Abstract
Objective:
Clinical application of autologous fat grafting (AFG) is quickly expanding. Despite the widely acceptance, long-term survival rate (SR) of AFG remains a question not yet solved. Meanwhile, although rare, severe complications related to AFG including vision loss, stroke even death could be seen in the literature.
Data Sources:
A comprehensive research of PubMed database to June 2013 was performed according to guidelines of the American Society of Plastic Surgeons Fat Graft Task Force Assessment Methodology. Articles were screened using predetermined inclusion and exclusion criteria.
Study Selection:
Data collected included patient characteristics, surgical technique, donor site, recipient site, graft amount, and quantified measurement methods. Patient cohorts were pooled, and SR was calculated. All the severe complications were also summarized according to the different clinical characteristics.
Results:
Of 550 articles, 16 clinical articles and 10 animal studies met the inclusion criteria and provided quantified measurement methods. Totally, 596 patients were included. SR varied from 34% to 82% in breast and 30–83% in the facial area. Nude mice were applied to investigate human fat grafting SR (38.3–52.5% after 15 weeks). Rabbits were commonly used to study animal AFG SR (14.00–14.56% after 1-year). Totally, 21 severe complications were reported, including death (2), stroke (10), vision loss (11, 8 of which accompanied with stroke), sepsis (3), multiple abscess (1) and giant fat necrotic cyst (2). Ten of these complications happened within 10 years.
Conclusions:
There is no unified measurement method to evaluate fat graft SR until now and no clinical evidence to show better SR according to different donor and recipient cite. Body mass index change between pre- and postoperation may be the bias factor in evaluating fat SR. Fat embolisms of the ophthalmic artery and the middle cerebral artery are the most severe complication of AFG and still lack of effective treatment.
Keywords: Autologous Fat Grafting, Complication, Fat Survival
INTRODUCTION
Neuber first described autologous fat grafting (AFG) in 1893 for unilateral facial atrophy.[1] With the continuous technical progress in the last century, there was an increased trend in replacement of soft tissue volume with AFG nowadays. However, assessment of the outcome after fat grafting has been criticized for lack of quantified evidence of fat graft survivability and predictability of volume restoration.[2] Researchers demonstrated that lipoaspiration caused more initial damage than scraping, but may yield better long-term viability based on increased proliferation.[3] Gir et al. compared various fat graft techniques into the following topics: fat harvesting, fat processing, fat reinjection and fat storage;[4] and demonstrated there was no evidence supported one harvesting or processing technique above another as superior. Pu[5] reviewed the studies related to fat grafting research, and one in vivo study shows that more adipose-derived stem cells are found within the fat of lower abdomen or inner thigh.[6] In spite of the numerous clinical reports regarding fat graft technique, the specific techniques of graft harvesting, preparation and injection are still selected according to a surgeon's individual preference, since quantitative evidence of clinical fat survivability and predictability of volume restoration does not exist in spite of the evidence-based practice recommendations.[7] The aim of this study was to provide an extensive study according to different recipient sites and fat graft survival rate (SR). The most severe complications of AFG are also summarized and critically reviewed.
METHODS
Literature search
A comprehensive search of PubMed database to June 2013 was performed according to the guidelines of the American Society of Plastic Surgeons Fat Graft Task Force Assessment Methodology.[7,8] The following search terms were used to obtained all the related English-language literature: AFG, autogenous fat grafting, autologous fat transfer, autogenous fat transfer, autologous fat filler, autogenous fat filler, fat harvest, adipocyte harvest, lipoaspirate, lipotransfer, lipoinjection, lipoinfiltration, fat augmentation, adipose augmentation, adipocyte augmentation, and adipocyte graft.
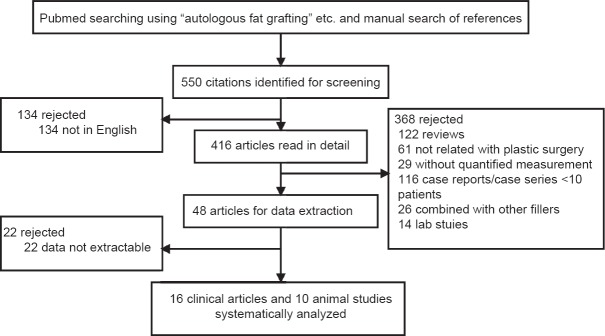
Articles with full text accessible were further evaluated and addressing fat grafting with other types of grafts and for nonplastic surgery applications were excluded. Also excluded were non-English articles, reviews, case reports or case series <10 patients. The inclusion and exclusion criteria are shown in Figure 1.
Figure 1.
Flow chart of the study.
