Abstract
Background:
No national research on maternal and fetal complications and outcomes has been carried out in the mainland of China in recent years. This study was to provide a scientific basis for better control of obstetrical and neonatal diseases and better allocation of medical resources by analyzing the epidemiological characteristics of obstetrical diseases in the mainland of China.
Methods:
Hospitalized obstetrical cases from 19 tertiary and 20 secondary hospitals in 14 provinces (nationally representative) during the period January 1, 2011 to December 31, 2011 were randomly selected. The general condition, pregnancy complications, and perinatal outcomes of the patients were studied.
Results:
The top five medical and surgical complications of pregnant women in the mainland of China were anemia (6.34%), uterine fibroids (2.69%), thyroid disease (1.11%), thrombocytopenia (0.59%), and heart disease (0.59%). The incidences of premature rupture of membranes (PROM), preterm birth, prolonged pregnancy, hypertensive disorders complicating pregnancy (HDCP), multiple pregnancy, intrahepatic cholestasis of pregnancy (ICP), placenta previa, placental abruption, postpartum hemorrhage, and amniotic fluid embolism were 15.27%, 7.04%, 6.71%, 5.35%, 1.57%, 1.22%, 1.14%, 0.54%, 3.26% and 0.06%, respectively. The incidences of anemia and prolonged pregnancy were significantly lower in tertiary than secondary hospitals (P < 0.001), whereas the incidence of uterine fibroids, thyroid diseases, thrombocytopenia, heart disease, PROM, preterm birth, HDCP, multiple pregnancy, ICP, placenta previa, and placental abruption were significantly higher in tertiary than secondary hospitals (P < 0.001). The cesarean section (CS) rate was 54.77%. The newborn sex ratio was 119:100, and 1.03% of the neonates were malformed. The percentages of low birth weight and fetal macrosomia in full-term babies were 2.10% and 7.09%, respectively.
Conclusions:
The incidence of some obstetrical diseases is still high in the mainland of China. The CS rate is much higher than World Health Organization recommendations, in which CS delivery by maternal request (CDMR) accounted for a large proportion. The government should propose solutions to reduce CS rate, especially the rate of CDMR. Most obstetrical complications have higher incidence in tertiary hospitals compared with secondary hospitals. It is important to manage the health of pregnant women systematically, especially those with high-risk factors.
Keywords: Disease Spectrum, Perinatal Outcomes, Pregnancy Complications
INTRODUCTION
Although there have been reports on the maternal and fetal complications and outcomes in some separate provinces or cities of the mainland of China, no national research has been carried out, thus it is difficult for health administration departments to obtain data on the national rates of obstetrical disease. Consequently, a study of the obstetric disease epidemiology in the mainland of China is important for health administration departments to devise policies for the control of obstetrical and neonatal diseases. In 2011, 16 million babies were born in the mainland of China.[1] In this study, we collected and analyzed 112,441 obstetrical cases during the period January 1, 2011 to December 31, 2011 from 19 tertiary and 20 secondary hospitals in 14 provinces (nationally representative) to study the obstetrical disease spectrum in China.
Disease epidemiology can reflect healthcare development and societal demand for healthcare. The obstetrical disease spectrum will change greatly with economic development and improvement in living standards, and will vary according to hospital grade. This study reflects the morbidity and geographical differences of the common obstetrical diseases in China. It provides a reliable basis for health administrative departments to devise policies to control obstetrical and neonatal diseases, and to allow better allocation of medical resources.
METHODS
Subjects
This study was a multi-center, large-sample, cross-sectional study performed in the mainland of China. In this study, 112,441 obstetrical cases from 39 hospitals in 14 provinces, municipalities and autonomous regions (Shanghai, Jilin, Liaoning, Jiangsu, Sichuan, Beijing, Shanxi, Hebei, Shaanxi, Hubei, Guangdong, Inner Mongolia, Shandong, and Xinjiang) of the seven regions in the mainland of China were retrospectively analyzed. The hospitals comprised 12 tertiary general hospitals, seven tertiary specialized hospitals, eight secondary general hospitals and 12 secondary specialized hospitals. The aforementioned hospitals were required to sign an agreement that they would inform us of all the obstetrical cases throughout 2011.
Information about the cases was recorded, including general condition, medical history, previous pregnancy history, medical and surgical complications (anemia, uterine fibroids, thyroid disease, thrombocytopenia, heart disease, etc.), obstetrical complications (premature rupture of membranes [PROM], preterm labor, prolonged pregnancy, hypertensive disorders complicating pregnancy [HDCP], multiple pregnancy, intrahepatic cholestasis, placenta previa, placental abruption, etc.), delivery mode, and maternal and neonatal outcomes. To ensure patient privacy, names, phone numbers, and home addresses of the mothers were not recorded.
Of the 112,441 cases collected, 674 cases were excluded because of abortion before 28 weeks’ gestation, labor induction for reasons of fetal malformations, occurrence of intrauterine fetal death, and incomplete information. Thus, a total of 111,767 cases with complete information were included, of which 79,418 were delivered in tertiary hospitals and 32,349 in secondary hospitals. The data are presented in Figure 1.
Figure 1.
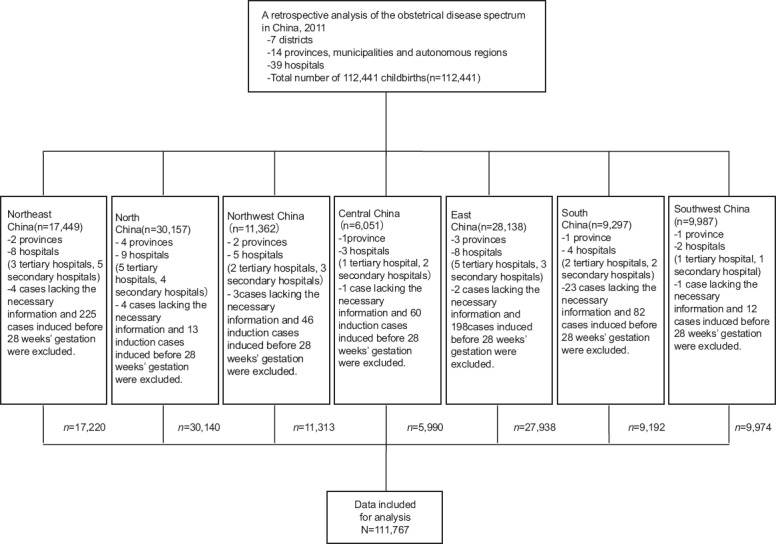
A retrospective analysis performed on 112,441 childbirths in 39 hospitals of different grades in the mainland of China.
