Abstract
Background
Insomnia is a common comorbidity associated with COPD. Although benzodiazepines (BZDs) can have adverse effects on respiratory response in COPD patients, these are the most common hypnotics. The aim of this study was to examine by meta-analysis the efficacy and safety of BZD to treat insomnia in COPD patients.
Materials and methods
Electronic databases (PubMed, China National Knowledge Infrastructure, Cochrane clinical trials database) were searched. Studies were eligible if they compared the effects of BZD versus placebo on insomnia in COPD patients. Two reviewers extracted data independently. Disagreements were resolved by discussion with another reviewer until a consensus was achieved. Data that included objective and subjective sleep evaluation and respiratory function variables were extracted. Data were analyzed by the methods recommended by Review Manager 5.3 software.
Results
A total of 233 records were identified through the initial search; of these, five studies were included in the meta-analysis. When BZD was compared with placebo, objective sleep quality was significantly improved, including total sleep time (95% confidence interval [CI] 0.54–1.14, P<0.00001), sleep efficiency (95% CI 0.48–1.16, P<0.00001), sleep latency (95% CI −18.24 to −4.46, P=0.001), and number of arousals/hour of sleep (95% CI −0.72 to −0.07, P=0.02). Otherwise, subjective sleep quality was not improved remarkably. Apart from maximum transcutaneous carbon dioxide pressure increase during sleep (95% CI 0.05–0.28, P=0.006), BZD administration had no effect on respiratory assessment.
Conclusion
In this meta-analysis, the results suggested BZDs might be efficient and safe hypnotics. Compared with placebo, BZD improved sleep quality partly, and significantly increased maximum transcutaneous carbon dioxide pressure during sleep. More randomized controlled trials are necessary to determine the potential effect of BZD in COPD patients with insomnia.
Keywords: benzodiazepine, COPD, insomnia, efficacy, safety, meta-analysis
Introduction
COPD, which is defined as a progressive and irreversible limitation of functional airflow, encompasses a range of chronic respiratory diseases. The sleep quality of patients with COPD is often poor. Insomnia is a common symptom accompanying COPD. Sleep disturbances are known to have a negative impact on a range of clinical outcomes in COPD.1–4 These patients report sleep-related complaints characterized by longer sleep latency, more frequent arousals and awakenings, and more daytime sleepiness.1,4,5 To improving patients’ quality of life, physicians often prescribe hypnotic drugs to their patients with COPD. Benzodiazepines (BZDs) are proven to influence sleep quality beneficially, and are the first-choice hypnotics nowadays.5
However, BZD may cause hypoventilation in patients with COPD, probably due to the decrease in the respiratory response to carbon dioxide and the increase in muscular relaxation, especially among older adults with COPD.6,7 On the other hand, the use of hypnotics or sedatives seems to be a cause of acute respiratory failure in COPD. In recent years, some population-based case-control studies to evaluate the effects of BZD on the risks of adverse respiratory events in COPD patients have been reported. The results of these epidemiological studies suggested that BZD increased the risks.8–10 Joint American Thoracic Society/European Respiratory Society guidelines recommend that BZD use must be cautious and especially be avoided in patients with severe COPD.11 In a recent study from 2015, the dispensing of BZD to COPD patients and clinical characteristics were investigated.12 BZDs are still widely used, despite the adverse pulmonary effects that could potentially lead to poor outcomes in the COPD population. Meanwhile, the results, among which BZD use had no adverse effect on respiratory function in COPD patients, have also been reported.13 Therefore, the safety and efficacy of BZD use among COPD patients with insomnia are very important, but controversial.
This meta-analysis aimed to study the effect of BZD on insomnia in patients with COPD, including the benefits of BZD use determined by objective and subjective changes in sleep quality, and the risks of respiratory adverse effects.
Materials and methods
Search strategy for identification of relevant studies
A search of the following databases was conducted: PubMed, China National Knowledge Infrastructure, and the Cochrane clinical trials database. The following keywords were used: “chronic obstructive pulmonary disease (COPD)” AND “BZD” AND “insomnia”. The literature language and type were not restricted. For each citation identified, we scanned titles or abstracts, or both, to identify randomized controlled trials.
Study selection
One reviewer screened the search results, and the full-text manuscripts of all potentially eligible studies were acquired. Then, all of the articles were reviewed by two reviewers independently in accordance with the inclusion criteria. Disagreements between the two reviewers were resolved by consensus and discussion with a third reviewer. When the “same author” or “same data” issue was confronted, the latest published study was included.
Inclusion and exclusion criteria
We included trials with the following features: randomized controlled clinical and crossover trials; trials including adult populations with COPD; patients given BZD for sedative medication; trials studying effect of BZD on sleep and respiratory function in patients with COPD; and outcomes of objective sleep evaluation (total sleep time [TST], sleep efficiency, sleep latency, number of arousals/hour during sleep), subjective sleep evaluation (sleep quality), and respiratory function in sleep state (Apnea–Hypopnea Index [AHI]), desaturation-event duration, sleep apnea, and increase in transcutaneous carbon dioxide pressure (tcPCO2).
