% | 149 | 74 | 75 | Group IIb/IIIa antagonist (abciximab) | 1 |
| Guan et al. 2012[12] | RCT | ACS (refractory angina pectoris, NSTEMI, or STEMI) | LTA | LTA >55% | 840 | 560 | 280 | Clopidogrel 300 mg, then cilostazol 50–100 mg if LTA >55% | 1 |
| Price et al. 2011[7] | RCT | Stable CAD, NSTE-ACS, or STEMI | VerifyNow | PRU ≥230 | 2214 | 1109 | 1105 | Clopidogrel 600 mg LD, 150 mg daily for 6 months | 6 |
| Gremmel et al. 2012[19] | RCT | CAD | VerifyNow VASP MEA | PRU >235 PRI >50% AU ≥47 | 44 | 21 | 23 | Clopidogrel 150 mg | 3 |
| Tang et al. 2012[20] | RCT | CAD | TEG | Inhibition rate <50% | 60 | 30 | 30 | Clopidogrel 150 mg | 12 |
| Trenk et al. 2012[8] | RCT | Stable CAD | VerifyNow | PRU >208 | 423 | 212 | 211 | Prasugrel 10 mg | 6 |
| Wang et al. 2010[21] | RCT | Refractory angina pectoris, silent ischemia, or NSTEMI | VASP | PRI ≥50% | 298 | 147 | 151 | Clopidogrel 75–375 mg | 12 |
| Paarup Dridi et al. 2015[10] | RCT | Stable angina pectoris or non-ST elevation ACS | MEA | Multiplate- ADP >70 U | 237 | 123 | 114 | Prasugrel, ticagrelor, or clopidogrel 150 mg | 1 |
| Samardzic et al. 2014[22] | RCT | ACS | MEA | Platelet reactivity ≤46 U | 87 | 43 | 44 | Increasing clopidogrel dose | 12 |
RCT: Randomized clinical trials; HOPR: High on_treatment platelet reactivity; IAT: Intensified antiplatelet treatment; CAT: Conventional antiplatelet treatment; NSTEMI: Non-ST-segment elevation myocardial infarction; STEMI: ST-segment elevation myocardial infarction; CAD: Coronary artery disease; ACS: Acute coronary syndrome; PCI: Percutaneous coronary intervention; LD: Loading dose; LTA: Light transmission aggregometry; VASP: Vasodilator-stimulated phosphoprotein; MEA: Multiple electrode aggregometry; PRU: P2Y12 reaction units; PRI: Platelet reactivity index; AU: Aggregation unit; TEG: Thrombelastography; ADP: Adenosine diphosphate.
Table 2.
Quality assessment of included studies
| Authors | Year | Quality assessment | ||||
|---|---|---|---|---|---|---|
| Concealed allocation | Randomization | Blinding | Withdrawals and dropouts | Jadad score | ||
| Aradi et al.[16] | 2011 | 2 | 2 | 2 | 1 | 7 |
| Ari et al.[17] | 2012 | 1 | 2 | 0 | 1 | 4 |
| Bonello et al.[18] | 2008 | 1 | 1 | 2 | 1 | 5 |
| Bonello et al.[9] | 2009 | 1 | 1 | 2 | 1 | 5 |
| Cuisset et al.[11] | 2008 | 1 | 2 | 0 | 1 | 4 |
| Guan et al.[12] | 2012 | 1 | 2 | 0 | 1 | 4 |
| Price et al.[7] | 2011 | 2 | 2 | 2 | 1 | 7 |
| Gremmel et al.[19] | 2012 | 0 | 1 | 2 | 1 | 4 |
| Tang et al.[20] | 2012 | 1 | 2 | 0 | 1 | 4 |
| Trenk et al.[8] | 2012 | 1 | 2 | 2 | 1 | 6 |
| Wang et al.[21] | 2010 | 1 | 1 | 2 | 1 | 5 |
| Paarup Dridi et al.[10] | 2014 | 1 | 2 | 2 | 0 | 5 |
| Samardzic et al.[22] | 2014 | 2 | 2 | 0 | 1 | 5 |
For the numbers, 2 indicates that the method of randomization or concealed allocation or blinding was described in the paper, and it was appropriate; 1 indicates that the method of randomization or concealed allocation or blinding or withdrawals and dropouts were described but were inappropriate; 0 indicates that randomization, concealed allocation, blinding or withdrawals, and dropouts were not mentioned in the paper.
