Abstract
Substance use among adolescents with one or more psychiatric disorders is a significant public health concern. In this study, 151 psychiatrically hospitalized adolescents, ages 13-17 with comorbid psychiatric and substance use disorders, were randomized to a two-session Motivational Interviewing intervention to reduce substance use plus treatment as usual (MI) vs. treatment as usual only (TAU). Results indicated that the MI group had a longer latency to first use of any substance following hospital discharge relative to TAU (36 days versus 11 days). Adolescents who received MI also reported less total use of substances and less use of marijuana during the first 6 months post-discharge, although this effect was not significant across 12 months. Finally, MI was associated with a significant reduction in rule-breaking behaviors at 6-month follow-up. Future directions are discussed, including means of extending effects beyond 6 months and dissemination of the intervention to community-based settings.
Keywords: Substance Abuse, Adolescents, Psychiatric Comorbidity, Motivational Interviewing, Externalizing Symptoms, Moderators
1. Introduction
Substance use and misuse among adolescents is a significant public health concern in light of its high prevalence (Johnston, O'Malley, Bachman, & Schulenberg, 2012; Roberts, Roberts, & Xing, 2007; SAMHSA, 2012) and associated negative consequences including traffic deaths (Kokotailo, 1995; Shope, Waller, Raghunathan, & Patil, 2001), delinquent behavior (D'Amico, Edelen, Miles, & Morral, 2008; Jessor, 1987; Myers, Stewart, & Brown, 1998), risky sexual behavior (Y. F. Chan, Passetti, Garner, Lloyd, & Dennis, 2011; MacKenzie, 1993), and elevated health care costs (Drug Abuse Warning Network, 1996; Parthasarathy & Weisner, 2006). Substance use disorders (SUDs) in adolescents are associated with high rates of psychiatric comorbidity (Kandel et al., 1997; Kandel et al., 1999; Lewinsohn, Rohde, & Seeley, 1995; Roberts et al., 2007) and suicidality (D'Eramo, Prinstein, Freeman, Grapentine, & Spirito, 2004; Fowler, Rich, & Young, 1986; Nock et al., 2013; Ramchand, Griffin, Harris, McCaffrey, & Morral, 2008). The most common comorbid disorders in adolescents with SUDs are externalizing disorders such as attention deficit hyperactivity disorder (ADHD), conduct disorder (CD), and oppositional defiant disorder (ODD), but rates of internalizing disorders including depression and anxiety are also elevated (Armstrong & Costello, 2002; Y. Chan, Dennis, & Funk, 2008).
Psychiatric disorders among substance-abusing adolescents complicate the clinical presentation of these youth and contribute to poor treatment outcomes (Boon & de Boer, 2007; Chi, Sterling, Campbell, & Weisner, 2013; Grella, Hser, Joshi, & Rounds-Bryant, 2001; King, Gaines, Lambert, Summerfelt, & Bickman, 2000; Rowe, Liddle, Greenbaum, & Henderson, 2004; Subramaniam, Stitzer, Clemmey, Kolodner, & Fishman, 2007; Tomlinson, Brown, & Abrantes, 2004; Vourakis, 2005). There appears to be some evidence that treating one disorder may have a beneficial impact on the other comorbid disorder (e.g., Kaminer, Burleson, Blitz, Sussman, & Rounsaville, 1998). Therefore, an intervention that results in decreases in substance involvement or related problems could also be expected to have a secondary benefit on psychiatric symptoms.
Unfortunately, many adolescents with comorbid disorders do not receive any treatment due to stigma or other barriers. Furthermore, the treatment they do receive often does not adequately address their needs because traditionally the treatment for mental health and substance use disorders has occurred in separate settings that differ in provider training and beliefs, which hinders communication and coordination of care (Hawkins, 2009). Mirroring the separation of psychiatric and substance use treatment services in the community, only a few treatment studies have included samples of adolescents with comorbid psychiatric and substance disorders (e.g., Cornelius et al., 2010; Cornelius et al., 2009; Deas, Randall, Roberts, & Anton, 2000; Thurstone, Riggs, Salomonsen-Sautel, & Mikulich-Gilbertson, 2010). In these studies, fluoxetine, sertraline, and atomoxetine hydrochloride, respectively, were compared to placebo in adolescents with major depressive disorder or ADHD and a substance use disorder. All participants also received behavioral treatment; results indicated that none of the medications was more efficacious than placebo in reducing psychiatric symptoms or substance use.
When adolescents with SUDs present for treatment, especially those with comorbid psychiatric disorders, they typically present in mental health rather than in substance abuse settings (Merikangas et al., 2011; SAMHSA, 2012). Among adolescents hospitalized for a primary psychiatric problem, 17% to 50% also meet criteria for one or more SUDs (Deas-Nesmith, Campbell, & Brady, 1998; Grilo et al., 1995; McDonell, Hsiao, Russo, Pasic, & Ries, 2011; Weaver et al., 2007). In our previous study involving psychiatrically hospitalized adolescent smokers, 71.2% also met criteria for a (non-nicotine) SUD (Brown et al., 2003).
Motivational Interviewing (MI) (Miller & Rollnick, 1991, 2002) is a client-centered counseling style that has recently been described (Miller & Rollnick, 2013) as “a collaborative conversation style for strengthening a person's own motivation and commitment to change” (p. 12). MI has been demonstrated to increase motivation to change substance use behavior among adults (Hettema, Steele, & Miller, 2005; Lundahl, Kunz, Brownell, Tollefson, & Burke, 2010). Less research has focused on adolescents; a meta-analysis of 21 studies indicated that to date, MI interventions for adolescents have produced significant but small effects on substance use behavior (Jensen et al., 2011). As explicated by Baer and Peterson (2002) and Naar-King (2011), MI seems particularly well-suited for use with teens because adolescence is a developmental period characterized by the need to develop autonomy and individuation, as well as the tendency to question and resist authority figures. Adolescents are likely to respond well to the spirit of the MI style, which respects their autonomy, provides choices and not only acknowledges ambivalence, but capitalizes upon it and “empathizes” with it (Naar-King, 2011,p. 653) to decrease resistance and develop motivation for change. MI also supports personal goal choice, which should logically promote greater follow-through and maintenance, since the goals are self-chosen (see also Tevyaw & Monti, 2004).
We are unaware of any MI interventions targeting adolescent substance use implemented in inpatient psychiatric settings with the exception of our previous study that targeted cigarette smoking in this population (Brown et al., 2003), nor are we aware of any existing MI interventions for adolescents with co-occurring psychiatric and substance use disorders with a focus on the effects of substance use on psychiatric symptoms. While the intervention in our previous study yielded no lasting effects on tobacco use (Brown et al., 2003), a treatment by time interaction emerged such that substance use significantly increased in the control (brief advice) condition 6 months following hospitalization, but did not increase significantly in the MI condition (Brown et al., 2009). This finding, while somewhat unanticipated, provided evidence that a motivational intervention with adolescent psychiatric inpatients could result in significant and lasting changes in substance use behaviors and led to the development of the intervention evaluated in the current study.
