Abstract
Background:
Surgical resection is generally considered the main curative treatment for intrahepatic biliary cystadenocarcinoma (IBCA) or suspected IBCAs, but controversy exists regarding the prognosis for IBCAs. This study aimed to describe the clinicopathological characteristics of IBCA and identify prognostic factors that may influence the survival of patients treated with surgical procedures.
Methods:
Thirty-four patients with histologically confirmed IBCA treated between January 2000 and June 2014 were included. The clinical characteristics of patients with IBCA were compared with those of 41 patients with intrahepatic biliary cystadenoma (IBC); factors that significant difference were analyzed for prognosis analysis of IBCA using multivariate/univariate Cox proportional hazards regression models. Survival curves were constructed using the Kaplan–Meier method and compared using the log-rank test.
Results:
IBCAs had a strong female predominance, and the most common presenting symptoms were abdominal pain or discomfort. Compared with IBCs, IBCAs occurred in older patients, in more male patients, and were associated statistically significant abnormal increase in alanine aminotransferase (P = 0.01) and total bilirubin (P = 0.04). Mural nodules were more frequently seen with IBCAs and may associate with malignancy. It was difficult to differentiate between IBC and IBCA based on laboratory examination and imaging findings. Although complete resection is recommended, enucleation with negative margins also achieved good outcomes. Median overall patient survival was 76.2 months; survival at 1, 3, and 5 years was 88.0%, 68.7%, and 45.8%, respectively. Radical resection and noninvasive tumor type were independent prognostic factors for overall survival.
Conclusions:
It remains difficult to distinguish between cystadenomas and cystadenocarcinomas based on laboratory examination and image findings. Complete resection is recommended for curative treatment, and patients should be closely followed postoperatively, particularly those with invasive tumors.
Keywords: Intrahepatic Biliary Cystadenocarcinoma, Intrahepatic Biliary Cystadenoma, Liver Surgery, Prognosis, Radiologic Findings, Survival
INTRODUCTION
Intrahepatic biliary cystic tumors (IBCTs) are rare cystic liver neoplasms that reportedly represent 5% of all intrahepatic cystic diseases.[1] IBCTs can be divided into two histologic categories: Intrahepatic biliary cystadenoma (IBC) and intrahepatic biliary cystadenocarcinoma (IBCA). With the increasing recognition of IBCTs and advancements in imaging modalities, an increasing number of reports addressing these tumors have been published.[2,3,4] Generally, the diagnosis of IBC is based on specific radiological features, such as multilocular cysts, thickened and irregular walls, internal septa, and mural nodules, and satisfying outcomes can be achieved if complete resections are performed.[3,5] Although the clinical features and prognosis of IBCs have been fully illustrated, few reports have focused on the clinical features and prognosis of IBCAs because of their rarity.[5,6,7]
Surgical resection is generally considered the main curative treatment for IBCAs or suspected IBCAs, but controversy exists regarding the prognosis for IBCAs. Some surgeons have suggested that long-term survival remains unsatisfying even after radical resections and that the prognosis is similar to that associated with cholangiocarcinoma[3,4] while others have reported satisfying outcomes after complete resection (i.e., low mortality and recurrence rates).[8,9] Clinicopathologic features reported to be associated with prognosis include male sex,[2] certain tumor growth types,[10] lymphatic invasion,[11] presence of mesenchymal stroma,[5,12] and surgery type.[3,13] However, these reports are not consistent with each other, and few of them aimed to assess the prognosis and prognostic factors associated with IBCAs.
Accordingly, in this study, we compared the clinicopathologic features of IBCA with those of IBC to identify possible prognostic factors, which were subsequently analyzed along with previously reported prognostic factors using Cox proportional hazards regression models. Our aim was to clarify the factors that influence the survival of patients treated with surgical procedures.
METHODS
Patients with pathologically confirmed IBCA, who underwent surgery between January 2000 and February 2014 at the Department of Hepatobiliary Surgery of the Chinese People's Liberation Army General Hospital, were included in this study. Their demographics, clinical presentations, liver function tests, tumor markers, radiological characteristics, pathological evaluation, surgical information, and outcomes were reviewed and analyzed. Liver function tests included alanine aminotransferase (ALT, normal: 0–40 U/L) and total bilirubin (TBIL, normal: 0–21 μmol/L). Tumor markers included carbohydrate antigen (CA19–9, normal: 0.1–37 U/ml) and carcinoembryonic antigen (CEA, normal: 0–5.0 μg/L).