Meta-analysis results
Heterogeneity assessment
The studies were homogeneous when evaluated for the heterogeneity of both a single event and the combined events. Similarly, the studies showed homogeneity when evaluated for the heterogeneity of different platelet function assays, such as LTA, VerifyNow, and MEA, except for the studies that showed heterogeneity in the VASP data (I2= 80%), in which the random-effects model was used for the VASP analysis. The funnel plots for CV death, nonfatal MI, ST, and bleeding are presented in Supplementary Figure 1 (662.4KB, tif) .
Funnel plots for evaluating publication bias of clinical events. (a-d) Represent CV death, nonfatal MI, ST, and bleeding, respectively. X axis shows the RR value of effect size from each study. Y axis shows the standard error (SE) of log (RR) of effect size from each study. CV: Cardiovascular; MI: Myocardial infarction; ST: Stent thrombosis RR: Risk ratio.
Risk ratio for ischemic events and bleeding
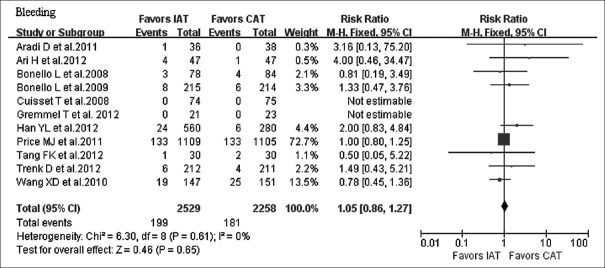
The incidence of combined ischemic endpoints was significantly lower in the IAT arm compared to the CAT arm (RR = 0.45, 95% CI: 0.36–0.57, P < 0.000,01) [Figure 2]. Separately, the incidence of CV death was significantly lower in the IAT arm compared to the CAT arm (RR = 0.46, 95% CI: 0.28–0.75, P = 0.002). The incidence of MI was significantly lower in the IAT arm compared to the CAT arm (RR = 0.49, 95% CI: 0.35–0.69, P < 0.0001), and the incidence of ST was also significantly lower in the IAT arm compared to the CAT arm (RR = 0.44, 95% CI: 0.27–0.73, P = 0.001). The incidence of bleeding was comparable between the two arms (RR = 1.05, 95% CI: 0.86–1.27, P = 0.65) [Figure 3].
Figure 2.
