Abstract
Invasive infections of the central nervous system or digestive tract caused by commensal fungi of the genus Candida are rare and life-threatening. The known risk factors include acquired and inherited immunodeficiencies, with patients often displaying a history of multiple infections. Cases of meningo-encephalitis and/or colitis caused by Candida remain unexplained. We studied five previously healthy children and adults with unexplained invasive disease of the central nervous system, or the digestive tract, or both, caused by Candida spp. The patients were aged 39, 7, 17 37, and 26 years at the time of infection and were unrelated but each born to consanguineous parents of Turkish (two patients), Iranian, Moroccan or Pakistani origin. Meningo-encephalitis was isolated in three patients, associated with colitis in a fourth patient, and the fifth patient suffered from isolated colitis. Inherited CARD9 deficiency was recently reported in otherwise healthy patients with other forms of severe disease caused by Candida, Trichophyton, Phialophora, and Exophiala, including meningo-encephalitis, but not colitis, caused by Candida and Exophiala. We therefore sequenced CARD9 in the five patients. All were found to be homozygous for rare and deleterious mutant CARD9 alleles: R70W and Q289* for the three patients with isolated C. albicans meningo-encephalitis, R35Q for the patient with meningo-encephalitis and colitis caused by C. glabrata, and Q295* for the patient with C. albicans colitis. Regardless of their levels of mutant CARD9 protein, the patients’ monocyte-derived dendritic cells responded poorly to CARD9-dependent fungal agonists (curdlan, heat-killed C. albicans, Saccharomyces cerevisiae and Exophiala dermatitidis). Invasive infections of the CNS or digestive tract caused by Candida in previously healthy children and even adults may be caused by inherited CARD9 deficiency.
Keywords: Inborn error of immunity, primary immunodeficiency, invasive fungal diseases, inherited CARD9 deficiency, central nervous system, colitis, Candida spp, human
Introduction
Candida spp. are commensal yeasts colonizing the skin and digestive tract of most healthy individuals. However, they can cause mucocutaneous candidiasis, which, when chronic (CMC), is commonly observed in patients with broad and profound acquired or inherited T-cell disorders. These patients typically display various other infections 1, 2. Syndromic CMC, in which CMC is a key element of the description of the clinical entity (e.g. autosomal recessive autoimmune polyendocrinopathy type 1), and isolated CMC, which generally affects otherwise healthy individuals (e.g. autosomal dominant CMC disease), have both been reported to result from primary immunodeficiencies (PIDs) impairing IL-17 immunity 2–11. Candida spp. may also cause severe invasive infections. Candidemia, the most frequent clinical form of invasive candidiasis 12, 13, is the fourth most frequent cause of bloodstream infection in hospitals and is classically reported in patients with neutropenia and/or patients with a central catheter receiving broad-spectrum antibiotics and/or parenteral nutrition. In contrast Candida spp. infection of the central nervous system (CNS) is rare. Candida meningitis is reported principally in preterm neonates (0.59% of neonates weighing < 1,000 g at birth develop Candida meningitis), possibly due to immaturity of the blood-brain barrier 14. Neurosurgery, abdominal surgery, intravenous catheter use, intravenous drug use, HIV infection, immunosuppressive treatments, and cancer have been associated with the few reported cases of Candida infection of the CNS 15–18. Candida CNS infections have also been observed in a few patients with inherited chronic granulomatous disease (CGD) 19, 20. Finally, 39 patients with isolated Candida CNS infection and no known underlying risk factors have been reported 21–48, although CGD was not excluded in most of these cases. In these latter patients, the median age at CNS infection was 27 years [1–54 years], 62% were male and 55% died of infection. Interestingly, Candida meningitis has been reported in five patients with inherited CARD9 deficiency 49–51, whereas two other patients with CARD9 deficiency developed brain abscesses, caused by Exophiala in one patient 52 and possibly by Trichophyton in the other 15. Candida causes colitis even less frequently than meningo-encephalitis, with only 25 cases reported, in a context of neutropenia, cancer, lymphoma, systemic lupus erythematosus, acquired immunodeficiency syndrome, corticosteroid treatment or preterm neonates 53–61. We therefore sequenced CARD9 in five unrelated patients: four with Candida spp. infections of the CNS, associated with proven Candida colitis in one patient, the final patient having isolated proven Candida colitis.
Methods
Patients
We recruited five patients with a history of proven meningo-encephalitis and/or colitis caused by Candida spp. (Table 1) and with no known underlying associated condition. Diagnosis was based on the revised EORTC/MSG criteria 62. This study was conducted in accordance with the Helsinki Declaration. All patients and their relatives provided written informed consent for participation in the study.
Table 1.
Characteristics of the five patients with invasive fungal infection and homozygous CARD9 mutations.
| Patient | Age at onset, y | Age at last follow-up, y | Sex | Country of origin | Organ involvement | Associated CMC | Fungus | Status | CARD9 mutation |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 39 | 41 | F | Turkey | CNS | Yes | C. albicans | Alive | R70W/R70W |
| P2 | 7 | 8 | F | Turkey | CNS | Yes | C. albicans | Alive | R70W/R70W |
| P3 | 17 | 28 | M | Iran | CNS, sinus, digestive tract | No | C. glabrata | Alive | R35Q/R35Q |
| P4 | 37 | 37 | F | Morocco | CNS | Yes | C. albicans | Alive | Q289*/Q289* |
| P5 | 26 | 34 | M | Pakistan | Digestive tract | No | C. albicans | Alive | Q295*/Q295* |
CNS: central nervous system, y: years, CMC: chronic mucocutaneous candidiasis
Molecular genetics
Genomic DNA was isolated from whole blood cells. CARD9 was amplified with specific primers (PCR amplification conditions and primer sequences are available upon request). PCR products were sequenced with the Big Dye Terminator cycle sequencing kit (Applied Biosystems, Foster City, CA), and analyzed on an ABI Prism 3700 apparatus (Applied Bio-systems, Foster City, CA) 49.
Controls
We sequenced exons 2, 3 and 6 of the CARD9 gene for all 1,050 healthy unrelated control individuals from the Human Genome Diversity Cell Line Panel (HGDP-CEPH), originating from 52 different ethnic groups initially sampled for population genetics studies63, and 30 individuals from Iran, 90 from Turkey and a total of 83 individuals from Algeria.
Whole-blood cell and monocyte-derived dendritic cell stimulation
Whole blood was diluted 1:2 and incubated for 24 or 48 hours with RPMI medium alone, zymosan (5 μg/ml), heat-killed C. albicans (106 particles/ml), heat-killed Saccharomyces cerevisiae (106 particles/ml), heat-killed Exophiala dermatitidis (106 particles/ml), lipopolysaccharide (LPS, 100 ng/ml), heat-killed Staphylococcus aureus (107 particles/ml), phorbol 12-myristate 13-acetate (PMA) plus ionomycin (0.2 μg/ml and 2 × 10−4 μg/ml, respectively), vesicular stomatitis virus (VSV) (106/ml) or BCG, for P1, P2, and P5, as well as P2’s father and 7 healthy controls. The production of IL-6 or TNF-α was assessed by determining the levels of these cytokines in supernatants by ELISA, according to the kit manufacturer’s instructions (Sanquin). Human peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by Ficoll-Hypaque density gradient centrifugation (Amersham Pharmacia Biotech, Sweden). Monocyte-derived dendritic cells (MDDCs) were derived from Peripheral Blood Mononuclear Cells (PBMCs) following the positive selection of CD14+ cells with CD14 MicroBeads (Miltenyi Biotec), by differentiation in the presence of GM-CSF (50 ng/ml) and IL-13 (20 ng/ml). After 6 days, 30,000 cells/well were plated in 96-well plates and stimulated for 24 hours with curdlan (25 μg/ml), zymosan (25 μg/ml), heat-killed S. cerevisiae (106 or 107 particles/ml), heat-killed C. albicans (106 or 107 particles/ml), heat-killed E. dermatitidis (107 particles/ml), heat-killed S. aureus (2.108 particles/ml), and LPS (100 ng/ml), for P1, P2 and P5, P2’s father and mother and 6 healthy controls tested in parallel. TNF-α production was evaluated by ELISA, according to the manufacturer’s instructions.