Because patients with IBC have a better prognosis, we also reviewed the data of 41 IBC patients who underwent surgical resection; we then compared the data between the two patient populations to identify differences. Considering the clinicopathologic variables that a significant difference between patients with IBCA and those with IBC might be closely related to prognosis, such variables were subsequently analyzed using Cox proportional hazards regression models. Also included in this analysis were several variables previously shown to be related to IBCA prognosis: Surgery type, presence of lymphatic invasion, presence of mesenchymal stroma, and tumor growth type.
Follow-up data were collected from clinical records or telephone interviews. The follow-up period was defined as the interval between the operation date and the patient's death or the time of last follow-up. Statistical analyses were conducted using SPSS software (SPSS Inc., Chicago, IL, USA), version 20.0, and differences were considered significant when the P < 0.05. Categorical data were compared using Chi-square analysis or Fisher's exact test; continuous data were compared using Student's t-test and the Wilcoxon signed rank sum test and were further categorized according to cut-off values determined using receiver operating characteristic (ROC) curves. Univariate and multivariate Cox proportional hazards regression models were used to determine associations between clinicopathologic variables and prognosis. Variables that showed a significant difference in univariate analysis were subsequently included in a multivariate analysis. Survival curves were constructed using the Kaplan–Meier method and were compared using the log-rank test.
RESULTS
Patient characteristics
Thirty-four patients with a mean age of 57.5 ± 10.9 years were included in the study. There was a strong female predominance: Totally, 21 patients (61.8%) were women. The most common presenting symptoms were abdominal pain or discomfort (n = 24, 70.6%), and jaundice was observed in nine patients (26.5%). Six patients (17.6%) were asymptomatic; their tumors were accidentally detected on routine physical examination. For symptomatic patients, the median symptom duration was 57.3 ± 85.6 months. The clinical characteristics of patients with IBCA are presented in Table 1. Six patients had undergone previous inappropriate treatments because of misdiagnosis (lesions were diagnosed as simple hepatic cysts or other hepatic cystic lesions), including percutaneous transcatheter drainage (n = 1), laparoscopic fenestration (n = 2), open fenestration (n = 2), internal Roux-en-Y drainage (n = 1), and partial resection (n = 1).
Table 1.
Clinical characteristics of IBC and IBCA patients
| Parameters | IBC subgroup | IBCA subgroup | P |
|---|---|---|---|
| Sex (n) | |||
| Male | 4 | 13 | 0.01* |
| Female | 37 | 21 | |
| Mean age (years) | 45.4 ± 17.0 | 57.5 ± 10.9 | 0.02† |
| Presenting symptoms (n) | |||
| Abdominal pain/discomfort | 28 | 24 | 0.16* |
| Asymptomatic | 14 | 6 | |
| Abdominal mass | 2 | 1 | |
| Weight loss | 9 | 5 | |
| Jaundice/occasional cholangitis | 3 | 9 | |
| Back pain | 1 | 0 | |
| Melena/hematemesis | 2 | 0 | |
| Duration of symptoms (months) | 69.2 ± 88.2 | 57.3 ± 85.6 | 0.12‡ |
| Serum CA19-9 (U/ml) | 193.8 ± 428.7 | 1103 ± 4249.8 | 0.32‡ |
| Serum CEA (ng/ml) | 1.59 ± 0.86 | 18.4 ± 76.3 | 0.36‡ |
| ALT (U/L) | 29.8 ± 29.7 | 88.3 ± 114.2 | 0.01‡ |
| TBIL (μmol/L) | 18.9 ± 15.1 | 28.9 ± 88.4 | 0.04‡ |
| Tumor location (n) | 0.24* | ||
| Left laterality | 21 | 22 | |
| Right laterality | 14 | 6 | |
| Bilateral laterality | 5 | 6 | |
| Tumor size (cm) | 11.7 ± 5.7 | 7.1 ± 4.3 | 0.00† |
| Radiological findings (n) | |||
| Wall (thickened, irregular/thin) | 33/8 | 30/4 | 0.36* |
| Tumor capsule (complete/discontinuity) | 36/5 | 26/8 | 0.19* |
| Internal septa (yes/no) | 29/12 | 26/8 | 0.58* |
| Mural nodules (yes/no) | 26/15 | 29/5 | 0.03* |
| Septal enhancement (yes/no) | 32/9 | 30/4 | 0.25* |
| Calcifications (yes/no) | 6/35 | 7/27 | 0.50* |
| Communication with biliary duct (yes/no) | 8/33 | 7/27 | 0.91* |
| Mesenchymal stroma (yes/no) | 24/17 | 18/16 | 0.63* |
*Chi-square test; †Student’s t-test; ‡Wilcoxon rank sum test. IBC: Intrahepatic biliary cystadenoma; IBCA: Intrahepatic biliary cystadenocarcinoma; CEA: Carcinoembryonic antigen; ALT: Alanine aminotransferase; TBIL: Total bilirubin; CA: Carbohydrate antigen.
