Abstract
Background
Equine neuroaxonal dystrophy/equine degenerative myeloencephalopathy (NAD/EDM) is a neurodegenerative disorder affecting genetically predisposed foals maintained on α‐tocopherol (α‐TP)‐deficient diet.
Objective
Intramuscular α‐TP and selenium (Se) administration at 4 days of age would have no significant effect on serum or cerebrospinal fluid (CSF) α‐TP in healthy foals. Serum and CSF α‐TP, but not Se, would be significantly decreased in NAD/EDM‐affected foals during first year of life.
Animals
Fourteen Quarter horse foals; 10 healthy foals supplemented with 0.02 mL/kg injectable α‐TP and Se (n = 5) or saline (n = 5) at 4 days of age and 4 unsupplemented NAD/EDM‐affected foals.
Methods
Complete neurologic examinations were performed, blood and CSF were collected before (4 days of age) and after supplementation at 10, 30, 60, 120, 180, 240, and 360 days of age. Additional blood collections occurred at 90, 150, 210, and 300 days. At 540 days, NAD/EDM‐affected foals and 1 unsupplemented healthy foal were euthanized and necropsies performed.
Results
Significant decreases in blood, CSF α‐TP and Se found in the first year of life in all foals, with most significant changes in serum α‐TP from 4–150 days. Dam α‐TP and Se significantly influenced blood concentrations in foals. Injection of α‐TP and Se did not significantly increase CSF Se, blood or CSF α‐TP in healthy foals. NAD/EDM‐affected foals had significantly lower CSF α‐TP through 120 days.
Conclusions and Clinical Importance
Injection of α‐TP and Se at 4 days of age does not significantly increase blood or CSF α‐TP. Despite all 14 foals remaining deficient in α‐TP, only the 4 genetically predisposed foals developed NAD/EDM.
Keywords: Ataxia, Equine, Genetics, Vitamin E
Abbreviations
- α‐TP
α‐tocopherol
- CI
confidence interval
- CSF
cerebrospinal fluid
- EDM
equine degenerative myeloencephalopathy
- NAD
neuroaxonal dystrophy
- QH
Quarter Horse
- RRR‐α‐TP
natural (or ‐d) α‐tocopherol
- Se
selenium
- VitE
vitamin E
The major dietary source of vitamin E (vitE) in horses is grazing pasture, providing approximately 2,000 IU/day.1 With recent drought conditions,2 pasture has become scarce in many regions of the United States and the amount and quality of hay, the alternate source of vitE, has decreased dramatically. According to the 2007 National Research Council (NRC), the dietary requirements of vitE for horses range from 1–2 IU/kg body weight,3 which is not provided by the quality of forage currently available in many regions or in most commercial feeds for horses.
Vitamin E refers to a closely related family of 8 fat‐soluble naturally occurring compounds.4 The family consists of 2 subgroups: tocopherols (saturated) and tocotrienols (unsaturated). Within each subgroup, there are 4 individual isoforms (α, β, γ and δ). Alpha‐tocopherol (α‐TP) is the most biologically available and most potent antioxidant.5 When concentrations of vitE are measured in biological samples, α‐TP typically is the isoform measured.6
Neuroaxonal dystrophy/equine degenerative myeloencephalopathy (NAD/EDM) is a neurologic condition that develops in genetically predisposed foals maintained on an α‐TP‐deficient diet.7, 8 Although the etiology of NAD/EDM remains unknown, the disease appears to be prevented, or at least minimized, if pregnant mares and genetically susceptible foals are supplemented with α‐TP.7, 8 In particular, injectable vitE supplementation (amount and type not specified) was found to be protective against the development of NAD/EDM in a bivariate screening analysis.9 The disease appears to develop during the first year of life10 and, in humans, there is strong evidence that the developing nervous system is particularly at risk from α‐TP deficiency.11
Studies assessing serum α‐TP concentrations in late‐term broodmares not maintained on pasture have found that many mares are deficient in α‐TP, with concentrations ranging from 1.37–1.93 μg/mL without any clinical signs attributable to deficiency.12, 13, 14 When access to fresh pasture is limited, many breeders supplement neonatal foals with α‐TP. Daily oral supplementation of α‐TP to neonatal foals is difficult and labor‐intensive on large‐scale breeding farms. In addition, supplementation of dams with α‐TP during gestation is unlikely to cause substantial increases in the foal's α‐TP status in utero because α‐TP does not cross the placenta.12 Therefore, supplementation in α‐TP‐deficient foals typically consists of an intramuscular injection of d‐alpha‐tocopheryl acetate, a synthetic formulation of α‐TP, which is combined with selenium (Se), another potent antioxidant.15 At this time, E‐Se® 1 is the only FDA‐approved injectable α‐TP and Se supplement for horses.
The purpose of this study was to determine the concentrations of α‐TP and Se during the first year of life in foals without access to pasture and describe the effects of a single injection of α‐TP/Se administered at 4 days of age. Because the impact of α‐TP deficiency lies in neural tissues,16 CSF concentrations were also evaluated. We hypothesized that administration of injectable vitE and Se would have no significant effect on serum or CSF α‐TP concentrations, whereas significantly increasing the whole blood and CSF Se concentrations in healthy foals. The second objective was to compare these measurements to those collected from 4 genetically susceptible NAD/EDM foals and monitor the progression of the disease during the first year of life. We hypothesized that the concentrations of serum and CSF α‐TP, but not Se, would be significantly decreased in NAD/EDM‐affected foals throughout the first year of life.
Materials and Methods
The study was divided among 3 foaling seasons (2010–12).