Data extraction
For each citation, data listed in Table 1 (clinical articles) or Table 2 (animal studies) was extracted. Reports were reviewed manually for patient demographics, operation related technique, and clinical outcomes. Studies with objective outcome efficacy as measured by tissue volume before and after filling treatment were calculated. All articles contained extractable data were analyzed. The data were pooled, and the fat SR was calculated. Reports of severe complications (with systemic manifestations and necessary medical intervention) were summarized according to the publication year, original country, fat inject area, inject volume, onset time and location of the complication, onset symptoms, final diagnosis, treatment and clinical outcomes as shown in Table 3.
Table 1.
Data extracted from clinical articles
| Patient characteristics |
| Average age |
| BMI |
| Operation related technique |
| Donor site |
| Harvest technique |
| Fat purifying method |
| Recipient site |
| Fat volume |
| Supplementary procedures |
| Injection technique |
| Clinical outcome |
| Follow-up time |
| Measurement technique |
| Complications |
| Fat survival rate |
BMI: Body mass index.
Table 2.
Data extracted from animal studies
| Animal type |
| Donor site |
| Harvest technique |
| Recipient site |
| Fat purifying method |
| Fat volume |
| Supplementary procedures |
| Injection technique |
| Follow-up time |
| Measurement technique |
| Fat survival rate |
Table 3.
Severe complications of autologous fat grafting
| Year | Author | Country | Injection area | Inject volume | Complication onset time | Onset symptom | Diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 2012 | David et al. | Canada | Lateral aspect of nose | N/A | 90 min | Vision loss | Retinal artery embolism | Lower intraocular pressure | Vision loss |
| 2011 | Chang et al. | Korea | Periocular | N/A | 2 h | Vision loss, skin color change of the nose | Ophthalmic artery and middle cerebral artery infarction | Intravenous mannitolization | Vision loss |
| 2011 | Keu et al. | Korea | Breast | 250 cc each side | 1 day | Erythema, tenderness | Sepsis, multiple breast abscesses | Drainage, antibiotics | Recover with no deformity |
| 2011 | Jun H | China | Temporal area | N/A | Immediately | Drowsy, aphasia, right hemiparesis | Middle cerebral artery occlusion | Mannitol, hydrocortisone, antiplatelet, hyperbaric oxygen | Function loss, National Institutes of Health Stroke Scale 6 |
| 2011 | Yoo et al. | Korea | Forehead | N/A | 1 day | Swelling, vision loss, right hemiparesis | Ophthalmic artery and middle cerebral artery infarction | Methylprednisolone | Vision loss |
| 2011 | James | United Arab Emirates | Earlobe | 1 ml | 9 weeks | Tenderness, serous discharge in earlobes and thigh | Atypical mycobacteria infection | Curettage, antibiotics | Hyperpigmentation |
| 2011 | Sang et al. | Korea | Nasal area | N/A | Immediately | Pain and vision loss | Ophthalmic artery occlusion | Pharmacomechanical thrombolysis | Vision loss |
| 2011 | Catherine et al. | UK | Medial cheeks, temple | 35 ml | 2 h | Dizzy, progressive respiratory failure | Pulmonary arteriole obstruction | Invasive ventilator support | Dearth |
| 2010 | Simon et al. | US | Breast and buttock | 100 ml | Several days | Erythema, pain | Sepsis | Drainage | Contour deformity |
| 2004 | Olivier et al. | France | Left face | 17 ml | Immediately | Hypertonia, confusion | Bilateral anterior cerebral arteries embolism | Reintubation | Mutism, paraplegia |
| 2003 | Sung et al. | Korea | Glabella | 5 ml | 1 min | Mental change, hemiplegia | Internal carotid artery embolism | Artificial ventilation, dexamethasone | Left eye necrosis then death |
| 2001 | Helen et al. | US | Nasolabial fold, lips, paranasal defect | 6.5 ml | 10 min | Pain, disoriented, vision loss, hemiparesis | Middle cerebral, ophthalmic, central retinal artery embolism | N/A | Patchy necrosis, vision loss |
| 1998 | Feinendegen | UK | Nasolabial fold, lower lip, chin | N/A | 7 h | Global aphasia, hemiparesis | Left middle cerebral artery infarction, right retinal fundus embolism | N/A | Fluent speech aphasia |
| Periorbital area | N/A | Immediately | Eye pain, headache, unresponsive, right hemiplegia, global aphasia | Ophthalmic artery occlusion, deep venous thrombosis | N/A | Vision loss, able to walk, global aphasia | |||
| 1998 | Jose | Spain | Breast | N/A | 10 months | Enlarged, painful lump | Liponecrotic pseudocyst | Lumpectomy | Recovered |
| 1997 | Baumgartner | Switzerland | Nasolabial fold, lower lip and chin | N/A | 7 h | Global aphasia, hemiparesis | Right retinal artery embolism, left middle cerebral artery infarction | N/A | N/A |
| 1996 | Kyung | Korea | Nasolabial fold | 0.5 ml | Immediately | Headache, dyspnea, unconscious | Ophthalmic artery, middle cerebral artery infarction | Carbon dioxide and oxygen | Vision loss |
| 1993 | Jose | Spain | Glabellar | N/A | During the procedure | Pain, vision loss, hemiplegia | Ophthalmic artery, middle cerebral embolism artery infarction | N/A | Vision loss, partially function recovery |
| 1990 | Vizcaino | Spain | Breast | 120 ml | Immediately | Enlarged lump | Liponecrotic pseudocyst | Surgical excision | Recovered |
| 1989 | Neil | US | Forehead | N/A | Immediately | Headache, vision loss | Ophthalmic artery embolism | N/A | Vision loss |
| 1988 | Bahman | US | Glabellar | N/A | During the procedure | Eye pain, vision loss | Retinal arterial occlusion | N/A | Vision loss |
N/A: Not applicable.