Laboratory data were available for 32 patients and revealed normal liver function in 11; obvious liver dysfunction mostly occurred in patients with obstructive jaundice. Tumor marker serum CA19-9 levels were available for all patients and were elevated in 14; the average value was 1103 ± 4249.8 U/ml. A variety of radiological examinations were performed preoperatively, including computed tomography (CT; n = 31), ultrasonography (n = 17), magnetic resonance imaging (n = 4), magnetic resonance cholangiopancreatography (n = 2), and positron emission tomography (n = 2). CT was the most commonly used imaging modality, and the typical findings associated with IBCA included multilocular cysts with thickened and irregular walls (30/34, 88.2%), internal septa (26/34, 76.5%), and mural nodules (29/34, 85.3%). Internal septa and mural nodules showed mild or marked contrast enhancement in most patients (30/34, 88.2%; Figure 1). The mean tumor size was 7.1 ± 4.3 cm; 64.7% of tumors were located in the left liver lobe.
Figure 1.
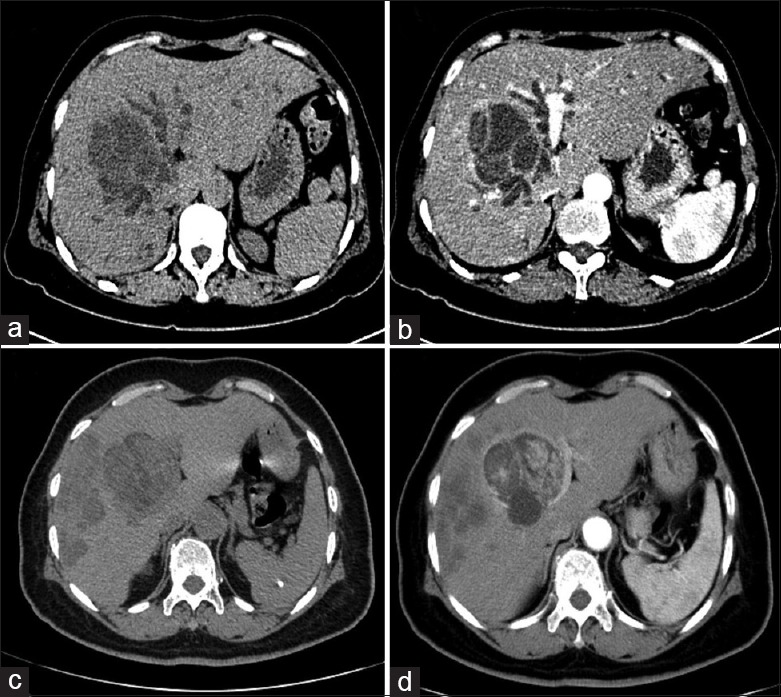
(a) and (b) Computed tomography (CT) shows intrahepatic biliary cystadenoma in liver segment IV with intrahepatic bile duct dilation due to tumor compression; thin internal septa were observed in the tumor; (c) and (d) Intrahepatic biliary cystadenocarcinoma located in the left hepatic lobe with multiple metastases in the right lobe; internal septa and mural nodules showed mild contrast enhancement on venous phase CT.
Surgical procedures
Surgeries were successfully performed in all patients: Laparoscopic enucleation (n = 1), open enucleation (n = 2), enucleation combined with cholangiojejunostomy (n = 1), left hemihepatectomy (n = 6), left hemihepatectomy with T-tube drainage (n = 2), left bisegmentectomy (n = 1), left segmentectomy (n = 7), right hemihepatectomy (n = 4), right segmentectomy (n = 4), and partial tumor resection or biopsy (n = 6). The three patients who underwent partial tumor resection did not receive radical excision because of peritoneal or distant metastasis.
No patients died perioperatively. Perioperative complications occurred in 12 patients and included intra-abdominal abscess (n = 2), postincision infection (n = 2), intra-abdominal bleeding (n = 3), bile leakage (n = 3), gastrointestinal bleeding (n = 2), and pleural effusion (n = 6). Only one patient suffering from intra-abdominal bleeding required reoperation; the other complications resolved with conservative management. The mean hospital stay was 9.6 ± 6.8 days.
