Abstract
The ratio of glutathione disulfide (GSSG) to reduced glutathione (GSH) in biological samples is a frequently used parameter of oxidative stress. As a result, many methods have been developed to measure GSSG. The most popular and convenient of these relies on enzymatic cycling following the chemical masking of GSH in the sample using 2-vinylpyridine (2VP). However, 2VP is a slow reactant and its use may result in artificially high GSSG values due to oxidation of the sample over time. Faster-reacting reagents like N-ethylmaleimide (NEM) may provide more accurate results. We have performed a direct comparison of methods using 2VP and NEM. With 2VP, the percentage of total glutathione (GSH+GSSG) in the oxidized form was significantly higher in all tested tissues (kidney, lung, and liver) compared to the same procedure performed using NEM. We conclude that NEM, when coupled with a simple solid phase extraction procedure, is more accurate for determination of GSSG. We also tested the effects of various handling and storage conditions on GSSG. A detailed description and a discussion of other methods are also included.
Keywords: Glutathione, GSSG, N-ethylmaleimide, 2-vinylpyridine, liver, Tietze assay
INTRODUCTION
Glutathione is a ubiquitous thiol-containing tripeptide with normal intracellular concentration in the millimolar range. Among other roles, the chemical properties and relative abundance of this molecule makes it well-suited to function as an antioxidant. In such cases, a portion of the reduced glutathione (GSH) in the cell can donate hydrogen to reduce peroxides and free radicals, becoming oxidized to glutathione disulfide (GSSG). Combined with the protective effects of reversible protein S-glutathionylation, this scavenging behavior effectively saves other molecules in the cell from the same fate. Because of this, the ratio of GSSG to GSH in biological samples is often used as an indicator of oxidant stress and injury in toxicological studies (Meister and Anderson, 1983; Jaeschke, 1990; Jones, 2002; Knight et al., 2001; 2003; Valko et al., 2007).
Early methods used to measure GSSG involved the chemical masking of GSH in the samples to be tested, followed by enzymatic cycling of the GSSG to GSH by glutathione reductase (GR) and NADPH. Measurement of GSSG was based on oxidation of NADPH (Guntherberg and Rost, 1966; Srivastava and Beutler, 1967) or formation of 5-thio-2-nitrobenzoic acid (TNB), the reduced form of dithionitrobenzene (DTNB), in the cycling assay (Fig. 1) (Tietze, 1969). Early practitioners used the fast-reacting N-ethylmaleimide (NEM) as their masking agent (Guntherberg and Rost, 1966). However, NEM is known to inhibit GR and efforts to remove excess NEM proved largely impractical. To circumvent this difficulty, NEM was substituted with 2-vinylpyridine (2VP) as the GSH masking agent (Griffith, 1980). This method has since become standard for determination of GSSG in most biological samples. This approach has several advantages over more recent analytical methods including low cost and convenience of use. However, a potential problem with this method is that 2VP reacts with GSH very slowly. At room temperature, complete masking of GSH takes approximately 1 h. During this time, it is possible for GSH in the sample to become oxidized due simply to exposure to ambient conditions, yielding reports of erroneously high GSSG/GSH values.
Figure 1.
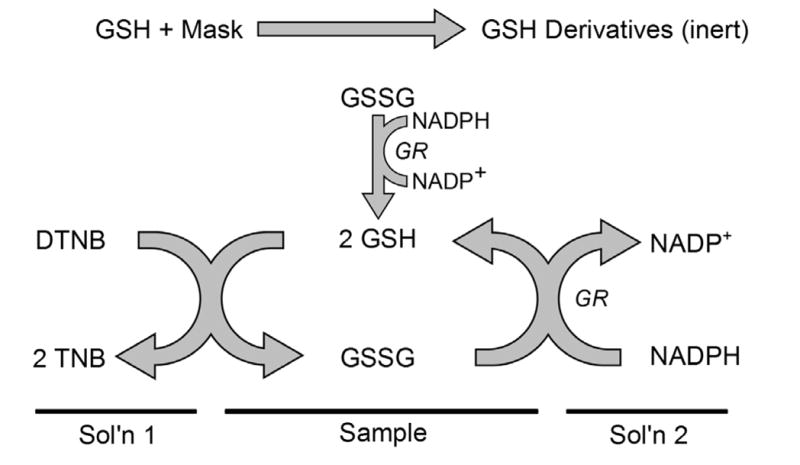
A proposed mechanism of enzymatic cycling for detection of oxidized glutathione. GSH in the sample reacts with the masking agent (NEM or 2-VP) and is effectively removed from the reaction. The remaining glutathione is in the oxidized form (GSSG). After reaction of GSH and the masking agent is complete and, in some cases, removal of the GSH derivative, the GSSG is converted to GSH by glutathione reductase (GR). The GSH then reacts with a colorimetric reagent, such as 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB; Ellman’s reagent). The GSSG can then be recycled by GR to continue the reaction. The absorbance of TNB (412 nm) at a specific reaction time is directly proportional to the concentration of GSSG in the sample. Lines indicate which reagents provide the above reaction constituents.
