Abstract
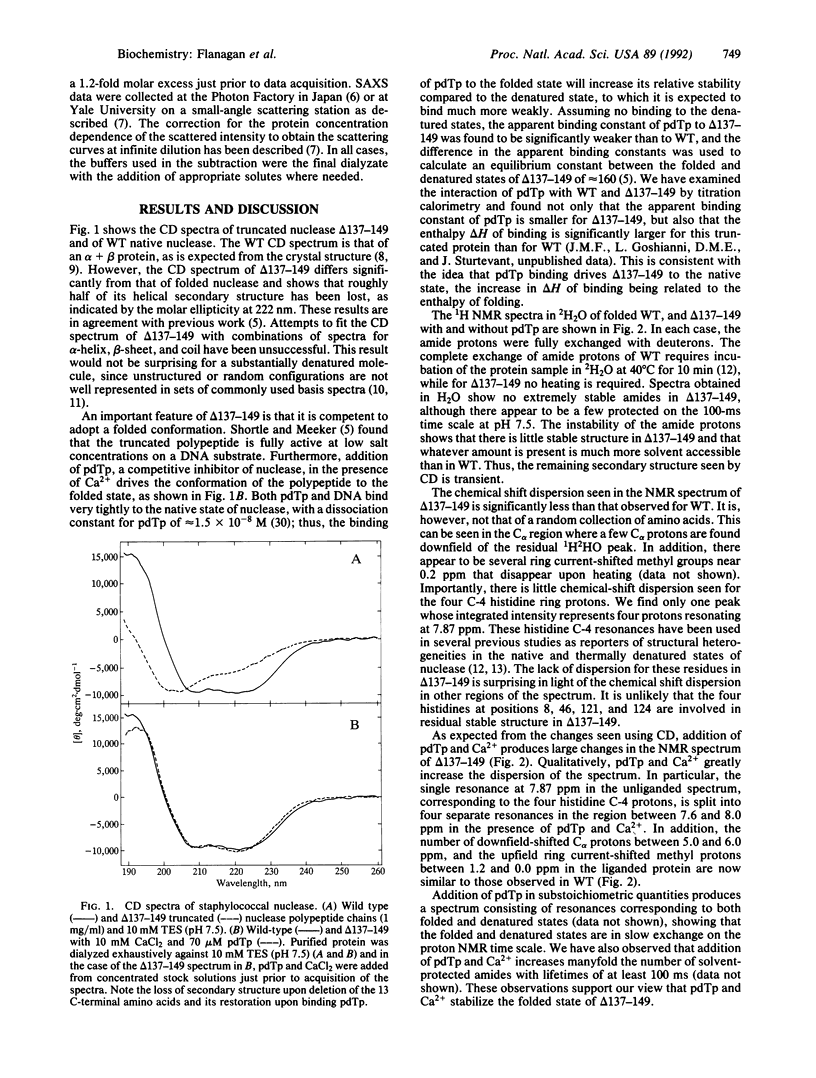
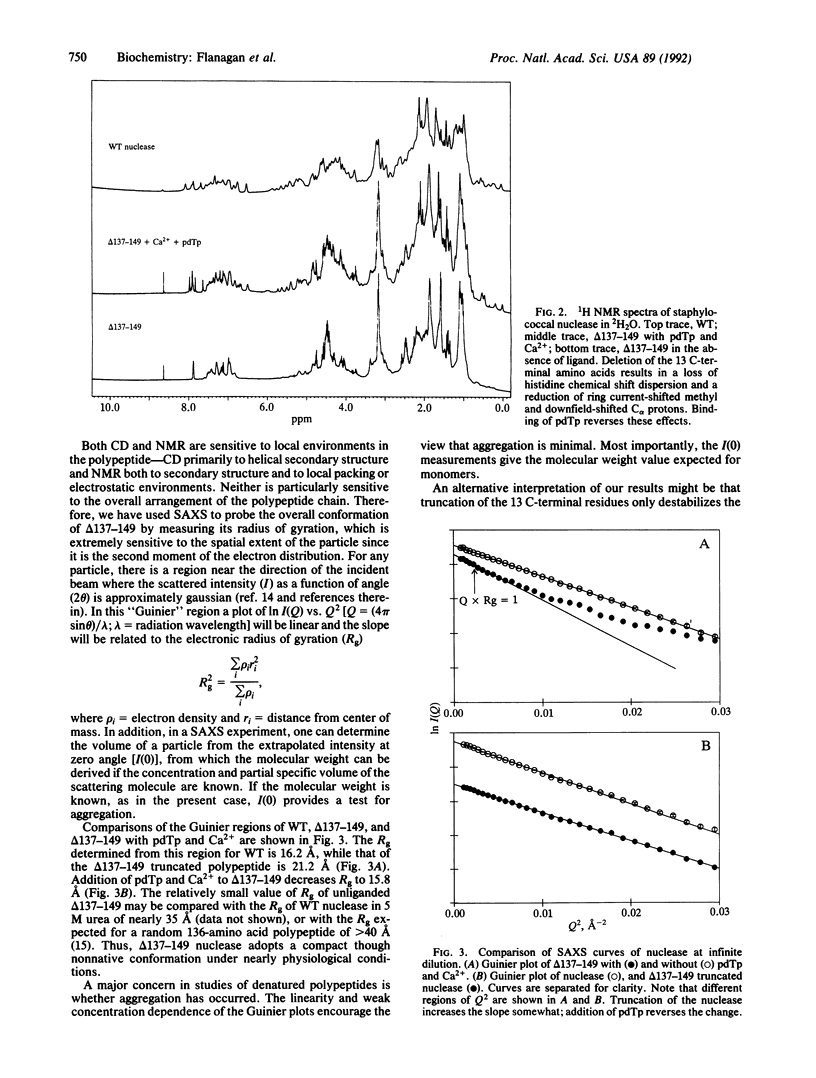
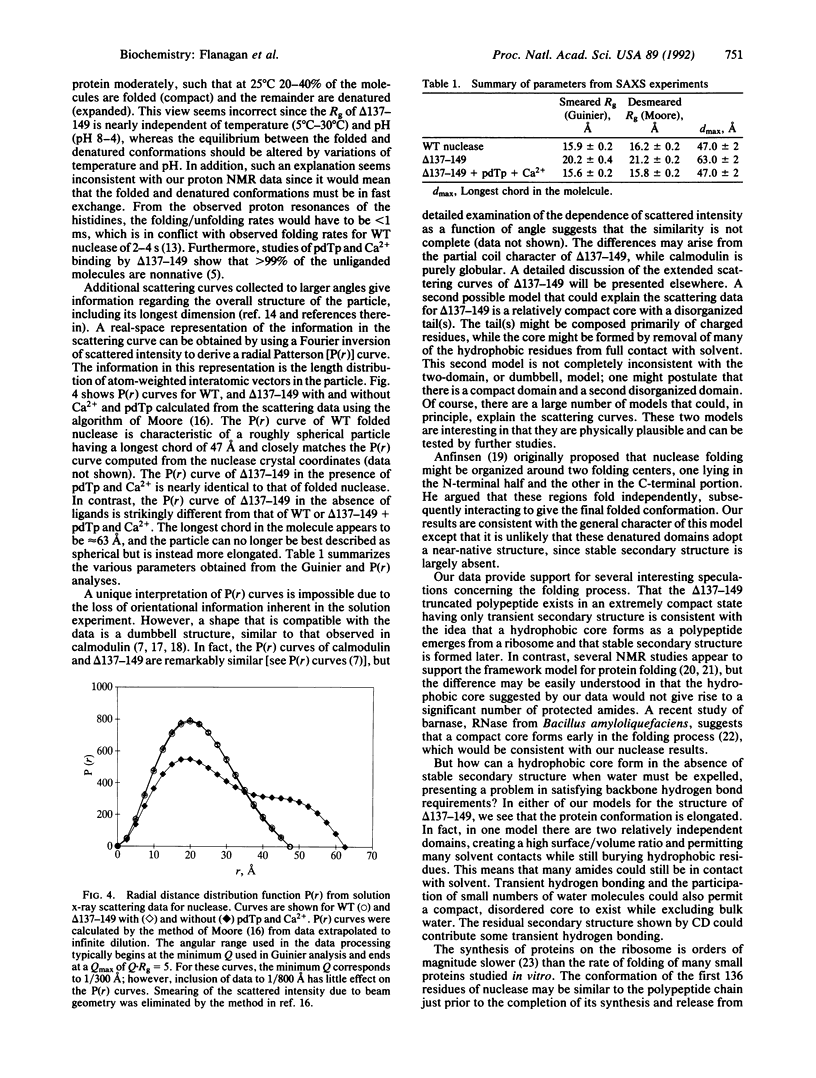
Deletion of 13 amino acids from the carboxyl terminus of the 149-amino acid staphylococcal nuclease molecule results in a denatured, partly unfolded molecule that lacks persistent secondary structure but is compact under physiological conditions. Since the modification is a carboxyl-terminal deletion, it is argued that the state resembles a peptide emerging from the ribosome just before the complete folding pathway is initiated. In this paper, we characterize the molecule by nuclear magnetic resonance, circular dichroism, and small-angle x-ray scattering measurements. The truncated nuclease shows wild-type levels of activity in the presence of calcium and is found to fold into a native-like conformation in the presence of 3',5'-bisphospho-2'-deoxythymidine, a potent inhibitor. Thus, the truncated molecule retains the capacity to fold. Our results suggest that extensive solvent exclusion generates a compact polypeptide chain prior to the development of persistent secondary structural features as a protein folds during biosynthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandrescu A. T., Hinck A. P., Markley J. L. Coupling between local structure and global stability of a protein: mutants of staphylococcal nuclease. Biochemistry. 1990 May 15;29(19):4516–4525. doi: 10.1021/bi00471a003. [DOI] [PubMed] [Google Scholar]
- Anfinsen C. B. The formation and stabilization of protein structure. Biochem J. 1972 Jul;128(4):737–749. doi: 10.1042/bj1280737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum J., Dobson C. M., Evans P. A., Hanley C. Characterization of a partly folded protein by NMR methods: studies on the molten globule state of guinea pig alpha-lactalbumin. Biochemistry. 1989 Jan 10;28(1):7–13. doi: 10.1021/bi00427a002. [DOI] [PubMed] [Google Scholar]