Follow-up data
Follow-up data were available for 31 patients; the median follow-up time was 39.1 ± 32.7 months (range, 6–123 months). Three patients were lost to follow-up at 6, 18, and 21 months postoperatively, respectively. At the last follow-up, 16 patients had no evidence of disease, two patients survived with the disease, and three patients had undergone reoperation because of tumor recurrence but were still alive. Thirteen patients died during the follow-up period. The median overall survival was 76.2 months; survival at 1, 3, and 5 years was 88.0%, 68.7%, and 45.8%, respectively.
Comparison of patient characteristics between those with intrahepatic biliary cystadenoma and those with intrahepatic biliary cystadenocarcinoma
Because patients with IBC who undergo complete resection can achieve a better prognosis than those with IBCA, in order to identify prognostic factors for survival, we first compared the clinical characteristics of IBC patients with those of IBCA patients to identify risk factors associated with malignancy, considering that clinical variables showing a significant difference might also be factors associated with prognosis. Clinical variables showing a significant difference between these two patient populations were sex, mean age, ALT, TBIL, tumor size, and the presence of mural nodules [Table 1]. Patients with IBCA showed greater elevations in ALT (P = 0.01) and TBIL (P = 0.04) than those with IBC. Serum CA19-9 (P = 0.32) and CEA (P = 0.36) showed no significant difference.
Univariate and multivariate analysis using Cox proportional hazards regression models
Although we did not detect a significant difference in the presence of mesenchymal stroma between patients with IBC and those with IBCA, this was identified as an important factor associated with prognosis by previous studies,[5,12] so we included it in subsequent analyses. Other variables, reported to be associated with prognosis by previous studies that is surgical type,[3,13] presence of lymphatic invasion,[11] and tumor growth type,[5,10] were also included.
According to the cut-off values determined using ROC curves, patients were further categorized as 60 years or >60 years [Figure 2a], tumor size as ≤7.5 cm or >7.5 cm [Figure 2b], TBIL as ≤ 23.5 μmol/L or >23.5 μmol/L [Figure 2c], and ALT as ≤98.5 μmol/L or >98.5 μmol/L [Figure 2d]. In univariate analyses, age (P = 0.029), surgical type (P = 0.007), lymphatic invasion (P = 0.046), and tumor growth type (P = 0.038) all showed significant differences, while sex, tumor size, TBIL, ALT, presence of mural nodules, and presence of mesenchymal stroma did not [Table 2]. However, multivariate analysis found only radical resection (P = 0.002; hazard ratio [HR]: 0.079; 95% confidence interval [CI]: 0.016–0.378) and noninvasive tumor type (P = 0.009; HR: 0.161; 95% CI: 0.042–0.629) to be independent prognostic factors for survival.
Figure 2.
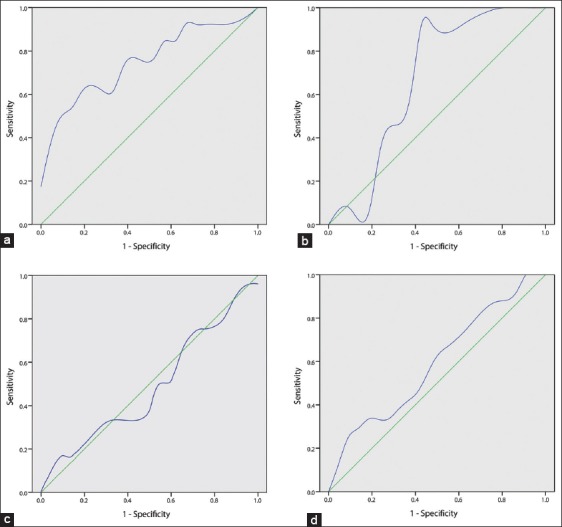
Receiver operating characteristic curves for dead and live patients. (a) Age (dead patients vs. live patients); area under the curve (AUC): 0.751; standard error (SE): 0.092; P = 0.015; sensitivity: 53.8%; specificity: 90.5%; (b) Tumor size; AUC: 0.678; SE: 0.093; P = 0.086; sensitivity: 92.3%; specificity: 57.1%; (c) Total bilirubin; AUC: 0.483; SE: 0.108; P = 0.871; sensitivity: 16.7%; specificity: 95.5%; (d) Alanine aminotransferase; AUC: 0.593; SE: 0.103; P = 0.377; sensitivity: 25.0%; specificity: 95.5%.
Table 2.