Adams et al. (1983) introduced a method for determination of the GSSG content using NEM with solid phase extraction. This method was later modified to reduce certain errors (Jaeschke and Mitchell, 1990). In this paper, we describe the modified method more fully and directly compare it with the more common approach that uses 2VP. Our results show the superiority of NEM with solid phase extraction to the current standard. While newer methods for determination of glutathione content than those mentioned have been reported in the literature, most of these require expensive equipment or kits, questionable handling of material, or are laborious and time-intensive. We believe that the procedure described here can provide physiologically accurate results without any of these specific disadvantages. Because it was clear from our comparison of NEM and 2VP that sample handling may also affect GSSG results, we have tested the effect of storage conditions and time-to-storage on GSSG values.
METHODS
Animals
Wildtype C57BL/6J were purchased from Jackson Laboratories (Bar Harbor, ME). All animals were housed in an environmentally controlled room with 12-hr light/dark cycle and allowed free access to food and water. Animals were euthanized under anesthesia by exsanguination and diaphragmatic rupture. Organs were excised and rinsed in saline. Except where noted otherwise, livers were freeze-clamped immediately following removal and stored at −80°C. Other organs were snap frozen in liquid nitrogen and also stored at −80°C.
Reagents and Chemicals
Mono- and di- potassium phosphate were purchased from J.T. Baker (Phillipsburg, NJ). All other chemicals were purchased from Sigma (St. Louis, MO). The following reagents were used in experiments: Solution 1: 600 mM potassium phosphate (pH 7.2), 1.2 mM DTNB, 30 mM EDTA, and 0.08% BSA. Solution 2: 100 mM Imidazole (pH 7.2), 2 mM EDTA, and 0.04% BSA. Reaction Mix: 0.5 mg/mL NADPH and 1.60 U/mL of Glutathione Reductase added to appropriate volume of Solution 2 (Fig. 1).
Sample Processing
Frozen tissue was weighed on ice and rapidly homogenized in ice cold 3% sulfosalicylic acid (SSA) containing 0.1mM EDTA with a PowerGen Model 125 blade-type homogenizer to yield a 10% (w/v) homogenate. 200 μL of this homogenate was immediately transferred to 1 mL of a 10 mM NEM solution prepared in 100 mM potassium phosphate buffer on ice. Another aliquot of the homogenate was diluted 1:50 in 0.01 N HCl in H2O for use in determination of total glutathione. Tubes were then centrifuged at 4°C, 14,000 × g, for 4 minutes and the deproteinized supernatants were transferred to fresh tubes. All samples were kept on ice. Removal of excess NEM was accomplished by chromatographic separation using a small C18 column (Sep-Pak; Waters Associates, Millipore Corporation, Milford, PA). 700 μL of sample was passed through the column (~1 drop/sec) by injection and into a fresh tube, followed by 1 mL of 100 mM potassium phosphate buffer. Regeneration of the column for use with subsequent samples was accomplished with a 6 mL methanol rinse using a fresh syringe. To prevent interference due to methanol in the enzymatic assay, the column was air dried using the same syringe, rinsed with H2O, and air dried again, before applying the next sample. In all, the process required < 5 minutes per sample. Between experiments, the C18 cartridges were stored in methanol. Determination of GSSG using 2VP as the GSH masking agent was done as described (Griffith, 1980), in parallel with the above experiments. Briefly, 200 μL of the deproteinized supernatant was mixed with 4 μL 2VP and 12 μL triethanolamine (to pH 7.0–7.5) and incubated at room temperature for 1 h. Following the 1 h incubation, 2VP treated samples were diluted 1:30 in 100 mM potassium phosphate buffer. GSH sample supernatants in HCl were further diluted 1:26 in 100 mM potassium phosphate before measurement. NEM treated samples were used without further dilution.
Enzymatic Determination of Glutathione
150 μL of Solution 1 was added to each reaction tube, followed by 500 μL of either blank (100 mM potassium phosphate), GSH standard, or diluted sample. Timing of the reaction began with the addition of 150 μL of the enzyme-containing reaction mix. For each standard and sample, absorbance at 412 nm was recorded at exactly 10 minutes using a Shimadzu UV-1601 spectrophotometer. Sample concentrations were determined using a 7-point 0.1 – 1 μmol/L standard curve.
Statistical Analysis
Data represent means ± SE for multiple experiments. Data were analyzed using the Student’s t-test. P values < 0.05 were considered significant.
RESULTS
Using healthy livers from 18 C57Bl/6J mice, the intra- and inter-assay coefficients of variation (CV) were found to be 3.3% and 11.1% for GSSG measurement using NEM as the masking agent. To compare the accuracy of GSSG measurement when GSH was masked with NEM compared to 2VP, freeze-clamped or snap frozen tissues from healthy mice were weighed on ice and quickly homogenized in ice cold 3% SSA and aliquots were added to a solution containing NEM at pH 6.5. Alternatively, neat 2VP was added to the supernatants of sample homogenates and incubated for 1 h following adjustment of pH to 7.0–7.5 using triethanolamine, as described (Griffith, 1980). NEM immediately reacts with GSH, without need for prolonged incubation. Removal of NEM from samples was accomplished with a simple chromatographic procedure using a C18 column. GSH and GSSG following NEM removal or incubation with 2VP were measured using an enzymatic cycling method (Tietze, 1969).