Animals and Diet
Fourteen breedings were performed. Twelve Quarter horse (QH) mares (4 in 2009, 6 in 2010, and 4 in 2011 [2 mares bred in 2009 and 2011]) were bred to 1 of 4 stallions (1 Thoroughbred, 3 QHs; Fig 1). Before breeding, a complete neurologic examination was performed on each mare and 3 of the 4 stallions (1 QH stallion unavailable) by one of the authors (CF). The 3 neurologically abnormal mares used in this study were a subset of potential NAD/EDM horses from a previous study8 that were subsequently donated to the UC Davis Center for Equine Health. These mares previously had produced postmortem confirmed NAD/EDM‐affected foals.8 The mares' diets were adjusted to meet their dietary energy and protein requirements at each stage of gestation and throughout lactation, and α‐TP and Se were measured in the grass hay and concentrate2 each year (Tables 1 and 2). The diets were designed to be deficient in vitE with adequate Se concentrations, and all mares and foals were maintained on the same type of hay and concentrate2 fed according to body weight (hay) and label recommendations (concentrate2); (Table 2). Hay was stored in a covered barn and protected from sunlight throughout the study period. Mares had no access to pasture at any time during gestation.
Figure 1.
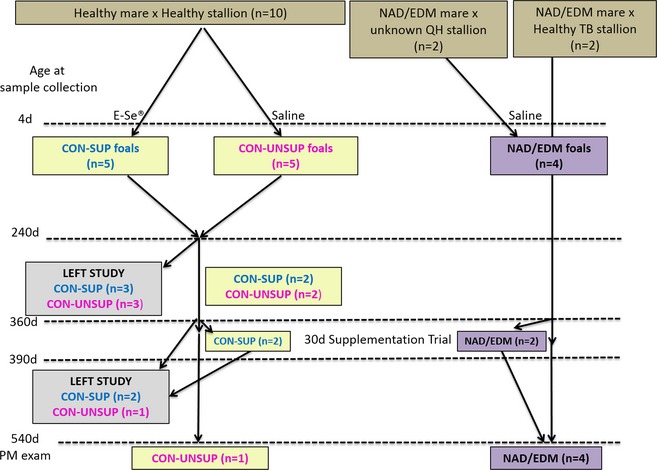
Groups of foals in study. E‐Se® (0.02 mL/kg) was administered after the 4 d sampling time point. NAD/EDM = neuroaxonal dystrophy/equine degenerative myelencephalopathy, QH = Quarter horse, TB = Thoroughbred, d = days, CON‐SUP = healthy foals supplemented with E‐Se®, CON‐UNSUP = healthy foals supplemented with saline, PM = post‐mortem.
Table 1.
Dietary analysis of timothy hay (over 3 years; performed by Dairy One11) and estimated concentratea analysis per manufacturer's label calculated on a dry matter basis
| Component | Timothy Grass Hay | Concentratea | ||
|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | ||
| % Dry matter | 90.2 | 76 | 89 | 85 |
| DE (Mcal/kg DM) | 2.38 | 2.31 | 2.42 | 3.08 |
| Crude protein (g/kg DM) | 190 | 180 | 199 | 89 |
| Vitamin E (IU/kg DM) | 22.4 | 25.6 | 23.6 | 4.41 |
| Selenium (mg/kg DM) | 0.17 | 0.31 | 0.22 | 0.35 |
DM, dry matter. aFarmers Best Sweet Cob, Keyes, CA.
Table 2.
Dietary analysis of timothy hay and concentratea calculated on a dry matter basis. Hay energy, protein, vitamin E and selenium values are represented as the median and range over the 3 years (see Table 1 for analysis per year). Nutritional Research Council dietary energy, total protein and total Se concentrations were met or exceeded for each life stage, whereas total vitE concentrations were deficient
| Stage | Approximate Weight (kg) | Hay (% BW) | Grain (kg) | Calculated and Reported on a Dry Matter Basis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total MCal | DE Requirementb (MCal)/day | Total Protein (g) | CP Requirementb (g/day) | Total vitE (IU) | VitE Requirementb (IU/day) | Total Se (mg) | Se Requirementb (mg/day) | ||||
| Gestation: Months 1–8 | 454 | 2 | 0.91 | 22.8 (19–22.8) | 15–17 | 1,697 (1,368–1,760) | 622–689 | 218 (208–237) | 500 | 2.36 (2.2–3.2) | 2.2–3 |
| Gestation: Months 9–11 | 514 | 2 | 1.82 | 26.8 (22.8–26.9) | 18–20 | 1,899 (1,544–1,968) | 723–811 | 272 (259–295) | 500 | 3.15 (2.5–4.2) | 2.2–3 |
| Lactation | 454 | 2.5 | 2.72 | 31.5 (27–31.6) | 27–29 | 2,151 (1,758–2,226) | 1,269–1,394 | 280 (267–303) | 1,000 | 3.52 (2.8–4.5) | 2.2–3 |
| Growing foal: 4‐6 months | 200 | 2.5 | 0.91 | 13.12 (12–13.2) | 12–14 | 926 (753–959) | 607–614 | 122 (116–132) | 306–392 | 1.44 (1.2–1.9) | 0.31–0.39 |
| Growing foal: 7–12 months | 300 | 2.5 | 0.91 | 18.5 (15.5–18.5) | 14–17 | 1,354 (1,095–1,404) | 614–768 | 181 (116–196) | 392–583 | 2.00 (1.6–2.7) | 0.39–0.58 |
BW, body weight; CP, crude protein; vitE, vitamin E; IU, international units; Se, selenium. aFarmers Best Sweet Cob, Keyes, CA. bNutritional Research Council. Nutrient Requirements of Horses 2007; 6th edition, Washington, D.C.
All foals were born between February and May (7 colts and 7 fillies). Each foaling was attended and every foal received a veterinary examination, including a SNAP® Foal IgG test3 to verify passive transfer of colostral antibodies. Day 0 for each foal was defined as its date of birth. Each mare and foal pair remained in a stall, and turnout consisted of a dry lot. At 4 months of age, foals were weaned, housed together in separate dry lot paddocks and fed the same timothy grass hay at 2.5% of their body weight and grain2 to meet their dietary energy requirements (Table 2). Pasture was not provided. All protocols were approved by the UCD Institutional Animal Care and Use Committee (Protocol # 15866).