In the current study, we report the results of a randomized clinical trial that compared the effect of a motivational interviewing intervention to change substance use behavior plus treatment as usual (MI) vs. treatment as usual alone (TAU) on substance use and psychiatric symptom outcomes among psychiatrically hospitalized adolescents who had both a SUD and another Axis I psychiatric disorder. We hypothesized that adolescents who received MI in the current study would have a longer latency to first substance use after hospital discharge, a lower number of days per month on which substances were used, and fewer substance-related consequences, during the 12 months after hospital discharge compared to those who received only TAU. MI was also hypothesized to reduce psychiatric symptoms (i.e., externalizing and internalizing symptoms) compared to TAU alone.
2. Materials and methods
2.1 Overview of study design
Adolescents with comorbid psychiatric and substance use disorders (SUDs) were recruited during inpatient psychiatric hospitalization and randomly assigned to a motivational interviewing intervention to reduce substance use plus treatment as usual (MI) vs. treatment as usual only (TAU). All adolescents (MI and TAU) completed assessments at baseline (i.e., during their hospital stay), end of hospital stay (i.e., at time of discharge), and at 1-, 6-, and 12-months after discharge. If coming to the hospital to complete the post-discharge assessments was not possible, the assessments were completed by phone. All participants were also interviewed briefly via telephone at 3- and 9-months post-discharge. Patients received $50, $25, $35, $50, and $50 in the form of gift certificates to a local mall for completion of baseline, end of hospital, and 1-month, 6-month, and 12-month follow-up assessments respectively.
2.2 Participants
2.2.1 Inclusion and exclusion criteria
Participants were recruited from the adolescent inpatient units at Butler Hospital, a private psychiatric hospital in Providence, RI, and Bradley Hospital, a private psychiatric hospital for children and adolescents in East Providence, RI. Eligible patients were 13 to 17 years of age, met DSM-IV criteria for a non-nicotine substance use disorder (SUD) during the past 12 months and one or more additional current Axis I psychiatric disorders (other than a SUD), and had access to a telephone. Patients were excluded if they had a current DSM-IV diagnosis of a psychotic disorder, mental retardation, or pervasive developmental disorder.
2.2.2 Screening and recruitment
This study was approved by the Institutional Review Boards of Butler Hospital and Lifespan, the parent corporation of Bradley Hospital. Per the policies of both hospitals and made known in writing to all adolescents and parents during the admission process, medical records were subject to screening by research staff for possible research study recruitment. In this case, study staff pre-screened the medical records of admitted patients for evidence of substance use and consulted with unit staff to learn about patients who might be eligible. The parents of eligible patients were contacted to obtain their written informed consent and permission to approach their child about participating. Patients were then given a detailed explanation of study procedures and provided written assent.
2.3 Baseline assessment and randomization
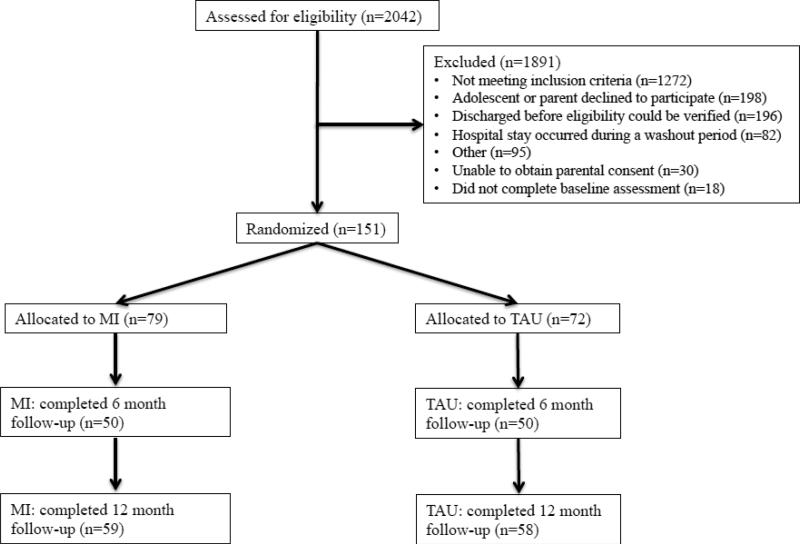
After completing the baseline assessment, eligible patients were assigned to either MI or TAU. To avoid potential intervention contamination during hospitalization, group assignment was done in cohorts determined randomly before initiation of the study, with a washout period between cohorts. Initially, 161 adolescent patients provided assent for this study. Ten adolescents subsequently withdrew their assent to participate. The remaining 151 adolescents comprise the final sample for the current analyses (see Figure 1).
Figure 1.
CONSORT Diagram.
2.4 Treatment conditions
2.4.1 Treatment as Usual (TAU)
Adolescents assigned to TAU received no study intervention of any kind. TAU was intended to address the patient's most acute problems. All patients were assigned a psychiatrist who coordinated the assessment and treatment planning activities of a multidisciplinary team. Treatment included pharmacotherapy, individual and family sessions with clinical staff, and psychoeducational groups on various topics, including one weekly 45-minute group in which substance use-related issues were discussed. Substance use was addressed on an individual basis at the discretion of the patient's psychiatrist.
2.4.2 Motivational Interviewing plus Treatment as Usual (MI)
Adolescents assigned to MI received an MI intervention during their hospital stay consisting of two, 45-minute individual sessions. All adolescents assigned to MI also received TAU, and sessions were scheduled so as not to interfere with the patient's usual treatment on the unit. The general goals of the sessions were to build rapport, heighten patients’ awareness of the consequences of substance use, increase discrepancy between patients’ current substance use behavior and their goals, help patients re-evaluate their substance use behavior, increase self-efficacy for changing substance use, and assist with goal-setting and creating a change plan. Sessions were tailored to patients’ individual level of readiness to change, reasons for substance use, social influences, negative consequences of substance use, and interaction of substance use with the patient's specific psychiatric disorder(s). A significant portion of the first session was spent exploring the pros and cons of the patient's substance use. The therapist then provided feedback regarding the negative consequences of substance use that the patient endorsed on the baseline assessment measures. This feedback was tailored based on the extent to which the patient identified negative consequences during the pros and cons discussion (i.e., if patient articulated numerous negative consequences during pros and cons discussion, less feedback was provided). The first session ended with a discussion of the patient's goals and a summary of the session. The discussion of goals was intended to build motivation for change by highlighting discrepancy between the patient's current behavior (i.e., substance use) and short- (e.g., graduating high school) and long-term (e.g., career) goals. During the second session, the patient was given additional feedback, including national normative data on adolescent substance use and information about the effects of substance use on the patient's psychiatric symptoms. About halfway through the session, the therapist verbally assessed the patient's readiness and confidence to change on a scale from 1 to 10, with further discussion as appropriate to enhance readiness and confidence. Finally, therapists assisted patients in creating a change plan that included specific behavioral goals, names of individuals who would provide social support for change and how to overcome barriers to change for those at higher levels of readiness to change and more preparatory, behavioral goals for those at lower levels of readiness. As the intervention emphasized personal goal choice, therapists did not insist on a goal of abstinence although abstinence was implied as a preferred goal.