RESULTS
The original search resulted in 550 articles with 416 in English. Totally, 122 reviews, 61 articles not related to plastic surgery, 29 articles without quantified measurement and 116 case reports with <10 patients and 22 articles with data not extractable were excluded.
After the application of the exclusion/inclusion criteria, a total of 16 clinical studies and 10 animal research articles were included in this review [Figure 1].
Animal research
Nude mice were used as the model to study human fat SR. Common recipient sites were dorsal area[9,10,11] and scalp.[12,13] The SR varied from 38.3% to 57.84% with 4–15 weeks follow-up period, with the highest rate reported by Matsumoto et al. (57.84% after 4 weeks)[10] and longest follow-up time reported by Shoshani et al. (51.5% after 15 weeks)[12] without any intervention to the fat cells. Protocols to help the fat survive, such as insulin, adipose-derived stem cell, and hyperbaric oxygen, were given by different authors with a highest SR when grafted with adipose-derived stem cell reported by Matsumoto et al. (79.14% after 4 weeks).[10] Rabbits were also commonly used for autologous fat SR. The donor sites included shoulder, inguinal, inter-scapular and groin area, and recipient sits included scalp, ear and rectus muscle. Autologous fat SR varied from 14.56% to 56% with 3–12 months follow-up period without any intervention. Epidermal growth factor, insulin, and other methods to increase fat SR have been studies on rabbit, with the highest SR reported by Fagrell et al.[14] with fat cylinder transplantation (99% after 6 months) and longest follow-up time as 1-year reported by Viterbo et al.[15] and Marques et al.[16] (with a SR as 14.56% and 35%, respectively). Dogs were also studied by some researchers while only for vocal fold reconstruction.[17,18] Grafted fat volume varied from 0.5 ml to 2 ml, and the most commonly used operation method was Coleman's technique. Kruschewsky et al. performed the only three-dimensional objective fat volume measured experiment in 2007.[17] They tested fat SR on canine vocal fold and performed magnetic resonance imaging (MRI) on the animal pre- and 3 months post-operation. However, the SR was only 18% if not combined with any intervention method.
Clinical studies
According to the 16 clinical studies included, fat SR varied from 15% to 83% with 6 months to 3.7 years of follow-up period in average. Zocchi and Zuliani reported the largest case series (181 patients) in 2008[19] with the biggest volume of grafted fat (375 ml) in a single session. They combined AFG with mesenchymal stem cells and Brava technique in breast augmentation, and gained a SR as 55% at 1-year postoperation. Tanna et al.[20] reported the highest fat SR as 83% in craniofacial microsomia soft-tissue reconstruction. However, the final result was achieved by 3–5 sessions’ fat graft with 33 ml in average of each session. Intervention methods to increase fat SR included enhanced stromal vascular fraction[21] (63% fat survived after 1-year), platelet-rich plasma[21,22] (69% and 77% fat survived after 1-year respectively), progenitor-supplemented adipose tissue[23] (also named as cell-assisted lipotransfer, with 56% fat survived after 6 months), enriched cell culture medium[24] (50–60% fat survived after 6 months), platelet rich fibrin[22] (82% fat survived after 1-year), and mesenchymal stem cells[19] (55% fat survived after 1-year). Khouri et al.[25] reported the longest average follow-up time as 3.7 years in autologous fat breast augmentation patients with an average of 277 ml fat graft in one session. They also combined Brava technique with AFG in part of their patients and measured the result by MRI. The SR was 55% without Brava and 82% with Brava according to this prospective multicenter study. The most commonly used graft method is Coleman's technique, with frequently used donor sites as abdomen and thighs. The grafted fat volume varied from 50 ml to 375 ml in breast and 6–50 ml in the facial area.