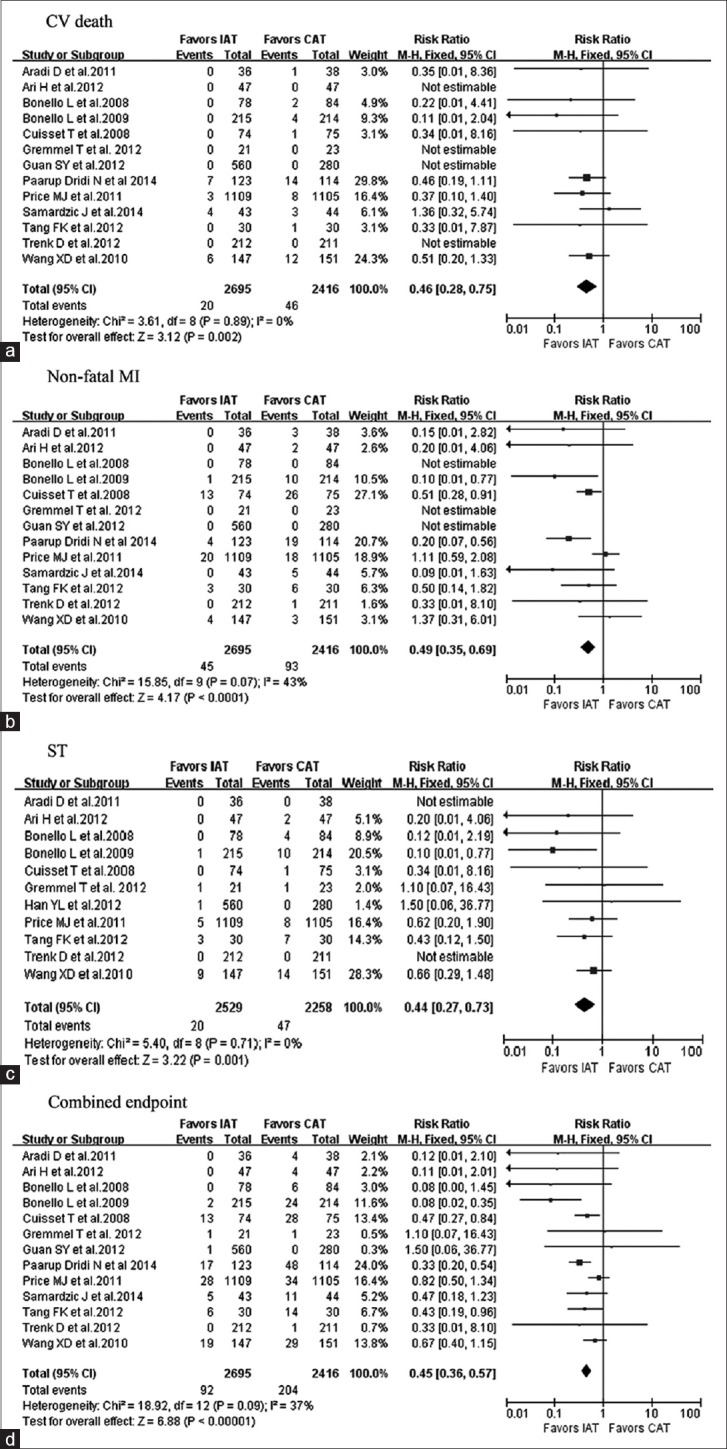
Forest plots for the effect of IAT versus CAT. (a-d) Represent for CV death, nonfatal MI, ST, and the combined endpoints, respectively. IAT: Intensified antiplatelet treatment; CAT: Conventional antiplatelet treatment; CV: Cardiovascular; MI: Myocardial infarction; ST: Stent thrombosis; CI: Confidence interval.
Figure 3.
Forest plot for the effect of IAT versus CAT on bleeding. IAT: Intensified antiplatelet treatment; CAT: Conventional antiplatelet treatment; CI: Confidence interval.
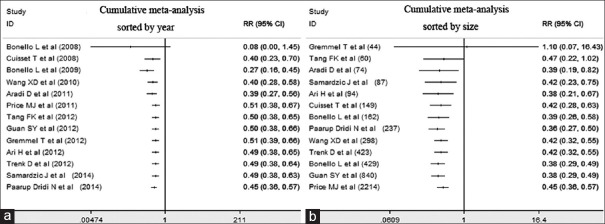
Cumulative meta-analyses results
Cumulative meta-analyses were performed to evaluate the effectiveness of IAT sorted by year. The results showed that the cumulative effect of IAT on the incidence of combined endpoints was superior to that of CAT from the initial trial in 2008 (RR = 0.40, 95% CI: 0.23–0.70). Later trials showed a consistent and significant benefit of IAT [Figure 4a]. If the trials were sorted by the population size, the overall effect of IAT remained relatively stable with similar point estimates, whereas the later studies had an increased population size and narrowed the CIs [Figure 4b].