Univariate analysis of the prognostic factors for survival (n = 34)
| Items | Dead | Surviving | HR | HR (95% CI) | P |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 8 | 5 | 1.923 | 0.611–6.057 | 0.264 |
| Female | 5 | 16 | |||
| Age group | |||||
| ≤60 years old | 7 | 19 | 3.566 | 1.142–11.135 | 0.029 |
| >60 years old | 6 | 2 | |||
| Tumor size | |||||
| ≤7.5 cm | 4 | 12 | 5.752 | 0.447–23.107 | 0.104 |
| >7.5 cm | 9 | 9 | |||
| ALT level | |||||
| ≤98.5 μmol/L | 3 | 3 | 0.374 | 0.048–2.890 | 0.346 |
| >98.5 μmol/L | 10 | 18 | |||
| TBIL level | |||||
| ≤23.5 μmol/L | 10 | 15 | 0.484 | 0.124–1.885 | 0.296 |
| >23.5 μmol/L | 3 | 6 | |||
| Mural nodules | |||||
| Yes | 12 | 17 | 1.425 | 0.179–11.321 | 0.738 |
| No | 1 | 4 | |||
| Surgical type | |||||
| Radical resection | 8 | 19 | 0.155 | 0.067–0.863 | 0.007 |
| Palliative surgery | 5 | 1 | |||
| Lymphatic invasion | |||||
| Yes | 2 | 1 | 5.209 | 1.029–26.370 | 0.046 |
| No | |||||
| Mesenchymal stroma | |||||
| Yes | 6 | 12 | 0.776 | 0.246–2.444 | 0.665 |
| No | 7 | 9 | |||
| Tumor growth type | |||||
| Invasive type | 7 | 6 | 0.266 | 0.076–0.930 | 0.038 |
| Noninvasive type | 6 | 15 |
CI: Confidence interval; ALT: Alanine aminotransferase; TBIL: Total bilirubin; HR: Hazard ratio.
For patients who underwent radical resection, the Kaplan–Meier estimated survival rates at 1, 3, and 5 years were 84.9%, 72.7%, and 66.1%, respectively. For those who underwent palliative resection, the estimated survival rates at 1, 3, and 5 years were 33.3%, 33.3%, and data not available, respectively [Figure 3a]. The median survival times of patients who underwent radical or palliative resections were 84.4 months and 18.3 months, respectively; the difference was significant according to log-rank analysis of the Kaplan–Meier model (P = 0.002). For patients with noninvasive tumors, the survival rates at 1, 3, and 5 years were 90.5%, 77.7%, and 62.1%, respectively; for those with invasive tumors, the survival rates at 1, 3, and 5 years were only 83.9%, 44.1%, and 22.0%, respectively [Figure 3b]. The median survival times of patients with invasive and noninvasive tumors were 32.4 months and 92.7 months, respectively; the difference was significant (P = 0.025).
Figure 3.
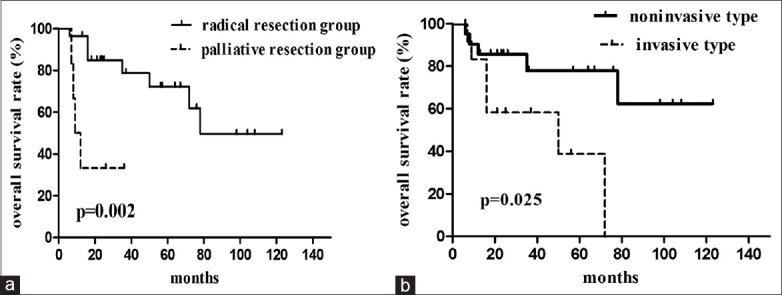
(a) Overall survival curves for the radical and palliative resection groups; (b) Overall survival curves for the invasive and noninvasive tumor types.
DISCUSSION
Intrahepatic biliary cystic tumors are reported to represent only 5% of all intrahepatic cysts of biliary origin,[1] but their actual incidence is likely much higher because patients are sometimes misdiagnosed with simple hepatic cysts or other cystic lesions.[14] In our study, six patients had undergone previous inappropriate treatment because of misdiagnosis. The accurate diagnosis of IBCA is important in optimizing clinical decisions and patient prognosis.
Previous studies have shown that complementary imaging modalities may contribute to correct the diagnosis and provide information useful in formulating appropriate treatment strategies.[7,8,15] IBCAs in our study were mainly located in the left liver lobe, which is consistent with other reports; this localization may be related to embryonic gallbladder development.[8] The presence of multilocular cysts, thickened and irregular walls, internal septa, mural nodules, and enhancement of internal septa and mural nodules are radiological characteristics of IBCA that can be helpful in differentiating them from biliary intraductal papillary neoplasm and simple hepatic cysts.[12,16] However, distinguishing between IBC and IBCA remains difficult because these lesions have similar imaging characteristics [Figure 1].