Investigative methods
Questionnaire
The questionnaire included the mother's age, ethnic group, educational level, marital status, employment status, medical history, parity, pregnancy history, pregnancy comorbidities and complications, method of delivery, and maternal and neonatal outcomes. Obstetric experts and statistical experts together finalized the questionnaire after repeated discussion with regard to its feasibility and accordance with ethical standards.
Training of investigators
The sub-centers of every province/municipality/autonomous region sent 2–3 inspectors and the person with overall responsibility for their study to the investigation center to receive training in questionnaire completion and data entry.
Training of data-entry clerks
Inspectors from every province/municipality/autonomous region trained their own data-entry clerks. The data were first recorded on a paper form, and then entered into a computer and uploaded to a database.
Quality control
One or two people who had been trained uniformly were in charge of the initial quality control in every province/municipality/autonomous region. After the data had been uploaded to the database, the specialized staff in the investigation center performed the second quality control process.
Classification of diseases
The International Classification of Diseases, 10th version, Clinical Modification (ICD-10-CM) was used to classify diseases statistically. The ICD-10-CM codes for diseases in this study ranged from 000.001 to 099.853.
Data collection
Data were first collected from inpatients’ medical records and entered onto the paper forms from January to April 2012. The data were then entered into computers and uploaded to the network database from May to June 2012. In the meantime, quality control was conducted. Each participant hospital was responsible for its own case collection and data-entry.
Diagnosis of relevant complications
Preterm birth
Preterm birth was defined as birth after 28 weeks (≥196 days) and before 37 completed weeks (≤258 days) of gestation.[2] Last menstrual period (LMP) was used to calculate gestational age. If a woman had irregular menstruation or her LMP was unknown, the fetal size in early pregnancy, and the fetal size and growth rate in mid-trimester, as measured by ultrasound, were used together to calculate gestational age.
Perinatal period
Perinatal period was defined as the period after birth of an infant born after 28 weeks and ending at 7 days after birth. When perinatal rates were based on birth weight, rather than gestational age, it was recommended[2] that the perinatal period be defined as commencing at 1000 g.
Diagnosis of medical and surgical complications (anemia, uterine fibroids, thyroid disease, intrahepatic cholestasis, thrombocytopenia, heart disease, and reproductive tract malformations) and of obstetrical complications (PROM prolonged pregnancy, HDCP, multiple pregnancy, placenta previa, and placental abruption) was performed in accordance with the standards in the 23rd edition of Williams Obstetrics.
Statistical analysis
Statistical analysis was performed using SPSS software (version 16.0; SPSS Inc., Chicago, IL, USA). The Chi-square test and Fisher's exact test were used to analyze enumeration data as appropriate. P <0.05 was considered statistically significant.
Ethics statement
The study was approved by the Institutional Review Boards of the Beijing Obstetrics and Gynecology Hospital and all the other participating hospitals.
RESULTS
Subjects
Maternal age
Age records were available for 110,919 of 111,767 pregnant women, and these were divided into six groups: ≤17 years (0.17%), 18–24 years (22.94%), 25–29 years (42.07%), 30–34 years (24.76%), 35–39 years (8.29%), and ≥40 years (1.77%). The minimum and maximum ages of delivery were 14 and 54 years, respectively. The results showed that maternal age at delivery in the mainland of China fell mostly in the range of 25–29 years. The peak maternal ages of delivery were 25–29 years in tertiary hospitals and 18–24 years in secondary hospitals. The constituent ratio of numbers of pregnant women in tertiary hospitals is significantly different from that in secondary hospitals (P < 0.001). The constituent ratio of numbers of pregnant women whose ages at delivery were < 24 years was significantly lower in tertiary than secondary hospitals (P < 0.001), whereas the constituent ratio of numbers of pregnant women whose ages at delivery were between 25 and 40 years was significantly higher in tertiary than secondary hospitals (P < 0.001). There was no significant difference between tertiary and secondary hospitals in the constituent ratios of pregnant women whose delivery age was >40.
Parity
Primiparas accounted for 81.12% of pregnant women while multiparas accounted for 18.88%, of whom 10.80% had delivered three times or more. The proportion of primiparas in tertiary hospitals is significantly higher than that in secondary hospitals (P < 0.001).
Blood type distribution
The A-B-O and the Rh blood type systems were used to analyze the blood type distribution of the cases. Of the 111,767 cases of pregnant women, 111,072 had blood type recorded. For the A-B-O blood type system, types A, B, O, and AB accounted for 36.17%, 27.18%, 27.75%, and 8.92%, respectively. For the Rh blood type system, Rh-negative accounted for 1.11%. The proportion of pregnant women with Rh-negative blood type in tertiary hospitals is significantly higher than that in secondary hospitals (P < 0.001).
Hepatitis B virus infection
The number of cases with records of hepatitis B surface antigen (HBsAg) was 111,092. In the cases with records of HBsAg, 3717 (3.35%) cases were HBsAg-positive. HBsAg seroprevalence was 3.49% in tertiary hospitals which is significantly higher than that in secondary hospitals (3.02%) (P < 0.001).
Mode of conception
The data showed that women who conceived naturally and who conceived by assisted reproductive technology (ART) accounted for 98.99% and 1.01%, respectively. The proportion of women who conceived by ART in tertiary hospitals is significantly higher than that in secondary hospitals (P < 0.001). For the women who conceived by ART, the cesarean section (CS) rate was 85.61%. The proportion of cases that conceived by ART and delivered in tertiary hospitals was 98.94% while only 1.06% delivered in secondary hospitals.
Data of maternal age, parity, blood type, carrying of HBsAg, and mode of conception are shown in Table 1.
Table 1.