Total glutathione (GSH+GSSG) and GSSG levels were measured in liver, lung and kidney tissue from mice. GSSG levels were substantially higher when 2VP was used as the masking agent for GSH (Table 1). In particular, the percentage of GSH+GSSG in the form of GSSG in healthy livers was found to be much lower using NEM (Fig. 2). These data clearly demonstrate that the prolonged reaction time of 2VP with GSH results in oxidation of unreacted GSH that leads to erroneously high GSSG values. A literature search yielded similar results from other laboratories (Table 2). While considerably less work appears to have been done using NEM as the GSH masking agent, available data are consistent with our results (Table 2).
TABLE 1.
PERCENTAGE OF GLUTATHIONE IN THE OXIDIZED FORM IN VARIOUS TISSUES FROM HEALTHY MICE USING DIFFERENT MASKING AGENTS.
| Tissue | GSSG nmol / g tissue | GSH + GSSG μmol / g tissue | % GSSG |
|---|---|---|---|
| Mouse Liver | |||
| NEM | 31.9 ± 15.4 | 7.23 ± 0.39 | 0.44 ± 0.07 |
| 2VP | 251 ± 52.8* | 3.85 ± 0.28* | |
| Mouse Lungs | |||
| NEM | 23.9 ± 3.2 | 2.50 ± 0.34 | 0.99 ± 0.13 |
| 2VP | 89.3 ± 11* | 3.58 ± 0.08* | |
| Mouse Kidney (Left) | |||
| NEM | 6.48 ± 1.8 | 2.90 ± 0.50 | 0.21 ± 0.01 |
| 2VP | 55.1 ± 5.1* | 1.90 ± 0.16* | |
| Mouse Kidney (Right) | |||
| NEM | 6.16 ± 1.9 | 3.54 ± 0.76 | 0.17 ± 0.02 |
| 2VP | 41.0 ± 5.1* | 1.21 ± 0.16* |
For GSSG measurement, GSH was trapped using either NEM or 2-VP and GSSG was measured. Data reported as GSH equivalents, mean ± SEM of n = 3–5.
P < 0.05.
Figure 2.
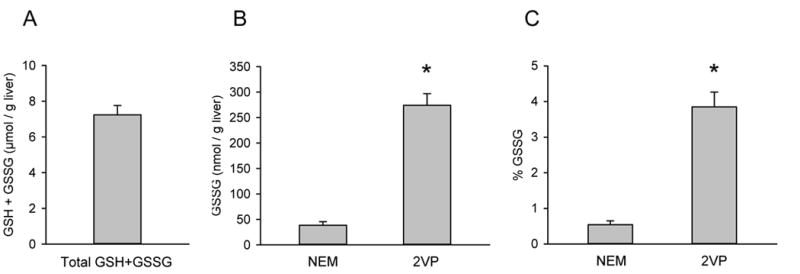
Direct comparison of the effectiveness of NEM and 2VP as thiol-masking reagents in the measurement of GSSG/GSH. Liver samples were collected from healthy C57BL/6J mice and assayed for (A) total GSH (GSH+GSSG), (B) GSSG and (C) GSSG/GSH+GSSG using either NEM or 2VP as the GSH masking agent. Data represent mean ± SE of n = 3–5. *p < 0.05 vs. NEM.
TABLE 2.
2-VP: LITERATURE VALUES FOR APPARENT PERCENTAGE OF GLUTATHIONE IN THE OXIDIZED FORM IN VARIOUS TISSUES FROM HEALTHY OR CONTROL ANIMALS.
| Tissue | GSSG | GSH+GSSG | %GSSG | Ref. |
|---|---|---|---|---|
| Mouse | ||||
| Liver | 135 ± 12 | 8920 ± 760 | ~1.5 | Santra et al., 2006 |
| 161.33 ± 17.8 | 8420 ± 111 | ~2 | Chowdhury et al., 2006 | |
| 2.5 ± 0.3‡ | 30.4 ± 3.3‡ | ~8.2 | Hung et al., 2006 | |
| Rat | ||||
| Liver | 712 ± 98 | 8901 ± 1310 | ~8 | Ghanem et al., 2009 |
| 490 ± 85 | 8093 ± 1310 | ~6 | Ghanem et al., 2009 | |
| 109 ± 30 | 4544 ± 579 | ~2.4 | Villanueva et al., 2006 | |
| ~2.7‡ | ~25‡ | ~10.8 | Morrison et al., 2005 | |
| ~0.3‡ | ~30‡ | ~1 | Caraceni et al., 2005 | |
| Kidney | 18.1 ± 1 | 462 ± 35 | ~3.9 | Villaueva et al., 2006 |
| Average | ~4.9 |
Data derived from references listed. In each study, 2-VP was listed as the GSH masking agent in the Methods section Most values are reported as nmol/g liver.
nmol/mg protein. ~ indicates estimation from available data.
Because the longer reaction time with 2VP resulted in higher GSSG levels, we hypothesized that anything that prolongs exposure of the sample to ambient conditions could result in artifactual elevations of GSSG. To test this, we measured the effects of different storage temperatures and of time from excision of tissue to freeze-clamping on determination of GSSG/GSH+GSSG. Healthy livers were excised and immediately cut into sections. Some sections were stored at different temperatures for 3 days. Importantly, tissue stored at −20°C before testing showed a large increase in GSSG/GSH+GSSG compared with those stored at −80°C for the same length of time, and compared with fresh tissue homogenized immediately following excision and freeze-clamping (Fig. 3). Surprisingly, sections held at room temperature for 5 or 10 minutes before freeze-clamping did not show a significant increase in GSSG/GSH (Fig. 4).