Trials were excluded if any of the following applied: not published as original article; inability to acquire the full-text article; not adult patients; not published in English; histories of hypoxemic hypercapnic respiratory failure with long-term oxygen therapy; and histories of diseases interfering with the absorption or metabolism of BZD (eg, chronic hepatic disease, chronic kidney disease).
Quality assessment and publication bias
The quality of each article was assessed by two reviewers independently. Disagreements were resolved by consulting a third reviewer. This scale included the method of randomization, blinding, and loss to follow-up. In addition, sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other biases were inspected to assess the risk of bias. The latter were reported as low risk, unclear risk, or high risk for each trial. Low risk was defined as low risk of bias in all domains. Unclear risk was defined as unclear risk of bias in at least one or more domains. Publication bias was assessed by funnel-plot techniques with Review Manager 5.3, Begg’s funnel plot, and Egger’s test with Stata software (version 12.0; StataCorp LP, College Station, TX, USA).
Data extraction and management
Using a data-extraction table, two reviewers independently extracted data. Disagreements were resolved by discussion with another two reviewers until a consensus was achieved. Then, data were proofread by another reviewer. Benefits were measured by participants’ perceived change in sleep. The variables that we considered were objective sleep evaluation (TST, sleep efficiency, sleep latency, and the number of arousals/hour during sleep) and subjective sleep evaluation (sleep quality). All variables were analyzed separately. To measure risks, we determined the reverse respiratory effects in the sleep state (AHI, desaturation-event duration, sleep apnea, maximum tcPCO2).
Statistical analysis
Data were mostly analyzed by Review Manager 5.3. Outcome variables were continuous data that were combined to weighted mean difference (WMD) or standardized MD (SMD). In total, five potentially eligible studies were identified for inclusion. Standard deviation (SD) was used in four articles and standard error (SE) in one. In according to SD = SE × n½, SE was transformed to SD. The statistical heterogeneity of the data was explored and quantified using the I2 test. Heterogeneity was predefined as I2>50%. The random-effect model was used if heterogeneity was observed; otherwise, the fixed-effect model was used. P<0.05 was considered statistically significant. Publication bias and layered analysis were done with the Stata software.
Results
Study selection and characteristics
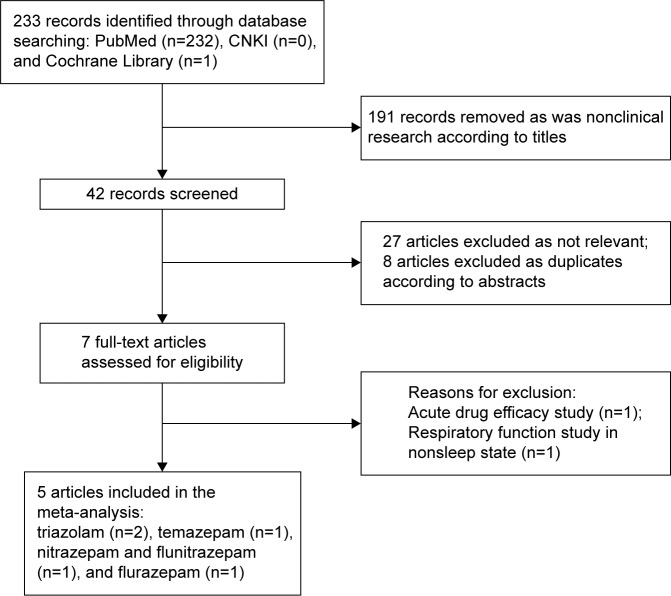
A total of 233 records were identified through the initial search, and 191 records were removed as nonclinical. The remainders of the 42 records were screened. After assessment of the titles and abstracts, eight articles were removed as duplicates and 27 articles were excluded as not relevant. After reading full texts, two studies were removed, which were of acute pharmacodynamics and respiratory function in nonsleep status. In total, five potentially eligible studies were identified for inclusion. The flow diagram is presented in Figure 1.
Figure 1.
Flow diagram of the search process and study selection.
Abbreviation: CNKI, China National Knowledge Infrastructure.
The studies included, from five countries, were published from 1984 to 2010.13–17 They were written in English, and were randomized, double-blind, placebo-controlled, and crossover clinical trials. The purposes of these studies were to see the efficacy of BZD on sleep and the effect on respiratory function in patients with COPD. The enrolled patients with insomnia and COPD of different degrees (mild, moderate, and severe) were without chronic carbon dioxide retention or an exacerbation in the previous 6 weeks. The characteristics of the included studies are shown in Table 1. Quality assessments of the included studies are shown in Table 2.
Table 1.