Maternal characteristics, by hospital grade
| Characteristic | Total n (%) | Tertiary hospitals n (%) | Secondary hospitals n (%) | Statistics | P |
|---|---|---|---|---|---|
| Age (years) | 110,919 (100.00) | 78,885 (100.00) | 32,034 (100.00) | ||
| ≤17 | 194 (0.17) | 96 (0.12) | 98 (0.31) | −69.921 | <0.001 |
| 18–24 | 25,449 (22.94) | 12,803 (16.23) | 12,646 (39.47) | ||
| 25–29 | 46,659 (42.07) | 35,416 (44.90) | 11,243 (35.10) | ||
| 30–34 | 27,466 (24.76) | 22,010 (27.90) | 5456 (17.03) | ||
| 35–39 | 9192 (8.29) | 7071 (8.96) | 2121 (6.62) | ||
| ≥40 | 1959 (1.77) | 1489 (1.89) | 470 (1.47) | ||
| Number of deliveries | 111,767 (100.00) | 79,418 (100.00) | 32,349 (100.00) | ||
| Primiparas | 90,665 (81.12) | 66,643 (83.91) | 24,022 (74.26) | 1399.16 | <0.001 |
| Multiparas | 21,102 (18.88) | 12,775 (16.09) | 8327 (25.74) | ||
| 2 deliveries | 18,975 (16.98) | 11,409 (14.37) | 7566 (23.39) | ||
| ≥3 deliveries | 2127 (1.903) | 1366 (1.72) | 761 (2.35) | ||
| Blood type | 111,072 (100.00) | 78,811 (100.00) | 32,261 (100.00) | ||
| A-B-O blood system | |||||
| Type A | 40,171 (36.17) | 26,410 (33.51) | 13,761 (42.66) | 905.40 | <0.001 |
| Type B | 30,174 (27.18) | 22,218 (28.19) | 7956 (24.66) | ||
| Type 0 | 30,819 (27.75) | 23,179 (29.41) | 7640 (23.68) | ||
| Type AB | 9908 (8.92) | 7004 (8.89) | 2904 (9.00) | ||
| Rh blood system | |||||
| Rh-positive | 109,838 (98.89) | 77,637 (98.51) | 32,201 (99.81) | 119.86 | <0.001 |
| Rh-negative | 1234 (1.11) | 1048 (1.49) | 186 (0.19) | ||
| HBsAg | 111,092 (100.00) | 78,897 (100.00) | 32,195 (100.00) | ||
| Positive | 3717 (3.35) | 2826 (3.58) | 891 (2.77) | 46.89 | <0.001 |
| Negative | 107,375 (96.65) | 76,071 (96.42) | 31,304 (97.23) | ||
| Conception mode | 111,767 (100.00) | 79,418 (100.00) | 32,349 (100.00) | ||
| Natural | 110,640 (98.99) | 78,303 (98.60) | 32,337 (99.96) | 430.24 | <0.001 |
| ART | 1127 (1.01) | 1115 (1.40) | 12 (0.04) |
HBsAg: Hepatitis B surface antigen; ART: Assisted reproductive technology.
Medical and surgical complications
The data showed that the incidence of medical and surgical illnesses complicating pregnancy was 11.72%. The top five medical and surgical complications of pregnant women in the mainland of China were anemia (7087 cases, 6.34%), uterine fibroids (3007 cases, 2.69%), thyroid diseases (1241 cases, 1.11%), thrombocytopenia (660 cases, 0.59%), and heart disease (654 cases, 0.59%). The incidence of anemia in pregnant women was significantly lower in tertiary than secondary hospitals (P < 0.001), and the incidence of uterine fibroids, thyroid diseases, thrombocytopenia, and heart disease was significantly higher in tertiary than secondary hospitals (P < 0.001) [Table 2].
Table 2.
Medical and surgical complications, by hospital grade
| Complications | Total n (%) | Tertiary hospitals n (%) | Secondary hospitals n (%) | Statistics | P |
|---|---|---|---|---|---|
| Anemia | 7087 (6.34) | 4297 (5.41) | 2790 (8.62) | 399.83 | <0.001 |
| Uterine fibroids | 3002 (2.69) | 2772 (3.49) | 230 (0.71) | 679.35 | <0.001 |
| Thyroid diseases | 1241 (1.11) | 1208 (1.52) | 33 (0.10) | 421.56 | <0.001 |
| Thrombocytopenia | 660 (0.59) | 627 (0.79) | 33 (0.10) | 185.07 | <0.001 |
| Heart disease | 654 (0.59) | 628 (0.79) | 26 (0.08) | 199.40 | <0.001 |
Obstetrical complications
In this study, 43,487 (38.67%) cases had one or more obstetrical complications. The incidence of PROM was 15.27%, and the incidence of preterm PROM (PPROM) was 2.75%. There were 1751 cases of multiple pregnancy, in which 1694 cases were twin pregnancies, 55 triplet pregnancies, and two quadruplet pregnancies. Of these, 301 (17.77%) cases of twin pregnancy and seven (12.5%) of the triplet pregnancy were conceived by ART. The incidence of preterm birth, prolonged pregnancy, HDCP, multiple pregnancy, intrahepatic cholestasis of pregnancy (ICP), placenta previa, and placental abruption was 7.04%, 6.71%, 5.35%, 1.57%, 1.22%, 1.14%, and 0.54%, respectively. The incidence of prolonged pregnancy was significantly lower in tertiary than secondary hospitals (P < 0.001), and the incidence of PROM, preterm birth, HDCP, multiple pregnancy, ICP, placenta previa, and placental abruption was significantly higher in tertiary than secondary hospitals (P < 0.001) [Table 3].
Table 3.
Obstetrical complications, by hospital grade
| Complications | Total n (%) | Tertiary hospitals n (%) | Secondary hospitals n (%) | Statistics | P |
|---|---|---|---|---|---|
| PROM | 17,064 (15.27) | 13,954 (17.57) | 3110 (9.61) | 1124.82 | <0.001 |
| Preterm birth | 7872 (7.04) | 7126 (8.97) | 746 (2.31) | 1560.39 | <0.001 |
| Prolonged pregnancy | 7501 (6.71) | 5049 (6.36) | 2452 (7.58) | 54.85 | <0.001 |
| HDCP | 5982 (5.35) | 5122 (6.45) | 860 (2.66) | 652.09 | <0.001 |
| Multiple pregnancy | 1751 (1.57) | 1626 (2.05) | 125 (0.39) | 411.23 | <0.001 |
| ICP | 1276 (1.14) | 1102 (1.39) | 174 (0.54) | 147.05 | <0.001 |
| Placenta previa | 1367 (1.22) | 1228 (1.55) | 139 (0.43) | 237.20 | <0.001 |
| Placenta abruption | 607 (0.54) | 540 (0.68) | 67 (0.21) | 95.14 | <0.001 |
PROM: Premature rupture of membranes; HDCP: Hypertensive disorders complicating pregnancy; ICP: Intrahepatic cholestasis of pregnancy.