MI therapists were doctoral-level clinical psychologists, psychology post-doctoral fellows at Alpert Medical School of Brown University, and a masters-level clinician. All therapists were provided with a copy of Motivational Interviewing: Preparing People for Change (Miller & Rollnick, 2002) and the study therapist manual, and attended an intensive two-day training workshop in MI led by RAB and TRA. Throughout the study, therapists received weekly group supervision and feedback from RAB and TRA, based upon review of session audio recordings.
MI fidelity was assessed with the Motivational Interviewing Treatment Integrity Scale (MITI 3.1.1, Moyers, Martin, Manual, & Miller, 2010). The MITI uses seven therapist behavior count items (i.e., Giving Information; MI Adherent; MI non-Adherent; Closed and Open Questions; and Simple and Complex Reflections) and three global session ratings (MI Spirit, Direction, Empathy, range 1-5), and was designed to measure treatment integrity in clinical trials of MI. Two trained raters double-coded a 30% random selection of all available MI sessions, resulting in n =32 sessions. Overall, therapists showed high levels of MI-Adherent Behaviors (98%) and Complex Reflections (56%), as well as high scores on Global Direction (M = 4.56[0.56]), Empathy (M = 3.96[1.37]), and MI-Spirit (M = 4.00[0.62]). Therapists asked more Open Questions (56%) than closed questions, and offered about one Reflection for each Question asked. Study therapists exhibited minimal MI Non-Adherent Behaviors (less than 1 on average per session).
2.5. Measures
2.5.1 Overview
Assessment focused on a number of domains: diagnoses, days of use by specific substance (alcohol, marijuana, other substances) tagged to each calendar day, psychiatric symptoms, negative consequences and other treatment received. Assessment measures were administered by bachelors or masters level research assistants who were trained in their administration by RAB and AMA. Research assistants were blind to participant study condition.
2.5.2 Diagnostic and screening measures
The Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime (K-SADS-PL) (Kaufman et al., 1997) was administered to participants at baseline to determine study eligibility and to provide descriptive clinical information about the sample.
2.5.3 Primary outcome measures
Self-reports of daily alcohol and drug use (up to 6 classes) were collected from participants using the Timeline Followback (TLFB) interview (Sobell & Sobell, 1996) at baseline (to assess the 3 months prior to hospitalization) and at all follow-ups (including via phone at 3- and 9-months) to cover the time period since the previous assessment. The TLFB interview has also been shown to be a reliable method of quantifying alcohol and cannabis use among adolescents (Levy et al., 2004). Consistent with existing literature, primary outcomes were operationalized as 1) latency to first use and 2) days of use per month (during months 1-12 of follow-up). Outcomes included three categories of substance use: 1) any substance use, 2) alcohol use, and 3) marijuana use. For the sake of consistency throughout, “substance use” will refer to use of any substance, including alcohol, marijuana, and other drugs. In order to take into account days in which participants were in a restricted environment (e.g., residential treatment, inpatient hospitalization) where substance use was not permitted or possible, we first calculated the valid percent days per month of substance use as the number of days of substance use reported divided by the number of reported days, excluding days in a restricted environment. We then multiplied this valid percent days used by 30 to extrapolate the number of days used per month. The same method was used to calculate participants’ baseline substance use (i.e., the average numbers of days per month across 3 months before enrollment in the study, taking into account days in a restricted environment).
Participants were asked to provide urine samples for toxicology screening at 1-, 6-, and 12-month follow-ups. Drugs tested included tetrahydrocannabinol (THC), amphetamines, barbiturates, benzodiazepines, cocaine, methadone, opiates, and phencyclidine.
2.5.4. Secondary outcome measures
The Adolescent Problem Use Scale (APUS) (Chassin, Rogosch, & Barrera, 1991) was administered at baseline and all follow-ups to assess the occurrence of negative (social, health, and legal) consequences resulting from alcohol and drug use (separate scales). In this study, the Cronbach alpha for the alcohol subscale was 0.81, and for the drug subscale was 0.82.
The Youth Self Report (YSR) (Achenbach & Rescorla, 2001) is a widely used self-report instrument for youths aged 11 through 18 to assess psychiatric symptomatology and problem behaviors. The YSR was administered at baseline and at 6 and 12-month follow-ups. The YSR has two main scales, internalizing and externalizing, and eight symptom subscales. Within the internalizing scale, there are three subscales (somatic complaints, depressed/withdrawn and depressed/anxious subscales) and within the externalizing scale there are two subscales (aggressive behavior and rule-breaking behavior subscales). The rule-breaking behavior subscale is a revision of the “delinquent behavior” subscale from the 1991 version of the YSR. The other symptom subscales include thought problems, attention problems and social problems. The Cronbach alphas for both main scales range from .89 to .90 (Achenbach & Rescorla, 2001).
2.6 Statistical Approach
2.6.1 Primary outcomes
Cox proportional hazards analyses were used to examine MI effects on time to first substance use, controlling for age and gender. Separate survival analyses were fit for (a) any substance use, (b) alcohol use, and (c) marijuana use.
MI effects on the number of days of substance use per month (i.e., any substance, alcohol, and marijuana, separately) during months 1-12 follow-up was tested using multilevel modeling for count data, controlling for age, gender, and baseline use of the corresponding substance(s). Prior to running the multilevel models, we tested the distributional properties of each dependent variable. All dependent variables were count variable and over-dispersed (over-dispersion parameters = 1.24 – 1.52, ps < .001) indicating that a negative binomial distribution better fit the data, compared to a Poisson distribution. The negative binomial dispersion parameter for each model (over-dispersion parameters = 2.00 – 6.96, SE = 0.39 – 1.30) also confirmed that the data were better estimated using negative binomial models than Poisson models. Therefore, we specified the outcome variables as negative binomial, which is considered best practice for highly skewed count data (Neal & Simons, 2007).
We then ran one multilevel model per dependent variable (i.e., any substance, alcohol, and marijuana) predicting use from treatment condition, controlling for age, gender, and time as months 1-6, then repeated the analyses with time as months 7-12. The intercept and slope (time) were allowed to vary across subjects as long as doing so improved model fit, indicated by significant reduction in deviance scores (Raudenbush & Bryk, 2002). All models, except the models of marijuana and any substance use over months 7-12 (random intercept only models), were estimated with random intercept and slope. Missing data were handled with a maximum likelihood using the Laplace approximation that uses all available data from each participant and assumes that data are missing at random
2.6.2 Secondary outcomes
In line with our analyses for number of days per month of substance use, we conducted separate multilevel analyses to test for MI effects on the number of alcohol consequences, the number of drug consequences, and total number of substance consequences (sum of alcohol and drug problems) assessed at months 1, 3, 6, 9, and 12, controlling for time, age, gender and the corresponding baseline scores. Here, Poisson distribution was specified for all models as the outcome variables were not over-dispersed (over-dispersion parameters = 0.007 - 0.014, ps >.01). For each model, only the intercept was allowed to vary across subjects as specifying slope (time) as random did not improve model fit.
We tested for MI effects on changes in two main YSR psychiatric scales (i.e., internalizing and externalizing psychiatric symptoms), as well as the eight YSR symptom subscales, with path analyses that simultaneously estimated all regression coefficients to control for Type I error and allowed for the inclusion of gender as a covariate. Overall model fit for path analyses was assessed following recommendations by Hu and Bentler (1999).