Study Design and Sample Collection
At foaling, colostral samples were collected from 6/14 mares. At 4 days, a complete neurologic examination was performed on each foal and video‐recorded. Serum samples (light‐protected on ice) and EDTA whole blood were collected from the dam. The foals were sedated with diazepam4 (0.2 mg/kg IV) and an IV catheter5 was placed. At 4 and 10 days of age, anesthesia in foals consisted of premedication with an additional 0.1 mg/kg diazepam4 IV and 0.25 mg/kg xylazine6 IV, and anesthesia was induced with ketamine hydrochloride (1.5 mg/kg IV). The atlanto‐occipital region was used for CSF collection to minimize blood contamination. Cerebrospinal fluid (6–8 mL) was aliquotted into plastic light‐protected vials on ice and centrifuged at 4°C (2,000 × g for 15 min) within 1 h of collection. Supernatant was collected into precooled cryovials, immediately flash frozen in liquid nitrogen, and maintained at −80°C until analysis. Serum and EDTA whole blood samples were collected from each foal via the IV catheter after removal of 6 mL of heparinized blood. Serum samples were processed in an identical manner as CSF. After sample collection at this time point (4 days), foals from healthy mares were randomly divided into 2 groups (Fig 1). The supplemented group (CON‐SUP, n = 5) received 0.02 mL/kg (equivalent to 1.5 IU/kg d‐alpha tocopheryl acetate and 0.055 mg/kg Se selenite) of an injectable α‐TP and Se solution (E‐SE® 1) IM into the right semimembranosus muscle. The unsupplemented group (CON‐UNSUP, n = 5) received an equivalent volume of saline. Four foals born from neurologically abnormal mares (NAD/EDM) were given the saline placebo. After the sample collections were completed, flunixin meglumine7 (1.1 mg/kg IV) was administered and the foal was recovered from anesthesia. The same collection procedure was used on each foal at the following days of age: 10, 30, 60, 120, 180, and 240. At 30 days, the anesthetic protocol was changed to premedication with xylazinef (1.0 mg/kg IV) followed by 0.1 mg/kg diazepam4 and 2.2 mg/kg ketamine hydrochloride8 IV. Six foals (3 CON‐SUP and 3 CON‐UNSUP) were adopted out of the research herd after 240 days of age, whereas 8 foals (2 CON‐SUP, 2 CON‐UNSUP and 4 NAD/EDM) were available for additional CSF collections at 360 days (Fig 1).
Alpha‐TP Supplementation
In 2 CON‐SUP foals and 2 NAD/EDM foals, after the 360‐day sampling time point, supplementation with natural (or ‐d) α‐tocopherol (RRR‐α‐TP9) (6.67 IU/kg po q24 h) was implemented for 30 days. Repeat sampling was performed in an identical manner at 15 and 30 days postsupplementation. Supplementation was subsequently discontinued and NAD/EDM foals were maintained on the diet described previously until euthanasia at 1.5 years of age.
Postmortem Evaluation
At 1.5 years of age, 4 NAD/EDM foals and 1 CON‐UNSUP foal were euthanized with an overdose of pentobarbital10 (100 mg/kg IV) and a complete postmortem evaluation was performed as previously described.8 Liver samples were collected and stored at –80°C until micronutrient analysis was performed.
Alpha‐TP and Se Analyses
Serum, whole blood and CSF samples were analyzed within 6 months of collection. Concentrations of α‐TP in serum samples, in pulverized fresh‐frozen liver samples collected at necropsy and in grain and hay were determined by high‐performance liquid chromatography with fluorescence detection as previously described.8 Whole blood, CSF, liver, hay, and grain samples were prepared and analyzed by inductively coupled argon plasma spectrometry according to standard protocols for Se.17 Submitted hay and grain samples were analyzed for percent moisture and vitE and Se analytes were converted to a dry matter basis for calculations. VitE and Se analytes in hay were measured once, at the time of foaling, throughout each 12‐month period. Protein and energy analysis of hay, sampled with a core sampler (10 bales), was performed on a dry matter basis by a forage laboratory11 and protein and energy contents of the concentrate were estimated from the manufacturer's analysis.
Statistical Analysis
Concentrations of serum and CSF α‐TP and whole blood and CSF Se each were log‐transformed and modeled with linear mixed models. Fixed effects were group status, time (as a linear effect) with different slopes for each group status, sex, and year, and random effects included both an intercept and a slope for time for each subject. A secondary model was fitted for each variable that also included serum α‐TP or Se concentration of the dam as a fixed effect; this was not included in the primary model because the dam measurements were made over a much smaller set of time points. For the same reason, sex and year were not included in the primary model assessing dam serum α‐TP or Se concentrations as the variable of interest. Visual inspection of residual plots did not identify any obvious deviations from homoscedasticity or normality. For each model, P values for each term of interest were calculated using Type II F‐tests using Kenward–Rogers degrees of freedom; for the time/group differences, tests included the difference between CON‐SUP and CON‐UNSUP (for both intercept and slope together) and the difference between NAD/EDM and the average of CON‐SUP and CON‐UNSUP (again for both intercept and slope together). Confidence intervals for parameters of interest were computed. To explore differences further, t‐tests between the NAD group and a combined control group (CON‐SUP and CON‐UNSUP) and regressions on dam α‐TP or Se concentration were performed for each variable at each time point, with P values corrected across time points using the Bonferroni–Holm adjustment. Models were fit in R18 using the lme419 package with tests performed using the car and lmerTest packages.