Flow cytometry
The antibody against human CARD9 (Epitomics, 5281), an isotype control antibody and a secondary goat anti-rabbit-Alexa-488 antibody (Epitomics 3064-1) were used in accordance with the manufacturers’ protocols.
Cell transfections
The full-length wild-type (WT) CARD9 cDNA was inserted into a pcDNA3.1 expression vector such that the protein was produced with a V5 tag at the C-terminus. The c.G104A (p.R35Q), c.C208T (p.R70W), c.C865T (p.Q289*) and c.C883T (p.Q295*) mutations were introduced into the cDNA with the QuikChange II XL site-directed mutagenesis kit from Stratagene (200522-5), according to the manufacturer’s instructions. Plasmids containing the WT or mutated CARD9 sequences were then amplified and purified with the QIAprep Spin Miniprep Kit from Qiagen (27106). The resulting plasmids were used to transfect HEK-293T cells in 6 cm-diameter plates, with a calcium phosphate transfection kit (Invitrogen 278001) and a cyan fluorescent protein (CFP) plasmid, according to the manufacturer’s instructions.
Western blotting
Total extracts of HEK-293 T cells were prepared 48 hours after transfection. Proteins were separated by electrophoresis and transferred to a membrane, which was then probed with anti-V5 (Invitrogen 46–0708), anti-CARD9 H-90 (Sc-99054), anti-CFP or anti-GAPDH (Sc-25778) antibodies.
NF-κB-luciferase reporter assay
We plated 105 HEK-293 cells in DMEM supplemented with 10% FBS in 96-well plates. These cells were incubated for 6 hours and then transfected in the presence of Lipofectamine® LTX with PLUS™ Reagent (Invitrogen), according to the manufacturer’s protocol. The cells were transfected with 6 ng of Dectin1-, SYK- and BCL10-expressing pcDNA3 constructs, with or without CARD9 wild-type (WT) or CARD9 R35Q, R70W, Q289* or Q295* constructs, and with 100 ng of NF-κB-firefly luciferase vector and 40 ng of pRL-SV40 reporter vector, used as an internal control and expressing the Renilla gene under control of the SV40 promoter. Cells were then left unstimulated or were stimulated with 25 μg/ml of curdlan, or 107 particles/ml of S. cerevisiae or of C. albicans. After 24 hours, cells were lysed and both firefly and Renilla luciferase activities were determined with the Dual-Glo Luciferase Assay System (Promega). Results are expressed as means ± SEM of the ratio of firefly and Renilla luciferase activities adjusted to 1. We used Student’s t-test to determine the significance of differences. Statistics were calculated with GraphPad Prism version 5 (GraphPad Software).
IL-17 production
We evaluated IL-17A production by whole blood ex vivo after 24 hours of stimulation with PMA/ionomycin, by ELISA, according to the manufacturer’s recommendations, for P1, P2, P5, P2’s father and 7 healthy controls. We also determined the percentages of CD3+/IL-17A+ cells by flow cytometry after 12 hours of stimulation with PMA/ionomycin, as previously described 9, for P1, P2 and P5, as well as P2’s father and 10 healthy controls.
Results
Case reports
We describe here five patients with proven Candida spp. infections of the CNS and/or of the digestive tract, from five unrelated consanguineous families originating from Turkey (two), Iran Morocco and Pakistan (Table 1).
Kindred A
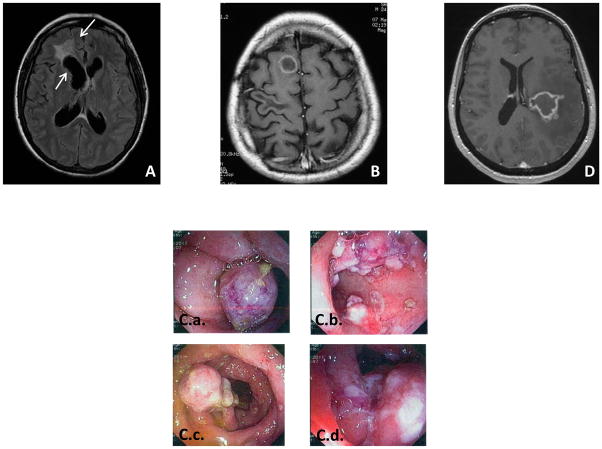
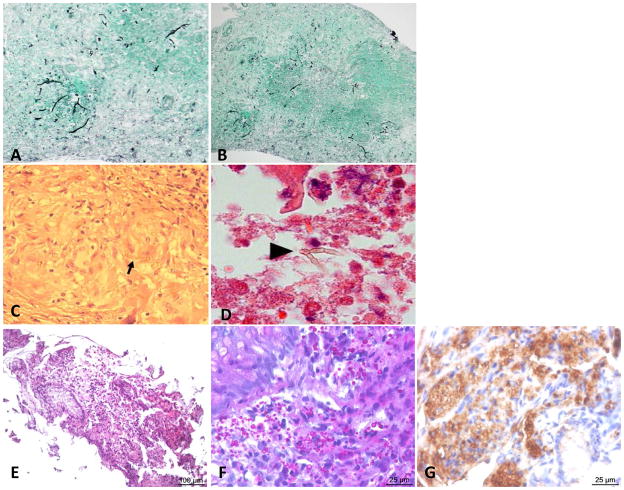
A 42-year-old woman (P1, A.II.2, Figure 1.A), living in France and born to consanguineous Turkish parents, presented at 39 years with C. albicans meningitis and brain abscesses. She had suffered from recurrent vulvo-vaginal candidiasis, with episodes occurring about five times per year since the age of 36 years. She presented with headache, persistent fever and vomiting. She then displayed an altered mental state, right arm paresis and facial palsy. Brain Magnetic Resonance Imaging (MRI) provided evidence of an infiltrative frontal lesion with a mass effect and contrast enhancement with ventricle dilation (Figure 2.A). Lumbar puncture revealed the presence in the cerebrospinal fluid (CSF) of 1,100 leukocytes/mm3, with 80% lymphocytes and 16% eosinophils, together with hyperproteinorachia of up to 1.53 g/l, and hypoglycorachia at 1.5 mmol/l. CSF pressure was high, at up to 25 cm H2O. Three lumbar punctures were performed and C. albicans grew from the CSF samples collected. Brain biopsy showed yeasts and numerous pseudohyphae in giant cell granulomas, with necrosis (Figure 3.A–D). A culture of the biopsy sample was positive for C. albicans. Abdominal and thoracic computed tomography (CT) and transesophageal echocardiography provided no evidence for the dissemination of C. albicans infection. Immunological explorations showed normal CD4+ T, CD8+ T, and NK lymphocyte counts, and B-cell lymphopenia at 4% (56 cells/μl). T lymphocyte proliferations were normal in response to phytohemagglutinin (PHA) and antigens (tuberculin and candidin). Leukocytes oxidative burst, assessed by dihydrorhodamine (DHR) tests, was normal, and IgG, IgA and IgM levels were also normal. The infection was cured by two months of combined intravenous (IV) antifungal therapy combining liposomal amphotericin B and 5 fluorocytosine, subsequently replaced with oral fluconazole. A cerebral shunt was performed to treat intracranial hypertension. Two years later, fluconazole treatment is continuing and the patient is alive, without sequel. Neither her parents nor her siblings and children have suffered from any severe infectious disease.