Figure 4.
Cumulative meta-analysis sorted by year (a) and sample size (b) to show the effect of IAT versus CAT on the combined endpoints. RR: Risk ratio; CI: Confidence interval.
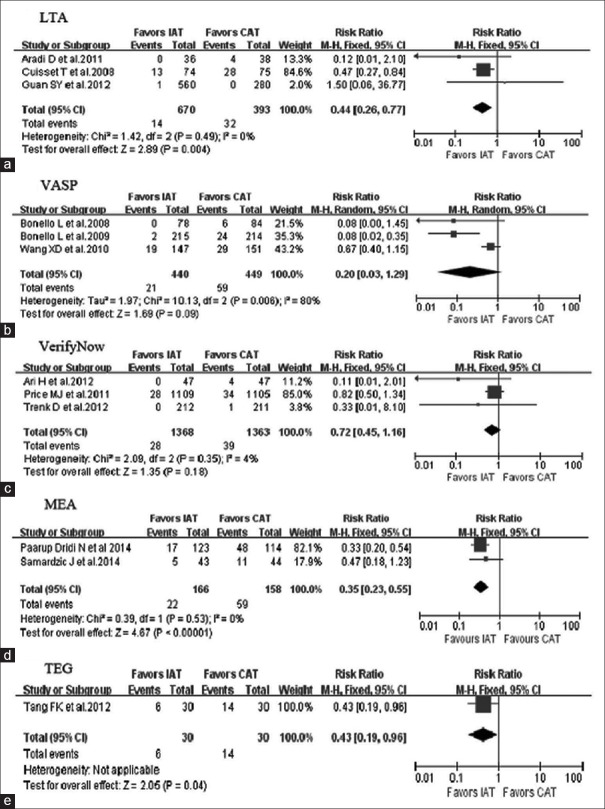
Subgroup meta-analysis results
Trials were grouped according to different methods of platelet function assay. Subgroup analysis showed that the combined endpoints were significantly decreased in the IAT arm compared to the CAT arm as guided by the LTA and MEA (P = 0.004 and P < 0.000,01, respectively) [Figure 5a–5d]. One trial adopted TEG to determine the IAT, which resulted in decreased combined endpoints in the IAT arm compared to the CAT arm [P = 0.04; Figure 5e]. However, there were no significant differences in the combined endpoints between the two arms guided by either VASP or VerifyNow [P = 0.09 and P = 0.18, respectively; Figure 5b and 5c].
Figure 5.
Subgroup analysis according to platelet function assays. LTA: Light transmission aggregometry subgroup (a); VASP: Vasodilator-stimulated phosphoprotein subgroup (b); VerifyNow and MEA: Multiplate analyzer subgroup (c) and subgroup (d); TEG: Thrombelastography hemostasis analyzer subgroup (e). IAT: Intensified antiplatelet treatment; CAT: Conventional antiplatelet treatment; CI: Confidence interval.
DISCUSSION
This study revealed that platelet function assay-guided IAT significantly decreased the incidences of CV death, MI, and ST without an increased risk of bleeding compared to CAT in CAD patients undergoing PCI. The result of this meta-analysis might provide evidence-based science for clinical guideline development.