Results
None of the foals suffered any adverse effects from the multiple CSF testing procedures. Cerebrospinal fluid cytology was normal (total nucleated cell count <5/μL and normal cytologic differential cell count) in all samples obtained with the exception of blood contamination (2,621 ± 2,860 RBCs/μL) in 11/106 CSF collections. Blood contamination of CSF up to 9,550 RBCs/μL did not appear to alter CSF α‐TP and Se concentrations; these samples therefore remained in the analyses. Repeat CSF sampling did not affect the CSF total nucleated cell count. Body weight of CON and NAD/EDM foals did not differ significantly at any time point (P adj > 0.05). One CON‐SUP foal (2010) had missing values for the 4‐day collection of CSF and 2 CON‐SUP foals (2010) had missing whole blood Se concentrations at each time point. These 2 CON‐SUP foals were excluded from the blood Se analysis.
Neurologic Evaluation
Neurologic deficits were not observed in the 3 stallions and 9/12 mares. Three QH mares (NAD/EDM group; 1 mare bred both in year 1 and year 3) demonstrated general proprioceptive ataxia (grade 2–3/520), without evidence of paresis, as previously described.8 Neurologic deficits did not develop in any of the CON foals. All 4 NAD/EDM foals developed neurologic deficits, with general proprioceptive abnormalities characterized by hypermetria, interference during circling and abnormal posture. Consistent neurologic deficits were first observed at 4 months (n = 2) and 6 months (n = 2) of age, with scores ranging from 2 to 2.5/520 (Table S1). The menace response was only evaluated in foals >1 month of age and found to be normal in all CON foals. An inconsistent menace response, as previously reported,8 was apparent in 3 of 4 NAD/EDM foals by 2 (n = 1), 4 (n = 1), or 6 (n = 1) months of age. Foals with NAD/EDM resulted from both the affected QH mares × unaffected QH stallion and affected QH mares × unaffected Thoroughbred stallion crosses.
Colostrum Samples
Colostrum samples were available from 6 mares (4 CON and 2 NAD/EDM). Colostrum α‐TP concentrations were lower in NAD/EDM mares (0.54 and 0.64 μg/mL) than in dams of CON foals (median, 1.45; range, 0.7–2.2 μg/mL). Colostrum Se concentrations of NAD/EDM mares (0.024 and 0.027 μg/mL) were comparable to concentrations in the samples from dams of CON foals (median, 0.031; range, 0.026–0.05 μg/mL).
Foal Serum and CSF α‐TP Concentrations
For both serum and CSF α‐TP, there was no significant difference between CON‐SUP and CON‐UNSUP foals, and therefore no effect of a single E‐Se® injection (P = .30 and P = .58, respectively). Therefore, CON‐SUP and CON‐UNSUP foals were combined into a single CON group. There was a significant (P < .0001) decrease in serum (Fig 2) and CSF (Fig 3) α‐TP concentration over time. This time effect was different between the combined CON group and the NAD/EDM group for both serum and CSF α‐TP concentrations (P = .0027 and P = .033, respectively), with a steeper early decrease evident in the CON group. For serum, the t‐tests between the groups were significant (P adj < 0.05) between 4 and 150 days (except for day 120), with average NAD/EDM concentrations ranging from 43 to 65% of the average combined CON concentrations. For CSF α‐TP concentration, although the mixed model showed a significant difference, adjusting the t‐tests for multiple comparisons lost enough power that none of the adjusted P values were significant. However, the unadjusted t‐tests for differences between the groups were significant up to day 120, with average NAD/EDM CSF α‐TP concentrations ranging from 47 to 73% of the average combined CON concentrations.
Figure 2 and 3.
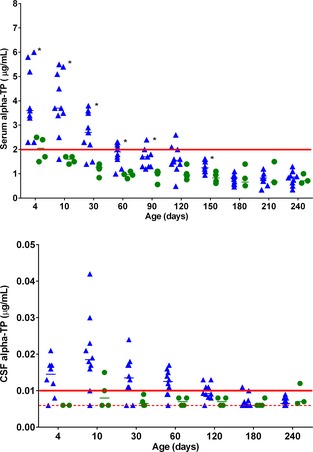
Individual scatter plot and of serum (Fig. 2) and cerebrospinal fluid (CSF; Fig. 3) alpha‐tocopherol (α‐TP) concentrations in foals during the first 240 days of life. Data for healthy supplemented (CON‐SUP) and healthy unsupplemented (CON‐UNSUP) were combined (blue triangles, n = 10). Foals that developed neuroaxonal dystrophy/equine degenerative myeloencephalopathy (NAD/EDM) are represented in green circles (n = 4). Median values are denoted by a horizontal bar. Normal reference ranges are denoted by the red solid line. The red hatched line represents the limit of detection of the CSF assay (0.07 μg/mL). The level of *P adj < .05 is based on a Bonferroni‐adjusted t‐test (CON versus NAD/EDM).
In addition, dam serum α‐TP concentration was significantly associated with foal serum α‐TP concentration (P < .0001), but not foal CSF α‐TP concentration (P = .31). A doubling of the dam serum α‐TP concentration resulted in an average increase of 1.88 times (95% CI, 1.48, 2.41) for the foal's serum α‐TP concentration. For both serum and CSF α‐TP concentrations, there was no significant difference between year (P = .94 and P = .74) or sex (P = .87 and P = .82).
Foal Whole Blood and CSF Se Concentrations
For CSF Se concentration, there was no significant difference between CON‐SUP and CON‐UNSUP foals and therefore no effect of a single E‐Se® injection (P = .17). An effect of an E‐Se® injection on whole blood Se concentration could not be assessed because of the missing data points in 2 of the CON‐SUP foals. Data for whole blood and CSF Se was analyzed with 3 groups (CON‐SUP, CON‐UNSUP, and NAD/EDM).
There was a significant decrease in whole blood (P < .001; Fig 4) and CSF (P < .0001; Fig 5) Se concentration over time. There was no difference in the rate of decrease between the combined CON group and the NAD/EDM groups. For whole blood and CSF Se, the t‐tests between the groups were not significant (P adj > .05).
Figure 4 and 5.