Complications of autologous fat grafting
Multiple complications were reported in the literature, including infection, bleeding, calcification, fat necrosis, unspecified superficial lumps, less than expected beneficial outcome, fat embolism, systemic infection, etc. Totally, 21 severe complications of AFG were reported, including death (2), stroke (10), vision loss (11, 8 of which accompanied with stroke), sepsis (3), multiple abscess (1) and giant fat necrotic cyst (2). The details of included publications are listed in Table 3.
DISCUSSION
In 1987, the American Society of Plastic and Reconstructive Surgeons reported the problems and difficulties that have been encountered with autologous fat transplantation and concludes that only 30% of injected fat can be expected to survive for 1-year.[26] Doubts have been expressed about the maintenance of the volume grafted and the possibility of determining the actual surviving volume.
Animal research
Measurement tools of fat SR in animals include weight, volume, computer image analysis, and dermal thickness. It still lacks of strong enough research evidence to measure fat SR in the subcutaneous tissue, no matter animal AFG or human fat graft on nude mice, due to the less quantified measurement pre- and postoperation.
Clinical studies
Few studies have addressed the issue of the rate of AFG absorption and the factors that may be associated with a higher or lower graft survival volume in the literature. Also, the mechanism of fat tissue survival and absorption is not fully understood. It is believed that fat tissue survives by nutrient diffusion from the serum within the first 48 h and then starts to regenerate.[13]
The average percentage of fat obtained after each centrifugation was 70.1% without any significant difference in relation to the donor site.[27] There is no evidence to support the belief that a specific donor site is optimal for a specific recipient site. Some authors also believed that the SR was related to the amount of fat injected in the certain volume, which caused the rapid increase in local tissue pressure.[25] According to the included articles, the amount of autologous fat injected in the breast is 50–277 ml, and 1–33 ml in the facial area. However, there is no linear relation between the fat graft volume and SR according to the included studies, no matter where the recipient cite was.
It is believed that fat graft results are dependent on technique and surgeon expertise, also body weight fluctuations can affect graft volume over time.[28] However, among the 16 included studies, only 3 of them mentioned the average body mass index (BMI) of patients, as 19.8, 23.5 and 19.7 respectively.[25,29,30] One study used dual-energy X-ray absorptiometry scans during the screening visit and at weeks 24 and 48, to measure body fat variations throughout the follow-up period. The authors mentioned in the event that as body fat increased, facial improvement after infiltrations were considered with caution,[31] but the results focused on patient satisfaction and improvement of quality of life without any quantified measurement. Further animal researches and clinical studies are required to demonstrate the correlation between BMI (as well as its variation pre- and post-AFG) and fat SR to reduce the bias of the results.
Mechanical washing of fat with ringer lactate is supported by studies that showed no damage to adipocyte integrity and protection of AFG from the negative action of hematic cells and components.[32,33] Also, low-pressure suction by means of larger bore cannulas, centrifugation with a force <3000 r/min, and 2.5 mm diameter injection cannulas (compared with smaller diameter) with syringe aspiration were suggested.[2,4] Coleman is frequently cited for his description of a structural fat grafting technique.[34] His success is attributed to a technique using 10-ml syringe fat harvest, isolation of fat by centrifugation, and 3-ml syringe injection of approximately 0.2 ml of fat in at least two directions per cannula pass. Coleman stresses that fat should be layered in many different levels, with limited parenchymal infiltration. This time-consuming technique (2 h for the first 100 ml of injections) and avoidance of “transplanting fat in large clumps” are reported to be critical to graft survival and prevention of fat necrosis and consequent infection. Thus, Coleman's technique is the most commonly performed method in both animal research and clinical studies reviewed in this article.
Researchers demonstrated that fat injection produces a considerable fat volume loss, which may be attributed to the partial fat liquefaction produced by the pressure of syringe.[17] Thus, many articles are based on the principle that the volume placed in the syringe and presumably injected is the real volume may not be correct.
In regard to the method used to determine the initial and final volume of the graft, MRI has been established as the method of choice because of its excellent qualities for the study of fatty tissue.[17] It is difficult to compare preoperative and postoperative photographs, even though they were taken with the same camera, same light, and the same distance.[35] However, some authors argued that MRI was found to be not sensitive in determining breast volume and detecting defects in the superior and lateral periphery of the breast.[36] Computed tomography (CT) scans can distinguish fat density from all other tissues and was chosen over MRI because CT measurements are more exact than those of MRI.[37] Nguyen et al. observed the tissue sample at 9 months postfat injection and demonstrated that it was possible that fibrous connective tissues are the main components that maintain the bulk and tissue volume after long-term adipose tissue transplantation.[38] Above all, a wide-accepted, objective, quantified method to confirm the fat volume is required during the study of AFG.