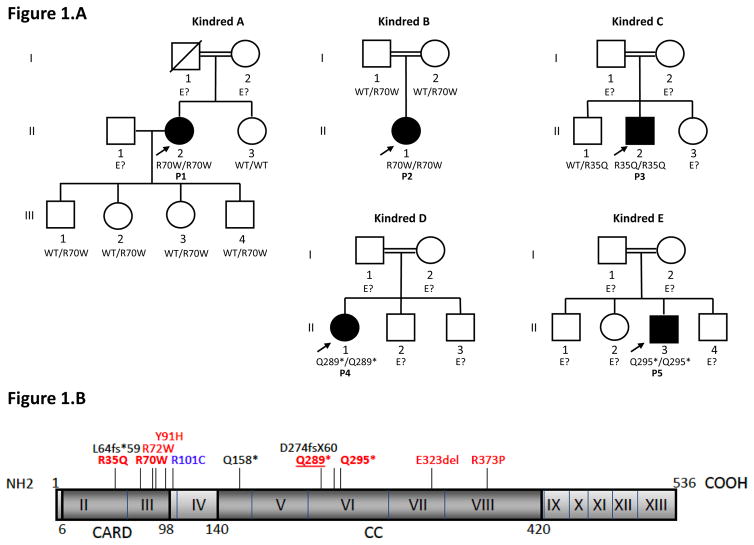
Figure 1.
A. Pedigrees of five kindreds with invasive fungal infection and CARD9 mutations
Each kindred is designated by a letter (A–E), each generation is designated by a Roman numeral (I–III) and each individual by an Arabic numeral (1–4). Patients with invasive fungal infections are indicated in black. The probands are indicated by arrows. The genotype of CARD9 is indicated below each individual. E? indicates that no DNA was available.
B. Schematic diagram of the human CARD9 (isoform 1) gene and its mutations
The human CARD9 isoform 1 gene is shown, with its known pathogenic mutations. Coding exons are numbered with roman numerals. Regions corresponding to the CARD domain and coiled-coil (CC) domain are indicated. Mutations associated with invasive fungal (Candida spp., Exophiala spp.) infections are indicated in red. Mutations associated with invasive fungal (Candida spp.) infections or deep dermatophytosis are underlined. Mutations previously reported in patients with deep dermatophytosis are indicated in blue or underlined, and mutations associated with subcutaneous phaeohyphomycosis are indicated in black.
Figure 2. Clinical, pathological and radiological features of patients.
A. Brain abscess of P1, and B. Brain computed tomography scan of P3, and C.a–d Colonoscopy results for P3, and D Brain MRI of P4
Figure 3. Histology A–D.
Histology results for the brain biopsy carried out on P1. Arrows indicate fungal agents. A–B. Epithelioid granuloma containing pseudohyphae and displaying Gomori-Grocott staining. C. Epithelioid granuloma containing pseudohyphae, stained with hematoxylin-eosin-safran and D. Periodic acid-Schiff stain. E–G. Histology results for the ileum mucosa biopsy of patient P2. E,F. Round yeasts, measuring up to 4 μ;m in diameter, identified by PAS staining. G. Anti-Candida immunohistochemistry.
Kindred B
P2 is a seven-year-old girl born to a Turkish kindred (P2, kindred B, B.II.1 Figure 1.A), living in Belgium. She has been suffering from chronic thrush and onychomycosis since the age of five years. At the age of seven years, she developed fever for several weeks, with headache and vomiting. Cerebrospinal fluid analysis provided evidence of C. albicans meningitis, with 920 cells/mm3, 20% of which were eosinophils (fluconazole MIC=0.5 mg/l). Brain MRI revealed the presence of two lesions of 11 mm and 6 mm in diameter, respectively. Medullary MRI revealed several enhancing lesions. C. albicans grew from nail and buccal samples. The patient was treated with liposomal amphotericin B for two weeks, then fluconazole (12 mg/kg/d), with a positive outcome. Soon after fluconazole treatment, a relapse occurred, with fever, headache and vomiting. CSF culture was sterile, but with 1520 cells/mm3, mostly eosinophils (60%). Symptoms improved with liposomal amphotericin B treatment, replaced after five months with fluconazole because of renal failure related to liposomal amphotericin B treatment. Lesions were controlled 6 months after starting fluconazole as shown by MRI. Brain lesions were in regression while medullar lesions remained unchanged. The patient’s parents did not suffer from any severe infectious disease.
Kindred C
P3 is a 28-year-old man from a consanguineous Iranian kindred (P3, kindred C, C.II.2, Figure 1.A), living in Iran. He had a history of left hemiplegia at the age of 17 years. MRI and CT scans revealed a brain abscess (Figure 2.B). Candida spp. grew from the surgical biopsy specimen obtained. The patient was subsequently discharged on oral fluconazole treatment. At the age of 20 years, he developed fever and right ptosis. Cerebral CT scan showed soft tissue opacities and calcifications in the sphenoids, ethmoids, left maxillary and frontal sinuses, with two regions of bones erosion in the median wall of the right orbit, adjacent to the right orbital apex. There was a 6 × 4 mm region of high-density soft tissue, which extended to the frontal sinus. Bone attenuation was observed at the superolateral left orbital rim and the posterolateral wall of the left sphenoid tissue, suggesting fungal sinonasal infection with orbital and intracranial extension. The patient underwent sinus surgery and the cultures obtained from the surgical specimen were positive for Candida spp. He was discharged on oral itraconazole treatment. At the age of 22 years, the patient developed bloody diarrhea complicated with anemia. Colonoscopy revealed extensive linear ulcers throughout the colon, a low level of vascular development, low levels of haustration and many sessile and ulcerative polyps, with a larger number of small polyps and ulcerations in the terminal ileum (Figure 2.C.a–d). Two gut biopsies were carried out on the terminal ileum. Histopathological analysis of the biopsy specimens revealed a diffuse inflammatory lesion, characterized by an infiltration of macrophages and eosinophils, associated with the presence of round yeasts, identified by periodic-acid Schiff (PAS) and Gomori-Grocott staining and measuring up to 4 μm in diameter without pseudo-hyphae (Figure 3.E–F). Anti-Candida immunohistochemistry results were positive (Figure 3.G). Collectively, morphological and immunohistochemical data suggested invasive colonic Candida spp. infection. C. glabrata grew from a cultured biopsy specimen. Immunological explorations were carried out: neutrophils, CD4+ T, CD8+ T, B and NK lymphocyte blood counts, DHR test results and IgG, IgA and IgM plasmatic levels were normal. IgE levels were high, at 1.7 mg/ml. Eosinophil counts were also high, at up to 1500/mm3. Neither the patient’s parents nor his siblings had suffered from any severe infectious disease.
Kindred D
P4 is a 37-year-old woman from a consanguineous Moroccan kindred (P4, kindred D, D.II.1, Figure 1.A.). Three years before presentation, she had developed thrush, with recurrent episodes occurring about three times per year. At the age of 37 years, she suddenly developed severe headache, vomiting and right hemiparesis. Fundus examination provided evidence of papillary edema. Cerebral MRI showed a 30 × 40 mm left temporo-parietal lesion (Figure 2.D) with several small peripheral nodules, displaying peripheral enhancement after contrast medium injection, and a large perilesional edema with a mass effect on the left ventricle. C. albicans grew from a CNS biopsy specimen. Histological examination revealed the presence of a lymphoplasmocytic infiltrate around the vessels and Gomori-Grocott staining revealed the presence of pseudohyphae. Abdominal and thoracic CT and transesophageal echocardiog-raphy provided no evidence for the dissemination of C. albicans infection. Immunological explorations were carried out: neutrophils, CD4+ T, CD8+ T, B and NK lymphocyte counts, DHR test results and IgG, IgA and IgM plasmatic levels were normal. Serological tests for HIV were negative and the patient did not have diabetes mellitus. The infection was cured after treatment for 15 days with a combination of liposomal amphotericin B and 5-fluorocytosine, followed by fluconazole treatment, which is still underway after 10 months. The patient’s father had a history of recurrent skin dermatophytosis. Neither her mother nor her siblings had suffered from any severe infectious disease.