Gestational outcome
Method of delivery
The data showed that the rate of CS in this study was 54.77%, of which CS delivery by maternal request (CDMR) constituted 31.40%. Among the vaginal delivery cases, the proportion of forceps delivery was 2.08%, assisted breech delivery 0.21%, vacuum extraction traction 0.22%, and buttock traction 0.10%. The rate of CS in tertiary hospitals is significantly higher in tertiary hospitals than that in secondary hospitals (P < 0.001).
Intrapartum and postpartum complications
The data showed that 28,885 (25.84%) cases were complicated by umbilical cord entanglement. In addition, 3639 (3.26%) cases were complicated by postpartum hemorrhage and 65 (0.06%) cases by amniotic fluid embolism. The incidence of postpartum hemorrhage and amniotic fluid embolism in tertiary hospitals is significantly higher than that in secondary hospitals (P < 0.001).
Data of delivery method, intrapartum and postpartum complications are shown in Table 4.
Table 4.
Delivery method, intrapartum and postpartum complications by hospital grade
| Delivery method and complications | Total n (%) | Tertiary hospitals n (%) | Secondary hospitals n (%) | Statistics | P |
|---|---|---|---|---|---|
| All cases | 111,767 (100.00) | 79,418 (100.00) | 32,349 (100.00) | ||
| Delivery method | |||||
| Cesarean section | 61,215 (54.77) | 44,662 (56.24) | 16,553 (51.17) | 238.19 | <0.001 |
| Vaginal delivery | 50,552 (45.23) | 34,756 (43.76) | 15,796 (48.83) | ||
| Forceps delivery | 1053 (0.94) | 998 (1.26) | 55 (0.17) | ||
| Vacuum extraction traction | 111 (0.10) | 82 (0.10) | 29 (0.09) | ||
| Assisted breech delivery | 105 (0.09) | 72 (0.09) | 33 (0.10) | ||
| Buttock traction | 49 (0.04) | 45 (0.06) | 4 (0.01) | ||
| Umbilical cord entanglement | 28,885 (25.84) | 20,547 (25.87) | 8338 (25.78) | 0.11 | 0.737 |
| Postpartum hemorrhage | 3639 (3.26) | 3088 (3.89) | 551 (1.70) | 348.40 | <0.001 |
| Amniotic fluid embolism | 65 (0.06) | 59 (0.07) | 6 (0.02) | 12.29 | <0.001 |
Newborns
In the study, 111,767 pregnant women gave birth to 113,597 children, comprising 61,834 boys and 51,763 girls, giving a ratio of 119:100. Fetuses whose Apgar score at 1 min was <7 accounted for 2.37% of all births. There were 3351 (2.94%) fetuses with neonatal complications. In which pathologic jaundice, neonatal respiratory distress syndrome (NRDS), pneumonia, infections, and birth trauma accounted for 1.17%, 0.39%, 0.12%, 0.08%, and 0.3%, respectively. The neonatal death rate in this study was 0.44%. Of the 103,895 full-term babies, 2159 (2.10%) cases had low birth weight, and 7368 (7.09%) had fetal macrosomia. There were 1175 (1.03%) cases of malformations, of which 46 (0.04%) cases had multiple malformations. The neonatal death rate and proportion of neonates whose Apgar score at 1 min was <3 was significantly higher in tertiary hospitals than that in secondary hospitals, while the proportions of fetuses with pathologic jaundice, NRDS, low birth weight and macrosomia are significantly lower in tertiary hospitals than that in secondary hospitals (P < 0.001). The characteristics of neonates are shown in Table 5.
Table 5.
Characteristics of newborns by hospital grade
| Characteristics | Total n (%) | Tertiary hospitals n (%) | Secondary hospitals n (%) | Statistics | P |
|---|---|---|---|---|---|
| All babies | 113,597 (100.00) | 81,100 (100.00) | 32,497 (100.00) | ||
| Sex | |||||
| Male | 61,834 (54.43) | 43,956 (54.20) | 17,878 (55.01) | 6.21 | 0.013 |
| Female | 51,763 (45.57) | 37,144 (45.80) | 14,619 (44.99) | ||
| Apgar score at 1 min | |||||
| 1–3 | 421 (0.37) | 370 (0.46) | 51 (0.16) | 78.43 | <0.001 |
| 4–7 | 2 272 (2.00) | 1721 (2.12) | 551 (1.70) | ||
| 8–10 | 110,904 (97.63) | 79,009 (97.42) | 31,895 (98.15) | ||
| Neonatal death | 504 (0.44) | 411 (0.51) | 93 (0.29) | 25.56 | <0.001 |
| Neonatal complications | |||||
| None | 110,246 (97.05) | 79,102 (97.54) | 31,144 (95.84) | 23.42 | <0.001 |
| Pathologic jaundice | 1330 (1.17) | 747 (0.92) | 583 (1.79) | 152.79 | <0.001 |
| NRDS | 439 (0.39) | 283 (0.35) | 156 (0.48) | 10.36 | 0.001 |
| Pneumonia | 212 (0.12) | 156 (0.19) | 56 (0.17) | 0.500 | 0.480 |
| Infection | 88 (0.08) | 69 (0.09) | 19 (0.06) | 2.12 | 0.145 |
| Birth trauma | 34 (0.03) | 21 (0.03) | 13 (0.04) | 1.54 | 0.214 |
| Others | 1336 (1.18) | 792 (0.98) | 544 (1.67) | 97.10 | <0.001 |
| Birth weight for full-term infants | 103,895 (100.00) | 71,660 (100.00) | 32,235 (100.00) | ||
| Low birth weight | 2159 (2.08) | 1258 (1.76) | 901 (2.80) | 292.94 | <0.001 |
| Appropriate for gestational age | 94,368 (90.83) | 65,809 (91.84) | 28,559 (88.60) | ||
| Fetal macrosomia | 7368 (7.09) | 4593 (6.41) | 2775 (8.61) | ||
| Presence of malformations | 113,895 (100.000) | 81,100 (100.000) | 32,497 (100.000) | ||
| None | 112,422 (98.707) | 80,125 (98.798) | 32,297 (99.380) | 78.04 | <0.001 |
| Chromosome abnormality | 68 (0.060) | 59 (0.073) | 9 (0.028) | 7.87 | 0.005 |
| Neural tube defect | 22 (0.019) | 20 (0.025) | 2 (0.006) | 4.10 | 0.043 |
| Congenital heart disease | 207 (0.182) | 149 (0.184) | 58 (0.178) | 0.04 | 0.851 |
| Cheilopalatognathus | 79 (0.070) | 71 (0.088) | 8 (0.025) | 13.22 | <0.001 |
| Microtia/anotia | 24 (0.021) | 17 (0.021) | 7 (0.022) | 0.00 | 0.952 |
| Esophageal stenosis/atresia | 17 (0.015) | 15 (0.018) | 2 (0.006) | 2.36 | 0.124 |
| Imperforate anus | 19 (0.017) | 16 (0.020) | 3 (0.009) | 1.53 | 0.216 |
| Hypospadia | 22 (0.019) | 18 (0.022) | 4 (0.012) | 1.17 | 0.279 |
| Exstrophy of bladder | 7 (0.006) | 4 (0.005) | 3 (0.009) | - | 0.416 |
| Talipesequinovarus | 28 (0.025) | 23 (0.028) | 5 (0.015) | - | 0.208 |
| Polydactyly | 64 (0.056) | 47 (0.058) | 17 (0.052) | - | 0.717 |
| Syndactylia | 16 (0.014) | 12 (0.015) | 4 (0.012) | - | 1.000 |
| Limb shortening | 11 (0.010) | 10 (0.012) | 1 (0.003) | - | 0.196 |
| Congenital diaphragmatic hernia | 9 (0.008) | 9 (0.011) | 0 (0.000) | - | 0.068 |
| Acromphalus | 1 (0.001) | 1 (0.001) | 0 (0.000) | - | 1.000 |
| Gastroschisis | 5 (0.004) | 5 (0.006) | 0 (0.000) | - | 0.331 |
| Others | 649 (0.571) | 579 (0.714) | 70 (0.215) | 23.20 | <0.001 |
NRDS: Neonatal respiratory distress syndrome.