Characteristics of the studies included in the meta-analysis
| Study | Region | Study design | n | FEV1% predicted (mean ± SD) | Trial drug (dose) | Trial time | Outcome |
|---|---|---|---|---|---|---|---|
| Block et al14 | USA | DB, COS, R, PC | 20 | 29–99 (53±21) | Flurazepam (30 mg) | 2 consecutive nights | TST, apnea |
| Timms et al15 | UK | DB, COS, R, PC | 10 | 17–76 (38±19) | Triazolam (0.125 mg, 0.25 mg) | 3 nights within a2-week period | TST, sleep duration, arousals/hour of sleep, percentage of time below 90% SaO2, time spent in stage 2 non-REM sleep, subjective sleep quality, mean SaO2, minimum SaO2, number of apneic and hypopneic events |
| Midgren et al16 | Sweden | DB, COS, R, PC | 14 | 31±11 | Nitrazepam (5 mg) flunitrazepam (1 mg) | 3 nights within a1-week period | Sleep latency, TST, SE, periods of wakefulness, apneas, maximum tcPCO2 increase |
| Steens et al17 | Canada | DB, COS, R, PC | 23 | 40–80 (61±12) | Triazolam (0.25 mg) | 4 consecutive nights | TST, sleep latency, arousals/total sleep time, SE, sleep quality (VAS) |
| Stege et al13 | the Netherlands | DB, COS, R, PC | 14 | 33.5±9.2 | Temazepam (10 mg) | 1 week | TST, SE, NWAK, sleep quality (VAS) (AHI, %TST) with SaO290% |
Abbreviations: DB, double-blind; COS, crossover study; R, randomized; PC, placebo-controlled; TST, total sleep time; SaO2, arterial oxygen saturation; REM, rapid eye movement; SE, sleep efficiency; tcPCO2, transcutaneous carbon dioxide pressure; VAS, visual analog scale; NWAK, number of awakenings; AHI, Apnea–Hypopnea Index.
Table 2.
Quality assessments of the studies included
| Study | Random-sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Block et al14 | Unclear | Unclear | Low | Unclear | Low | Low | Low |
| Midgren et al16 | Unclear | Unclear | Low | Unclear | Low | Low | Low |
| Stege et al13 | Low | Unclear | Low | Unclear | Low | Low | Low |
| Timms et al15 | Unclear | Unclear | Low | Unclear | Low | Low | Low |
| sSteens et al17 | Unclear | Unclear | Low | Unclear | Low | Low | Low |
Objective assessment of sleep
Sleep time
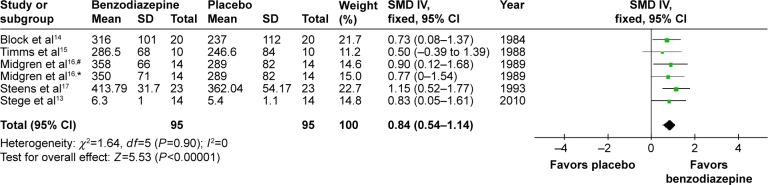
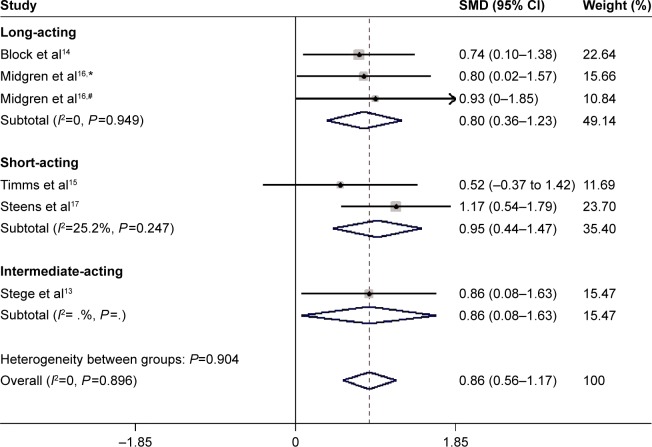
The effects of BZD on TST were estimated from all five trials (two kinds of drugs involved in one of them),13–17 which included a total of 190 patients (Figure 2). We detected no evidence of a publication bias after funnel-plot analysis (Figure 3), Begg’s test (P=0.851), and Egger’s test (P=0.335). Heterogeneity was determined to be nonsignificant (P=0.90, I2=0), and thus the fixed-effects model was used. Minutes were a measure in four articles and hours in one article. Total effects were merged to SMD, which was 0.84 (95% confidence interval [CI] 0.54–1.14, P<0.00001) (Figure 2). When BZD was compared with placebo, TST was significantly longer. According to layered research, TST was increased by all BZD administration. SMDs were shown as 0.80 (95% CI 0.36–1.23, P=0.949) for long-acting BZD, 0.86 (95% CI 0.08–1.63) for intermediate-acting BZD, and 0.95 (95% CI 0.44–1.47, P=0.247) for short-acting BZD (Figure 4).