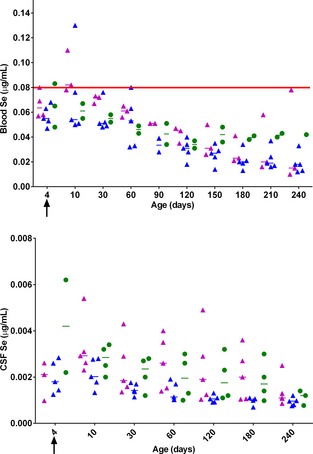
Individual scatter plot of whole blood (Fig. 2) and cerebrospinal fluid (CSF; Fig. 3) selenium (Se) concentrations in foals during the first 240 days of life; CON‐SUP (purple triangles, n = 5; not all data points available), CON‐UNSUP (blue triangles, n = 5) and NAD/EDM (green circles, n = 4; not all data points available). Median values are denoted by a horizontal bar. Arrow depicts the time point that E‐Se® (0.2 mL/kg) or saline was administered. The normal reference range is denoted by the red solid line.
In addition, dam Se was significantly associated with whole blood (P = .013), but not CSF (P = .37) Se concentration. A doubling of the dam Se concentration resulted in an average increase of 1.31 times (95% CI, 1.09, 1.58) for the foal's whole blood Se concentration. For both serum and CSF Se, there was no significant difference between sexes (P = .94 and .32, respectively). A significant difference was observed for CSF (P = .025) but not whole blood (P = .53) Se concentration, with slightly higher CSF Se concentrations in the combined CON foals from year 1 versus year 2.
Postfoaling Dam α‐TP and Se Concentrations
Throughout the first 60 days postpartum, there was a significant decrease in serum α‐TP (P = .0058; Fig 6), but not whole blood Se (P = .16; Fig 7) concentrations. The significant time effect for dam serum α‐TP concentration was different between the combined CON group and the NAD/EDM groups (P = .032).
Figure 6 and 7.
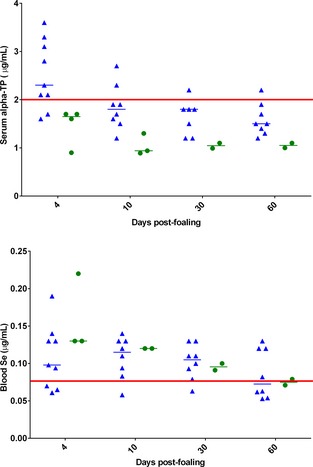
Individual scatter plot of dams' serum alpha‐tocopherol (α‐TP; Fig. 6) and whole blood selenium (Se; Fig. 7) concentrations from 4–60 days postfoaling. Data for dams of healthy supplemented (CON‐SUP) and healthy unsupplemented (CON‐UNSUP) were combined (blue triangles, n = 10; not all data points available). Dams of foals that developed neuroaxonal dystrophy/equine degenerative myeloencephalopathy (NAD/EDM) are represented in green circles (n = 4; not all data points available). Normal reference ranges are denoted by the red solid line.
Alpha‐TP Supplementation
After supplementation with 6.67 IU/kg of RRR‐α‐TPi, α‐TP concentrations increased in both serum and CSF in foals affected with NAD/EDM at concentrations comparable to age‐matched control foals (Table S2). No improvement was noted in their overall neurologic status.
Postmortem Evaluation
The 4 NAD/EDM foals were found to have lesions consistent with NAD (n = 3) or EDM (n = 1) as previously described.8 Subclinical histologic evidence of NAD/EDM was not observed in the CON‐UNSUP foal. Hepatic α‐TP concentrations were low (reference range, >4.5 μg/mL; limit of detection, 0.17 μg/mL wet weight) in the NAD/EDM foals (median, 0.65 μg/mL; range, <0.17–2.2) and in the CON‐UNSUP foal (1 μg/mL). Hepatic Se concentrations were low (reference range, 0.3–1 μg/mL) in the NAD/EDM foals (median, 0.062 μg/mL; range, 0.02–0.11) and in the CON‐UNSUP foal (0.072 μg/mL).
Discussion
Although previous studies have evaluated the effect of age on foal serum α‐TP and Se concentrations, foals were maintained on pasture and a strong seasonal effect was apparent, with increased concentrations occurring during the summer months, coinciding with the highest concentrations of α‐TP in the pasture.14, 21 Because adequate pasture has become less available in many regions, we sought to evaluate the effect of age on α‐TP and Se concentrations in foals maintained without pasture. Ours is the first study to report decreasing serum and CSF α‐TP and Se concentrations in healthy foals maintained without pasture during the first year of life. In addition, a single injection of α‐TP and Se, administered to healthy foals at 4 days, will cause a transient and limited increase in whole blood Se, but not CSF Se and serum and CSF α‐TP concentrations. Foals affected with NAD/EDM have decreased concentrations of serum and CSF α‐TP, but the lower concentrations of serum α‐TP are associated with lower serum dam α‐TP.
In our study, the pregnant mares and their foals were maintained on a diet that met or exceeded their dietary energy, crude protein and Se requirements, but was deficient in vitE according to the 2007 NRC recommendations.3 The mares were marginal or deficient in their serum α‐TP concentrations at 4 days postpartum and these concentrations decreased over time. Although only 4/10 CON dams were deficient in serum α‐TP concentration at 4 days postpartum, 8/10 were deficient by 30 days postpartum and 9/10 were deficient by 60 days postpartum with no change in diet or management. Although Se concentration decreased in some dams during this postpartum period, the effect was not as pronounced as for α‐TP. Previous studies in horses have identified no significant change in serum α‐TP or whole blood Se concentrations during the prepartum period and a similar decrease in serum α‐TP concentration during the first 2 weeks of lactation.21 In humans, infants typically are born with lower plasma α‐TP concentrations than their mothers22 because transplacental transfer of α‐TP is limited and selective for the natural RRR stereoisomer of α‐TP.23 In horses, as a result of their placentation, there is even more limited transfer of fat‐soluble components to the uterus and embryo.12 The concentration of α‐TP in equine colostrum is 5.7 times higher than in mature milk sampled at 21 days postpartum.12 In many other species, the accumulation of fat‐soluble vitamins in colostrum can be observed.24, 25 Similar to cows26 and ewes,27 mare milk α‐TP concentrations have been reported to be highest at 12 h postpartum with a progressive decline over the next 30 days.21 Interestingly, CON foals had higher serum α‐TP concentrations than their dams. This was a transient effect, with concentrations decreasing to those comparable to their dams by 60 days. It has been previously reported that α‐TP concentrations in mares and their foals are not well correlated, with some mares having a low α‐TP concentrations, whereas their foals had high concentrations and vice versa.28 In our study, the serum α‐TP concentration of the dam had a highly significant effect on foal serum α‐TP concentration. This finding suggests that supplementing mares with α‐TP during late gestation may provide the foal with additional α‐TP in the postnatal period, most likely through increased colostral concentrations.