3. Results
3.1 Participant characteristics
The sample (N = 151) was 64.9% female with a mean age of 15.8 (SD = 1.0) years, 88.4% white (130/147), 6.1% African-American (9/147), and 5.4% as other races or more than one race (8/147). Additionally, 6.6% were Hispanic (10/151). Please see Table 1 for more detail about demographic characteristics of the study sample and alcohol and drug use across treatment conditions. As assessed by the K-SADS, common SUD diagnoses in the sample included cannabis abuse or dependence (95% of the sample) and alcohol abuse or dependence (73%). Please see Table 2 for more details on the prevalence of abuse and dependence diagnoses for this sample. The most common Axis-I disorders were major depressive disorder (57%), conduct disorder (56%), post traumatic stress disorder (34%), attention deficit hyperactive disorder (31%) and generalized anxiety disorder (17%). Other less common diagnoses included social phobia (11%), oppositional defiant disorder (9%), panic disorder (8%), OCD (6%), mania (5%), anorexia (4%), agoraphobia (3%), bulimia (3%) and dysthymia (1%).
Table 1.
Baseline demographic, alcohol and drug use across conditions
| MI (n=79) | TAU (n=72) | p-value | |
|---|---|---|---|
| Mean (%/SD) | Mean (%/SD) | ||
| Female | 46 (58.2%) | 52 (72.2%) | 0.10 |
| Age | 15.85 (1.06) | 15.85 (1.00) | 0.99 |
| White | 66 (83.5%) | 64 (88.9%) | 0.36 |
| Hispanic | 6 (7.6%) | 4 (5.6%) | 0.75 |
| Baseline (past 3 months) | |||
| Alcohol Use | 3.70 (5.55) days | 2.3 5(3.49) days | 0.08 |
| Marijuana Use | 14.9 (10.1) days | 14.6 (10.8) days | 0.85 |
| Any Substance Use | 15.9 (9.73) days | 15.7 (10.16) days | 0.87 |
Table 2.
Prevalence of Substance Use Disorder (SUD) Diagnoses in the Sample
| Substance | Abuse | Dependence | Either |
|---|---|---|---|
| Alcohol | 31% | 42% | 73% |
| Cannabis | 25% | 70% | 95% |
| Stimulants | 10% | 9% | 19% |
| Sedatives/Anxiolytics | 10% | 4% | 14% |
| Cocaine | 8% | 11% | 19% |
| Opioids | 8% | 10% | 18% |
| PCP | 0% | 1% | 1% |
| Hallucinogens | 7% | 1% | 8% |
| Solvents/Inhalants | 3% | 3% | 6% |
| Other | 5% | 12% | 17% |
| Any | 66% | 81% | 100% |
The MI and TAU groups were compared on demographics and baseline values of all primary outcomes and secondary outcomes. Across these pairwise comparisons, no significant differences were detected. Table 1 presents demographic characteristics and baseline use of substances for each treatment group. In addition, there were no differences in demographic characteristic between participants who completed the 12-month follow-up and those who were lost to follow-up (p > .104).
The mean duration of inpatient treatment among study participants was 6.99 days (SD = 9.77 days). The retention rates at the 1, 3, 6, 9, and 12-month follow-ups, respectively, were: 90%, 85%, 83%, 80%, and 76%. Rates of missing data were not significantly different across MI and TAU conditions.
3.2. Primary outcomes
3.2.1. Time to first substance use
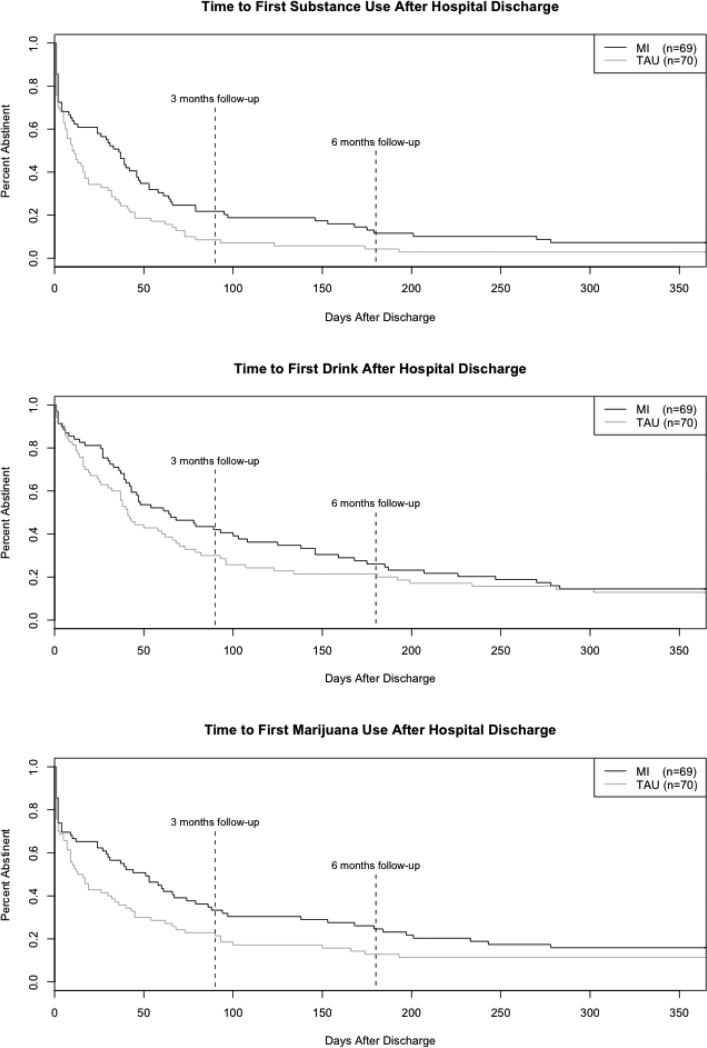
Figure 2 presents the survival functions by treatment condition paneled for each substance use category. Survival analyses included 139 participants (MI: n = 69, TAU: n = 70) who provided follow-up data.
Figure 2.
Days to first use of any substance after hospital discharge.
In the model predicting time to any substance use, 132 of 139 participants eventually returned to using. Participants in the MI condition showed a significantly longer latency to first use of any substance (HR = .62, 95% CI = .44, .88, p = .008), suggesting that the MI intervention delayed a return to any substance use compared to TAU (medians: MI = 36 days vs. TAU = 11 days). Neither age (HR = 1.09, 95% CI = .94, 1.27, p = .247) nor gender (HR = .76, 95% CI = .53, 1.09, p = .130) predicted time to first use of any substance.
In the model predicting time to alcohol use, 120 of 139 participants eventually drank. While those in the MI condition had a longer median time to first drink relative to TAU (MI = 64 days vs. TAU = 41 days), this difference was nonsignificant (HR = .81, 95% CI = .56, 1.16, p = .242). Nor did the variables in the model significantly predict a return to alcohol use (age: HR = 1.09, 95% CI = .93, 1.29, p = .285; gender: HR = .79, 95% CI = .55, 1.16, p = .228).