Figure 2.
Effect of BZD on TST in COPD patients with insomnia.
Notes: #Flunitrazepam; *nitrazepam. Data was analyzed by Review Manager 5.3. Heterogeneity was predefined as I2>50%. The random-effect model was used if heterogeneity was observed. Otherwise, the fixed-effect model was used.
Abbreviations: BZD, benzodiazepine; TST, total sleep time; SD, standard deviation; SMD, standardized mean difference; IV, independent variable; CI, confidence interval.
Figure 3.
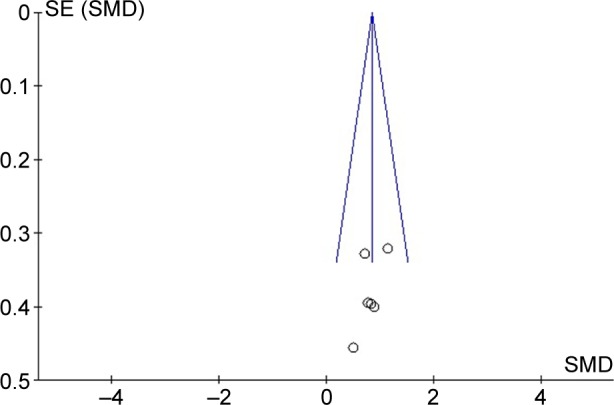
Funnel plot of published studies in relation to TST meta-analysis.
Abbreviations: TST, total sleep time; SE, sleep efficiency; SMD, standardized mean difference.
Figure 4.
Subgroup meta-analysis of different half-life of BZD on TST in COPD patients with insomnia.
Notes: #Flunitrazepam; *nitrazepam. As there is limited data, there is no value from statistical software in this section.
Abbreviations: SMD, standardized mean difference; CI, confidence interval.
Sleep efficiency
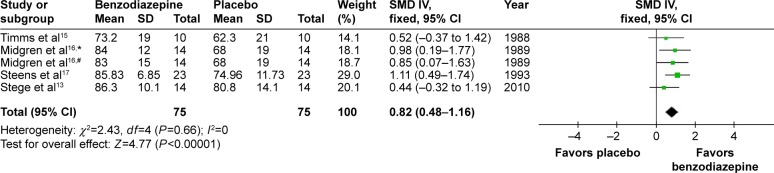
The effects of BZD on sleep efficiency were estimated from four studies with five types of BZD.13,15–17 SMD was 0.82 (95% CI 0.48–1.16, P<0.00001), with testing for heterogeneity (I2=0, P=0.66) (Figure 5). Compared with placebo, BZD improved sleep efficiency.
Figure 5.
Effect of BZD on SE in COPD patients with insomnia.
Notes: #Flunitrazepam; *nitrazepam. Data was analyzed by Review Manager 5.3. Heterogeneity was predefined as I2>50%. The random-effect model was used if heterogeneity was observed. Otherwise, the fixed-effect model was used.
Abbreviations: BZD, benzodiazepine; SE, sleep efficiency; SD, standard deviation; SMD, standardized mean difference; IV, independent variable; CI, confidence interval.
Sleep latency
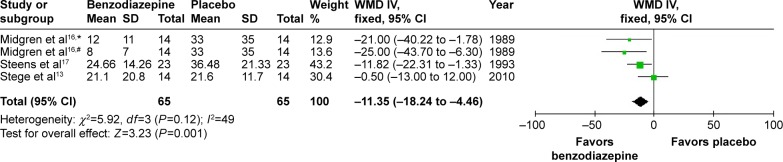
In three studies, the effects of four types of BZD on sleep latency were investigated. WMD was −11.35 (95% CI −18.24 to −4.46, P=0.001), with testing for heterogeneity (I2=49%, P=0.12) (Figure 6). As shown in the data, compared with placebo, BZD decreased sleep latency.
Figure 6.
Effect of BZD on sleep latency in COPD patients with insomnia.
Notes: #Flunitrazepam; *nitrazepam.
Abbreviations: BZD, benzodiazepine; SD, standard deviation; WMD, weighted mean difference; IV, independent variable; CI, confidence interval.
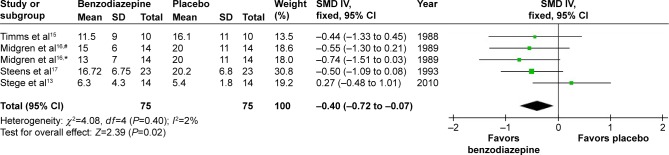
Number of arousals/hour of sleep
In four studies,14–17 the effects of five types of BZD on arousals/hour of sleep were studied. SMD was −0.40 (95% CI −0.72 to −0.07, P=0.02), with testing for heterogeneity (I2=2%, P=0.40) (Figure 7). As shown in the data, compared with placebo, BZD reduced the number of arousals/hour of sleep.
Figure 7.