A reference range of 4.1–13.5 ng/mL for CSF α‐TP concentration in adult horses has been suggested based on a recent study.29 In this study, however, horses were deficient in serum α‐TP (mean, 1.1 ± 0.35 μg/mL). If a CSF reference range for adult horses was based upon a normal serum α‐TP reference range (ie, the 10 days time point in the same study when horses had a mean serum α‐TP concentration of 2.96 ± 1.05 μg/mL), the CSF range would be set at 9.4–25.4 ng/mL. A reference range has not been determined previously in healthy neonatal foals. Because most of the CON foals maintained adequate serum α‐TP concentration until 30 days of age, the reference range of 9.4–25.4 ng/mL may be suitable for foals as well as adults. In healthy foals, serum and CSF α‐TP and Se concentrations were well correlated in our study, which is in agreement with a previous study that assessed concentrations post‐supplementation in adult horses.30
Whole blood Se concentrations of all the dams in this study remained above the reference range in the majority of mares until 60 days post‐foaling, whereas most foals were Se deficient (<0.08 μg/mL) throughout the first 8 months of life. Dam Se concentrations significantly affected whole blood Se concentration in foals, but did not significantly influence CSF Se concentration in the final model. Although the foals in this study remained deficient in Se for an extended period of time, there was no clinical evidence of nutritional myodegeneration. Nutritional myodegeneration, or white muscle disease, is because of a primary Se deficiency3 and many foals have normal α‐TP concentrations.31 It may be that additional genetic factors are required for the development of nutritional myodegeneration. A reference range for CSF Se concentrations in horses has not yet been established.
The majority of foals remained deficient in both α‐TP and Se from 60 to 240 days of age. Foals maintained on pasture throughout the first year of life do not demonstrate these deficiencies.21 Despite the strong evidence for synergy, deficiencies of both α‐TP and Se often are uncorrelated. In a study of horses on Prince Edward Island, 79% (159/201) were Se deficient, whereas only a subset (15/201) were vitE deficient.32 Therefore, despite the evidence of synergy between both of these antioxidants, it is recommended that α‐TP and Se status be assessed independently.
A single injection of E‐Se®, administered at 4 days, did not provide adequate α‐TP supplementation. Supplementation with E‐Se® increased whole blood Se in 3/5 CON‐SUP for which samples were available, but the effect was short‐lived and blood Se concentrations were comparable to the CON‐UNSUP group by 60 days of age. Although a single IM injection of E‐Se® increased CSF Se concentrations in CON‐SUP foals, the dams' Se status had a more pronounced effect on the foals' blood and CSF Se concentrations. Therefore, it may be more prudent to focus on α‐TP and Se supplementation of pregnant mares during late gestation rather than rely on a single dose of α‐TP and Se for supplementation. If Se supplementation is required in a neonate, it is recommended to repeat an E‐Se® injection again at 7–10 days after the initial dose to prevent the decrease observed between the 10 and 30 days time points. Oral supplementation with RRR‐α‐TP should be considered to effectively increase serum α‐TP concentrations in neonates.
Concentrations of colostral α‐TP and serum α‐TP from NAD/EDM dams at 4 and 10 days postpartum were lower than those of CON dams. We were unable to determine if NAD/EDM‐affected mares transport less α‐TP into their colostrum. However, the pronounced effect of dam serum α‐TP on foal serum α‐TP concentrations most likely resulted in the lower serum α‐TP concentrations in NAD/EDM foals because, once this was accounted for in the final model, there was no significant effect of disease on serum α‐TP status. In our study, the 3 dams of the 4 NAD/EDM‐affected foals were clinically affected with NAD/EDM. The NAD/EDM‐affected mare that was bred to a QH stallion in year 1 and bred to the Thoroughbred stallion in year 3 produced NAD/EDM‐affected foals with both breedings. Previous research supports an incompletely penetrant autosomal dominant mode of inheritance33 for NAD/EDM. Our study is consistent with this proposed mode of inheritance, with breedings of 2 NAD/EDM‐affected QH mares to a healthy Thoroughbred stallion producing NAD/EDM‐affected foals. It is unlikely that an autosomal recessive mode of inheritance would have resulted in 2 NAD/EDM‐affected foals when these 2 breeds were crossed.