DISCUSSION
Geographical differences can reflect some environmental risk factors for disease, but studies based only on geographical differences do not consider that hospital could influence the incidence of diseases. In this study, an epidemic observation was carried out on 111,767 obstetrical cases throughout the mainland of China, and the following points are highlighted as noteworthy.
Maternal age
In this study, women aged 25–29 years (considered the prime childbearing age) formed the majority of mothers, which is consistent with data over the same period in America (28.5%).[3] The proportions of younger teenage (≤17 years) pregnancy in the mainland of China (0.33%) was much lower than that in America (2.517%).[3] and 10.05% of cases were <40 years old, which is also lower than that in America (14.679%).[3]
Studies show that teenage pregnancy and advanced maternal age are associated with increased risk of adverse pregnancy outcomes.[4,5] In China, a developing country, the main risk factors for teenage pregnancy include poverty, early marriage, low level of education, and low level of contraceptive use. Chinese people hold conservative views on sexual morality which leads to poor sexual health education in China. A survey of 440 college students shows that 47% had received no school-based education.[6] Lack of sexual knowledge leads to unprotected intercourse and unwanted pregnancy. Most teenage pregnancies in China are terminated. Official statistics[1] suggest that there were 6,361,539 cases of induced abortions in China in 2011, which did not include cases performed by mobile clinics, and 10 million abortion pills are reportedly sold each year. Thus, the incidence of teenage pregnancy could be much higher than the figure got out by this study (0.33%).
The proportion of women of advanced maternal age was 10.05%, which was higher than that in 2007 (8.56%).[7] A retrospective analysis of 6,308,594 parturient women between 1996 and 2007 showed that the proportion of advanced maternal women had a secular increasing trend.[8] This trend has also been observed in America[9,10] and England.[11] The Chinese government instituted a policy in 2014 to allow more than one child when one or both of the parents come from a single-child family. Predictably, with this relaxation of “one-child policy,” the incidence of advanced maternal age will rise further. To reduce the incidence of complications associated with younger and advanced maternal age, health administrative departments should build an effective monitoring system for pregnant women to ensure that all women of younger and advanced maternal age are systematically managed, and receive effective and accurate prenatal screening and diagnosis.
Hepatitis B virus infection
Mother to child transmission is the main route of transmission of hepatitis B virus (HBV). However, maternal HBV infection is often asymptomatic and found only by routine prenatal screening. Therefore, it is necessary to screen pregnant women routinely for HBsAg. The American College of Obstetricians and Gynecologists and the Centers for Disease Control and Prevention recommend prenatal screening of all pregnant women for HBV.[12,13] With early detection, doctors are able to carry out effective health interventions to keep the neonates of HBsAg-positive women free from HBV infection. Administration of HBV vaccine and hepatitis B immunoglobulin (HBIG) within 12–24 h after birth, followed by completion of a three-dose vaccine series, has been shown to be 89–98% effective in preventing acute and chronic HBV infection in infants born to women positive for both HBsAg and HBeAg.[14] In fact, after the HBV vaccine was recommended in China, the prevalence of HBsAg in the population aged 1–59 years decreased from 9.8% in 1992–7.2% in 2006, and to 1.0% for children under 5 years in 2006, 90% lower than that in 1992 (9.7%),[15] which may be ascribed to the Chinese government ensuring that all infant vaccinations were provided free of charge. Therefore, to reduce the incidence of vertical transmission further, pregnant women with risk factors for HBV positivity should be educated about related knowledge of HBV infection and systematically managed in order to provide effective health intervention during pregnancy, and ensure that HBIG combined with HBV vaccine injection is administered to at-risk neonates after birth.
Mode of delivery
The rate of CS has been increasing worldwide. One study showed that the rate has risen from 14.9% in 1993–54.1% in 2008 in China.[16] With the development of perinatology and the improvement in living standards, increasing numbers of Chinese women are choosing to deliver by CS without any medical indication for the procedure. Many studies have shown an inverse association between CS rates and maternal and infant mortality when CS rates fall below a certain level, whereas higher CS rates do not confer additional health gain but may increase maternal risks, have implications for future pregnancies, and have resource implications for health services.[17,18,19,20,21] In 1985, the World Health Organization (WHO) stated: “There is no justification for any region to have CS rates higher than 10–15%.”[22] The CS rate in this study was 54.60%, much higher than the WHO recommendations. Studies have showed that the highest indication of CS in China was CDMR, deliver by CS without medical necessities.[23,24,25,26] In this study, CDMR accounted for 31.40% among the cases delivered by CS. So why do so many women choose to deliver by CS without any maternal or fetal indications? With analyzing the reasons, it can be summarized as the following three ones. First, a common belief in Chinese society is that CS is safer, faster, and less painful than vaginal delivery.[27] There is a general perception that CS is much safer now than in the past. Second, some mothers choose CS with concerns about their subsequent living standard because they believe CS is less likely to affect the quality of sexual life and they are more likely to regain their prepregnancy shape after giving birth by CS than vaginal delivery. Finally, changes in obstetric management and the increasing autonomy of patients in deciding the mode of delivery may contribute to the increasing rate of CDMR.[28] China has such a high level of CS rate and CDMR rate, thus, it is essential to reduce the CS rates. Many researchers have studied the risk factors behind the rising CS rate in China, and proposed some solutions to reduce CS rate: (1) Provision of more widespread prenatal tests, and correct guidance about nutrition and exercise in pregnancy to reduce high-risk pregnancies; (2) Provision of knowledge to the general public about the advantages of vaginal delivery to reduce unnecessary CS by maternal request; (3) Improvement of the midwifery level of midwives and obstetrical doctors to deal with labor correctly; and (4) Action by the government to improve the doctor-patient relationship and thus alleviate medical workers’ concerns about medical disputes.