- Bergman L. W., Kuehl W. M. Formation of an intrachain disulfide bond on nascent immunoglobulin light chains. J Biol Chem. 1979 Sep 25;254(18):8869–8876. [PubMed] [Google Scholar]
- Compton L. A., Johnson W. C., Jr Analysis of protein circular dichroism spectra for secondary structure using a simple matrix multiplication. Anal Biochem. 1986 May 15;155(1):155–167. doi: 10.1016/0003-2697(86)90241-1. [DOI] [PubMed] [Google Scholar]
- Cotton F. A., Hazen E. E., Jr, Legg M. J. Staphylococcal nuclease: proposed mechanism of action based on structure of enzyme-thymidine 3',5'-bisphosphate-calcium ion complex at 1.5-A resolution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2551–2555. doi: 10.1073/pnas.76.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill K. A. Dominant forces in protein folding. Biochemistry. 1990 Aug 7;29(31):7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- Dill K. A. Theory for the folding and stability of globular proteins. Biochemistry. 1985 Mar 12;24(6):1501–1509. doi: 10.1021/bi00327a032. [DOI] [PubMed] [Google Scholar]
- Dolgikh D. A., Abaturov L. V., Bolotina I. A., Brazhnikov E. V., Bychkova V. E., Gilmanshin R. I., Lebedev YuO, Semisotnov G. V., Tiktopulo E. I., Ptitsyn O. B. Compact state of a protein molecule with pronounced small-scale mobility: bovine alpha-lactalbumin. Eur Biophys J. 1985;13(2):109–121. doi: 10.1007/BF00256531. [DOI] [PubMed] [Google Scholar]
- Dolgikh D. A., Gilmanshin R. I., Brazhnikov E. V., Bychkova V. E., Semisotnov G. V., Venyaminov SYu, Ptitsyn O. B. Alpha-Lactalbumin: compact state with fluctuating tertiary structure? FEBS Lett. 1981 Dec 28;136(2):311–315. doi: 10.1016/0014-5793(81)80642-4. [DOI] [PubMed] [Google Scholar]
- Evans P. A., Kautz R. A., Fox R. O., Dobson C. M. A magnetization-transfer nuclear magnetic resonance study of the folding of staphylococcal nuclease. Biochemistry. 1989 Jan 10;28(1):362–370. doi: 10.1021/bi00427a050. [DOI] [PubMed] [Google Scholar]