Complications
Chronic edema, calcification, fibrosis, acne, headache, dysesthesia, drooping, and irregularity after full-face fat injection were observed as moderate complications in most of the publications. The severe complications reviewed in this article happened in different decades [Figure 2] and countries [Figure 3] since the invention of AFG technique. Totally, 10 of these complications happened within 10 years, which is correlated with the rapid increasing number of publications [Figure 4] and citations [Figure 5] on this topic simultaneously according to the web of science. It is noticed that for the most severe complications such as vision loss and middle cerebral artery embolism, there was still lack of effective treatment and usually led to poor clinical outcomes.
Figure 2.
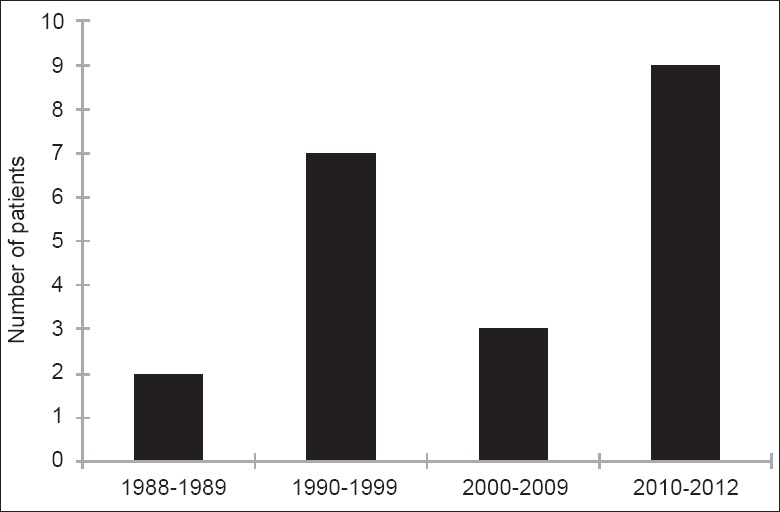
Publication decades of the severe complications related with autologous fat grafting.
Figure 3.
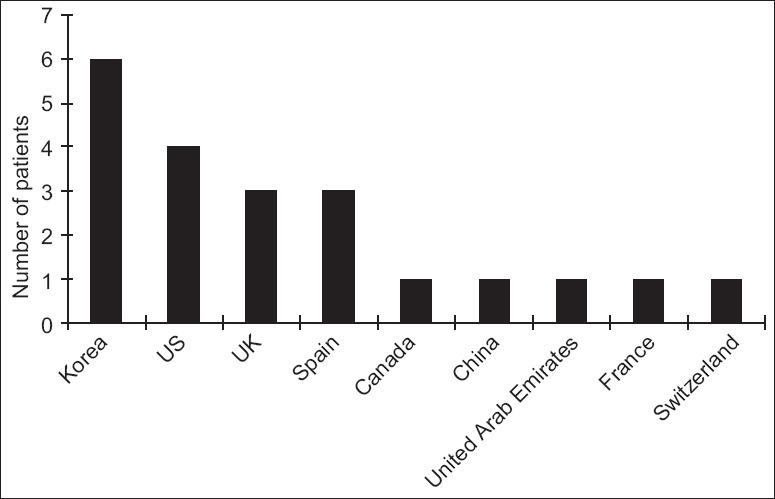
Original countries of the severe complications related with autologous fat grafting.
Figure 4.
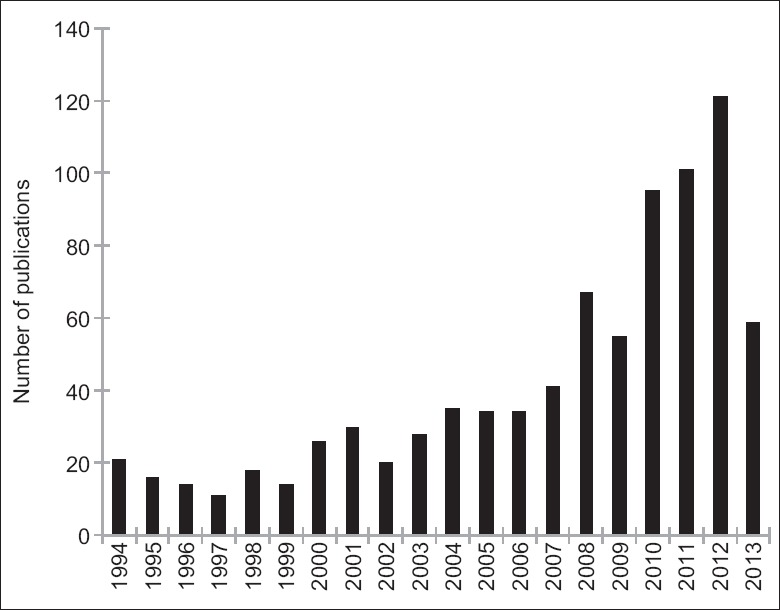
Number of publications on the topic of autologous fat grafting in each year according to the web of science.