Figure 3.
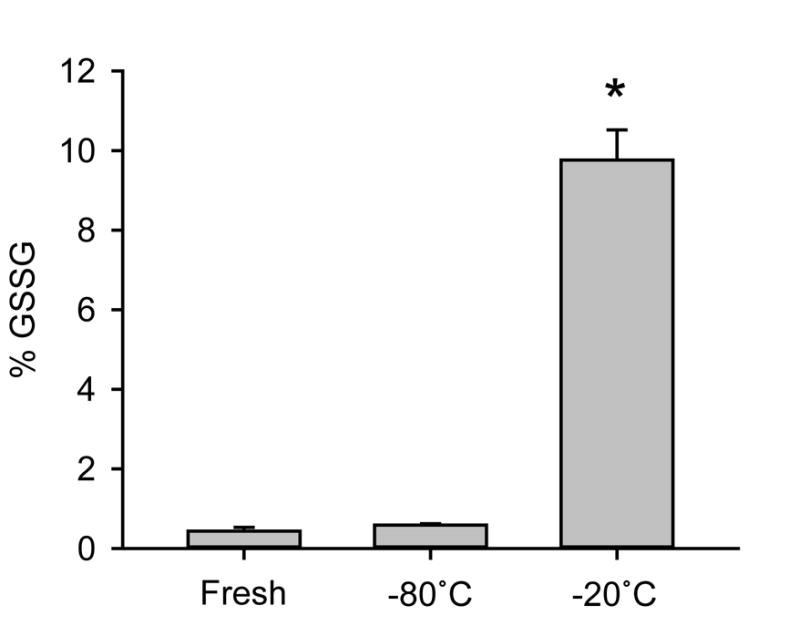
Comparison of storage conditions on GSSG/GSH values. Livers were freeze-clamped and assayed for GSSG/GSH either fresh or after 3 days of storage at the indicated temperatures. Data represent mean ± SE of n = 3–5. *p < 0.05 vs. fresh.
Figure 4.
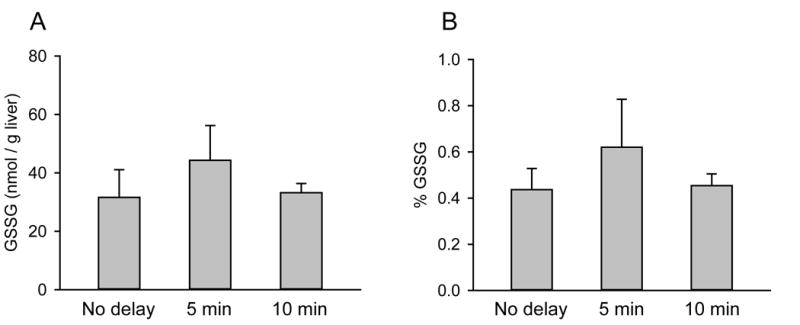
Comparison of the effects of delayed storage and storage temperature on GSSG/GSH. Livers were kept in ambient conditions for the indicated times before freeze-clamping and measurement of (A) GSSG and (B) GSSG/GSH. Data represent mean ± SE of n = 3. *p< 0.05 vs. fresh.
DISCUSSION
The ratio of GSSG to GSH is a frequently used indicator of oxidant stress in cells and tissues (Smith, 1989). However, due to the very low concentrations of GSSG relative to GSH in many tissues, it can be difficult to obtain accurate and precise measurements of GSSG alone. As a result, many methods have been introduced. While each technique has a unique set of pros and cons, the method demonstrated here has the advantages of being affordable, accessible, and capable of providing physiologically accurate results for multiple samples in parallel in a short amount of time.
The earliest methods used to measure GSSG in biological samples relied upon NEM to remove the reduced form of glutathione from the reaction mixture (Guntherberg and Rost, 1966). However, excess NEM inhibits glutathione reductase and immediately traps any GSH, preventing GSSG cycling. Adams et al. (1983) introduced the use of a C18 column to remove the NEM before assay. While this was effective, a difficulty with their protocol was the use of very small sample volumes with much larger volumes of reaction buffer. Even small pipetting errors during sample handling could have serious effects on the reaction rate and thus the results. Compounding this, three different reaction rates were needed to accurately determine each sample concentration: a baseline rate without added sample, a reaction rate with sample, and a third reaction rate to determine recovery after spiking the sample with a known amount of GSH. The concentration was calculated by comparison of the difference between the baseline rate and the reaction rate with a standard curve, then adjusted using the percentage of recovery determined from the third reaction rate. By modifying the method to allow much greater sample dilution, it became possible to use larger sample volumes (Jaeschke and Mitchell, 1990). Also, such high dilutions reduce concern over reaction interference caused by other sample or homogenate components, reducing the need for a recovery test for every sample.