In our study, sex, mean age, ALT, TBIL, tumor size, and the presence of mural nodules showed a significant difference between patients with IBC and those with IBCA. As reported by previous studies, almost all IBCs occurred in middle-aged females (mean age, 45.4 ± 17.0 years), while IBCAs tended to occur in older patients (mean age, 58.7 ± 11.9 years; P = 0.02) and in more male patients (13/34 vs. 4/41; P = 0.01). Most male patients were older than 60 years, which is consistent with studies by Wang et al. and Sang et al.[3,17] The older age of IBCA patients may support the hypothesis that cystadenomas can undergo malignant transformation into cystadenocarcinomas over a long time. Sang et al. also previously reported that nine of 11 male patients with cystic tumors had IBCAs.[3] Thus, IBCA should be suspected in an older male patient with such a lesion, especially when typical signs are present, e.g., multilocular cysts, internal septa, and mural nodules.
As with IBC, the most common symptom associated with IBCA is abdominal pain or discomfort. We did not find tumor size to correlate with symptoms (10.7 ± 5.3 cm for symptomatic patients vs. 13.2 ± 6.2 cm for asymptomatic patients; P = 0.37). Adjacent organ compression, intracystic hemorrhage and infection, and biliary obstruction may relate to clinical symptoms;[17,18,19,20] jaundice is a less common symptom. In our study, jaundice was found in nine patients and was caused by the following: Extrinsic compression or invasion by the tumor (n = 5), biliary thrombi (n = 3), and viscid intraductal mucin (n = 1).
Although patients with IBCA had smaller tumors, their serum liver enzyme values were higher than those of patients with IBCs, and the difference was statistically significant. One possible reason for this difference is that cystadenomas tend to be slow-growing and clinically silent, while cystadenocarcinomas are more inclined to invade the adjacent biliary tree or compress surrounding organs. Because IBCA is more likely to lead to persistent symptoms, earlier medical interventions are more likely to be performed in these patients, which may explain why IBCA patients had shorter symptom durations [Table 1] and presented with smaller tumors. Patients in the IBCA subgroup were more likely to have jaundice compared with the IBC subgroup (9/31 vs. 3/41, respectively); this may be another important reason for the elevated hepatic enzymes in these patients (obvious liver dysfunction was mostly found in patients with obstructive jaundice).
Mural nodules arise from epithelial tissue, as does ductal adenocarcinoma, and are more commonly seen in patients with IBCA.[16,18] Martel et al. identified 2 IBCAs out of 13 biliary cystic tumors, both of which presented with small foci of malignant cells in mural nodules.[21] Mural nodules were more commonly seen among patients with IBCA in our study; although there appears to be an association between these nodules and malignancy, clarification of this relationship requires additional studies involving larger sample sizes and larger pathological specimens.
Because it is difficult to make a definitive preoperative diagnosis of IBCA and because IBC also has malignant potential, complete resection is recommended for any suspected biliary cystadenoma.[8,13,15] Another main result of our study was that age, surgery type, the presence of lymphatic invasion, and tumor growth type were all related to IBCA prognosis while only surgery type and tumor growth type were independent prognostic factors. Earlier therapy, such as internal Roux-en-Y drainage, aspiration, sclerosis, and partial resection, resulted in high rates of recurrence and malignant degeneration; thus, any patient presenting with liver cyst recurrence after such treatments should be suspected of having a cystadenoma or cystadenocarcinoma.[8,9,13] In our study, radical resection was an independent prognostic factor for longer survival. For patients, who underwent radical resection, the 5-year survival rate was as high as 66.1%. Considering the favorable outcomes, it can produce, radical resection with wide margins (>2 cm) of normal liver tissue should be the goal of surgical procedures.[22] Cyst enucleation is only recommended when complete resection might jeopardize biliary radicles or vascular structures although satisfactory outcomes can be achieved if the surgical margins are negative. In our study, four patients underwent enucleation; as of the last follow-up, one patient had died, and the remaining patients were surviving disease-free. In univariate analysis, there was a significant difference in prognosis between patients who had local lymph node metastasis and those who did not. However, only three patients in our study had local lymph node metastasis, and complete resections could not be performed in two of them because of distant metastases and peritoneal metastasis; these patients died 6 and 9 months postoperatively, respectively. We may speculate that lymph node metastasis is an indication of an advanced tumor. In most patients, the tumor is confined to the cystic lesions or only invades the surrounding hepatic parenchyma, so extended lymph node resection is not warranted. Recurrence is not a contraindication for surgery. In our study, three patients with recurrence underwent reoperations; at the end of follow-up, only one of these patients had died, and the other two were surviving disease-free.