CLR is an independent risk factor for adverse clinical outcomes.[3,23,24] Theoretically, any intensified antiplatelet therapy that improves the platelet response should improve the patient's clinical outcome. However, most studies investigating the benefits of IAT yielded negative results,[7,12,19] and several critical issues regarding the design of these trials have been noted. First, the participants recruited in these studies were in stable condition. In the GRAVITAS, non-ST-segment elevation myocardial infarction and ST-segment elevation myocardial infarction, patients accounted for only 10% and 0.4% of the study population, respectively.[7] In the TRIGGER-PCI, 100% of the participants were stable CAD patients, and the low incidence of the primary endpoint (2.3% vs. the expected 7% rate) resulted in the early discontinuation of the trial.[8,25] Therefore, the low-risk population with a low incidence of primary endpoints might contribute to the negative results of these trials. Second, randomization was performed late during the GRAVITAS and TRIGGER-PCI trials (12–24 h after PCI and 2–7 h after the patients were first administered clopidogrel, respectively); therefore, some periprocedural events might have not been included in these trials. Third, inappropriate cutoff values for CLR with less IAT might also contribute to the negative results. In the GRAVITAS trial, CLR was defined as ≥230 platelet reaction units (PRU) using the VerifyNow P2Y12 test.[7] However, Price et al.[26] performed a post hoc analysis and found that using PRU <208 as a cutoff level, the IAT was associated with a lower risk of adverse clinical events. Finally, CLR patients did not receive an ideal IAT regimen in most trials. Currently available potent antiplatelet agents, such as ticagrelor and prasugrel, were not available or used in most of the recruited trials. In fact, most studies used an increased dose of clopidogrel as IAT [Table 1]; however, 40% of the CLR patients remained low response to clopidogrel although the dose of clopidogrel was doubled.[25]
Our study results were verified by cumulative meta-analysis, which indicated that IAT significantly diminished the major CV events, whereas the study sample size was increased to a statistically reasonable level. However, future studies should also consider recruiting higher risk patients and using more intensified antiplatelet agents. Hopefully, current ongoing studies, such as ANTARCTIC and TROPICAL-ACS, will increase our knowledge of the value of IAT guided by platelet function assays. We performed a subgroup analysis on different platelet function assays and found that LTA and MEA-guided IATs improved the combined endpoints of the CLR patients, whereas VASP- and VerifyNow-guided IAT did not. These results are consistent with reports that LTA and MEA are highly correlated to ischemic events[27,28,29,30] although Tang et al.[20] found that TEG-guided IAT also decreased the incidence of ischemic CV events compared to CAT.
It should be noted that some important trials were not recruited in our meta-analysis because they did not meet our inclusion criteria. The MEA in Patients Receiving Dual Antiplatelet Therapy to Guide Treatment with Novel Platelet Antagonists (MADONNA) study[31] found that personalized antiplatelet treatment according to platelet function testing with MEA resulted in an improved efficacy with equal safety compared to the standard treatment. However, it was excluded because it was a cohort study rather than an RCT. Although the Assessment by a Double Randomization of a Conventional Antiplatelet Strategy Versus a Monitoring-guided Strategy for Drug-Eluting Stent Implantation and of Treatment Interruption versus Continuation 1 year after Stenting (ARCTIC) study[32] was an RCT, it randomized all recruited patients, not only the CLR patients, into the IAT and CAT arms, which did not meet our inclusion criteria.
To the best of our knowledge, only one similar meta-analysis was reported by Aradi et al.[33] However, several differences existed between the study of Aradi et al. and ours. First, to ensure the quality of the studies and to diminish the heterogeneity, all RCTs included in our study must randomize the CLR patients into the IAT and CAT arms. The study of Hazarbasanov et al.[30] was recruited in the study of Aradi et al., but was excluded in our study for the same reason as the ARCTIC study. We proposed that because both arms involve patients who normally responded to clopidogrel such as in these studies, this would dilute the efficacy of the IAT causing the results to be less reliable. Second, we recruited the latest RCTs[12,19,20] that were not included in the study of Aradi et al. Third, in addition to the conventional meta-analysis, we also performed a cumulative meta-analysis, which further confirmed our study results.
Our study had potential limitations. First, different platelet function assays were using in the recruited studies. However, the studies were homogeneous for the evaluation of heterogeneity of different platelet function assays, such as LTA and VerifyNow. Although the studies showed heterogeneity in the VASP analysis, the random-effects model was used for the VASP analysis. Second, the cutoff values for CLR varied between studies [Table 1], which might result in different strengths of the IATs. Third, the follow-up period of some recruited studies was relatively short (one month), and late adverse events might have not occurred during the study period.