- Heidorn D. B., Trewhella J. Comparison of the crystal and solution structures of calmodulin and troponin C. Biochemistry. 1988 Feb 9;27(3):909–915. doi: 10.1021/bi00403a011. [DOI] [PubMed] [Google Scholar]
- Jeng M. F., Englander S. W., Elöve G. A., Wand A. J., Roder H. Structural description of acid-denatured cytochrome c by hydrogen exchange and 2D NMR. Biochemistry. 1990 Nov 20;29(46):10433–10437. doi: 10.1021/bi00498a001. [DOI] [PubMed] [Google Scholar]
- Kataoka M., Head J. F., Seaton B. A., Engelman D. M. Melittin binding causes a large calcium-dependent conformational change in calmodulin. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6944–6948. doi: 10.1073/pnas.86.18.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loll P. J., Lattman E. E. The crystal structure of the ternary complex of staphylococcal nuclease, Ca2+, and the inhibitor pdTp, refined at 1.65 A. Proteins. 1989;5(3):183–201. doi: 10.1002/prot.340050302. [DOI] [PubMed] [Google Scholar]
- Matouschek A., Kellis J. T., Jr, Serrano L., Bycroft M., Fersht A. R. Transient folding intermediates characterized by protein engineering. Nature. 1990 Aug 2;346(6283):440–445. doi: 10.1038/346440a0. [DOI] [PubMed] [Google Scholar]
- Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989 Sep 14;341(6238):125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr, Davidson L. K. The biosynthesis of rat serum albumin. In vivo studies on the formation of the disulfide bonds. J Biol Chem. 1982 Aug 10;257(15):8847–8853. [PubMed] [Google Scholar]
- Purvis I. J., Bettany A. J., Santiago T. C., Coggins J. R., Duncan K., Eason R., Brown A. J. The efficiency of folding of some proteins is increased by controlled rates of translation in vivo. A hypothesis. J Mol Biol. 1987 Jan 20;193(2):413–417. doi: 10.1016/0022-2836(87)90230-0. [DOI] [PubMed] [Google Scholar]