Pregnancy complications
No comprehensive data on the incidence of pregnancy complications in the mainland of China have been reported up to now. We reviewed the literature on obstetrical complications for comparison with the current study.
Premature rupture of membranes and preterm birth
According to a number of literature resources, PROM occurs in 5–10% of pregnancies.[29] Based on the current study, PROM is the most common pregnancy complication in the mainland of China. The total incidence of PROM was 15.27%: 13.46% in full-term delivery and 39.10% in preterm delivery. The incidence of preterm birth was 7.04%, which is lower than the worldwide incidence (11.1%[30]). This difference may be related to the different definition of the perinatal period for various regions of the world. Data from developed countries suggested an incidence of PROM of 1–3% of all pregnancies,[31] which is much lower than that seen in the current study. The incidence of PROM has been suggested to be higher in developing countries (8.09% in Baroda, India[32] and 6.91% in Tehran, Iran[28]). Studies have shown that PROM increases the risk of maternal and neonatal complications, and the risk factors for PROM include intrauterine infection at early gestational age, lower socioeconomic status of the pregnant woman, inadequate prenatal care and inadequate nutrition during pregnancy, and presence of sexually transmitted infections, vaginal bleeding, and smoking during pregnancy.[33,34] PPROM is responsible for approximately one-third of all preterm births,[31] so it is necessary to popularize prepregnancy checks and pregnancy guide and management in the mainland of China.
Hypertensive disorders complicating pregnancy
Hypertensive disorders of pregnancy affect 5–10% of pregnancies and remain a major driver of adverse maternal and neonatal outcomes, mainly associated with intrauterine growth restriction and iatrogenic prematurity.[35,36] The reported incidence of HDCP varies greatly. In the current study, we found that the incidence of HDCP in the mainland of China was 5.35%, which is the same as that reported by WHO in 2000 (5.35%).[37] Studies have shown that HDCP increases maternal and neonatal morbidity and mortality.[36,38,39] Risk factors for HDCP include nulliparity, maternal age > 35 or < 20 years, family history of pregnancy-induced hypertension (PIH), PIH in previous pregnancy, maternal obesity, and medical conditions including chronic hypertension, gestational diabetes, Type 1 diabetes, and renal disease.[40] A monitoring system for systematically managing HDCP could enable early detection and treatment of HDCP complications, which might greatly improve maternal and neonatal outcomes in patients with HDCP.
Multiple pregnancies
The risk of various obstetrical complications and adverse pregnancy outcomes is higher in multiple than singleton pregnancies, so multiple pregnancy is considered to be a high-risk pregnancy. In recent years, with widespread use of ART and ovulation-inducing drugs, the incidence of multiple pregnancy has increased significantly in developed countries. The incidence of multiple pregnancy in England increased from 1% in 1980 to 1.6% in 2009[41] and the birth data from America showed that the incidence of twin pregnancy increased from 1.89% in 1980 to 3.32% in 2011.[3] A large-sample test survey showed that the incidence of twin pregnancy in China was 1.0–1.2%.[42] In the current study, we found that the incidence of multiple pregnancy in the mainland of China was 1.57%, which is higher than in the past. The data showed that 17.77% of twin pregnancies and 12.5% of triplet pregnancies were conceived by ART; these percentages were 19.2% and 32.5%, respectively, in America.[43] Predictably, with increasing numbers of women of advanced maternal age, widespread use of infertility treatment and ART, and people's perception that having two or more children reduces the physical and economic burden, the incidence of multiple pregnancy is likely to continue to rise in the future. To reduce the risk of adverse pregnancy outcomes from multiple pregnancy, there are some points to be emphasized. First, the risk of multiple pregnancy should be explained in detail to couples that seek infertility treatment. To reduce the risks of multiple pregnancy, especially of triplet or greater pregnancy, the indications for ovulation-inducing drugs should be strictly controlled, the number of blastocysts implanted in the uterus should be reduced, and fetal reduction should be conducted in a timely fashion. For cases that are too late for fetal reduction, more frequent prenatal care, appropriate mode of delivery, and timely termination of pregnancy are recommended.
Obstetrical disease spectrum in different grades of hospitals
Tertiary hospitals are better able to deal with serious problems than secondary hospitals. The standardized extent of prenatal care was different in the different grades of hospitals. The results showed that the incidence of anemia and prolonged pregnancy complicating pregnancies was significantly lower in tertiary than secondary hospitals, whereas the incidence of other complications was significantly higher in tertiary than secondary hospitals. These differences could be attributable to the following points. First, women who receive prenatal care in tertiary hospitals may acquire better knowledge about the importance of prenatal care and thus are more likely to cooperate in consultations and in visiting their obstetrician more regularly. This is important for early discovery and treatment of pregnancy complications and timely termination of pregnancy. Thus, pregnancies complicated by anemia could be corrected by nutrition guidance and iron supplementation in tertiary hospitals. Second, a large proportion of high-risk pregnant women were transferred to tertiary hospitals because of the referral system in China, which resulted in the incidence of most pregnancy complications being significantly higher in tertiary than secondary hospitals. The main tasks of secondary hospitals are to offer healthcare for normal pregnancies, and thus the staffs are likely to have limited knowledge of obstetrical diseases. Therefore, obstetricians, especially those in secondary hospitals, should be formally trained to implement the guide of prenatal care more normatively and deal with complications of pregnancy. Moreover, obstetricians and the public should be acquainted with the importance of regular prenatal care for early discovery and treatment of pregnancy complications.
Study limitations
A significant proportion of gestational diabetes mellitus (GDM) data were missing because of differences in the criterion for GDM used in China in 2011.