Histologically, cystadenomas and cystadenocarcinomas can be divided into two subgroups according to the presence of ovarian stroma between the inner epithelial lining and the outer connective tissue capsule.[2,8] Those with ovarian stroma occur exclusively in women and have an indolent course with a good prognosis, while those without ovarian stroma more frequently occur in middle-aged men and more easily undergo malignant transformation, carrying a poor prognosis. Although a previous multi-institutional study concluded that long-term outcomes were associated with the presence of ovarian stroma,[5] our study did not find this to be true in either univariate or multivariate analysis. This may have been because only IBCAs were included, so we were unable to differentiate between tumors derived from malignant degeneration of cystadenomas and de novo cancer; these different origins may influence prognosis.[23] Our limited number of cases is another potential explanation, and further investigation is needed.
Nakajima et al. divided IBCAs into two growth types according to clinicopathologic features: Noninvasive (carcinoma cells confined to the cystic lesions) and invasive (carcinoma cells extending into the hepatic parenchyma or neighboring organs).[10] In their study, patients with noninvasive tumors had a lower recurrence rate and better long-term outcome than those with invasive tumors. We also found noninvasive tumor growth type to be an independent predictor of better outcome. For patients with invasive tumors, 5-year survival was only 22.0%, similar to that of patients with cholangiocarcinoma. Thus, close follow-up is recommended for patients with invasive tumors even after radical resection.
Our study had some limitations. First, this was a single-center study with a relatively small sample size, thus, additional studies with larger sample sizes or involving multiple medical centers should be performed to confirm our results. Second, patients who had undergone surgery in recent years had a relatively short follow-up period, a longer follow-up period may have influenced their prognosis. Third, few patients (4/34) received adjuvant chemotherapy or radiotherapy after surgical procedures. It is unknown whether these treatments influence patient outcomes, and we have little experience with this.
In conclusion, patients with IBCA were older, and more males were represented compared with IBC. The most common presenting symptoms were abdominal pain or discomfort. IBCAs more commonly led to liver dysfunction and were more frequently associated with mural nodules. Distinguishing between cystadenomas and cystadenocarcinomas based on laboratory examination and imaging findings remains difficult. Radical resection and noninvasive tumor type are independent prognostic factors for overall survival; accordingly, complete resection is recommended for curative treatment, and patients should be closely followed postoperatively, particularly those with invasive tumors.
Footnotes
Edited by: Jian Gao and Yuan-Yuan Ji
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Tsiftsis D, Christodoulakis M, de Bree E, Sanidas E. Primary intrahepatic biliary cystadenomatous tumors. J Surg Oncol. 1997;64:341–6. doi: 10.1002/(sici)1096-9098(199704)64:4<341::aid-jso17>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Devaney K, Goodman ZD, Ishak KG. Hepatobiliary cystadenoma and cystadenocarcinoma. A light microscopic and immunohistochemical study of 70 patients. Am J Surg Pathol. 1994;18:1078–91. [PubMed] [Google Scholar]
- 3.Sang X, Sun Y, Mao Y, Yang Z, Lu X, Yang H, et al. Hepatobiliary cystadenomas and cystadenocarcinomas: A report of 33 cases. Liver Int. 2011;31:1337–44. doi: 10.1111/j.1478-3231.2011.02560.x. [DOI] [PubMed] [Google Scholar]
- 4.Delis SG, Touloumis Z, Bakoyiannis A, Tassopoulos N, Paraskeva K, Athanassiou K, et al. Intrahepatic biliary cystadenoma: A need for radical resection. Eur J Gastroenterol Hepatol. 2008;20:10–4. doi: 10.1097/MEG.0b013e3282f16a76. [DOI] [PubMed] [Google Scholar]
- 5.Arnaoutakis DJ, Kim Y, Pulitano C, Zaydfudim V, Squires MH, Kooby D, et al. Management of biliary cystic tumors: A multi-institutional analysis of a rare liver tumor. Ann Surg. 2015;261:361–7. doi: 10.1097/SLA.0000000000000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu J, Wang Y, Yu X, Liang P. Hepatobiliary mucinous cystadenoma and cystadenocarcinoma: Report of six cases and review of the literature. Hepatogastroenterology. 2010;57:451–5. [PubMed] [Google Scholar]