- Roder H., Elöve G. A., Englander S. W. Structural characterization of folding intermediates in cytochrome c by H-exchange labelling and proton NMR. Nature. 1988 Oct 20;335(6192):700–704. doi: 10.1038/335700a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Seaton B. A., Head J. F., Engelman D. M., Richards F. M. Calcium-induced increase in the radius of gyration and maximum dimension of calmodulin measured by small-angle X-ray scattering. Biochemistry. 1985 Nov 19;24(24):6740–6743. doi: 10.1021/bi00345a002. [DOI] [PubMed] [Google Scholar]
- Serpersu E. H., Shortle D., Mildvan A. S. Kinetic and magnetic resonance studies of effects of genetic substitution of a Ca2+-liganding amino acid in staphylococcal nuclease. Biochemistry. 1986 Jan 14;25(1):68–77. doi: 10.1021/bi00349a011. [DOI] [PubMed] [Google Scholar]
- Shortle D., Meeker A. K. Residual structure in large fragments of staphylococcal nuclease: effects of amino acid substitutions. Biochemistry. 1989 Feb 7;28(3):936–944. doi: 10.1021/bi00429a003. [DOI] [PubMed] [Google Scholar]
- Tanford C., Kawahara K., Lapanje S. Proteins in 6-M guanidine hydrochloride. Demonstration of random coil behavior. J Biol Chem. 1966 Apr 25;241(8):1921–1923. [PubMed] [Google Scholar]
- Udgaonkar J. B., Baldwin R. L. NMR evidence for an early framework intermediate on the folding pathway of ribonuclease A. Nature. 1988 Oct 20;335(6192):694–699. doi: 10.1038/335694a0. [DOI] [PubMed] [Google Scholar]
- Ueki T., Hiragi Y., Kataoka M., Inoko Y., Amemiya Y., Izumi Y., Tagawa H., Muroga Y. Aggregation of bovine serum albumin upon cleavage of its disulfide bonds, studied by the time-resolved small-angle X-ray scattering technique with synchrotron radiation. Biophys Chem. 1985 Nov;23(1-2):115–124. doi: 10.1016/0301-4622(85)80069-7. [DOI] [PubMed] [Google Scholar]
- Yang J. T., Wu C. S., Martinez H. M. Calculation of protein conformation from circular dichroism. Methods Enzymol. 1986;130:208–269. doi: 10.1016/0076-6879(86)30013-2. [DOI] [PubMed] [Google Scholar]