Despite progress with NEM-based techniques, the method of Griffith (1980) has remained the standard for determination of GSSG concentration. Unfortunately, the use of 2VP as a GSH masking agent is not devoid of problems. The slow reaction of 2VP with the initial GSH in the sample provides a window for auto-oxidation, allowing some of the GSH to be converted to GSSG and resulting in inaccurate data. GSSG/GSH+GSSG values in the 1–12% range are regularly reported for healthy or control tissue (Table 2). Consistent with this, we observed much higher values for GSSG/GSH+GSSG using 2VP than when using NEM (Fig. 2). Although 2VP does not appear to interfere strongly with the enzymatic cycling of GSSG, some inhibition has been noted (Griffith, 1980). Use of the C18 Sep-Pak column in the NEM method ensures the complete removal of NEM from the assay solution so that no inhibition can occur. Moreover, the use of 2VP can require the adjustment of pH with compounds that can definitely interfere with the enzymatic cycling. To account for this, one must either add a comparable amount of the compound to all GSH samples and standards, or prepare a separate standard curve. This is unnecessary for the method using NEM that we have demonstrated. Recently, use of the related compounds 1-methyl-2-vinylpyridinium trifluoromethane sulfonate (M2VP) and 1-methyl-4-vinylpyridinium trifluoromethane sulfonate (M4VP) to mask GSH has been described (Rahman et al., 2006; Araujo et al., 2008; Shaik and Mehvar, 2006). However, use of these compounds is so far uncommon and results reported in the literature generally do not appear to be any more accurate than the method demonstrated here (Robin et al., 2005).
Pre-analytic sample handling can provide additional opportunities for the introduction of error. Because initial sample processing sometimes involves several steps before freezing and storage, we decided to test the effect of prolonged exposure to ambient conditions following organ excision on the GSSG/GSH+GSSG ratio. Our tests did not show a significant increase in GSSG/GSH+GSSG up to 10 min. We also examined the effect of different storage temperatures on this parameter. Surprisingly, liver sections stored at −20°C for 3 days yielded much higher values of GSSG than those stored at −80°C. These data suggest that samples should be rapidly frozen and kept at or below −80°C for long-term storage.
Most GSSG detection methods begin with a few common steps. Samples must be collected and stored in appropriate conditions, as discussed. Before they can be assayed, tissue samples must be homogenized. The selection of homogenization method may be important. Certain instruments, such as a motorized pestle, may thaw and heat the sample during homogenization. This could provide an additional window for autooxidation. To avoid this, tissues should be kept cold and homogenized quickly. To ensure stability of the GSH and to prevent interference with the assay or damage to equipment, samples must be deproteinized following homogenization. This is usually accomplished by mixing or homogenization of the sample in acidic conditions, followed by centrifugation. The choice of protein precipitation solution has been debated (Monostori et al., 2009; Rossi et al., 2002). In this study, the frozen tissue was homogenized before thawing in ice cold 3% SSA using a blade-type motorized homogenizer. We have obtained satisfying results with this reagent. The focus of this study was largely on the selection of an appropriate derivatizing agent for GSH. For enzymatic cycling assays, derivatization is necessary to isolate GSSG. For some HPLC-based assays, derivatization is also necessary for detection. Because derivatization usually requires neutral to alkaline pH, this choice of reagent is critical. If the reaction is slow, the higher pH conditions will facilitate oxidation of the GSH. This is a major shortcoming of 2VP and other approaches.
The importance of sample handling, acidification reagent and GSH trapping agent has been shown in previous studies (Jones et al., 1998; Rossi et al., 2002; McDermott et al., 2011a,b; Giustarini et al., 2013). In general, the results of these studies agree with ours and show that samples should be quickly processed, kept cold and derivatized with NEM instead of other trapping reagents. However, in many of these studies the authors were interested in whole blood or plasma, rather than tissue. Researchers working with blood or plasma have the advantage that these samples can be mixed immediately with reagents in order to avoid artificial oxidation. On the other hand, tissue must be harvested, frozen, stored and homogenized – all of which take time and present opportunities for GSH oxidation. Our finding that GSSG levels in intact liver are stable at room temperature for as long as 10 min contrasts with previous results using blood samples, in which GSH is oxidized quickly at room temperature even when acidic (Rossi et al., 2002). The difference is likely due to protection of the interior of the intact organ from exposure to the ambient atmosphere. However, it is clear that the tissue must be stored long-term at −80°C or below.
Technological advancement and the need for greater sensitivity in clinical detection have led to the introduction of more advanced techniques for determination of GSSG. Detailed reviews have been published elsewhere (Monostori et al., 2009; Iwasaki et al., 2009). The most popular of these techniques make use of high-performance liquid chromatography (HPLC) separation with UV absorbance (Reed et al., 1980; Katrusiak et al., 2001; Bald and Glowacki, 2005), chemiluminescence (McDermott et al., 2011a,b), fluorescence (Imai et al., 1983; Martin and White, 1991; Paroni et al., 1995; Yang et al., 1995; Kand’ar et al., 2007), electrochemical (Rodriguez-Ariza et al., 1994; Rose and Bode, 1995; Lakritz et al., 1997; Potesil et al., 2005; Sequellerio et al. 2012), or mass spectrometry-based (Lafaye et al., 2005; Bouligard et al., 2006; Iwasaki et al., 2009; Squellerio et al., 2012; Moore et al., 2013; Zhang et al., 2015; Fahrenholz et al., 2015) detection. Even nanosensors for GSH and GSSG are now being developed (Xu et al., 2012; Moore et al., 2013; Ni et al., 2015). Although many of these methods provide excellent results, in many cases the high cost of necessary apparatus is an obvious and severely limiting disadvantage.