In the model predicting marijuana use, 120 of 139 participants eventually used marijuana. The model showed a trend level treatment effect (HR = .71, 95% CI = .49, 1.02, p = .063), such that those in the MI condition had longer latency to first use of marijuana relative to those in the TAU condition (medians: MI = 51 days vs. TAU = 15 days). None of the other variables in the model predicted a return to marijuana use (age: HR = 1.04, 95% CI = .88, 1.22, p = .635; gender: HR = .89, 95% CI = .61, 1.30, p = .551).
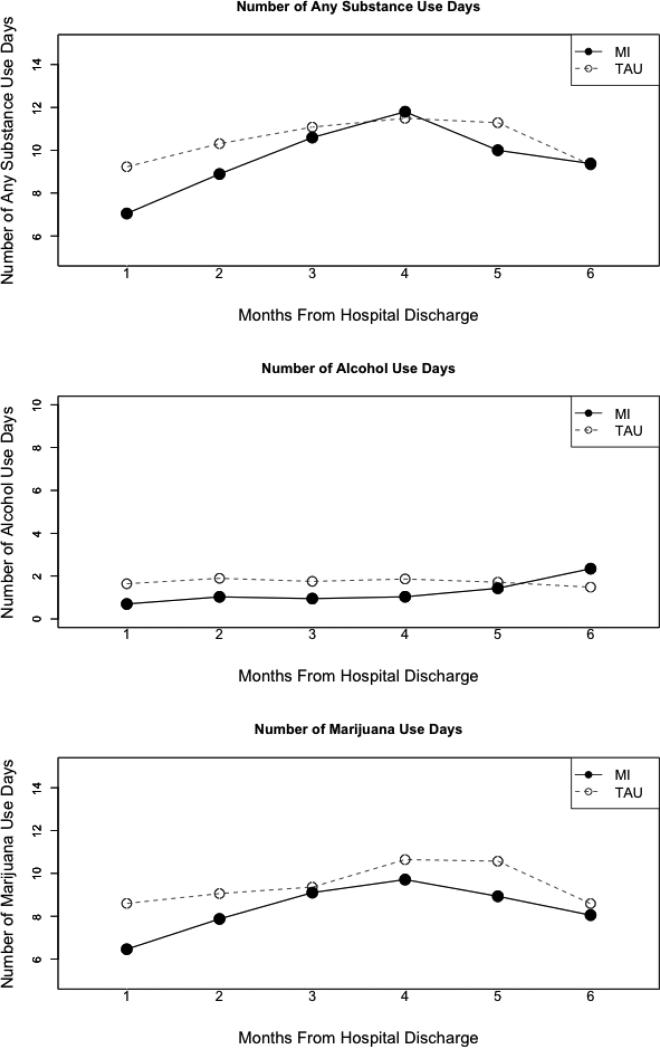
3.2.2. Number of days of substance use per month
The number of days of any substance, alcohol, or marijuana use reported each month across the first 6 months of follow-up by treatment condition is presented in Figure 3. Results showed that treatment condition predicted any substance use across the first six months (b = −.58, 95% CI = −1.16, −.01, p = .047), such that those in the TAU condition used substances more often than those in the MI condition, controlling for age, gender, time and baseline use of any substance. Again, none of the covariates, except for baseline use of any substance (b = .06, 95% CI = .03, .09, p < .001), predicted substance use across the first six months (ps > .05). Similarly, treatment condition predicted marijuana use in the first six months (b = −.81, 95% CI = −1.60, −.02, p = .044), such that those in the TAU condition used marijuana more often than those in the MI condition during the first 6 months controlling for gender, age, time, and baseline marijuana use. Baseline marijuana use predicted marijuana use in the first six months (b = .08, 95% CI = .04, .12, p < .001), but age, gender, and time did not (ps > .05).
Figure 3.
Percent days use per month in the first 6 months after hospital discharge.
Finally, the model for alcohol use showed a trend-level association (b = −.52, 95% CI =, −1.10, .06 p = .080), such that those in the MI condition reported less frequent alcohol use, compared to those in the TAU condition during the first six months. Among the covariates, time, age, and gender did not predict alcohol use in the first six months (ps > .05). However, baseline alcohol use (the average number of drinking days per month over 3 months pre-enrollment) significantly predicted alcohol use in the first six months (b = 0.09, 95% CI = 0.02, 0.16, p = .012).
In the models assessing the effect of treatment condition in months 7-12, treatment condition did not predict any of the dependent variables (ps > .05), indicating no significant effects of MI on substance use during months 7-12. Baseline use was a significant predictor of both marijuana (b = .08, 95% CI = .01, .15, p = .023) and any substance (b = .05, 95% CI = .01, .09, p = .017) use, but not for alcohol use (b = 0.06, 95% CI = −.02, .14, p = .16), in months 7-12, controlling for age, time, gender and condition. Finally, gender did not predict alcohol, marijuana, or any substance use (ps > .05) in months 7-12.
3.2.3. Urine toxicology
Valid urine toxicology results were obtained for 52.3%, 46.4%, and 50.3% of the sample at 1, 6, and 12-month follow-ups, respectively. Results revealed negative toxicology screens for all drugs on the following percentages of participants in the MI group versus the TAU group (1 month: 64.9% versus 50.0%, 6 months: 51.4% versus 42.4%, 12 months: 52.5% versus 36.1%). These differences between conditions were not statistically significant. Since THC stays in the urine longer than other drug metabolites, and since marijuana was the most prevalent drug of abuse, we verified the reliability of the self-report data collected on the TLFB with the THC urine results. Reported number of days using marijuana in the month (1-, 6-, and 12-month post-discharge) was highly related to whether THC was positive or negative in the urine, for all three months when the urine was collected, corresponding to point-biserial correlations (rpb) of 0.39, 0.60, and 0.66 (ps < .01), respectively.
3.3. Secondary outcomes
3.3.1. Substance use consequences
Results of multilevel analyses, controlling for age, gender, time, and the baseline score, showed that there were no differences in alcohol consequences (b = −.047, 95% CI = −.101, .008, p = .096), drug consequences (b = .040, 95% CI = −.026, 0.105, p = .238), or total substance consequences (b = −.000, 95% CI = −0.052, 0.051, p = .988) between conditions during the year following hospitalization.
3.3.2. Psychiatric symptoms
Mean YSR scale (i.e., internalizing and externalizing symptoms) and subscale (i.e., depression, anxiety, rule-breaking, aggression, thought problems, social problems, and attention problems) scores decreased from baseline to 6 months regardless of condition, indicating that psychiatric symptoms lessened following intensive inpatient psychiatric treatment. Specifically, on average YSR scales decreased by 4.40 (SD = 8.74, t(99) = −5.04, p < .01) points, and YSR subscales decreased by 1.43 (SD = 3.09, t(99) = −4.63, p < .01) points, from baseline to 6 month follow-up. We did not assess for differences in psychiatric symptoms by condition in the second six months due to the lack of difference in primary substance use outcomes during this time period.
We examined treatment condition effects on changes in YSR scales and subscales from baseline to 6-month follow-up (subtracting the baseline value from the 6-month value) using two path analyses controlling for gender. Overall model fit was excellent for all models based on recommendations by Hu and Bentler (1999). Specifically, all models had non-significant χ2 tests, Root Mean Squared Error of Approximation values less than .05, and Comparative Fit Indexes greater than .95.