Effect of BZD on arousal number during sleep in COPD patients with insomnia.
Notes: #Flunitrazepam; *nitrazepam.
Abbreviations: BZD, benzodiazepine; SD, standard deviation; SMD, standardized mean difference; IV, independent variable; CI, confidence interval.
Subjective assessment of sleep
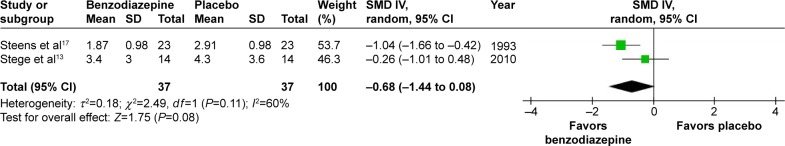
Three studies showed sleep-quality data for BZD versus placebo.13,15,17 Sleep quality was assessed with visual analog scales in two studies13,15 and digit–symbol substitution testing in one study.17 Scales used were the same in two of the studies.13,17 We combined the data of these two studies to see the effect of BZD versus placebo on sleep quality (SMD −0.68, 95% CI −1.44 to 0.08; P=0.08) (Figure 8). Testing of the two results for heterogeneity resulted in I2=60% (P=0.11), because of the different scales used. Meanwhile, the other scale, measuring the opposite direction, was analyzed separately (SMD 1.12, 95% CI −0.24 to 2.18).15 Results indicated that no significant improvement in subject sleep quality was shown with BZD use.
Figure 8.
Effect of BZD on subjective sleep quality in COPD patients with insomnia.
Abbreviations: BZD, benzodiazepine; SD, standard deviation; SMD, standardized mean difference; IV, independent variable; CI, confidence interval.
Respiratory assessment
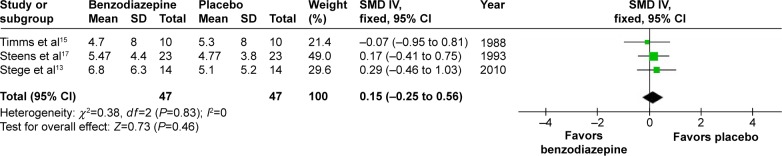
AHI and number of apneas
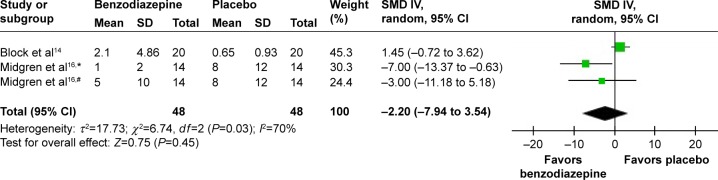
In three studies,13,15,17 the effects of BZD with two types on AHI were shown. The combined data indicated no significant increase (SMD 0.15, 95% CI −0.25 to 0.56; P=0.46) (Figure 9). In two studies,14,16 the effects of three types of BZD on apnea were shown. The combined data of apnea numbers showed no significant difference (SMD −2.20, 95% CI −7.94 to 3.54; P=0.45) (Figure 10). Therefore, BZD use did not increase AHI or number of apneas.
Figure 9.
Effect of BZD on AHI in COPD patients with insomnia.
Abbreviations: BZD, benzodiazepine; AHI, Apnea–Hypopnea Index; SD, standard deviation; SMD, standardized mean difference; IV, independent variable; CI, confidence interval.
Figure 10.
Effect of BZD on sleep apnea in COPD patients with insomnia.
Notes: #Flunitrazepam; *nitrazepam.
Abbreviations: BZD, benzodiazepine; SD, standard deviation; SMD, standardized mean difference; IV, independent variable; CI, confidence interval.
Percentage of time below 90% SaO2 and mean SaO2 in night sleep
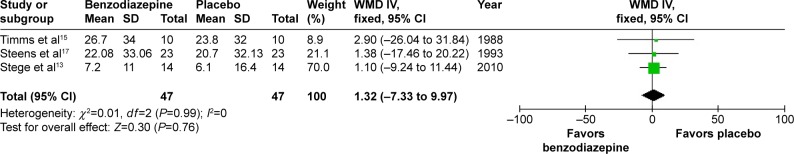
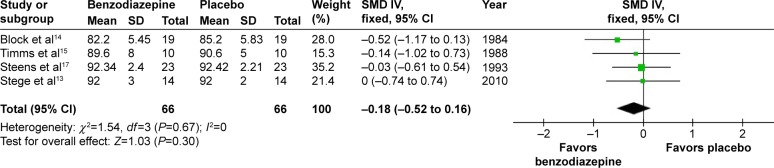
In three studies,13,15,17 the effects of three types of BZD on percentage of time below 90% arterial oxygen saturation (SaO2) were shown. The combined data revealed a WMD of 1.32 (95% CI −7.33 to 9.97, P=0.76) (Figure 11). In four studies,13–15,17 the effects of three types of BZD on mean SaO2 in night sleep were shown. The combined data revealed an SMD of −0.18 (95% CI −0.52 to 0.16, P=0.30) (Figure 12). Therefore, BZD use had no impact on percentage of time below 90% SaO2 or mean SaO2 in night sleep.