In conclusion, this study is the first national large-sample study of obstetrical cases in the mainland of China and reveals some demographic characteristics of obstetrical cases. The incidence of some obstetrical diseases is still high in the mainland of China. The CS rate is much higher than WHO recommendations, in which CDMR accounted for a large proportion. The government should propose solutions to reduce CS rate, especially the rate of CDMR. Most obstetrical complications have higher incidence in tertiary hospitals than secondary hospitals. It is important to manage the health of pregnant women systematically, especially those with high-risk factors.
ACKNOWLEDGMENTS
We thank the medical staff involved in this survey at the following hospitals: Capital Medical University Beijing Obstetrics and Gynecology Hospital, Capital Medical University Friendship Hospital, Obstetrics and Gynecology Hospital of Fudan University, First Affiliated Hospital of Medical College of Xi’an Jiaotong University, Nanjing Drum Tower Hospital, Shandong Provincial Hospital, West China Second University Hospital, Shengjing Hospital of China Medical University, Nanfang Hospital of Nanfang Medical University, the Second Hospital of Jilin University, the First Affiliated Hospital of Xinjiang Medical University, Hebei Cangzhou Central Hospital, the First Affiliated Hospital of Inner Mongolia Medical University, Hubei Xinhua Hospital, Beijing Daxing Maternal and Child Care Hospital, Tongzhou Maternal and Child Health Hospital of Beijing, Shanghai Changning Maternity and Infant Health Hospital, Shanghai Putuo Maternity and Infant Health Hospital, Shaanxi Zichang Hospital, Chenggu Maternal and Child Health Hospital, Xi’an Aerospace General Hospital, Wuxi Maternal and Child Health Hospital, Shandong Obstetrics and Gynecology Hospital, Dongming Maternal and Child Health Hospital of Heze, Pengzhou Maternal and Child Health Hospital, Benxi Center Hospital of Liaoning, Women and Infants Hospital of Liaohe Oilfield, Kaiyuan Hospital of Liaoning, Youyuan Maternal and Child Health Hospital, Wuhan No. 11 Hospital, The Third Affiliated Hospital of Southern Medical University, Shaoguan Hygienic Hospital of Women and Children, Foshan Maternity and Child Health Hospital, Nong’an People's Hospital of Jilin, Yushu Maternity and Child Health Hospital of Jilin Medical University, Maternal and Child Health Hospital of Inner Mongolia, Erenhot Hospital of Inner Mongolia, Taiyuan Maternity and Child Health Hospital.
Footnotes
Edited by: Li-Shao Guo
Source of Support: This project was supported by a grant from Special Health Industry Funds from the Public Benefit Research Foundation at the Ministry of Health, China (No. 201002013).
Conflict of Interest: None declared.
REFERENCES
- 1.Beijing: Union Medical University Press; 2012. China. China Health Statistical Yearbook 2011. [Google Scholar]
- 2.Zhang JP. Preterm birth. In: Xing X, Wenli G, editors. Gynecology and Obstetrics. 8th ed. Beijing: People's Medical Publishing House; 2013. p. 59. [Google Scholar]
- 3.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Mathews TJ. Births: Final data for 2011. Natl Vital Stat Rep. 2013;62:1–69. 72. [PubMed] [Google Scholar]
- 4.Chen XK, Wen SW, Fleming N, Demissie K, Rhoads GG, Walker M. Teenage pregnancy and adverse birth outcomes: A large population based retrospective cohort study. Int J Epidemiol. 2007;36:368–73. doi: 10.1093/ije/dyl284. [DOI] [PubMed] [Google Scholar]
- 5.Finlay JE, Özaltin E, Canning D. The association of maternal age with infant mortality, child anthropometric failure, diarrhoea and anaemia for first births: Evidence from 55 low- and middle-income countries. BMJ Open. 2011;1:e000226. doi: 10.1136/bmjopen-2011-000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan HT, Farley MM, Craig A, Mohle-Boetani J, Harrison LH, Petit S, et al. Revisiting the need for vaccine prevention of late-onset neonatal group B streptococcal disease: a multistate, population-based analysis. Pediatr Infect Dis J. 2008;27:1057–64. doi: 10.1097/INF.0b013e318180b3b9. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Cottrell RR, Wagner DI, Ban M. Needs and preferences regarding sex education among Chinese college students: A preliminary study. Int Fam Plan Perspect. 2004;30:128–33. doi: 10.1363/3012804. [DOI] [PubMed] [Google Scholar]
- 8.Li YH, Wang YP, Dai L, Zhou GX, Liang J, Li Q, et al. The trend of national advanced maternal age woman proportion in hospital-based surveillance (in Chinese) Chin J Prevent Med. 2009;43:1073–6. [PubMed] [Google Scholar]
- 9.Matthews TJ, Macdorman MF. Infant mortality statistics from the 2007 period linked birthinfant death data set. Natl Vital Stat Rep. 2011;59:1–30. [PubMed] [Google Scholar]
- 10.Osterman MJ, Martin JA, Mathews TJ, Hamilton BE. Expanded data from the new birth certificate, 2008. Natl Vital Stat Rep. 2011;59:1–28. [PubMed] [Google Scholar]
- 11.Stakes. Parturients, Births and Newborns 2007. Statistical Summary 30/2008. National Research and Development Centre for Welfare and Health. 2008 [Google Scholar]
- 12.Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: Immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54:1–31. [PubMed] [Google Scholar]
- 13.Mast EE, Weinbaum CM, Fiore AE, Alter MJ, Bell BP, Finelli L, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: Immunization of adults. MMWR Recomm Rep. 2006;55:1–33. [PubMed] [Google Scholar]
- 14.Greenberg DP. Pediatric experience with recombinant hepatitis B vaccines and relevant safety and immunogenicity studies. Pediatr Infect Dis J. 1993;12:438–45. doi: 10.1097/00006454-199305000-00037. [DOI] [PubMed] [Google Scholar]