When examining the direct effects, in the YSR scales model there was a trend for condition predicting externalizing symptoms (b = 3.31, SE = 1.91, p = .08, 95% CI = −.043, 7.05), such that those in the MI condition reported greater changes in externalizing symptoms than those in the TAU condition. In the subscales model, condition predicted changes in rule breaking (b = 2.07, SE = 1.05, p = .05, 95% CI = 0.01, 4.13), with those in the MI condition reporting greater reductions in rule-breaking behavior than those in the TAU condition. Moreover, gender significantly predicted thought problems (b = 2.08, SE = .73, p < .01, 95% CI = 0.65, 3.51), with males reporting greater reductions in thought problems compared to females. All other direct effects in both models were non-significant (ps > .05).
4. Discussion
The current study compared the effect of a motivational interviewing intervention plus treatment as usual (MI) to treatment as usual alone (TAU) on substance use and psychiatric symptom outcomes among adolescents with comorbid psychiatric disorder and SUD who were receiving inpatient psychiatric treatment. Results revealed that MI was associated with a delay in time to first use of any substance after discharge, and reductions in number of days of any substance use and of marijuana use reported during the first 6 months following hospital discharge. Moreover, those in the MI condition reported significantly greater reductions in rule-breaking behaviors in the first 6 months, compared to the TAU group. These results suggest that that MI had positive effects on some of the substance use outcomes, and an added benefit of decreasing rule-breaking behaviors.
Treatment for psychiatric disorders and SUDs has historically occurred in separate settings, with limited communication and coordination of care (Hawkins, 2009; Sterling, Weisner, Hinman, & Parthasarathy, 2010). As most adolescents with SUDs who present for treatment are treated in psychiatric settings, there is a critical need to integrate treatment for substance use in these settings (Lichtenstein, Spirito, & Zimmermann, 2010; Sterling et al., 2010). Our MI intervention was intended to address this gap, and was unique in targeting the effects of substance use on psychiatric symptoms. A recent meta-analysis of MI interventions for substance use among adolescents found that these interventions have significant, but small effects (Jensen et al., 2011). However, this meta-analysis excluded studies conducted with inpatients, such as our previous study targeting cigarette smoking among inpatients (Brown et al., 2003; Brown et al., 2009). Findings from the current study suggest that MI interventions can have beneficial effects on substance use and some specific behaviors among adolescents who are receiving intensive inpatient psychiatric treatment.
Although MI both delayed a return to first use of any substance and decreased the frequency of any substance use in the first 6 months following hospital discharge, these effects were largely driven by changes in marijuana use; no significant effects of MI were found for alcohol use. In this sample, the overall frequency of alcohol use at baseline was low relative to marijuana use (mean ~3 days/month for alcohol vs. ~15 days/month for marijuana) and remained low at follow-up (mean of <2 days/month in both MI and TAU groups). Therefore, a floor effect is likely. Also, it is likely that adolescents viewed their alcohol use as less of a problem than their marijuana use given these differences in use frequency, and change plans discussed in MI sessions likely focused on marijuana use.
Regarding psychiatric symptoms, we had hypothesized that MI would have a positive impact on broadly defined constructs using the YSR scales (i.e., internalizing and externalizing symptoms) over and above TAU; however, our primary results found only partial support for this hypothesis. MI showed no effects on internalizing symptoms and was associated with trend-level greater reductions in externalizing symptoms compared to TAU during the first 6-month follow-up period. However, the externalizing symptoms scale is comprised of two subscales (rule-breaking and aggression), and we found that those in the MI condition reported significantly greater reductions in rule-breaking, but not aggression, compared to the TAU group. Therefore, lack of group differences in aggression explains why treatment effects on externalizing symptoms as a whole was not found. Recent evidence from longitudinal research suggests that aggression, but not rule-breaking, is associated with neurobiological changes (Platje et al., 2013). Platje and colleagues’ (2013) demonstration that aggression is linked to neurobiology may help explain why rule breaking, but not aggression, was amenable to change with brief MI.
With respect to the relationship between internalizing symptoms and substance use, extant evidence suggests a complicated, reciprocal relationship, such that internalizing symptoms most frequently predate substance use initiation (Deas-Nesmith, Brady, & Campbell, 1998; Deas-Nesmith, Campbell, et al., 1998), but that use of substances may also lead to internalizing symptoms due to negative psychosocial consequences associated with substance-involvement (e.g., association with deviant peer groups, and high-risk situations, Whitbeck, Hoyt, & Yoder, 1999) and/or the effect of regular substance use on the central nervous system (Clark & Neighbors, 1996). Thus, for the majority of participants, internalizing symptoms may have predated their substance use, and therefore reduction in substance use would not necessarily have served to reduce internalizing symptoms.
Overall, results of the current study indicate that our MI intervention shows promise for delaying and reducing substance use following psychiatric hospital discharge, potentially creating space for adolescents to look more closely at their behaviors and learn to make better choices with continued treatment. Given the increasingly brief duration of inpatient psychiatric hospitalization in today's health care environment (Case, Olfson, Marcus, & Siegel, 2007), MI is particularly well-suited to help adolescents address substance abuse issues in a comparatively time- and cost-efficient manner. More intensive substance abuse interventions would be impractical and difficult to implement in this inpatient setting. The MI approach also has the potential to motivate adolescents to engage in substance abuse specific treatment services upon hospital discharge, as has been found in adult studies (e.g., Swanson, Pantalon, & Cohen, 1999) and among incarcerated adolescents (Stein et al., 2006). Intervening with teenagers is potentially a very effective way to reduce healthcare costs and other societal costs (e.g., incarceration) that they might otherwise incur over the course of their lifetimes. At the same time, the effects of MI on substance use did not last beyond 6 months following the intervention. A recent meta-analysis indicated that brief motivational interventions for adolescent substance use have their biggest impact within the first six months (Jensen et al., 2011); as mentioned previously, trials involving inpatients were excluded from this review. Thus the results of our study are consistent with this review and extend this finding to adolescents following inpatient treatment. It is difficult to speculate on why the impact of MI did not extend beyond six months, and reinforcement via brief booster sessions or other continued follow-up may be required to sustain the effects of brief interventions over the long-term (Wutzke, Conigrave, Saunders, & Hall, 2002).
Strengths of this study include the use of an understudied, inpatient sample of adolescents with comorbid psychiatric and substance use disorders, randomization, the use of a treatment manual to ensure standardized delivery of the MI intervention, the intervention's focus on the effects of substance use on psychiatric symptoms, the number of post-treatment assessment points that extended through 12 months, and the use of psychometrically sound assessment measures. Limitations include the ethnically non-diverse sample, exclusion of adolescents with psychotic disorders who also have high rates of comorbid substance use disorders, that the groups (MI vs. TAU) were not equated for contact time, and that we did not include a measure of motivation to change substance use, such as assessing “change talk”, to determine if MI had effects on motivation as well as behavior. All participants had the opportunity to attend a group about substance use as part of TAU; however, we did not collect data on how much individual intervention relevant to substance use participants received in TAU and any such intervention was at the discretion of the attending psychiatrist and treatment team. Finally, it is important to acknowledge that the intervention was specialized for the inpatient psychiatric setting and was conducted with patients at two hospitals in one city in the northeastern USA; results may not generalize to other hospitals with different TAU procedures or to the larger population of adolescents with SUDs in the community and outpatient settings.