Figure 11.
Effect of BZD on percentage of time below 90% SaO2 in COPD patients with insomnia.
Abbreviations: BZD, benzodiazepine; SaO2, arterial oxygen saturation; SD, standard deviation; WMD, weighted mean difference; IV, independent variable; CI, confidence interval.
Figure 12.
The effect of BZD on mean SaO2 in night sleep in COPD patients with insomnia.
Abbreviations: BZD, benzodiazepine; SaO2, arterial oxygen saturation; SD, standard deviation; SMD, standardized mean difference; IV, independent variable; CI, confidence interval.
Max tcPCO2 increase during sleep
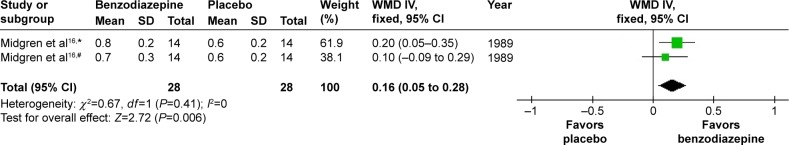
In one study,16 the effects of two types of BZD on maximum tcPCO2 increase during sleep were shown. The combined data revealed a WMD of 0.16 (95% CI 0.05–0.28, P=0.006) (Figure 13). Compared with placebo, BZD significantly increased maximum tcPCO2 during sleep.
Figure 13.
The effect of BZD on max tcPCO2 increase during sleep in COPD patients with insomnia.
Notes: #Flunitrazepam; *nitrazepam.
Abbreviations: BZD, benzodiazepine; tcPCO2, transcutaneous carbon dioxide pressure; SD, standard deviation; WMD, weighted mean difference; IV, independent variable; CI, confidence interval.
Discussion
COPD, a leading cause of mortality and morbidity worldwide, is a common disease in the respiratory system.18 Among the comorbidities accompanying COPD, insomnia is prevalent. The sleep of patients with COPD is often of poor quality. Sleep disturbances can substantially reduce patients’ quality of life. Over 50% of these patients report sleep-related complaints characterized by longer sleep latency, more frequent arousals and awakenings, and more daytime sleepiness.19 As a result, patients often consult their physicians for hypnotics. Among these, BZDs are proven to improve sleep quality, and are the first-choice hypnotics nowadays.
However, BZDs have been linked to adverse physiological respiratory outcomes in patients with COPD. They may decrease central sensitivity to hypoxic and hypercapnic stimuli, and then lead to exacerbation of respiratory failure. Also, they may increase upper-airway resistance, mainly due to muscular relaxation.20 Epidemiological research has also suggested that hypnotics increase the risk of adverse respiratory events (pneumonia, COPD with acute exacerbation, acute respiratory failure, and cardiopulmonary arrest) in patients with COPD.9 Therefore, administration of BZD might lead to potential respiratory adverse effects. Safe pharmacological treatment of insomnia must include consideration of whether the hypnotic drugs could potentially exacerbate the impaired gas exchange and atony problems that present during sleep in COPD patients. These complications highlight the need to reevaluate current insomnia treatment in COPD patients. Although several sets of results have been published, BZD use remains controversial.13–17 This meta-analysis showed the efficacy and safety of BZD to treat insomnia in COPD patients.
The most common drugs prescribed for insomnia with COPD are the BZD-receptor agonists (BZRAs), a group of drugs that function by binding to the BZD receptor at the γ-aminobutyric acid (GABA)-A complex. BZRAs include both traditional BZD and non-BZD BZRAs.6 Among them, the former is more common in clinical use. BZDs promote chloride-channel opening by combining with the GABA receptor, and then chloride influx, which leads to a sedative hypnotic effect. They distribute rapidly to tissues because of a high plasma protein-binding rate and lipid solubility. Meanwhile, the biotransformation is dependent on liver enzymes. The half-lives of most metabolites are longer than the originals. The drugs are inactivated by binding of the metabolites to glucuronic acid, and then eliminated through the kidneys. The drugs, with different half-lives, have different durations of action and characteristics.
According to polysomnography data, we carried out objective evaluations during sleep. Meta-analysis showed that BZD use could increase TST, improve sleep efficiency, shorten sleep latency, and reduce the number of arousals during sleep.13–17 Under layer analysis, BZD use was indicated to increase TST no matter how long the half-life was. BZD was able to improve objective indicators of sleep. However, it was shown that BZD was not able to improve subjective sleep quality, according to analysis with related data.13,15,17 As we know, different types of sleep disorders can present in COPD patients, including function disorders with sleep onset and sleep maintenance, early morning wakening or the presence of parasomnias, and refreshing sleep. Because of the limited number of clinical trials and participants, we were not able to analyze these. Despite the inconsistent results, our findings have some value. It seems that BZD may influence sleep quality.