In conclusion, this meta-analysis demonstrates that IAT guided by platelet function assays reduces the risk of CV death, nonfatal MI, and ST without an increased risk of bleeding.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
This work was supported by grants from the National Natural Science Funding of China (No. 81170181), and the Priority Academic Program Development of Jiangsu Higher Education Institutes (No. 81170181).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, et al. A CC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction –Summary article:A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina) J Am Coll Cardiol. 2002;40:1366–74. doi: 10.1016/s0735-1097(02)02336-7. doi:10.1016/S0735-1097(02)02336-7. [DOI] [PubMed] [Google Scholar]
- 2.Barragan P, Bouvier JL, Roquebert PO, Macaluso G, Commeau P, Comet B, et al. Resistance to thienopyridines:Clinical detection of coronary stent thrombosis by monitoring of vasodilator-stimulated phosphoprotein phosphorylation. Catheter Cardiovasc Interv. 2003;59:295–302. doi: 10.1002/ccd.10497. doi:10.1002/ccd.10497. [DOI] [PubMed] [Google Scholar]
- 3.Aradi D, Komócsi A, Vorobcsuk A, Rideg O, Tokés-Füzesi M, Magyarlaki T, et al. Prognostic significance of high on-clopidogrel platelet reactivity after percutaneous coronary intervention:Systematic review and meta-analysis. Am Heart J. 2010;160:543–51. doi: 10.1016/j.ahj.2010.06.004. doi:10.1016/j.ahj.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Cuisset T, Frere C, Quilici J, Barbou F, Morange PE, Hovasse T, et al. High post-treatment platelet reactivity identified low-responders to dual antiplatelet therapy at increased risk of recurrent cardiovascular events after stenting for acute coronary syndrome. J Thromb Haemost. 2006;4:542–9. doi: 10.1111/j.1538-7836.2005.01751.x. doi:10.1111/j.1538-7836.2005.01751.x. [DOI] [PubMed] [Google Scholar]
- 5.Combescure C, Fontana P, Mallouk N, Berdague P, Labruyere C, Barazer I, et al. Clinical implications of clopidogrel non-response in cardiovascular patients:A systematic review and meta-analysis. J Thromb Haemost. 2010;8:923–33. doi: 10.1111/j.1538-7836.2010.03809.x. doi:10.1111/j.1538-7836.2010.03809.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Gong X, Zhu T, Zhang Q, Zhang Y, Wang X, et al. Clopidogrel improves aspirin response after off-pump coronary artery bypass surgery. J Biomed Res. 2014;28:108–13. doi: 10.7555/JBR.28.20120139. doi:10.7555/JBR.28.20120139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention:The GRAVITAS randomized trial. JAMA. 2011;305:1097–105. doi: 10.1001/jama.2011.290. doi:10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- 8.Trenk D, Stone GW, Gawaz M, Kastrati A, Angiolillo DJ, Müller U, et al. Arandomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents:Results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. J Am Coll Cardiol. 2012;59:2159–64. doi: 10.1016/j.jacc.2012.02.026. doi:10.1016/j.jacc.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Bonello L, Camoin-Jau L, Armero S, Com O, Arques S, Burignat-Bonello C, et al. Tailored clopidogrel loading dose according to platelet reactivity monitoring to prevent acute and subacute stent thrombosis. Am J Cardiol. 2009;103:5–10. doi: 10.1016/j.amjcard.2008.08.048. doi:10.1016/j.amjcard.2008.08.048. [DOI] [PubMed] [Google Scholar]