- 15.Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et al. Epidemiological serosurvey of hepatitis B in China – declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550–7. doi: 10.1016/j.vaccine.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 16.Feng XL, Xu L, Guo Y, Ronsmans C. Factors influencing rising caesarean section rates in China between 1988 and 2008. Bull World Health Organ. 2012;90:30–9. doi: 10.2471/BLT.11.090399. 39A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Betrán AP, Merialdi M, Lauer JA, Bing-Shun W, Thomas J, Van Look P, et al. Rates of caesarean section: Analysis of global, regional and national estimates. Paediatr Perinat Epidemiol. 2007;21:98–113. doi: 10.1111/j.1365-3016.2007.00786.x. [DOI] [PubMed] [Google Scholar]
- 18.Althabe F, Sosa C, Belizán JM, Gibbons L, Jacquerioz F, Bergel E. Cesarean section rates and maternal and neonatal mortality in low-, medium-, and high-income countries: An ecological study. Birth. 2006;33:270–7. doi: 10.1111/j.1523-536X.2006.00118.x. [DOI] [PubMed] [Google Scholar]
- 19.Belizán JM, Althabe F, Cafferata ML. Health consequences of the increasing caesarean section rates. Epidemiology. 2007;18:485–6. doi: 10.1097/EDE.0b013e318068646a. [DOI] [PubMed] [Google Scholar]
- 20.Wray J. Review of the National Sentinel Caesarean Section Audit Report. Pract Midwife. 2001;4:24–5. [PubMed] [Google Scholar]
- 21.Bick D. National Collaborating Centre for Women's and Children’s Health, National Institute for Clinical Excellence. Caesarean Section. Clinical Guideline. National Collaborating Centre for Women's and Children's Health: Commissioned by the National Institute for Clinical Excellence. Worldviews Evid Based Nurs. 2004;1:198–9. doi: 10.1111/j.1524-475X.2004.04060.x. [DOI] [PubMed] [Google Scholar]
- 22.Appropriate technology for birth. Lancet. 1985;2:436–7. [PubMed] [Google Scholar]
- 23.Hong X. Factors related to the high cesarean section rate and their effects on the “price transparency policy” in Beijing, China. Tohoku J Exp Med. 2007;212:283–98. doi: 10.1620/tjem.212.283. [DOI] [PubMed] [Google Scholar]
- 24.Qin C, Zhou M, Callaghan WM, Posner SF, Zhang J, Berg CJ, et al. Clinical indications and determinants of the rise of cesarean section in three hospitals in rural China. Matern Child Health J. 2012;16:1484–90. doi: 10.1007/s10995-011-0913-7. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Liu Y, Meikle S, Zheng J, Sun W, Li Z. Cesarean delivery on maternal request in southeast China. Obstet Gynecol. 2008;111:1077–82. doi: 10.1097/AOG.0b013e31816e349e. [DOI] [PubMed] [Google Scholar]
- 26.Zhu YB, Li HT, Zhang YL, Li ZW, Zhang L, Liu JM. Secular trends of cesarean delivery and cesarean delivery on maternal request among primiparous women with singleton pregnancy in Southern and Northern China during 1993-2010 (in Chinese) Natl Med J China. 2012;92:1734–7. [PubMed] [Google Scholar]
- 27.Gao Y, Xue Q, Chen G, Stone P, Zhao M, Chen Q. An analysis of the indications for cesarean section in a teaching hospital in China. Eur J Obstet Gynecol Reprod Biol. 2013;170:414–8. doi: 10.1016/j.ejogrb.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Nili F, Ansari AAS. Neonatal complications of premature rupture of membranes. Acta Med Iran. 2003;41:175–9. [Google Scholar]
- 29.Duff P. Premature rupture of the membranes in term patients. Semin Perinatol. 1996;20:401–8. doi: 10.1016/s0146-0005(96)80007-3. [DOI] [PubMed] [Google Scholar]
- 30.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet. 2012;379:2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 31.Mercer BM. Preterm premature rupture of the membranes. In: Vincenzo B, editor. Preterm Birth: Prevention and Management. 5th ed. Oxford: Wiley-Blackwell; 2010. pp. 198–216. [Google Scholar]
- 32.Vaishnav J, Vaishnav G. A study of feto-maternal outcome in patients with prelabour rupture of membranes at term>37 weeks. Med Sci. 2012;1:118. [Google Scholar]
- 33.Dars S, Malik S, Samreen I, Kazi RA. Maternal morbidity and perinatal outcome in preterm premature rupture of membranes before 37 weeks gestation. Pak J Med Sci. 2014;30:626–9. doi: 10.12669/pjms.303.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seelbach-Goebel B. Antibiotic Therapy for premature rupture of membranes and preterm LABOR and effect on fetal outcome. Geburtshilfe Frauenheilkd. 2013;73:1218–1227. doi: 10.1055/s-0033-1360195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sibai BM, Gordon T, Thom E, Caritis SN, Klebanoff M, McNellis D, et al. Risk factors for preeclampsia in healthy nulliparous women: A prospective multicenter study. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1995;172:642–8. doi: 10.1016/0002-9378(95)90586-3. [DOI] [PubMed] [Google Scholar]
- 36.Ehrenthal DB, Jurkovitz C, Hoffman M, Jiang X, Weintraub WS. Prepregnancy body mass index as an independent risk factor for pregnancy-induced hypertension. J Womens Health (Larchmt) 2011;20:67–72. doi: 10.1089/jwh.2010.1970. [DOI] [PubMed] [Google Scholar]
- 37.Dolea C, Abouzahr C. Geneva: World Health Organization; 2003. Global burden of hypertensive disorders of pregnancy in the year 2000. Global Burden of Disease 2000. [Google Scholar]
- 38.Ananth CV, Basso O. Impact of pregnancy-induced hypertension on stillbirth and neonatal mortality. Epidemiology. 2010;21:118–23. doi: 10.1097/EDE.0b013e3181c297af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindheimer MD, Taler SJ, Cunningham FG. Hypertension in pregnancy. J Am Soc Hypertens. 2008;2:484–94. doi: 10.1016/j.jash.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Bulletins – Obstetrics Acog Committee on Practice. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 41.London: National Institute for Health and Care Excellence Online Resources, Inc; 2011. [Last updated on 2014 Jul; Last cited on 2014 Jul 09]. NICE-Org. Packham K. Multiple pregnancy: The management of twin and triplet pregnancies in the antenatal period; pp. 44–7. Available from: http://www.nice.org.uk/guidance/cg129 . [Google Scholar]
- 42.Dai ZY. Multiple pregnancy. In: Zhang XY, editor. Practical Obstetrics and Gynecology. 2nd ed. Beijing: People's Medical Publishing House; 2003. pp. 233–44. [Google Scholar]
- 43.Sunderam S, Kissin DM, Crawford S, Anderson JE, Folger SG, Jamieson DJ, et al. Assisted reproductive technology surveillance – United States, 2010. MMWR Surveill Summ. 2013;62:1–24. [PubMed] [Google Scholar]