In this study, we demonstrated the superiority of a method of GSSG measurement in biological samples. The technique is inexpensive (~$0.30 USD per GSSG/GSH determination when C18 cartridges are regenerated and reused across experiments), accessible (requiring only common laboratory equipment), and capable of producing physiologically accurate results when compared with other common procedures. While the high sensitivity that can be achieved with more advanced methods may necessitate their use in clinical chemistry, we believe the procedure described here is the best available for most common research applications.
TABLE 3.
NEM: LITERATURE VALUES FOR APPARENT PERCENTAGE OF GLUTATHIONE IN THE OXIDIZED FORM IN VARIOUS TISSUES FROM HEALTHY OR CONTROL ANIMALS OR HUMANS.
| Tissue | GSSG | GSH+GSSG | %GSSG | Ref. |
|---|---|---|---|---|
| Mouse | ||||
| Liver | ~60† | ~4,600† | ~1.3 | Knight et al., 2002 |
| ~40† | ~4,200† | ~0.95 | Williams et al., 2013 | |
| 0.283 ± 0.035‡ | 59.3 ± 3.3‡ | 0.58 | Jaeschke, 1990 | |
| Rat | ||||
| Liver | 74 ± 13.1† | 3800 ± 500† | ~1.9 | Nagy et al., 2007 |
| ~20† | ~8200† | ~0.24 | Geier et al., 2005 | |
| ~20† | NR | ~0.4 | Schauer et al., 2003 | |
| Blood | 0.0061 ± 0.004†† | 1.99 ± 0.9†† | 0.72 | Fahrenholz et al., 2015 |
| Average | ~0.9 |
Data derived from references listed. In each study, NEM was listed as the GSH masking agent in the Methods section.
nmol/g liver.
nmol/mg protein.
uM. ~ indicates estimation. NR, not reported.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health R01 DK102142 and R01 AA12916, and from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) of the National Institutes of Health. Additional support came from the “Training Program in Environmental Toxicology” T32 ES007079-26A2 (to M.R.M.) from the National Institute of Environmental Health Sciences.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors declare no competing financial interest.
References
- Adams JD, Jr, Lauterburg BH, Mitchell JR. Plasma glutathione and glutathione disulfide in the rat: regulation and response to oxidative stress. J Pharmacol Exp Ther. 1983;227:749–54. [PubMed] [Google Scholar]
- Araujo AR, S M, Lima JL. Determination of total and oxidized glutathione in human whole blood with a sequential injection analysis system. Talanta. 2008;74:1511–1519. doi: 10.1016/j.talanta.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Bald E, Glowacki R. Analysis of saliva for glutathione and metabolically related thiols by liquid chromatography with ultraviolet detection. Amino Acids. 2005;28:431–3. doi: 10.1007/s00726-005-0195-8. [DOI] [PubMed] [Google Scholar]
- Bouligand J, Deroussent A, Paci A, Morizet J, Vassal G. Liquid chromatography-tandem mass spectrometry assay of reduced and oxidized glutathione and main precursors in mice liver. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;832:67–74. doi: 10.1016/j.jchromb.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Caraceni P, Domenicali M, Vendemiale G, Grattagliano I, Pertosa A, Nardo B, Morselli-Labate AM, Trevisani F, Palasciano G, Altomare E, Bernardi M. The reduced tolerance of rat fatty liver to ischemia reperfusion is associated with mitochondrial oxidative injury. J Surg Res. 2005;124:160–8. doi: 10.1016/j.jss.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Chowdhury A, Santra A, Bhattacharjee K, Ghatak S, Saha DR, Dhali GK. Mitochondrial oxidative stress and permeability transition in isoniazid and rifampicin induced liver injury in mice. J Hepatol. 2006;45:117–26. doi: 10.1016/j.jhep.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Fahrenholz T, Wolle MM, Kingston HM, Faber S, Kern JC, 2nd, Pamuku M, Miller L, Chatragadda H, Kogelnik A. Molecular speciated isotope dilution mass spectrometric methods for accurate, reproducible and direct quantification of reduced, oxidized and total glutathione in biological samples. Anal Chem. 2015;87:1232–40. doi: 10.1021/ac503933t. [DOI] [PubMed] [Google Scholar]
- Geier A, Dietrich CG, Grote T, Beuers U, Prüfter T, Fraunberger P, Matern S, Gartung C, Gerbes AL, Bilzer M. Characterization of organic anion transporter regulation, glutathione metabolism and bile formation in the obese Zucker rat. J Hepatol. 2005;43:1021–30. doi: 10.1016/j.jhep.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Ghanem CI, Ruiz ML, Villanueva SS, Luguita M, Llesuy S, Catania VA, Benqochea LA, Mottino AD. Effect of repeated administration with subtoxic doses of acetaminophen to rats on enterohepatic recirculation of a subsequent toxic dose. Biochem Pharmacol. 2009;77:1621–8. doi: 10.1016/j.bcp.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–12. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Guntherberg H, Rost J. The true oxidized glutathione content of red blood cells obtained by new enzymic and paper chromatographic methods. Anal Biochem. 1966;15:205–10. doi: 10.1016/0003-2697(66)90025-x. [DOI] [PubMed] [Google Scholar]
- Giustarini D, Dalle-Donne I, Milzani A, Fanti P, Rossi R. Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nat Protoc. 2013;8:1660–1669. doi: 10.1038/nprot.2013.095. [DOI] [PubMed] [Google Scholar]