In conclusion, a motivational interviewing (MI) intervention targeting substance use that was provided to adolescents with comorbid psychiatric and substance use disorders during inpatient psychiatric hospitalization was associated with longer latency to first use of any substance following psychiatric hospital discharge, reduced frequency of marijuana and any substance use for 6 months post-discharge, and some effect on externalizing symptoms (specifically, reduction in rule-breaking behavior) but had no effect on internalizing symptoms for 6 months following treatment. Future research should aim to promote maintenance of these MI benefits, perhaps through the use of booster sessions, increased family involvement, or by incorporating recent technological advances in the development of computer and mobile phone-based treatments (Litvin, Abrantes, & Brown, 2013). These advances include therapist avatars (Ondersma, Chase, Svikis, & Schuster, 2005), text messaging (Riley, Obermayer, & Jean-Mary, 2008; Suffoletto, Callaway, Kristan, Kraemer, & Clark, 2012), and smartphone “apps” (Ly, Carlbring, & Andersson, 2012), which could replace and/or supplement in-person interventions to facilitate dissemination and implementation of this MI approach to community settings in a cost-effective manner. Also, mediators of MI effects, such as via increasing motivation, should be examined. In general, the development of efficacious approaches to motivate reduced substance use and maintenance of these changes in adolescents with psychiatric comorbidity are particularly important, especially given the significant deleterious consequences resulting from these combined morbidities and the paucity of available approaches that integrate treatment of substance abuse in psychiatric settings.
Highlights.
151 psychiatrically hospitalized adolescents, ages 13---17 with comorbid psychiatric and substance use disorders, were randomized to a two---session Motivational Interviewing intervention targeting substance plus treatment as usual (MI) vs. treatment as usual only (TAU)
Results indicated that the MI group had a longer latency to first use of any substance following hospital discharge relative to TAU (36 days versus 11 days).
Adolescents who received MI also reported less total use of substances and less use of marijuana during the first 6 months post---discharge, although this effect was not significant across 12 months.
MI was also associated with a significant reduction in rule---breaking behaviors at 6---month follow---up.
Future directions discussed include means of extending effects beyond 6 months and dissemination of the intervention to community---based settings.
Acknowledgements
This research was supported by the National Institute on Drug Abuse grant R01 DA016738 awarded to PI, Richard A. Brown, through Butler Hospital. We would like to thank Shelley Feuer, Cathryn Mainville, Mark Marinello, Chanta Pou, Jennifer Saritelli, Geneva Szulewski, Jessica Wolfe Molly Magill and Colleen Peterson for their assistance with this research. A subset of these data have been presented as: Brown, R.A. (2009, May). Motivational interviewing for substance abuse among adolescents with psychiatric co-morbidity. Invited paper presented as part of a symposium sponsored by the National Institute on Drug Abuse, entitled “Spotting the Wolf in Sheep's Clothing: Clinical Challenges Identifying and Treating Unpresented Co-morbidity”. Annual Meeting of the American Psychiatric Association, San Francisco, CA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach T, Rescorla L. Manual for the ASEBA School-Age Forms and Profiles. Research Center for Children, Youth and Families. University of Vermont Burlington; VT: 2001. [Google Scholar]
- Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. Journal of Consulting and Clinical Psychology. 2002;70(6):1224–1239. doi: 10.1037//0022-006x.70.6.1224. [DOI] [PubMed] [Google Scholar]
- Baer JS, Peterson PL. Motivational interviewing for adolescents and young adults. In: Miller WR, Rollnick S, editors. Motivational interviewing: Preparing people for change. 2nd edition The Guilford Press; New York: 2002. [Google Scholar]
- Boon AE, de Boer SB. Drug usage as a treat to the stability of treatment outcome: a one-year follow-up study of adolescent psychiatric patients. European Child and Adolescent Psychiatry. 2007;16(2):79–86. doi: 10.1007/s00787-006-0576-x. doi: 10.1007/s00787-006-0576-x. [DOI] [PubMed] [Google Scholar]
- Brown RA, Ramsey SE, Strong DR, Myers MG, Kahler CW, Lejuez CW, Abrams DB. Effects of motivational interviewing on smoking cessation in adolescents with psychiatric disorders. Tobacco Control. 2003;12(Suppl 4):IV3–10. doi: 10.1136/tc.12.suppl_4.iv3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Strong DR, Abrantes AM, Myers MG, Ramsey SE, Kahler CW. Effects on substance use outcomes in adolescents receiving motivational interviewing for smoking cessation during psychiatric hospitalization. Addictive Behaviors. 2009;34(10):887–891. doi: 10.1016/j.addbeh.2009.03.003. doi: 10.1016/j.addbeh.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case BG, Olfson M, Marcus SC, Siegel C. Trends in the inpatient mental health treatment of children and adolescents in US community hospitals between 1990 and 2000. Archives of General Psychiatry. 2007;64(1):89–96. doi: 10.1001/archpsyc.64.1.89. doi: 10.1001/archpsyc.64.1.89. [DOI] [PubMed] [Google Scholar]
- Chan Y, Dennis ML, Funk RR. Prevalence and comorbidity of major internalizing and externalizing problems among adolescents and adults presenting to substance abuse treatment. Journal of Substance Abuse Treatment. 2008;34:14–24. doi: 10.1016/j.jsat.2006.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YF, Passetti LL, Garner BR, Lloyd JJ, Dennis ML. HIV risk behaviors: risky sexual activities and needle use among adolescents in substance abuse treatment. AIDS and Behavior. 2011;15(1):114–124. doi: 10.1007/s10461-010-9702-3. doi: 10.1007/s10461-010-9702-3. [DOI] [PubMed] [Google Scholar]
- Chassin L, Rogosch F, Barrera M. Substance use and symptomatology among adolescent children of alcoholics. Journal of Abnormal Psychology. 1991;100(4):449–463. doi: 10.1037//0021-843x.100.4.449. [DOI] [PubMed] [Google Scholar]
- Chi FW, Sterling S, Campbell CI, Weisner C. 12-step participation and outcomes over 7 years among adolescent substance use patients with and without psychiatric comorbidity. Substance Abuse. 2013;34(1):33–42. doi: 10.1080/08897077.2012.691780. doi: 10.1080/08897077.2012.691780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D, Neighbors B. Adolescent substance abuse and internalizing disorders. Child and Adolescent Psychiatric Clinics of North America. 1996;5:45–58. [Google Scholar]