Kindred E
A 34-year-old man (P5, Kindred E, E.II.3, Figure 1.A) from a consanguineous Pakistani kindred had been living in the United Kingdom for the last eight years. While he was living in Pakistan, he was diagnosed with cervical lymphadenitis ascribed to tuberculosis although not microbiologically proven and had a full course of anti-tuberculous chemotherapy. Several months after completing his antituberculous treatment, he noticed bloody diarrhea, lost weight and became anemic. On initial assessment at the age of 26 years, the patient was found to have oral and esophageal candidiasis and florid right-side colitis with multiple pseudopolyps. Biopsies showed multiple fungal organisms. Chest X ray and abdominal CT scan showed no sign suggestive of tuberculosis, intra-abdominal lymphadenopathy or hepatosplenomegaly and colic biopsy results were consistently negative for tuberculosis. Initially, a diagnosis of histoplasmosis was retained. The patient was therefore treated with fluconazole for two years, followed by itraconazole. His symptoms improved. However, colonoscopy showed persistent infection, and the symptoms recurred when the treatment was stopped. The patient was HIV-seronegative, with normal lymphocyte subsets. At the age of 29 years, he was found to be anemic, with iron deficiency. Immunoglobulin levels (IgG, IgA and IgM) were within normal ranges, but IgE levels rose, at 4979 IU/ml. Following a diagnosis of possible histoplasmosis, the patient was treated with a short induction course of liposomal amphotericin B, followed by posaconazole. His symptoms improved, but following a colonoscopy performed in September 2009, obvious histological signs of ongoing infection were observed on colonic samples, and C. albicans resistant to triazoles, but susceptible to amphotericin B and caspofungin grew from cultured samples. The tissue samples were tested positive for Candida by PCR. Histoplasmosis complement fixation test, precipitin, and immunodiffusion antibody tests had all remained negative, and the serum Histoplasma antigen EIA performed in Indianapolis by the laboratory of Dr Joe Wheat was also negative, as was the test for cryptococcal serum antigen. This patient had no history of other recurrent infections, and there was no family history of susceptibility to any particular infection.
Identification of homozygous CARD9 mutations
We investigated these five patients from unrelated consanguineous kindreds. They displayed CNS candidiasis and/or Candida colitis, but no detectable immunodeficiency in the first round of routine immunological tests (HIV serology, neutrophil, T-, B-, NK-lymphocyte counts). We sequenced CARD9 exons and found homozygous CARD9 mutations in all five patients. P1 and P2 had a homozygous CARD9 missense mutation, c.208C>T, in exon 3, replacing the arginine residue in position 70 with a tryptophan (R70W), within the CARD domain of the CARD9 protein (Figure 1.B). P1 has four healthy children, all heterozygous for the mutation. The parents of P2 are heterozygous for the mutation. P3 had a homozygous CARD9 missense mutation, c.104G>A in exon 2, replacing the arginine residue in position 35 with a glutamine (R35Q), within the CARD domain of the CARD9 protein (Figure 1.B). His healthy brother is heterozygous for the mutation (WT/R35Q). P4 and P5 had homozygous c.865C>T and c.883C>T mutations in exon 6, resulting in premature termination codons at positions 289 (Q289*) and 295 (Q295*), respectively, in the region encoding the coiled-coil domain of CARD9 (Figure 1.B). Genotypes were not available for the other members of the family. The segregation of the four mutations in the five kindreds was consistent with autosomal recessive CARD9 deficiency with complete clinical penetrance. None of the mutations reported here was found in any of the various public databases checked (HGMD, Ensembl and 1000 Genomes, or our in-house whole-exome sequencing database (> 2,000 exomes)). We also sequenced 1,052 controls from the CEPH-HGD panel, and 30, 90, and 83 Iranian, Turkish and Algerian healthy controls, respectively, in whom we found none of the four variants described here. These data ruled out the possibility of R35Q, R70W, Q289* or Q295* being irrelevant polymorphisms. The two missense mutations were predicted to be probably damaging by PolyPhen 2 (with the highest possible score of 1) and damaging by SIFT (scores of 0 for R35Q and 0.02 for R70W). In addition, the Q289* and the Q295* mutations have already been reported and shown to be rare and deleterious CARD9 alleles 15, 49. Collectively, these data strongly suggest that all five patients tested are homozygous for rare and deleterious mutant CARD9 alleles.
Impact of CARD9 mutation on protein level and function
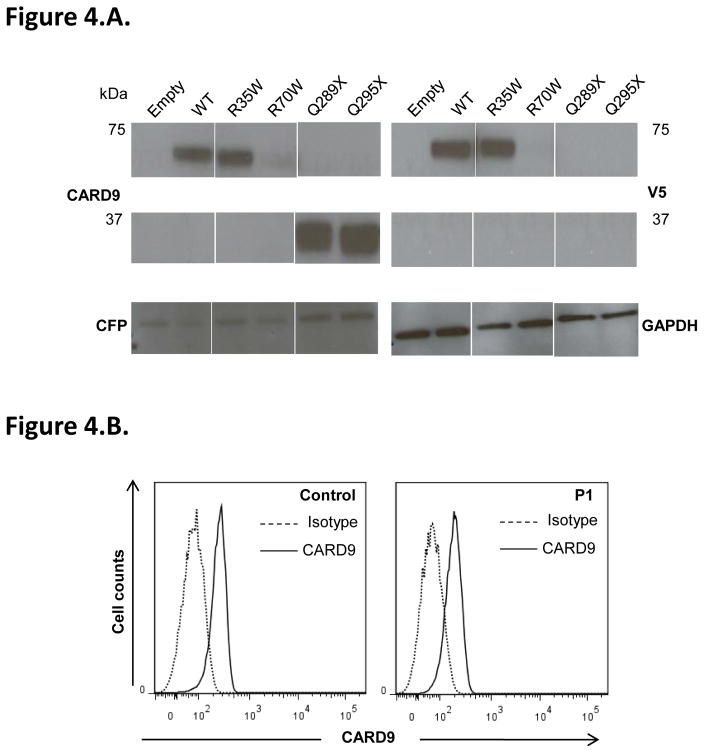
We investigated the consequences of these mutations for protein levels, by carrying out an immunoblot analysis for CARD9 on whole-cell extracts from HEK-293T cells transfected with a pcDNA3.1 V5 (C-terminal tagged) plasmid with no insert or carrying the WT (pcDNA3.1 V5 CARD9 WT) or one of the four mutant alleles of CARD9 (pcDNA3.1 V5 CARD9 R35Q, pcDNA3.1 V5 CARD9 R70W, pcDNA3.1 V5 CARD9 Q289* or pcDNA3.1 V5 CARD9 Q295*). In cells transfected with the CARD9 R35Q allele, CARD9 protein levels and molecular weight (MW) were similar to those in cells transfected with the WT allele, whereas cells transfected with the CARD9 R70W allele had reproducibly lower levels of a protein of normal size and cells transfected with the CARD9 Q289* and Q295* alleles had normal levels of a protein truncated by about 25 kDa, as previously reported15 (Figure 4.A). Flow cytometry analysis of CARD9 protein levels, carried out only on monocyte-derived dendritic cells (MDDCs) from P1 (R70W/R70W), showed this protein to be slightly less abundant than in MDDCs from a healthy control (63% of MDDCs were CARD9-positive in P1, whereas 87% of MDDCs were CARD9-positive in the control, tested in parallel) (Figure 4.B). Collectively, these data are consistent with the previously characterized pathogenic mutations of CARD915, 49–52, 64; nonsense mutations prevented the production of full-length CARD9, whereas missense mutations did not necessarily do so.
Figure 4. Impact of CARD9 mutations on CARD9 protein levels and function.
A–B. Impact of CARD9 mutations on CARD9 protein levels. A. Immunoblot analysis of CARD9 in whole-cell extracts of HEK-293T cells co-transfected with pcDNA3.1 V5 (C-terminally tagged), either empty or carrying the wild-type (WT) or mutant (R35Q, R70W, Q289* and Q295*) CARD9 alleles, together with a CFP plasmid, as a transfection control. Antibodies against CARD9, V5, CFP and GAPDH (as a loading control) were used. B. Flow cytometry analysis of CARD9 expression in monocyte-derived dendritic cells (MDDCs) from patient P1 and a control.