- 7.Lewin M, Mourra N, Honigman I, Fléjou JF, Parc R, Arrivé L, et al. Assessment of MRI and MRCP in diagnosis of biliary cystadenoma and cystadenocarcinoma. Eur Radiol. 2006;16:407–13. doi: 10.1007/s00330-005-2822-x. [DOI] [PubMed] [Google Scholar]
- 8.Vogt DP, Henderson JM, Chmielewski E. Cystadenoma and cystadenocarcinoma of the liver: A single center experience. J Am Coll Surg. 2005;200:727–33. doi: 10.1016/j.jamcollsurg.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Hansman MF, Ryan JA, Jr, Holmes JH, 4th, Hogan S, Lee FT, Kramer D, et al. Management and long-term follow-up of hepatic cysts. Am J Surg. 2001;181:404–10. doi: 10.1016/s0002-9610(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima T, Sugano I, Matsuzaki O, Nagao K, Kondo Y, Miyazaki M, et al. Biliary cystadenocarcinoma of the liver. A clinicopathologic and histochemical evaluation of nine cases. Cancer. 1992;69:2426–32. doi: 10.1002/1097-0142(19920515)69:10<2426::aid-cncr2820691007>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Simo KA, Mckillop IH, Ahrens WA, Martinie JB, Iannitti DA, Sindram D. Invasive biliary mucinous cystic neoplasm: A review. HPB (Oxford) 2012;14:725–40. doi: 10.1111/j.1477-2574.2012.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gómez-Martín C, Rodríguez A, Malón D, Cortés-Funes H. Biliary cystadenocarcinoma with mesenchymal stroma. Clin Transl Oncol. 2010;12:234–7. doi: 10.1007/s12094-010-0495-7. [DOI] [PubMed] [Google Scholar]
- 13.Teoh AY, Ng SS, Lee KF, Lai PB. Biliary cystadenoma and other complicated cystic lesions of the liver: Diagnostic and therapeutic challenges. World J Surg. 2006;30:1560–6. doi: 10.1007/s00268-005-0461-7. [DOI] [PubMed] [Google Scholar]
- 14.Kim HH, Hur YH, Koh YS, Cho CK, Kim JW. Intrahepatic biliary cystadenoma: Is there really an almost exclusively female predominance? World J Gastroenterol. 2011;17:3073–4. doi: 10.3748/wjg.v17.i25.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi HK, Lee JK, Lee KH, Lee KT, Rhee JC, Kim KH, et al. Differential diagnosis for intrahepatic biliary cystadenoma and hepatic simple cyst: Significance of cystic fluid analysis and radiologic findings. J Clin Gastroenterol. 2010;44:289–93. doi: 10.1097/MCG.0b013e3181b5c789. [DOI] [PubMed] [Google Scholar]
- 16.Mortelé KJ, Ros PR. Cystic focal liver lesions in the adult: Differential CT and MR imaging features. Radiographics. 2001;21:895–910. doi: 10.1148/radiographics.21.4.g01jl16895. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Miao R, Liu H, Du X, Liu L, Lu X, et al. Intrahepatic biliary cystadenoma and cystadenocarcinoma: An experience of 30 cases. Dig Liver Dis. 2012;44:426–31. doi: 10.1016/j.dld.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Seo JK, Kim SH, Lee SH, Park JK, Woo SM, Jeong JB, et al. Appropriate diagnosis of biliary cystic tumors: Comparison with atypical hepatic simple cysts. Eur J Gastroenterol Hepatol. 2010;22:989–96. doi: 10.1097/MEG.0b013e328337c971. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez M, Majno P, Terraz S, Morel P, Rubbia-Brandt L, Mentha G. Biliary cystadenoma revealed by obstructive jaundice. Dig Liver Dis. 2009;41:e11–3. doi: 10.1016/j.dld.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Facy O, Rode A, Mabrut JY. Segment I intrahepatic biliary cystadenocarcinoma impinging on the hepatic vein. J Visc Surg. 2012;149:423–5. doi: 10.1016/j.jviscsurg.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Martel G, Alsharif J, Aubin JM, Marginean C, Mimeault R, Fairfull-Smith RJ, et al. The management of hepatobiliary cystadenomas: Lessons learned. HPB (Oxford) 2013;15:617–22. doi: 10.1111/hpb.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu ZW, He Q, Lang R, Pan F, Jin ZK, Sheng QS, et al. Giant hepatobiliary cystadenoma in a male with obvious convex papillate. World J Gastroenterol. 2009;15:1906–9. doi: 10.3748/wjg.15.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawarada Y, Taoka H, Mizumoto R. A report of 5 cases of cystic bile duct carcinoma of the liver and proposal of a new classification. Gastroenterol Jpn. 1991;26:80–9. doi: 10.1007/BF02779514. [DOI] [PubMed] [Google Scholar]