The 4 genetically susceptible foals developed clinical signs of NAD/EDM by 6 months of age. Although foals that developed NAD/EDM had lower serum and CSF α‐TP concentrations than CON during the first 6 months of life, only CSF α‐TP concentrations remained significantly lower in the final model. This highlights the need to measure CSF, in addition to serum, α‐TP concentrations in young foals with suspected NAD/EDM. Of particular interest is that, after 6 months of age, there was no detectable difference in serum and CSF α‐TP concentrations between CON and NAD/EDM foals. Many studies comparing serum α‐TP concentrations between EDM‐affected foals and their healthy herd‐mates have reported no significant difference, but many of these foals were classified into groups that included foals >6 months of age when sampled.9 After 6 months of age, it would be difficult to determine if a foal is at an increased susceptibility to develop NAD/EDM based on a serum or CSF α‐TP concentration. Although whole blood Se concentrations were not available in all foals, there was no significant effect of NAD/EDM status on CSF Se concentrations. It has been previously reported that Se deficiency does not play a role in the development of NAD/EDM.7
All NAD/EDM foals developed symmetric ataxia, without evidence of paresis, consistent with NAD/EDM,8 by 4 months of age with the progression of signs and NAD/EDM confirmed at postmortem examination at 1.5 years of age. In 1 CON‐UNSUP foal that was examined in an identical manner, we did not observe any clinically apparent deficits or lesions consistent with NAD/EDM at necropsy. All other CON foals demonstrated no neurologic deficits at any time. Although NAD/EDM can develop between 2–3 years of age34 and the argument can therefore be made that this CON‐UNSUP foal could have developed disease later in life, careful observation of NAD/EDM in a large family of affected QHs has demonstrated that neurologic defects typically are present by 1 year of age.8 Therefore, we have validated that an α‐TP deficiency alone during the first year of life does not reliably cause NAD/EDM, a finding supported by other studies.14 Thirty‐day supplementation of 2 NAD/EDM yearlings with water‐dispersible RRR‐α‐TP resulted in serum and CSF α‐TP concentrations that were comparable to matched α‐TP‐deficient yearlings supplemented with the same dose of RRR‐ α‐TP. If the genetic defect underlying NAD/EDM is related to α‐TP transport, it appears that, with sufficient supplementation, affected foals can transport α‐TP into the CSF.
In conclusion, a single injection of α‐TP and Se, administered to neonatal foals during the first few days of life, will cause a transient and limited increase in whole blood, but not in CSF Se, or in serum and CSF α‐TP concentrations. Alpha‐TP supplementation of NAD/EDM‐affected mares during late gestation is warranted because neonatal foals appear to receive most of their α‐TP through the colostrum. In suspect NAD/EDM foals <6 months of age, measurement of both serum and CSF α‐TP concentrations is advised. In addition, supplementation of NAD/EDM genetically susceptible foals at birth and throughout the first year of life with a water‐dispersible formulation of RRR‐α‐TP is highly recommended.
Supporting information
Table S1. Neurologic examination results from the 4 NAD/EDM‐affected foals throughout the first 1.5 years of life.
Table S2. Serum and cerebrospinal fluid α‐tocopherol concentrations in 2 CON‐SUP and 2 NAD/EDM foals at 360 days of age and after 30 days of supplementation with RRR‐α‐tocopherol at 0.67 IU/kg PO once a day.
Acknowledgments
The authors acknowledge the large animal internal medicine residents, veterinary students and staff at the Center for Equine Health that assisted with this project.
Funding: This project was supported, in part, by the Center for Equine Health with funds provided by the State of California Pari‐Mutuel Fund and contributions by private donors. Dr. Finno's graduate work was supported, in part, by an NIH T32 grant (5 T32 DC 8072‐3) and her postdoctorate was supported by Morris Animal Foundation (D12EQ‐401) and NCATS (K01OD015134‐01A1).
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
All work was performed at the Center for Equine Health, University of California, Davis.
This work has not been presented at any meetings..
Footnotes
Schering‐Plough Animal Health Corp, Union, NJ.
Farmers Best Sweet Cob, Keyes, CA.
SNAP IgG Foal Kit; IDEXX Laboratories, Plainview, NY.
Valium® Hospira Inc, Lake Forest, IL.
Angiocath, Vascular Access; Becton, Dickinson & Co, Sandy, UT.
Rompun®; Mobay Corporation, Animal Health Division, Shawnee, KS.
Banamine®; Schering‐Plough, Madison, NJ.
Ketaject®; Phoenix Pharmaceutical, St. Joseph, MO.
EMCELLE Tocopherol; Stuart Products, Bedford, TX.
Euthasol®; Virbac AH, Fort Worth, TX.
Dairy One, Ithaca, NY.
References
- 1. Craig AM, Blythe LL. Antioxidants: Diseases associated with deficiencies and therapeutic usages in equine practice. 38th Annual Convention of the American Association of Equine Practitioners Orlando, FL 1992:579–580.
- 2. Heim R, Love‐Brotak L. U.S. Drought Monitor 2015. Available at: http://drought.unl.edu/dm. Accessed January 15, 2015.