BZDs can improve sleep quality partly, but are also thought to cause respiratory depression in patients with COPD. Because of abnormal respiratory function and insufficient compensation, new diseases and existing physical illnesses may be aggravated with BZD use. This study made an objective evaluation of respiratory status in COPD patients with BZD use. Meta-analysis showed that not all AHI scores, number of apneas, percentage of time below 90% SaO2, and mean SaO2 in night sleep were affected, but maximum tcPCO2 increase during sleep was affected. Therefore, these observed effects of respiratory function suggested that there might be safety concerns associated with the use of BZD in the COPD population.
However, in the included studies of the meta-analysis, all of the enrolled subjects had normocapnic COPD in a stable stage, and most of them were mild or moderate COPD patients. Most hypnotics used were short and medium half-life drugs, with oral route of administration, and the longest time duration was 1 week.13–17 Severity of illnesses, chemical characteristics, and time duration of drugs may all influence results. In addition, BZDs were associated with increased risks of several serious adverse respiratory outcomes among older adults with COPD.7,21 Also, BZD and cognitive function results suggested that BZD may not be the best choice for use in elderly COPD patients, who may be at increased risk of motor vehicle accidents, falls, fractures, and dementia.8,22 Other psychological and pharmacological aspects of BZD use in COPD patients, eg, anxiety, agitated depression,23 night cramping, pharmacological dependency, should also be considered. Therefore, decisions on BZD use to improve sleep in patients with COPD need to consider cautiously the severity of disease, type of BZD, dose, route, frequency of delivery, duration of treatment, and psychodynamic aspects of BZD use.
Administration of BZD must be cautious in COPD patients, especially for older patients with severe COPD, as these drugs suppress the respiratory response and exacerbate sleep-related disorders.8–10,24 Now, a newer group of more selective BZRAs called non-BZD BZRAs has come out. These drugs are more selective for a BZD-receptor subtype that is expressed in the central nervous system, and produce fewer adverse side effects of pulmonary function than the traditional BZRAs.6,10 In addition, another treatment option for insomnia in COPD patients is ramelteon, a MT1/MT2 melatonin-receptor agonist. By engaging signaling pathways downstream of these G-protein-coupled receptors, ramelteon is believed to decrease sleep latency and increase sleep efficiency. More clinical trials need to be done to assess the effects of ramelteon on respiratory function. In randomized studies, ramelteon has been shown to be safe in patients with mild-to-moderate, even to severe COPD patients.7
This meta-analysis has some limitations that should be noted. First, only five trials were included, and the total number of participants was limited. In recent years, very few randomized controlled clinical trials, but some population-based case-control studies, have been done on this topic.8–10 This has probably been confined because of ethical issues. Therefore, the trials included were few and old. High-quality studies were required. Second, the longest duration of drug use was 1 week in trials included, and most durations were short. Insomnia accompanying COPD is a chronic disease. Long-term administration of hypnotic drugs should be needed. Therefore, the clinical implications of this study are limited. Clinical signs of respiratory depression should be evaluated with long-term treatment with BZD in COPD patients. In addition, layer analysis with the various half-lives of different drugs was not done to lead to different adverse respiratory outcomes. Because of different metabolic times of different half-life drugs, the effect of drugs on respiratory depression is different. Very few trials were included, and these trials had small sample sizes. Therefore, layer analysis was not done. Finally, most of the subjects in the trials had mild and moderate COPD, and few had a severe status of pulmonary function. The severity of diseases could influence the results.
Conclusion
This manuscript focused on an ambitious clinical project: the efficacy and safety of BZD on insomnia in COPD patients. The results suggested BZDs might be efficient and safe hypnotics. Compared with placebo, BZD improved sleep quality partly and significantly increased maximum tcPCO2 during sleep. More randomized controlled trials are necessary. In spite of some limitations in this meta-analysis, the results, which show partly improved efficacy and safety, are valuable to guide further study for ethical issues. In addition, BZD use must be cautious in COPD patients with hypercapnia, and newer drugs should be developed.