Figure 5.
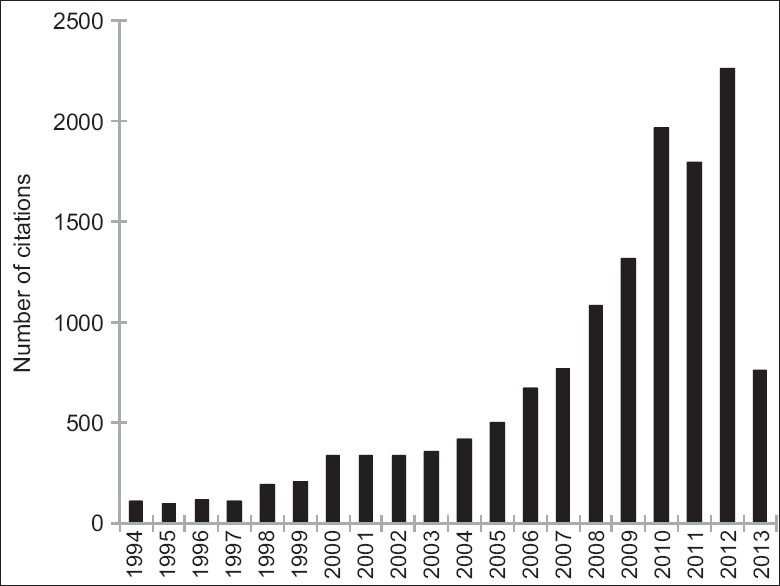
Number of citations on the topic of autologous fat grafting in each year according to the web of science.
Other clinical applications of autologous fat grafting
Rigotti et al. have previously demonstrated clinical improvement of chronic radiation-induced fibrosis following injection of processed autogenous lipoaspirate beneath fibrotic skin.[39] AFG was also widely used in laryngoplasy,[40] dural defect repair,[41] prostate-perineal fistula closure, and treating, etc.
The conclusion may not be drawn regarding the differences of fat long-term viability based on the donor site or recipient site. Despite the limited success and poor results in animal studies, the clinical result is optimistic. However, most of the clinical reports failed to objectively assess the outcomes. Longevity of grafted fat remains unknown, and additional treatments may be necessary to obtain more optimal results.
Footnotes
Edited by: Yuan-Yuan Ji
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Neuber G. Fet transplantation. Verh Dtsch Ges Chir. 1893;22:66. [Google Scholar]
- 2.Kaufman MR, Miller TA, Huang C, Roostaeian J, Wasson KL, Ashley RK, et al. Autologous fat transfer for facial recontouring: Is there science behind the art? Plast Reconstr Surg. 2007;119:2287–96. doi: 10.1097/01.prs.0000260712.44089.e7. [DOI] [PubMed] [Google Scholar]
- 3.Park H, Williams R, Goldman N, Choe H, Kobler J, Lopez-Guerra G, et al. Comparison of effects of 2 harvesting methods on fat autograft. Laryngoscope. 2008;118:1493–9. doi: 10.1097/MLG.0b013e3181735634. [DOI] [PubMed] [Google Scholar]
- 4.Gir P, Brown SA, Oni G, Kashefi N, Mojallal A, Rohrich RJ. Fat grafting: Evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast Reconstr Surg. 2012;130:249–58. doi: 10.1097/PRS.0b013e318254b4d3. [DOI] [PubMed] [Google Scholar]
- 5.Pu LL. Towards more rationalized approach to autologous fat grafting. J Plast Reconstr Aesthet Surg. 2012;65:413–9. doi: 10.1016/j.bjps.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 6.Padoin AV, Braga-Silva J, Martins P, Rezende K, Rezende AR, Grechi B, et al. Sources of processed lipoaspirate cells: Influence of donor site on cell concentration. Plast Reconstr Surg. 2008;122:614–8. doi: 10.1097/PRS.0b013e31817d5476. [DOI] [PubMed] [Google Scholar]
- 7.Gutowski KA. ASPS Fat Graft Task Force. Current applications and safety of autologous fat grafts: A report of the ASPS fat graft task force. Plast Reconstr Surg. 2009;124:272–80. doi: 10.1097/PRS.0b013e3181a09506. [DOI] [PubMed] [Google Scholar]
- 8.Villani F, Caviggioli F, Giannasi S, Klinger M, Klinger F. Current applications and safety of autologous fat grafts: A report of the ASPS Fat Graft Task Force. Plast Reconstr Surg. 2010;125:758–9. doi: 10.1097/PRS.0b013e3181c722cf. [DOI] [PubMed] [Google Scholar]
- 9.Sultan SM, Stern CS, Allen RJ, Jr, Thanik VD, Chang CC, Nguyen PD, et al. Human fat grafting alleviates radiation skin damage in a murine model. Plast Reconstr Surg. 2011;128:363–72. doi: 10.1097/PRS.0b013e31821e6e90. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto D, Sato K, Gonda K, Takaki Y, Shigeura T, Sato T, et al. Cell-assisted lipotransfer: Supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12:3375–82. doi: 10.1089/ten.2006.12.3375. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi M, Matsumoto F, Bujo H, Shibasaki M, Takahashi K, Yoshimoto S, et al. Revascularization determines volume retention and gene expression by fat grafts in mice. Exp Biol Med (Maywood) 2005;230:742–8. doi: 10.1177/153537020523001007. [DOI] [PubMed] [Google Scholar]