- 3. NRC . Nutrient Requirements of Horses, 6th ed. Washington, DC: NRC; 2007. [Google Scholar]
- 4. Schneider C. Chemistry and biology of vitamin E. Mol Nutr Food Res 2005;49:7–30. [DOI] [PubMed] [Google Scholar]
- 5. Mustacich DJ, Bruno RS, Traber MG. Vitamin E. Vitam Horm 2007;76:1–21. [DOI] [PubMed] [Google Scholar]
- 6. Finno CJ, Valberg SJ. A comparative review of vitamin E and associated equine disorders. J Vet Intern Med 2012;26:1251–1266. [DOI] [PubMed] [Google Scholar]
- 7. Mayhew IG, Brown CM, Stowe HD, et al. Equine degenerative myeloencephalopathy: A vitamin E deficiency that may be familial. J Vet Intern Med 1987;1:45–50. [DOI] [PubMed] [Google Scholar]
- 8. Aleman M, Finno CJ, Higgins RJ, et al. Evaluation of epidemiological, clinical, and pathological features of neuroaxonal dystrophy in Quarter Horses. J Am Vet Med Assoc 2011;239:823–833. [DOI] [PubMed] [Google Scholar]
- 9. Dill SG, Correa MT, Erb HN, et al. Factors associated with the development of equine degenerative myeloencephalopathy. Am J Vet Res 1990;51:1300–1305. [PubMed] [Google Scholar]
- 10. Mayhew IG, deLahunta A, Whitlock RH, et al. Spinal cord disease in the horse. Cornell Vet 1978;68(Suppl 6):1–207. [PubMed] [Google Scholar]
- 11. Muller DP, Goss‐Sampson MA. Neurochemical, neurophysiological, and neuropathological studies in vitamin E deficiency. Crit Rev Neurobiol 1990;5:239–263. [PubMed] [Google Scholar]
- 12. Schweigert FJ, Gottwald C. Effect of parturition on levels of vitamins A and E and of beta‐carotene in plasma and milk of mares. Equine Vet J 1999;31:319–323. [DOI] [PubMed] [Google Scholar]
- 13. Bondo T, Jensen SK. Administration of RRR‐alpha‐tocopherol to pregnant mares stimulates maternal IgG and IgM production in colostrum and enhances vitamin E and IgM status in foals. J Anim Physiol Anim Nutr 2011;95:214–222. [DOI] [PubMed] [Google Scholar]
- 14. Maenpaa PH, Koskinen T, Koskinen E. Serum profiles of vitamins A, E and D in mares and foals during different seasons. J Anim Sci 1988;66:1418–1423. [DOI] [PubMed] [Google Scholar]
- 15. Rotruck JT, Pope AL, Ganther HE, et al. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973;179:588–590. [DOI] [PubMed] [Google Scholar]
- 16. Muller DP. Vitamin E and neurological function. Mol Nutr Food Res 2010;54:710–718. [DOI] [PubMed] [Google Scholar]
- 17. Melton LA, Tracy ML, Moller G. Screening trace elements and electrolytes in serum by inductively‐coupled plasma emission spectrometry. Clin Chem 1990;36:247–250. [PubMed] [Google Scholar]
- 18. RDC T . R: A Language and Environment for Statistical Computing. Vienna, Austria: RDC T; 2005. [Google Scholar]
- 19. Bates D, Maechler M, Bolker B, et al. lme4: Linear Mixed‐Effects Models using Eigen and S4. R package version 1.1‐7 ed; 2014. https://CRAN.R-project.org/package=lme4. [Google Scholar]
- 20. Lunn DP, Mayhew IG. The neurologic evaluation of horses. Equine Vet Educ 1989;1:94–101. [Google Scholar]
- 21. Hargreaves BJ. Vitamin E status of thoroughbred horses and the antioxidant status of endurance horses In: Animal and Poultry Science. Blacksburg, VA: Virginia Polytech Institute and State University; 2002:217. [Google Scholar]
- 22. Debier C. Vitamin E during pre‐ and postnatal periods. Vitam Horm 2007;76:357–373. [DOI] [PubMed] [Google Scholar]
- 23. Acuff RV, Dunworth RG, Webb LW, et al. Transport of deuterium‐labeled tocopherols during pregnancy. Am J Clin Nutr 1998;67:459–464. [DOI] [PubMed] [Google Scholar]
- 24. Pazak HE, Scholz RW. Effects of maternal vitamin E and selenium status during the perinatal period on age‐related changes in heart, lung and liver microsomal lipid peroxidation in rat pups. Int J Vitam Nutr Res 1996;66:134–140. [PubMed] [Google Scholar]
- 25. Hidiroglou M, Farnworth E, Butler G. Vitamin E and fat supplementation of sows and the effect on tissue vitamin E concentrations in their progeny. Reprod Nutr Dev 1993;33:557–565. [DOI] [PubMed] [Google Scholar]
- 26. Deschuytere A, Vermeylen K, Deelstra H. Vitamin E and selenium concentrations in milk and milkfractions. Z Lebensm Unters Forsch 1987;184:385–387. [DOI] [PubMed] [Google Scholar]
- 27. Njeru CA, McDowell LR, Wilkinson NS, et al. Pre‐ and postpartum supplemental DL‐alpha‐tocopheryl acetate effects on placental and mammary vitamin E transfer in sheep. J Ani Sci 1994;72:1636–1640. [DOI] [PubMed] [Google Scholar]
- 28. Lindholm A. Vitamin E and certain muscular enzymes in the blood serum of horses. Acta Agric Scand 1973;Suppl 19:40–42. [Google Scholar]
- 29. Higgins JK, Puschner B, Kass PH, et al. Assessment of vitamin E concentrations in serum and cerebrospinal fluid of horses following oral administration of vitamin E. Am J Vet Res 2008;69:785–790. [DOI] [PubMed] [Google Scholar]
- 30. Pusterla N, Puschner B, Steidl S, et al. alpha‐Tocopherol concentrations in equine serum and cerebrospinal fluid after vitamin E supplementation. Vet Rec 2010;166:366–368. [DOI] [PubMed] [Google Scholar]
- 31. Wilson TM, Morrison HA, Palmer NC, et al. Myodegeneration and suspected selenium/vitamin E deficiency in horses. J Am Vet Med Assoc 1976;169:213–217. [PubMed] [Google Scholar]
- 32. Muirhead TL, Wichtel JJ, Stryhn H, et al. The selenium and vitamin E status of horses in Prince Edward Island. Can Vet J 2010;51:979–985. [PMC free article] [PubMed] [Google Scholar]
- 33. Finno CJ, Famula T, Aleman M, et al. Pedigree analysis and exclusion of alpha‐tocopherol transfer protein (TTPA) as a candidate gene for neuroaxonal dystrophy in the American Quarter Horse. J Vet Intern Med 2013;27:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blythe LL, Hultgren BD, Craig AM, et al. Clinical, viral, and genetic evaluation of equine degenerative myeloencephalopathy in a family of Appaloosas. J Am Vet Med Assoc 1991;198:1005–1013. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Neurologic examination results from the 4 NAD/EDM‐affected foals throughout the first 1.5 years of life.
Table S2. Serum and cerebrospinal fluid α‐tocopherol concentrations in 2 CON‐SUP and 2 NAD/EDM foals at 360 days of age and after 30 days of supplementation with RRR‐α‐tocopherol at 0.67 IU/kg PO once a day.