Acknowledgments
The article was financed by the National Natural Science Foundation of China (grant 81201489).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Budhiraja R, Siddiqi TA, Quan SF. Sleep disorders in chronic obstructive pulmonary disease: etiology, impact, and management. J Clin Sleep Med. 2015;11(3):259–270. doi: 10.5664/jcsm.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hynninen MJ, Pallesen S, Hardie J, et al. Insomnia symptoms, objectively measured sleep, and disease severity in chronic obstructive pulmonary disease outpatients. Sleep Med. 2013;14(12):1328–1333. doi: 10.1016/j.sleep.2013.08.785. [DOI] [PubMed] [Google Scholar]
- 3.Omachi TA, Blanc PD, Claman DM, et al. Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes. Sleep Med. 2012;13(5):476–483. doi: 10.1016/j.sleep.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budhiraja R, Parthasarathy S, Budhiraja P, Habib MP, Wendel C, Quan SF. Insomnia in patients with COPD. Sleep. 2012;35(3):369–375. doi: 10.5665/sleep.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth T. Hypnotic use for insomnia management in chronic obstructive pulmonary disease. Sleep Med. 2009;10(1):19–25. doi: 10.1016/j.sleep.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine use among older adults with chronic obstructive pulmonary disease. Drugs Aging. 2013;30(3):183–192. doi: 10.1007/s40266-013-0056-1. [DOI] [PubMed] [Google Scholar]
- 7.Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J. 2014;44(2):332–340. doi: 10.1183/09031936.00008014. [DOI] [PubMed] [Google Scholar]
- 8.Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. doi: 10.1136/bmj.e6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung WS, Lai CY, Lin CL, Kao CH. Adverse respiratory events associated with hypnotics use in patients of chronic obstructive pulmonary disease: a population-based case-control study. Medicine (Baltimore) 2015;94(27):e1110. doi: 10.1097/MD.0000000000001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen SJ, Yeh CM, Chao TF, et al. The use of benzodiazepine receptor agonists and risk of respiratory failure in patients with chronic obstructive pulmonary disease: a nationwide population-based case-control study. Sleep. 2015;38(7):1045–1050. doi: 10.5665/sleep.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 12.Halvorsen T, Martinussen PE. Benzodiazepine use in COPD: empirical evidence from Norway. Int J Chron Obstruct Pulmon Dis. 2015;10:1695–1702. doi: 10.2147/COPD.S83107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stege G, Heijdra YF, van den Elshout FJ, et al. Temazepam 10 mg does not affect breathing and gas exchange in patients with severe normocapnic COPD. Respir Med. 2010;104(4):518–524. doi: 10.1016/j.rmed.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Block AJ, Dolly FR, Slayton PC. Does flurazepam ingestion affect breathing and oxygenation during sleep in patients with chronic obstructive lung disease? Am Rev Respir Dis. 1984;129(2):230–233. [PubMed] [Google Scholar]
- 15.Timms RM, Dawson A, Hajdukovic RM, Mitler MM. Effect of triazolam on sleep and arterial oxygen saturation in patients with chronic obstructive pulmonary disease. Arch Intern Med. 1988;148(10):2159–2163. [PubMed] [Google Scholar]
- 16.Midgren B, Hansson L, Skeidsvoll H, Elmqvist D. The effects of nitrazepam and flunitrazepam on oxygen desaturation during sleep in patients with stable hypoxemic nonhypercapnic COPD. Chest. 1989;95(4):765–768. doi: 10.1378/chest.95.4.765. [DOI] [PubMed] [Google Scholar]
- 17.Steens RD, Pouliot Z, Millar TW, Kryger MH, George CF. Effects of zolpidem and triazolam on sleep and respiration in mild to moderate chronic obstructive pulmonary disease. Sleep. 1993;16(4):318–326. doi: 10.1093/sleep/16.4.318. [DOI] [PubMed] [Google Scholar]
- 18.Raherison C, Girodet PO. Epidemiology of COPD. Eur Respir Rev. 2009;18(114):213–221. doi: 10.1183/09059180.00003609. [DOI] [PubMed] [Google Scholar]
- 19.Urbano F, Mohsenin V. Chronic obstructive pulmonary disease and sleep: the interaction. Panminerva Med. 2006;48(4):223–230. [PubMed] [Google Scholar]
- 20.Berry RB, McCasland CR, Light RW. The effect of triazolam on the arousal response to airway occlusion during sleep in normal subjects. Am Rev Respir Dis. 1992;146(5 Pt 1):1256–1260. doi: 10.1164/ajrccm/146.5_Pt_1.1256. [DOI] [PubMed] [Google Scholar]
- 21.Ekström MP, Bornefalk-Hermansson A, Abernethy AP, Currow DC. Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ. 2014;348:g445. doi: 10.1136/bmj.g445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogunovic OJ, Greenfield SF. Practical geriatrics: use of benzodiazepines among elderly patients. Psychiatr Serv. 2004;55(3):233–235. doi: 10.1176/appi.ps.55.3.233. [DOI] [PubMed] [Google Scholar]
- 23.Asnaashari AM, Talaei A, Haghigh B. Evaluation of psychological states in patients with asthma and COPD. Iran J Allergy Asthma Immunol. 2012;11(1):65–71. [PubMed] [Google Scholar]
- 24.Chen SJ, Yeh CM, Chao TF, et al. The use of benzodiazepine receptor agonists and risk of respiratory failure in patients with chronic obstructive pulmonary disease: a nationwide population-based case-control study. Sleep. 2015;38(7):1045–1050. doi: 10.5665/sleep.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]