- 10.Paarup Dridi N, Johansson PI, Lønborg JT, Clemmensen P, Radu MD, Qayyum A, et al. Tailored antiplatelet therapy to improve prognosis in patients exhibiting clopidogrel low-response prior to percutaneous coronary intervention for stable angina or non-ST elevation acute coronary syndrome. Platelets. 2015;26:521–9. doi: 10.3109/09537104.2014.948837. doi:10.3109/09537104.2014.948837. [DOI] [PubMed] [Google Scholar]
- 11.Cuisset T, Frere C, Quilici J, Morange PE, Mouret JP, Bali L, et al. Glycoprotein IIb/IIIa inhibitors improve outcome after coronary stenting in clopidogrel nonresponders:A prospective, randomized study. JACC Cardiovasc Interv. 2008;1:649–53. doi: 10.1016/j.jcin.2008.08.018. doi:10.1016/j.jcin.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Guan SY, Han YL, Li Y, Guo L, Yang BS, Wang SL, et al. Effects of intensive antiplatelet therapy for patients with high on-treatment platelet reactivity after coronary stent implantation (in Chinese) Chin J Cardiol. 2012;40:25–9. [PubMed] [Google Scholar]
- 13.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. doi:10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med. 2001;135:982–9. doi: 10.7326/0003-4819-135-11-200112040-00010. doi:10.7326/0003-4819-135-11-200112040-00010. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609–13. doi: 10.1016/S0140-6736(98)01085-X. doi:10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 16.Aradi D, Rideg O, Vorobcsuk A, Magyarlaki T, Magyari B, Kónyi A, et al. Justification of 150 mg clopidogrel in patients with high on-clopidogrel platelet reactivity. Eur J Clin Invest. 2012;42:384–92. doi: 10.1111/j.1365-2362.2011.02594.x. doi:10.1111/j.1365-2362.2011.02594.x. [DOI] [PubMed] [Google Scholar]
- 17.Ari H, Ozkan H, Karacinar A, Ari S, Koca V, Bozat T. The effect of high-dose clopidogrel treatment in patients with clopidogrel resistance (the EFFICIENT trial) Int J Cardiol. 2012;157:374–80. doi: 10.1016/j.ijcard.2010.12.083. doi:10.1016/j.ijcard.2010.12.083. [DOI] [PubMed] [Google Scholar]
- 18.Bonello L, Camoin-Jau L, Arques S, Boyer C, Panagides D, Wittenberg O, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance:A multicenter randomized prospective study. J Am Coll Cardiol. 2008;51:1404–11. doi: 10.1016/j.jacc.2007.12.044. doi:10.1016/j.jacc.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 19.Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp CW. A high maintenance dose increases the inhibitory response to clopidogrel in patients with high on-treatment residual platelet reactivity. Int J Cardiol. 2012;160:109–13. doi: 10.1016/j.ijcard.2011.04.001. doi:10.1016/j.ijcard.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Tang FK, Lin LJ, Hua N, Lu H, Qi Z, Tang XZ. Earlier application of loading doses of aspirin and clopidogrel decreases rate of recurrent cardiovascular ischemic events for patients undergoing percutaneous coronary intervention. Chin Med J. 2012;125:631–8. [PubMed] [Google Scholar]
- 21.Wang XD, Zhang DF, Zhuang SW, Lai Y. Modifying clopidogrel maintenance doses according to vasodilator-stimulated phosphoprotein phosphorylation index improves clinical outcome in patients with clopidogrel resistance. Clin Cardiol. 2011;34:332–8. doi: 10.1002/clc.20884. doi:10.1002/clc.20884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samardzic J, Krpan M, Skoric B, Pasalic M, Petricevic M, Milicic D. Serial clopidogrel dose adjustment after platelet function testing improves outcome of acute coronary syndrome patients undergoing percutaneous coronary intervention with high on-treatment platelet reactivity. J Thromb Thrombolysis. 2014;38:459–69. doi: 10.1007/s11239-014-1087-0. doi:10.1007/s11239-014-1087-0. [DOI] [PubMed] [Google Scholar]