- 12.Shoshani O, Berger J, Fodor L, Ramon Y, Shupak A, Kehat I, et al. The effect of lidocaine and adrenaline on the viability of injected adipose tissue – An experimental study in nude mice. J Drugs Dermatol. 2005;4:311–6. [PubMed] [Google Scholar]
- 13.Atik B, Oztürk G, Erdogan E, Tan O. Comparison of techniques for long-term storage of fat grafts: An experimental study. Plast Reconstr Surg. 2006;118:1533–7. doi: 10.1097/01.prs.0000240806.19404.a8. [DOI] [PubMed] [Google Scholar]
- 14.Fagrell D, Eneström S, Berggren A, Kniola B. Fat cylinder transplantation: An experimental comparative study of three different kinds of fat transplants. Plast Reconstr Surg. 1996;98:90–6. doi: 10.1097/00006534-199607000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Viterbo F, Marques M, Valente M. Fat-tissue injection versus graft: Experimental study in rabbits. Ann Plast Surg. 1994;33:184–92. doi: 10.1097/00000637-199408000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Marques A, Brenda E, Saldiva PH, Amarante MT, Ferreira MC. Autologous fat grafts: A quantitative and morphometric study in rabbits. Scand J Plast Reconstr Surg Hand Surg. 1994;28:241–7. doi: 10.3109/02844319409022006. [DOI] [PubMed] [Google Scholar]
- 17.Kruschewsky Lde S, de Mello-Filho FV, dos Santos AC, Rosen CA. Autologous fat graft absorption in unilateral paralyzed canine vocal folds. Laryngoscope. 2007;117:96–100. doi: 10.1097/01.mlg.0000245013.98844.30. [DOI] [PubMed] [Google Scholar]
- 18.Mikus JL, Koufman JA, Kilpatrick SE. Fate of liposuctioned and purified autologous fat injections in the canine vocal fold. Laryngoscope. 1995;105:17–22. doi: 10.1288/00005537-199501000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Zocchi ML, Zuliani F. Bicompartmental breast lipostructuring. Aesthetic Plast Surg. 2008;32:313–28. doi: 10.1007/s00266-007-9089-3. [DOI] [PubMed] [Google Scholar]
- 20.Tanna N, Wan DC, Kawamoto HK, Bradley JP. Craniofacial microsomia soft-tissue reconstruction comparison: Inframammary extended circumflex scapular flap versus serial fat grafting. Plast Reconstr Surg. 2011;127:802–11. doi: 10.1097/PRS.0b013e3181fed6e4. [DOI] [PubMed] [Google Scholar]
- 21.Gentile P, Oriandi A, Scioli MG, Kurita M, Oshima Y, Sato K, et al. A comparative translational study: The combined use of enhanced stromal vascular fraction and platelet-rich plasma improves fat grafting maintenance in breast reconstruction. Stem Cell Transl Med. 2012;1:341–51. doi: 10.5966/sctm.2011-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keyhan SO, Hemmat S, Badri AA, Abdeshahzadeh A, Khiabani K. Use of platelet-rich fibrin and platelet-rich plasma in combination with fat graft: Which is more effective during facial lipostructure? J Oral Maxillofac Surg. 2013;71:610–21. doi: 10.1016/j.joms.2012.06.176. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura K, Asano Y, Aoi N, Kurita M, Oshima Y, Sato K, et al. Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J. 2010;16:169–75. doi: 10.1111/j.1524-4741.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- 24.Har-Shai Y, Lindenbaum ES, Gamliel-Lazarovich A, Beach D, Hirshowitz B. An integrated approach for increasing the survival of autologous fat grafts in the treatment of contour defects. Plast Reconstr Surg. 1999;104:945–54. doi: 10.1097/00006534-199909040-00008. [DOI] [PubMed] [Google Scholar]
- 25.Khouri RK, Eisenmann-Klein M, Cardoso E, Cooley BC, Kacher D, Gombos E, et al. Brava and autologous fat transfer is a safe and effective breast augmentation alternative: Results of a 6-year, 81-patient, prospective multicenter study. Plast Reconstr Surg. 2012;129:1173–87. doi: 10.1097/PRS.0b013e31824a2db6. [DOI] [PubMed] [Google Scholar]