- Hung YC, Guang GS, Sava VM, Blagodarsky VA, Hong MY. Protective effects of tea melanin against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity: antioxidant activity and aryl hydrocarbon receptor suppressive effect. Biol Pharm Bull. 2006;29:2284–91. doi: 10.1248/bpb.29.2284. [DOI] [PubMed] [Google Scholar]
- Imai K, T Toyo’oka, Watanabe Y. A novel fluorogenic reagent for thiols: ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonate. Anal Biochem. 1983;128:471–3. doi: 10.1016/0003-2697(83)90404-9. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y, Saito Y, Nakano Y, Mochuzuki K, Sakata O, Ito R, Saito K, Nakazawa H. Chromatographic and mass spectrometric analysis of glutathione in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3309–17. doi: 10.1016/j.jchromb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255:935–41. [PubMed] [Google Scholar]
- Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752–9. doi: 10.1016/0076-6879(90)86175-u. [DOI] [PubMed] [Google Scholar]
- Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- Jones DP, Carlson JL, Samiec PS, Sternberg P, Jr, Mody VC, Jr, Reed RL, Brown LA. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275:175–184. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- Kand’ár R, Záková P, Lotková H, Kucera O, Cervinková Z. Determination of reduced and oxidized glutathione in biological samples using liquid chromatography with fluorimetric detection. J Pharm Biomed Anal. 2007;43:1382–7. doi: 10.1016/j.jpba.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Katrusiak AE, Paterson PG, Kamencic H, Shoker A, Lyon AW. Pre-column derivatization high-performance liquid chromatographic method for determination of cysteine, cysteinyl-glycine, homocysteine and glutathione in plasma and cell extracts. J Chromatogr B Biomed Sci Appl. 2001;758:207–12. doi: 10.1016/s0378-4347(01)00182-7. [DOI] [PubMed] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther. 2002;303:468–75. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci. 2001;62:212–20. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- Lafaye A, Labarre J, Tabet JC, Ezan E, Junot C. Liquid chromatography-mass spectrometry and 15N metabolic labeling for quantitative metabolic profiling. Anal Chem. 2005;77:2026–33. doi: 10.1021/ac048657g. [DOI] [PubMed] [Google Scholar]
- Lakritz J, CG Plopper, Buckpitt AR. Validated high-performance liquid chromatography-electrochemical method for determination of glutathione and glutathione disulfide in small tissue samples. Anal Biochem. 1997;247:63–8. doi: 10.1006/abio.1997.2032. [DOI] [PubMed] [Google Scholar]
- Martin J, White IN. Fluorimetric determination of oxidised and reduced glutathione in cells and tissues by high-performance liquid chromatography following derivatization with dansyl chloride. J Chromatogr. 1991;568:219–25. doi: 10.1016/0378-4347(91)80356-h. [DOI] [PubMed] [Google Scholar]
- McDermott GP, Francis PS, Holt KJ, Scott KL, Martin SD, Stupka N, Barnett NW, Conlan XA. Determination of intracellular glutathione and glutathione disulfide using high performance liquid chromatography with acidic potassium permanganate chemiluminescence detection. Analyst. 2011a;136:2578–2585. doi: 10.1039/c1an00004g. [DOI] [PubMed] [Google Scholar]
- McDermott GP, Terry JM, Conlan XA, Barnett NW, Francis PS. Direct detection of biologically significant thiols and disulfides with manganese(IV) chemiluminescence. Anal Chem. 2011b;83:6034–6039. doi: 10.1021/ac2010668. [DOI] [PubMed] [Google Scholar]
- Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–60. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Monostori P, Wittmann G, Karg E, Túri S. Determination of glutathione and glutathione disulfide in biological samples: An in-depth review. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3331–46. doi: 10.1016/j.jchromb.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Moore T, Le A, Niemi AK, Kwan T, Cusmano-Ozog K, Enns GM, Cowan TM. A new LC-MS/MS method for the clinical determination of reduced and oxidized glutathione from whole blood. J Chromatogr B Analyt Biomed Life Sci. 2013;929:51–5. doi: 10.1016/j.jchromb.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Morrison JP, Coleman MC, Aunan ES, Walsh SA, Spitz DR, Kregel KC. Thiol supplementation in aged animals alters antioxidant enzyme activity after heat stress. J Appl Physiol. 2005;99:2271–7. doi: 10.1152/japplphysiol.00412.2005. [DOI] [PubMed] [Google Scholar]
- Nagy L, Nagata M, Szabo S. Protein and non-protein sulfhydryls and disulfides in gastric mucosa and liver after gastrotoxic chemicals and sucralfate: possible new targets of pharmacologic agents. World J Gastroenterol. 2007;13:2053–60. doi: 10.3748/wjg.v13.i14.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni P, Sun Y, Dai H, Hu J, Jiang S, Wang Y, Li Z. Highly sensitive and selective colorimetric detection of glutathione based on Ag [I] ion-3,3′,5,5′-tetramethylbenzidine (TMB) Biosensors and Bioelectronics. 2015;63:47–52. doi: 10.1016/j.bios.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Paroni R, De Vecchi E, Cighetti G, Arcelloni C, Fermo I, Grossi A, Bonini P. HPLC with o-phthalaldehyde precolumn derivatization to measure total, oxidized, and protein-bound glutathione in blood, plasma, and tissue. Clin Chem. 1995;41:448–54. [PubMed] [Google Scholar]