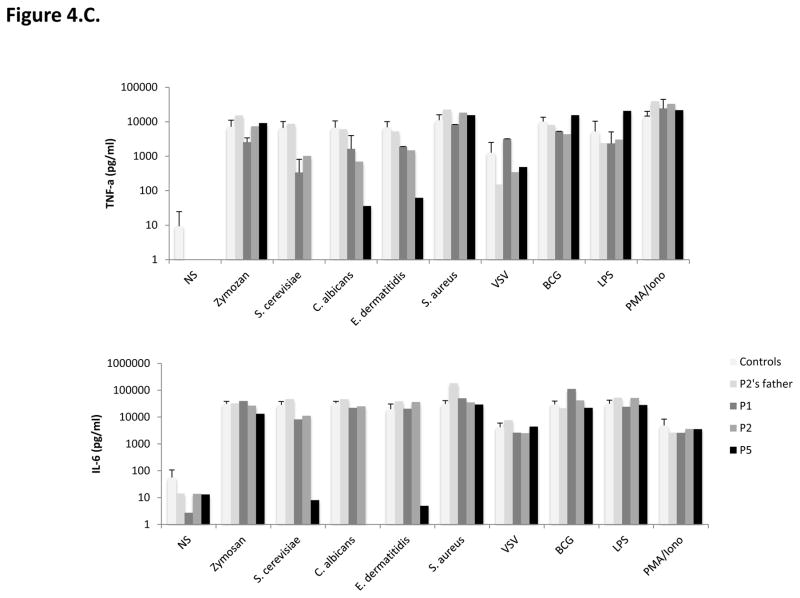
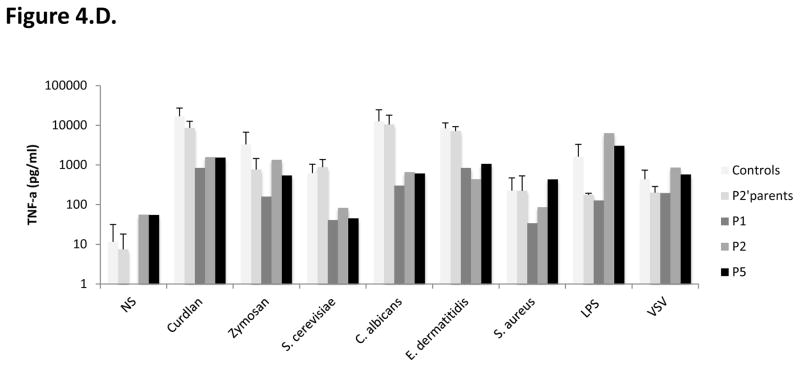
C–D. Impact of CARD9 mutations on CARD9 protein function. C. TNF-α (top panel) and Interleukin (IL)-6 (bottom panel) production by whole blood cells after 24 hours of stimulation with zymosan, heat-killed S. cerevisiae, C. albicans, E. dermatitidis, S. aureus, VSV, BCG, LPS, and PMA plus ionomycin for P1, P2, P5, P2’s father and 7 controls. D. TNF-α production after 24 hours of stimulation of MDDCs with curdlan, zymosan, S. cerevisiae, C. albicans, E. dermatitidis, S. aureus, LPS and VSV for P1, P2 and P5, P2’s parents, and 6 healthy controls tested in parallel.
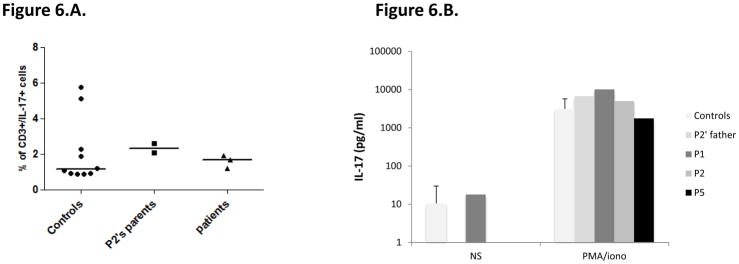
We then evaluated the functional consequences of the mutations, by studying the production of TNF-α and interleukin (IL)-6 by whole blood cells after 24 or 48 hours of stimulation with zymosan (an agonist of Dectin-1 and TLR2), heat-killed Saccharomyces cerevisiae, C. albicans, Exophiala dermatitidis, and Staphylococcus aureus, vesicular stomatitis virus (VSV), BCG, lipopolysaccharide (LPS) (TLR4 agonist), and PMA plus ionomycin. P1, P2 (both homozygous for the CARD9 R70W allele) and more strikingly P5 (Q295*/Q295*), presented impaired TNF-α production after 24 hours of stimulation with S. cerevisiae, C. albicans, and E. dermatiidis, in comparisons with the seven healthy controls tested in parallel or P2’s father (R70W/WT) (Figure 4C, top panel). P1 and P2 presented also reduced levels of IL-6 production after 24 hours of stimulation with S. cerevisiae, but subnormal to normal levels of IL-6 production in response to C. albicans, and E. dermatitidis. By contrast, P5 showed a marked impairment of IL-6 production in response to S. cerevisiae, C. albicans, and E. dermatitidis, in comparisons with the seven healthy controls tested in parallel or P2’s father (R70W/WT) (Figure 4C, bottom panel). Both TNF-α and IL-6 productions in response to zymosan, S. aureus, VSV, BCG, LPS, and PMA plus ionomycin were comparable in the three patients tested (P1, P2 and P5) and in healthy controls. Moreover, IL-6 production, tested after 48 hours (Supplementary Figure), on whole blood from P1 only, was strongly impaired after stimulation with heat-killed S. cerevisiae, C. albicans and, to a lesser extent, zymosan, but was normal after stimulation with LPS, S. aureus, VSV, BCG or PMA/ionomycin. Unfortunately, P3 (R35Q/R35Q) and P4 (Q289*/Q289*) could not been tested. We then assessed TNF-α production by monocyte-derived dendritic cells (MDDCs) from P1, P2 and P5, P2’s father and mother and six healthy controls, stimulated for 24 hours with curdlan and the same agonists as used in the whole blood assay (Figure 4.D.). The patients displayed a strong impairment of TNF-α production in response to stimulation with all fungal ligands used (curdlan, heat-killed S. cerevisiae, C. albicans, and E. dermatitidis and, to a lesser extent, zymosan), whereas it was within the range of healthy controls after stimulation with S. aureus, LPS and VSV. In addition, 293 HEK cells transfected with the R35Q and the R70W CARD9 alleles displayed impaired NF-κB transcriptional activity, as shown by NF-κB–luciferase reporter assays comparing these cells with cells transfected with the WT CARD9 allele, at the basal level and after stimulation with curdlan, S. cerevisae or C. albicans (Figure 5). Surprisingly, under these conditions, the two nonsense mutations (Q289* and Q295*) were associated with constitutive NF-κB–luciferase activity. These results can be accounted for by the production, in this overexpression system, of normal amounts of truncated CARD9 truncated proteins able to interact through their intact coiled-coil domain with the downstream partner, BCL10. Finally, we evaluated the proportion of ex vivo IL-17A-producing T cells by flow cytometry, as low proportions of IL-17 T cells have been reported in some 15, 49, 50, 64 but not all 51, 52 CARD9-deficient patients. Under these conditions, no differences were observed between the patients tested (P1, P2 and P5), P2’s parents (CARD9 R70W/WT) and the ten healthy controls tested in parallel (Figure 6A). Moreover, IL-17A production by whole blood cells after 24 hours of stimulation with PMA and ionomycin, as measured by ELISA, was similar for the three patients tested, P2’s father and seven healthy controls tested in parallel (Figure 6B). We therefore conclude that the homozygous R35Q and R70W mutations led to the production of normal or small amounts of loss-of-function CARD9 proteins, whereas the Q289* and Q295* mutations led to the absence of a normal CARD9 protein. This resulted in the impairment of pro-inflammatory cytokine production by CARD9-deficient whole blood cells and particularly by MDDCs, specifically in response to various fungal ligands, and an impairment of NF-kB transcriptional activity in transfected HEK cells, whereas IL-17 T-cell production was normal.