- 26.Report on autologous fat transplantation. ASPRS Ad-Hoc committee on new procedures, September 30, 1987. Plast Surg Nurs. 1987;7:140–1. [PubMed] [Google Scholar]
- 27.Mojallal A, Veber M, Shipkov C, Ghetu N, Foyatier JL, Braye F. Analysis of a series of autologous fat tissue transfer for lower limb atrophies. Ann Plast Surg. 2008;61:537–43. doi: 10.1097/SAP.0b013e318164088f. [DOI] [PubMed] [Google Scholar]
- 28.Nelson L, Stewart KJ. Psychological morbidity and facial volume in HIV lipodystrophy: Quantification of treatment outcome. J Plast Reconstr Aesthet Surg. 2012;65:439–47. doi: 10.1016/j.bjps.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48–55. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Negredo E, Higueras C, Adell X, Martinez JC, Martinez E, Puig J, et al. Reconstructive treatment for antiretroviral-associated facial lipoatrophy: A prospective study comparing autologous fat and synthetic substances. AIDS Patient Care STDS. 2006;20:829–37. doi: 10.1089/apc.2006.20.829. [DOI] [PubMed] [Google Scholar]
- 31.Shaw GY, Szewczyk MA, Searle J, Woodroof J. Autologous fat injection into the vocal folds: Technical considerations and long-term follow-up. Laryngoscope. 1997;107:177–86. doi: 10.1097/00005537-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Hörl HW, Feller AM, Biemer E. Technique for liposuction fat reimplantation and long-term volume evaluation by magnetic resonance imaging. Ann Plast Surg. 1991;26:248–58. doi: 10.1097/00000637-199103000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Coleman SR. Facial recontouring with lipostructure. Clin Plast Surg. 1997;24:347–67. [PubMed] [Google Scholar]
- 34.Ersek RA. Transplantation of purified autologous fat: A 3-year follow-up is disappointing. Plast Reconstr Surg. 1991;87:219–27. [PubMed] [Google Scholar]
- 35.Pérez-Cano R, Vranckx JJ, Lasso JM, Calabrese C, Merck B, Milstein AM, et al. Prospective trial of adipose-derived regenerative cell (ADRC)-enriched fat grafting for partial mastectomy defects: The RESTORE-2 trial. Eur J Surg Oncol. 2012;38:382–9. doi: 10.1016/j.ejso.2012.02.178. [DOI] [PubMed] [Google Scholar]
- 36.Fontdevila J, Serra-Renom JM, Raigosa M, Berenguer J, Guisantes E, Prades E, et al. Assessing the long-term viability of facial fat grafts: An objective measure using computed tomography. Aesthet Surg J. 2008;28:380–6. doi: 10.1016/j.asj.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen A, Pasyk KA, Bouvier TN, Hassett CA, Argenta LC. Comparative study of survival of autologous adipose tissue taken and transplanted by different techniques. Plast Reconstr Surg. 1990;85:378–86. [PubMed] [Google Scholar]
- 38.Rigotti G, Marchi A, Galiè M, Baroni G, Benati D, Krampera M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409–22. doi: 10.1097/01.prs.0000256047.47909.71. [DOI] [PubMed] [Google Scholar]
- 39.Leboulanger N, Blanchard M, Denoyelle F, Glynn F, Charrier JB, Roger G, et al. Autologous fat transfer in velopharyngeal insufficiency: Indications and results of a 25 procedures series. Int J Pediatr Otorhinolaryngol. 2011;75:1404–7. doi: 10.1016/j.ijporl.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Zaidi HA, Pendleton C, Cohen-Gadol AA, Quinones-Hinojosa A. Harvey Cushing's repair of a dural defect after a traumatic brain injury: Novel use of a fat graft. World Neurosurg. 2011;75:696–9. doi: 10.1016/j.wneu.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Palou J, Caparrós-Sariol J, Salvador-Bayarri J, Villavicencio-Mavrich H, Vicente-Rodriguez J. Polytetrafluoroethylene and autologous fat endoscopic injection for a prostato-perineal fistula. J Urol. 2001;165:882–3. [PubMed] [Google Scholar]