- Potesil D, Petrlova J, Adam V, Vacek J, Klejdus B, Zehnalek J, Trnkova L, Havel L, Kizek R. Simultaneous femtomole determination of cysteine, reduced and oxidized glutathione, and phytochelatin in maize (Zea mays L.) kernels using high-performance liquid chromatography with electrochemical detection. J Chromatogr A. 2005;1084:134–44. doi: 10.1016/j.chroma.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–65. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- Reed DJ, Babson JR, Beatty PW, Brodie AE, Ellis WW, Potter DW. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980;106:55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- Robin MA, Demeilliers C, Sutton A, Paradis V, Maisonneuve C, Dubois S, Poirel O, Lettéron P, Pessayre D, Fromenty B. Alcohol increases tumor necrosis factor alpha and decreases nuclear factor-kappab to activate hepatic apoptosis in genetically obese mice. Hepatology. 2005;42:1280–90. doi: 10.1002/hep.20949. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ariza A, Toribio F, Lopez-Barea J. Rapid determination of glutathione status in fish liver using high-performance liquid chromatography and electrochemical detection. J Chromatogr B Biomed Appl. 1994;656:311–8. doi: 10.1016/0378-4347(94)00111-1. [DOI] [PubMed] [Google Scholar]
- Rose RC, Bode AM. Analysis of water-soluble antioxidants by high-pressure liquid chromatography. Biochem J. 1995;306:101–5. doi: 10.1042/bj3060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi R, Milzani A, Dalle-Donne I, Giustarini D, Lusini L, Colombo R, Di Simplicio P. Blood glutathione disulfide: In vivo factor or in vitro artifact? Clinical Chemistry. 2002;48:742–753. [PubMed] [Google Scholar]
- Shaik IH, Mehvar R. Rapid determination of reduced and oxidized glutathione levels using a new thiol-masking reagent and the enzymatic recycling method: application to the rat liver and bile samples. Anal Bioanal Chem. 2006;385:105–13. doi: 10.1007/s00216-006-0375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra A, Chowdhury A, Ghatak S, Biswas A, Dhali GK. Arsenic induces apoptosis in mouse liver is mitochondria dependent and is abrogated by N-acetylcysteine. Toxicology and Applied Pharmacology. 2007;220:146–155. doi: 10.1016/j.taap.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Schauer RJ, Gerbes AL, Vonier D, op den Winkel M, Fruanberger P, Bilzer M. Induction of cellular resistance against Kupffer cell-derived oxidant stress: a novel concept of hepatoprotection by ischemic preconditioning. Hepatology. 2003;37:286–95. doi: 10.1053/jhep.2003.50064. [DOI] [PubMed] [Google Scholar]
- Smith CV. Correlations and apparent contradictions in assessment of oxidant stress status in vivo. Free Radic Biol Med. 1991;10(3–4):217–24. doi: 10.1016/0891-5849(91)90079-i. [DOI] [PubMed] [Google Scholar]
- Sqeullerio I, Caruso D, Porro B, Veglia F, Tremoli E, Cavalca V. Direct glutathione quantification in human blood by LC-MS/MS: comparison with HPLC with electrochemical detection. J Pharm Biom Anal. 2012;71:111–118. doi: 10.1016/j.jpba.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Srivastava SK, Beutler E. Permeability of normal and glucose-6-phosphate dehydrogenase deficient erythrocytes to glutathione. Biochem Biophys Res Commun. 1967;28:659–64. doi: 10.1016/0006-291x(67)90365-8. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–22. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Villanueva SS, Ruiz ML, Soroka CJ, Cai SY, Luguita MG, Torres AM, Sánchez Pozzi EJ, Pellegrino JM, Boyer JL, Catania VA, Mottino AD. Hepatic and extrahepatic synthesis and disposition of dinitrophenyl-S-glutathione in bile duct-ligated rats. Drug Metab Dispos. 2006;34:1301–9. doi: 10.1124/dmd.106.009415. [DOI] [PubMed] [Google Scholar]
- Williams CD, McGill MR, Farhood A, Jaeschke H. Fas receptor-deficient lpr mice are protected against acetaminophen hepatotoxicity due to higher glutathione synthesis and enhanced detoxification of oxidant stress. Food Chem Toxicol. 2013;58:228–35. doi: 10.1016/j.fct.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CS, Chou ST, Liu L, Tsai PJ, Kuo JS. Effect of ageing on human plasma glutathione concentrations as determined by high-performance liquid chromatography with fluorimetric detection. J Chromatogr B Biomed Appl. 1995;674:23–30. doi: 10.1016/0378-4347(95)00287-8. [DOI] [PubMed] [Google Scholar]
- Zhang F, Bartels MJ, LeBaron MJ, Schisler MR, Gollapudi BB, Moore NP. A novel approach for the concurrent quantitation of glutathione, glutathione disulfide, and 2-hydroxyethylated glutathione in lungs of mice exposed to ethylene oxide, using liquid chromatography-positive electrospray tandem mass spectrometry. Biomed Chromatogr. 2015 doi: 10.1002/bmc.3432. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