Figure 5. NF-κB transcriptional activity.
NF-κB–luciferase assay in 293 HEK cells transfected with NF-κB–luciferase and pRL-SV40 vectors alone (A); with DECTIN1, SYK, and BCL10 constructs (B); with DECTIN-1, SYK, BCL10, and CARD9 WT constructs; and with DECTIN-1, SYK, BCL10, and CARD9 mutant constructs (R35Q, R70W, Q289* or Q295*). Cells were left unstimulated or were stimulated with 25 μg/ml curdlan, or 107 particles/ml of S. cerevisae or C. albicans. Results are representative of two independent experiments performed and are expressed as means ± SEM of the ratio of Renilla luciferase and firefly control luciferase activities. Abbreviation: RLU, relative light units.
Figure 6. IL-17 production.
A. Percentage of CD3+/IL-17+ cells of 10 controls, P2’s parents (CARD9 R70W/WT), P1, P2 and P5 measured by flow cytometry after 12 hours of stimulation with PMA/ionomycin. B. IL-17A production by the whole blood of 7 controls, P2′ father, P1, P2 and P5 measured after 24 hours of stimulation with PMA/ionomycin by ELISA.
Discussion
Four of the five patients described here displayed Candida infections of the CNS, associated with Candida colitis in one case, whereas the fifth patient had Candida colitis solely. Our findings demonstrate that CARD9 deficiency is a genetic etiology of rare forms of invasive candidiasis and that CARD9 plays an essential role against Candida infection in the brain and colon. This is concordant with the recently reported role of Card9 in mouse antifungal immunity of the gut 65. Indeed, Card9-deficient mice were shown to display particularly strong fungal colonization of the digestive tract, with smaller than normal numbers of colonic IL-17-producing T cells and innate lymphoid cells, strongly suggesting a critical role for CARD9 in gut IL-17 immune responses and in fungal control.
The five unrelated patients described here are homozygous for four different CARD9 mutations, two of which have never before been described. In addition to the Q295* homozygous nonsense mutation, previously reported in a large multiplex consanguineous Iranian family 49, a homozygous missense (R101C) mutation found in a consanguineous Moroccan family 15, a homozygous nonsense (Q289*) mutation found in five Algerian and two Tunisian kindreds 15 and an Egyptian patient 66, compound heterozygous missense mutations (G72S and R373P) reported in a child of Korean origin50, a homozygous missense mutation (Y91H) reported in a French-Canadian patient 51, a homozygous missense mutation (R18W) found in a patient of Angolan origin 52, a homozygous in-frame deletion (E323del) in an Iranian patient 52, compound heterozygous nonsense mutations (L64fs*59 and Q158*) reported in a Chinese patient 64 and a homozygous frameshift mutation (D274fs*60) reported in three unrelated Chinese patients 64, we identified two new homozygous CARD9 missense mutations: R35Q and R70W, in an Iranian and two unrelated Turkish kindreds. We also report a patient from Morocco with the previously described Q289* CARD9 allele 15, 66, and a patient from Pakistan, with the previously described Q295* mutation 49. In all kindreds studied, none of the heterozygous individuals were symptomatic, whereas all homozygotes were symptomatic, consistent with an autosomal recessive mode of inheritance with complete clinical penetrance (albeit often late in adulthood, up to 39 years). In total, 38 patients from 23 families in nine countries have been identified with CARD9 deficiency due to 13 different alleles, mostly with invasive fungal infections (deep dermatophytosis in 17 patients15, superficial or extensive dermatophytosis in 4 patients49, 66, CMC in 15 patients15, 49, present report, CNS infection with Candida spp. in nine patients 49–51,present report, Candida colitis in two patients present report, Exophiala CNS and liver disease in one patient, lung and bone disease in another patient 52, and Phialophora verrucosa subcutaneous disease in four patients 64).
These mutations are all loss-of-function, with a partial or complete defect, as suggested by the whole blood TNF-α or IL-6 production upon stimulation with fungal agonists being more impaired in P5 (Q295*) than in P1 or P2 (R70W). However, this difference was no more observed when using MDDCs, suggesting some-cell specific compensatory mechanisms. None of these patients developed other fungal, parasitic, bacterial or viral infections, whereas CARD9-deficient mice are susceptible not only to Candida spp., but also to Mycobacterium tuberculosis and Listeria monocytogenes 67–69. Intriguingly, individual CARD9-deficient patients seem to be prone to a single type of invasive fungal disease 70. Patients with invasive dermatophytic disease do not suffer from invasive candidiasis, and vice versa. Moreover, none of these patients were reported with Aspergillus spp infections, concordant with a CARD9-independent neutrophil killing of Aspergillus 50. In any case, our report shows that Candida infection of the CNS is a major clinical phenotype in CARD9-deficient patients. Patients with Candida infections of the CNS should be explored for possible CARD9 deficiency, even if they are previously healthy adults. The same principle applies to rare patients with unexplained histologically-documented colitis caused by Candida. Finally, these studies add further weight to the idea that life-threatening infectious diseases, whether in children or adults, striking otherwise healthy individuals in the course of primary infection, may result from single-gene inborn errors of immunity 71, 72.
Supplementary Material
IL-6 production by whole blood cells after 48 hours of stimulation with zymosan, heat-killed C. albicans, S. cerevisiae, LPS, heat-killed S. aureus, VSV, BCG and PMA plus ionomycin.
Acknowledgments
We thank the patients and their families for participating in this study. We thank Dr Sylvain Poirée for radiological studies. We thank Dr Guillaume Laurent for his help in collecting a patient blood sample. We thank Maya Chrabieh and Malik Bensifi for their technical assistance, and Martine Courat, Yelena Nemirovskaya, Lahouari Amar and Eric Anderson for secretarial assistance. We thank the members of both branches of the Laboratory of Human Genetics of Infectious Diseases for helpful discussions.
Funding
This work was supported, in part, by the National Institutes of Health, the Rockefeller University, INSERM, Paris Descartes University, the St. Giles Foundation and l’Agence Nationale pour la Recherche (grant GENCMCD no 11-BSV3-005-01 to Anne Puel), and a Translational Research grant from The Jeffrey Modell Foundation (to Anne Puel). This study also received funding from the French Government as part of the Investissement d’Avenir program, Laboratoire d’Excellence “Integrative Biology of Emerging Infectious Diseases” (grant no. ANR-10-LABX-62-IBEID). F.L. was supported by a grant from the CMIT (French Faculties College of Infectious Diseases) and INSERM.