- 23.Sibbing D, Steinhubl SR, Schulz S, Schömig A, Kastrati A. Platelet aggregation and its association with stent thrombosis and bleeding in clopidogrel-treated patients:Initial evidence of a therapeutic window. J Am Coll Cardiol. 2010;56:317–8. doi: 10.1016/j.jacc.2010.03.048. doi:10.1016/j.jacc.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 24.Ellis KJ, Stouffer GA, McLeod HL, Lee CR. Clopidogrel pharmacogenomics and risk of inadequate platelet inhibition:US FDA recommendations. Pharmacogenomics. 2009;10:1799–817. doi: 10.2217/pgs.09.143. doi:10.2217/pgs.09.143. [DOI] [PubMed] [Google Scholar]
- 25.Siller-Matula JM, Jilma B. Why have studies of tailored anti-platelet therapy failed so far? Thromb Haemost. 2013;110:628–31. doi: 10.1160/TH13-03-0250. doi:10.1160/TH13-03-0250. [DOI] [PubMed] [Google Scholar]
- 26.Price MJ, Angiolillo DJ, Teirstein PS, Lillie E, Manoukian SV, Berger PB, et al. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention:A time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay:Impact on thrombosis and safety (GRAVITAS) trial. Circulation. 2011;124:1132–7. doi: 10.1161/CIRCULATIONAHA.111.029165. doi:10.1161/CIRCULATIONAHA.111.029165. [DOI] [PubMed] [Google Scholar]
- 27.LordkipanidzéM Pharand C, Schampaert E, Palisaitis DA, Diodati JG. Evaluation of the platelet count drop method for assessment of platelet function in comparison with “gold standard” light transmission aggregometry. Thromb Res. 2009;124:418–22. doi: 10.1016/j.thromres.2009.02.002. doi:10.1016/j.thromres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–33. doi: 10.1016/j.jacc.2010.04.047. doi:10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 29.Michelson AD. Platelet function testing in cardiovascular diseases. Circulation. 2004;110:e489–93. doi: 10.1161/01.CIR.0000147228.29325.F9. doi:10.1161/01.CIR.0000147228.29325.F9. [DOI] [PubMed] [Google Scholar]
- 30.Hazarbasanov D, Velchev V, Finkov B, Postadjian A, Kostov E, Rifai N, et al. Tailoring clopidogrel dose according to multiple electrode aggregometry decreases the rate of ischemic complications after percutaneous coronary intervention. J Thromb Thrombolysis. 2012;34:85–90. doi: 10.1007/s11239-012-0684-z. doi:10.1007/s11239-012-0684-z. [DOI] [PubMed] [Google Scholar]
- 31.Siller-Matula JM, Francesconi M, Dechant C, Jilma B, Maurer G, Delle-Karth G, et al. Personalized antiplatelet treatment after percutaneous coronary intervention:The MADONNA study. Int J Cardiol. 2013;167:2018–23. doi: 10.1016/j.ijcard.2012.05.040. doi:10.1016/j.ijcard.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 32.Collet JP, Cuisset T, Rangé G, Cayla G, Elhadad S, Pouillot C, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012;367:2100–9. doi: 10.1056/NEJMoa1209979. doi:10.1056/NEJMoa1209979. [DOI] [PubMed] [Google Scholar]
- 33.Aradi D, Komócsi A, Price MJ, Cuisset T, Ari H, Hazarbasanov D, et al. Efficacy and safety of intensified antiplatelet therapy on the basis of platelet reactivity testing in patients after percutaneous coronary intervention:Systematic review and meta-analysis. Int J Cardiol. 2013;167:2140–8. doi: 10.1016/j.ijcard.2012.05.100. doi:10.1016/j.ijcard.2012.05.100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel plots for evaluating publication bias of clinical events. (a-d) Represent CV death, nonfatal MI, ST, and bleeding, respectively. X axis shows the RR value of effect size from each study. Y axis shows the standard error (SE) of log (RR) of effect size from each study. CV: Cardiovascular; MI: Myocardial infarction; ST: Stent thrombosis RR: Risk ratio.