Abbreviations’ list
- C. albicans
Candida albicans
- CGD
chronic granulomatous disease
- CMC
chronic mucocutaneous candidiasis
- CNS
central nervous system
- CFP
cyan fluorescent protein
- CLR
C-type lectin receptors
- CSF
cerebrospinal fluid
- CT
computed tomography
- DHR
dihydrorhodamine
- E. dermatitidis
Exophiala dermatitidis
- HGDP-CEPH
Human Genome Diversity Cell Line Panel
- IL
interleukin
- LPS
lipopolysaccharide
- MDDCs
monocyte-derived dendritic cells
- MRI
Magnetic Resonance Imaging
- MW
molecular weight
- PAS
periodic-acid Schiff
- PBMC
Peripheral Blood Mononuclear Cells
- PHA
phytohemagglutinin
- PIDs
primary immunodeficiencies
- PMA
phorbol 12-myristate 13-acetate
- S. aureus
Staphylococcus aureus
- S. cerevisiae
Saccharomyces cerevisiae
- VSV
vesicular stomatitis virus
- WT
wild-type
References
- 1.Lanternier F, Cypowyj S, Picard C, et al. Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr. 2013;25(6):736–47. doi: 10.1097/MOP.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puel A, Cypowyj S, Marodi L, Abel L, Picard C, Casanova JL. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol. 2012;12(6):616–22. doi: 10.1097/ACI.0b013e328358cc0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Okada S, Kong XF, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208(8):1635–48. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puel A, Doffinger R, Natividad A, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207(2):291–7. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puel A, Picard C, Cypowyj S, Lilic D, Abel L, Casanova JL. Inborn errors of mucocutaneous immunity to Candida albicans in humans: a role for IL-17 cytokines? Curr Opin Immunol. 2010;22(4):467–74. doi: 10.1016/j.coi.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332(6025):65–8. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boisson B, Wang C, Pedergnana V, et al. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity. 2013;39(4):676–86. doi: 10.1016/j.immuni.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minegishi Y, Saito M, Tsuchiya S, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448(7157):1058–62. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 9.de Beaucoudrey L, Puel A, Filipe-Santos O, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205(7):1543–50. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandesris MO, Melki I, Natividad A, et al. Autosomal dominant STAT3 deficiency and hyper-IgE syndrome: molecular, cellular, and clinical features from a French national survey. Medicine (Baltimore) 2012;91(4):e1–19. doi: 10.1097/MD.0b013e31825f95b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland SM, DeLeo FR, Elloumi HZ, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357(16):1608–19. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 12.Lamagni TL, Evans BG, Shigematsu M, Johnson EM. Emerging trends in the epidemiology of invasive mycoses in England and Wales (1990–9) Epidemiol Infect. 2001;126(3):397–414. doi: 10.1017/s0950268801005507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lortholary O, Renaudat C, Sitbon K, et al. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010) Intensive care medicine. 2014;40(9):1303–12. doi: 10.1007/s00134-014-3408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117(1):84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 15.Lanternier F, Pathan S, Vincent QB, et al. Deep dermatophytosis and inherited CARD9 deficiency. N Engl J Med. 2013;369(18):1704–14. doi: 10.1056/NEJMoa1208487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen MH, Yu VL. Meningitis caused by Candida species: an emerging problem in neurosurgical patients. Clin Infect Dis. 1995;21(2):323–7. doi: 10.1093/clinids/21.2.323. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien D, Stevens NT, Lim CH, et al. Candida infection of the central nervous system following neurosurgery: a 12-year review. Acta Neurochir (Wien) 2011;153(6):1347–50. doi: 10.1007/s00701-011-0990-9. [DOI] [PubMed] [Google Scholar]
- 18.Casado JL, Quereda C, Corral I. Candidal meningitis in HIV-infected patients. AIDS Patient Care STDS. 1998;12(9):681–6. doi: 10.1089/apc.1998.12.681. [DOI] [PubMed] [Google Scholar]
- 19.Agus S, Spektor S, Israel Z. CNS granulomatosis in a child with chronic granulomatous disease. Br J Neurosurg. 2000;14(1):59–61. doi: 10.1080/02688690042951. [DOI] [PubMed] [Google Scholar]
- 20.Fleischmann J, Church JA, Lehrer RI. Primary Candida meningitis and chronic granulomatous disease. Am J Med Sci. 1986;291(5):334–41. doi: 10.1097/00000441-198605000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Belisle LG. Day treatment at the internal clinic of the department of child psychiatry of the Hopital Sainte Justine. Rev Neuropsychiatr Infant. 1968;16(9):763–71. [PubMed] [Google Scholar]
- 22.Emdin W, Finlayson MH. Moniliasis of the central nervous system in a child with recovery. S Afr Med J. 1954;28(41):868–71. [PubMed] [Google Scholar]
- 23.Fine JM, Franklin DA, Lieberthal AS. Mycotic meningitis due to candida albicans; a four year recovery. Neurology. 1955;5(6):438–43. doi: 10.1212/wnl.5.6.438. [DOI] [PubMed] [Google Scholar]
- 24.Halpert B, Wilkins H. Mycotic meningitis due to Candida. J Am Med Assoc. 1946;130:932–4. doi: 10.1001/jama.1946.02870140024008. [DOI] [PubMed] [Google Scholar]
- 25.Morris AA, Kalz GG, Lotspeich ES. Ependymitis and meningitis due to Candida (Monilia) albicans. Arch Neurol Psychiatry. 1945;54:361–6. doi: 10.1001/archneurpsyc.1945.02300110045006. [DOI] [PubMed] [Google Scholar]
- 26.Zimmerman SL, Frutchey L, Gibbes JH. Meningitis due to Candida (Monilia) albicans with recovery. J Am Med Assoc. 1947;135(3):145–7. doi: 10.1001/jama.1947.02890030013004. [DOI] [PubMed] [Google Scholar]
- 27.Craig WM, Gates EM. Metastatic mycotic abscesses of the brain. Arch Neurol Psychiatry. 1949;62(3):314–21. doi: 10.1001/archneurpsyc.1949.02310150061007. [DOI] [PubMed] [Google Scholar]
- 28.Janke D. Serology of moniliasis. Arch Klin Exp Dermatol. 1957;206:608–13. discussion 13–4. [PubMed] [Google Scholar]
- 29.Vorreith M, Bares L, Benes V, Vancurik J. Candidiasis of the central nervous system diagnosed by a bioptic test. Cas Lek Cesk. 1961;100:966–71. [PubMed] [Google Scholar]
- 30.Black JT. Cerebral candidiasis: case report of brain abscess secondary to Candida albicans, and review of literature. J Neurol Neurosurg Psychiatry. 1970;33(6):864–70. doi: 10.1136/jnnp.33.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White BE. Cerebral candidiasis. N Engl J Med. 1972;286(6):321. doi: 10.1056/NEJM197202102860616. [DOI] [PubMed] [Google Scholar]
- 32.Edelson RN, McNatt EN, Porro RS. Candida meningitis; with cerebral arteritis. N Y State J Med. 1975;75(6):900–4. [PubMed] [Google Scholar]
- 33.Holyst J, Majewski A, Tyszkiewicz S. Massive cerebellar abscess due to candida albicans. Neurochirurgia (Stuttg) 1976;19(3):126–9. doi: 10.1055/s-0028-1090401. [DOI] [PubMed] [Google Scholar]
- 34.Popow C, Haschke F, Maida E, et al. Combined Tb and Candida meningitis in an 8-year old boy (author’s transl) Klin Padiatr. 1981;193(5):401–3. doi: 10.1055/s-2008-1034509. [DOI] [PubMed] [Google Scholar]
- 35.Kauffman CA, Shea MJ, Frame PT. Invasive fungal infections in patients with chronic mucocutaneous candidiasis. Arch Intern Med. 1981;141(8):1076–9. [PubMed] [Google Scholar]
- 36.Wietholter H, Thron A, Scholz E, Dichgans J. Systemic Candida albicans infection with cerebral abscess and granulomas. Clin Neuropathol. 1984;3(1):37–41. [PubMed] [Google Scholar]
- 37.Vilella JM, Jacquemin JL, Brion S, et al. Candida albicans meningoencephalitis in an apparently non-immunosuppressed patient. Presse Med. 1985;14(17):980. [PubMed] [Google Scholar]
- 38.Ikeda K, Yamashita J, Fujisawa H, Fujita S. Cerebral granuloma and meningitis caused by Candida albicans: useful monitoring of mannan antigen in cerebrospinal fluid. Neurosurgery. 1990;26(5):860–3. [PubMed] [Google Scholar]
- 39.Vasylyk VU, Kokoshko VP, Kudla VM, Stempinskii EI. Candidal meningoencephalitis diagnosed as tuberculous meningoencephalitis. Probl Tuberk. 1992;(3–4):62–3. [PubMed] [Google Scholar]
- 40.Jamjoom A, al-Abedeen Jamjoom Z, al-Hedaithy S, Jamali A, Naim Ur R, Malabarey T. Ventriculitis and hydrocephalus caused by Candida albicans successfully treated by antimycotic therapy and cerebrospinal fluid shunting. Br J Neurosurg. 1992;6(5):501–4. doi: 10.3109/02688699208995043. [DOI] [PubMed] [Google Scholar]
- 41.Lisch S, Steudel WI. Unusual course of candidiasis of the central nervous system. Dtsch Med Wochenschr. 1994;119(1–2):13–8. doi: 10.1055/s-2008-1058655. [DOI] [PubMed] [Google Scholar]
- 42.Zheng L, Okabe S, Hino K, Kohno T, Kamata K. Intracranial fungal granuloma with CSF space dissemination: a case report. No Shinkei Geka. 1996;24(4):389–92. [PubMed] [Google Scholar]
- 43.Mason TB, 2nd, Chiriboga CA, Cargan AL, et al. Postinflammatory hydrocephalus and intracranial mass lesion from Candida in an immunocompetent child. J Child Neurol. 1996;11(4):336–41. doi: 10.1177/088307389601100413. [DOI] [PubMed] [Google Scholar]
- 44.Hanci M, Kafadar A, Sarioglu A, Islak C, Oz B. Cerebral candidiasis presenting as a mass lesion. Zentralbl Neurochir. 1998;59(2):129–31. [PubMed] [Google Scholar]
- 45.Akyuz M, Karpuzoglu G, Acikbas C, Tuncer R. Candida albicans granuloma imitate clivus chordoma. Acta Neurochir (Wien) 2002;144(5):505–6. doi: 10.1007/s007010200074. [DOI] [PubMed] [Google Scholar]
- 46.Choudhari YK, Choudhari KA. Life-threatening fungal meningitis-ventriculitis secondary to vaginal candidiasis. Br J Hosp Med (Lond) 2007;68(5):274–5. doi: 10.12968/hmed.2007.68.5.23337. [DOI] [PubMed] [Google Scholar]
- 47.Mathai AM, Khadilkar UN. Candidosis of brain. Indian J Pathol Microbiol. 2007;50(4):838. [PubMed] [Google Scholar]
- 48.Borha A, Parienti JJ, Emery E, Coskun O, Khouri S, Derlon JM. Candida albicans cerebral granuloma in an immunocompetent patient. A case report. Neurochirurgie. 2009;55(1):57–62. doi: 10.1016/j.neuchi.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Glocker EO, Hennigs A, Nabavi M, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361(18):1727–35. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drewniak A, Gazendam RP, Tool AT, et al. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood. 2013;121(13):2385–92. doi: 10.1182/blood-2012-08-450551. [DOI] [PubMed] [Google Scholar]
- 51.Gavino C, Cotter A, Lichtenstein D, et al. CARD9 deficiency and spontaneous central nervous system candidiasis: complete clinical remission with GM-CSF therapy. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lanternier F, Barbati E, Meinzer U, et al. Inherited CARD9 Deficiency in 2 Unrelated Patients With Invasive Exophiala Infection. J Infect Dis. 2014 doi: 10.1093/infdis/jiu412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Praneenararat S. The First Reported Case of Colonic Infection Caused by Candida tropicalis and a Review of the Literature. Case reports in gastroenterology. 2014;8(2):199–205. doi: 10.1159/000363566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kudo T, Aoyagi Y, Fujii T, Ohtsuka Y, Nagata S, Shimizu T. Development of Candida albicans colitis in a child undergoing steroid therapy for ulcerative colitis. Journal of pediatric gastroenterology and nutrition. 2010;51(1):96–9. doi: 10.1097/MPG.0b013e31818de1ba. [DOI] [PubMed] [Google Scholar]
- 55.Kitagawa KH, Kalb RE. Efalizumab treatment associated with Candida colitis. Journal of the American Academy of Dermatology. 2008;59(5 Suppl):S120–1. doi: 10.1016/j.jaad.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 56.Cimbaluk D, Scudiere J, Butsch J, Jakate S. Invasive candidal enterocolitis followed shortly by fatal cerebral hemorrhage in immunocompromised patients. J Clin Gastroenterol. 2005;39(9):795–7. doi: 10.1097/01.mcg.0000177237.82382.b8. [DOI] [PubMed] [Google Scholar]
- 57.Kouklakis G, Dokas S, Molyvas E, Vakianis P, Efthymiou A. Candida colitis in a middle-aged male receiving permanent haemodialysis. European journal of gastroenterology & hepatology. 2001;13(6):735–6. doi: 10.1097/00042737-200106000-00021. [DOI] [PubMed] [Google Scholar]
- 58.Jayagopal S, Cervia JS. Colitis due to Candida albicans in a patient with AIDS. Clin Infect Dis. 1992;15(3):555. doi: 10.1093/clind/15.3.555. [DOI] [PubMed] [Google Scholar]
- 59.Prescott RJ, Harris M, Banerjee SS. Fungal infections of the small and large intestine. Journal of clinical pathology. 1992;45(9):806–11. doi: 10.1136/jcp.45.9.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nadorra RL, Nakazato Y, Landing BH. Pathologic features of gastrointestinal tract lesions in childhood-onset systemic lupus erythematosus: study of 26 patients, with review of the literature. Pediatric pathology/affiliated with the International Paediatric Pathology Association. 1987;7(3):245–59. doi: 10.1080/15513818709177128. [DOI] [PubMed] [Google Scholar]
- 61.Adderson EE, Pappin A, Pavia AT. Spontaneous intestinal perforation in premature infants: a distinct clinical entity associated with systemic candidiasis. Journal of pediatric surgery. 1998;33(10):1463–7. doi: 10.1016/s0022-3468(98)90475-4. [DOI] [PubMed] [Google Scholar]
- 62.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cann HM, de Toma C, Cazes L, et al. A human genome diversity cell line panel. Science. 2002;296(5566):261–2. doi: 10.1126/science.296.5566.261b. [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Wang W, Lin Z, et al. CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J Allergy Clin Immunol. 2014;133(3):905–8. e3. doi: 10.1016/j.jaci.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 65.Sokol H, Conway KL, Zhang M, et al. Card9 Mediates Intestinal Epithelial Cell Restitution, T-Helper 17 Responses, and Control of Bacterial Infection in Mice. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jachiet M, Lanternier F, Rybojad M, et al. Posaconazole Treatment of Extensive Skin and Nail Dermatophytosis Due to Autosomal Recessive Deficiency of CARD9. JAMA Dermatology. 2014 doi: 10.1001/jamadermatol.2014.2154. [DOI] [PubMed] [Google Scholar]
- 67.Gross O, Gewies A, Finger K, et al. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature. 2006;442(7103):651–6. doi: 10.1038/nature04926. [DOI] [PubMed] [Google Scholar]
- 68.Dorhoi A, Desel C, Yeremeev V, et al. The adaptor molecule CARD9 is essential for tuberculosis control. J Exp Med. 2010;207(4):777–92. doi: 10.1084/jem.20090067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsu YM, Zhang Y, You Y, et al. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol. 2007;8(2):198–205. doi: 10.1038/ni1426. [DOI] [PubMed] [Google Scholar]
- 70.Lionakis MS, Holland SM. Human Invasive Mycoses: Immunogenetics on the Rise. J Infect Dis. 2014 doi: 10.1093/infdis/jiu411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alcais A, Quintana-Murci L, Thaler DS, Schurr E, Abel L, Casanova JL. Life-threatening infectious diseases of childhood: single-gene inborn errors of immunity? Ann N Y Acad Sci. 2010;1214:18–33. doi: 10.1111/j.1749-6632.2010.05834.x. [DOI] [PubMed] [Google Scholar]
- 72.Casanova JL, Abel L. The genetic theory of infectious diseases: a brief history and selected illustrations. Annu Rev Genomics Hum Genet. 2013;14:215–43. doi: 10.1146/annurev-genom-091212-153448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IL-6 production by whole blood cells after 48 hours of stimulation with zymosan, heat-killed C. albicans, S. cerevisiae, LPS, heat-killed S. aureus, VSV, BCG and PMA plus ionomycin.