- Cornelius JR, Bukstein OG, Douaihy AB, Clark DB, Chung TA, Daley DC, Brown SJ. Double-blind fluoxetine trial in comorbid MDD-CUD youth and young adults. Drug and Alcohol Dependence. 2010;112(1-2):39–45. doi: 10.1016/j.drugalcdep.2010.05.010. doi: 10.1016/j.drugalcdep.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius JR, Bukstein OG, Wood DS, Kirisci L, Douaihy A, Clark DB. Double-blind placebo-controlled trial of fluoxetine in adolescents with comorbid major depression and an alcohol use disorder. Addictive Behaviors. 2009;34(10):905–909. doi: 10.1016/j.addbeh.2009.03.008. doi: 10.1016/j.addbeh.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico EJ, Edelen MO, Miles JN, Morral AR. The longitudinal association between substance use and delinquency among high-risk youth. Drug and Alcohol Dependence. 2008;93(1-2):85–92. doi: 10.1016/j.drugalcdep.2007.09.006. doi: 10.1016/j.drugalcdep.2007.09.006. [DOI] [PubMed] [Google Scholar]
- D'Eramo KS, Prinstein MJ, Freeman J, Grapentine WL, Spirito A. Psychiatric diagnoses and comorbidity in relation to suicidal behavior among psychiatrically hospitalized adolescents. Child Psychiatry and Human Development. 2004;35(1):21–35. doi: 10.1023/b:chud.0000039318.72868.a2. [DOI] [PubMed] [Google Scholar]
- Deas D, Randall CL, Roberts JS, Anton RF. A double-blind, placebo-controlled trial of sertraline in depressed adolescent alcoholics: a pilot study. Human Psychopharmacology. 2000;15(6):461–469. doi: 10.1002/1099-1077(200008)15:6<461::AID-HUP209>3.0.CO;2-J. doi: 10.1002/1099-1077(200008)15:6<461::AIDHUP209>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Deas-Nesmith D, Brady KT, Campbell S. Comorbid substance use and anxiety disorders in adolescents. Journal of Psychopathology and Behavioral Assessment. 1998;20(2):139–148. [Google Scholar]
- Deas-Nesmith D, Campbell S, Brady KT. Substance use disorders in an adolescent inpatient psychiatric population. Journal of the National Medical Association. 1998;90(4):233–238. [PMC free article] [PubMed] [Google Scholar]
- Drug Abuse Warning Network . 1996 DAWN Report. Substance Abuse and Mental Health Service Administration; Washington, D.C.: 1996. [Google Scholar]
- Fowler RC, Rich CL, Young D. San Diego Suicide Study. II. Substance abuse in young cases. Archives of General Psychiatry. 1986;43(10):962–965. doi: 10.1001/archpsyc.1986.01800100056008. [DOI] [PubMed] [Google Scholar]
- Grella CE, Hser YI, Joshi V, Rounds-Bryant J. Drug treatment outcomes for adolescents with comorbid mental and substance use disorders. Journal of Nervous and Mental Disease. 2001;189(6):384–392. doi: 10.1097/00005053-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Becker DF, Walker ML, Levy KN, Edell WS, McGlashan TH. Psychiatric comorbidity in adolescent inpatients with substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34(8):1085–1091. doi: 10.1097/00004583-199508000-00019. [DOI] [PubMed] [Google Scholar]
- Hawkins EH. A tale of two systems: co-occurring mental health and substance abuse disorders treatment for adolescents. Annual Review of Psychology. 2009;60:197–227. doi: 10.1146/annurev.psych.60.110707.163456. doi: 10.1146/annurev.psych.60.110707.163456. [DOI] [PubMed] [Google Scholar]
- Hettema J, Steele J, Miller WR. Motivational interviewing. Annual Review of Clinical Psychology. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Jensen CD, Cushing CC, Aylward BS, Craig JT, Sorell DM, Steele RG. Effectiveness of motivational interviewing interventions for adolescent substance use behavior change: A meta-analytic review. Journal of Consulting and Clinical Psychology. 2011 doi: 10.1037/a0023992. doi: 10.1037/a0023992. [DOI] [PubMed] [Google Scholar]
- Jessor R. Problem-behavior theory, psychosocial development, and adolescent problem drinking. British Journal of Addiction. 1987;1987(82):331–342. doi: 10.1111/j.1360-0443.1987.tb01490.x. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2011. Institute for Social Research, The University of Michigan; Ann Arbor, MI: 2012. [Google Scholar]
- Kaminer Y, Burleson JA, Blitz C, Sussman J, Rounsaville BJ. Psychotherapies for adolescent substance abusers: a pilot study. Journal of Nervous and Mental Disease. 1998;186(11):684–690. doi: 10.1097/00005053-199811000-00004. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Johnson JG, Bird HR, Canino G, Goodman SH, Lahey BB. Psychiatric disorders associated with substance use among children and adolescents: Findings from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) study. Journal of Abnormal Child Psychology. 1997;25:121. doi: 10.1023/a:1025779412167. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Johnson JG, Bird HR, Weissman MM, Goodman SH, Lahey BB, Schwab-Stone ME. Psychiatric comorbidity among adolescents with substance use disorders: Findings from the MECA Study. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(6):693–699. doi: 10.1097/00004583-199906000-00016. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- King RD, Gaines LS, Lambert EW, Summerfelt WT, Bickman L. The cooccurrence of psychiatric and substance use diagnoses in adolescents in different service systems: Frequency, recognition, cost, and outcomes. Journal of Behavioral Health Services and Research. 2000;27(4):417–430. doi: 10.1007/BF02287823. [DOI] [PubMed] [Google Scholar]
- Kokotailo P. Physical health problems associated with adolescent substance abuse. NIDA Research Monograph. 1995;156:112–129. [PubMed] [Google Scholar]
- Levy S, Sherritt L, Harris S, Gates E, Holder D, Kulig JW, Knight JR. Test–retest reliability of adolescents’ self-report of substance use. Alcoholism: Clinical and Experimental Research. 2004;28:1236–1241. doi: 10.1097/01.alc.0000134216.22162.a5. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Rohde P, Seeley JR. Adolescent psychopathology: III. The clinical consequences of comorbidity. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34(4):510–519. doi: 10.1097/00004583-199504000-00018. [DOI] [PubMed] [Google Scholar]
- Lichtenstein DP, Spirito A, Zimmermann RP. Assessing and treating cooccurring disorders in adolescents: examining typical practice of community-based mental health and substance use treatment providers. Community Mental Health Journal. 2010;46(3):252–257. doi: 10.1007/s10597-009-9239-y. doi: 10.1007/s10597-009-9239-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvin EB, Abrantes AM, Brown RA. Computer and mobile technology-based interventions for substance use disorders: An organizing framework. Addictive Behaviors. 2013;38(3):1747–1756. doi: 10.1016/j.addbeh.2012.09.003. doi: 10.1016/j.addbeh.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Lundahl BW, Kunz C, Brownell C, Tollefson D, Burke BL. Meta-analysis of motivational interviewing: Twenty-five years of empirical studies. Research on Social Work Practice. 2010;20(2):137–160. [Google Scholar]
- Ly KH, Carlbring P, Andersson G. Behavioral activation-based guided self-help treatment administered through a smartphone application: study protocol for a randomized controlled trial. Trials. 2012;13:62. doi: 10.1186/1745-6215-13-62. doi: 10.1186/1745-6215-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie RG. Influence of drug use on adolescent sexual activity. Adolescent Medicine. 1993;4(2):417–422. [PubMed] [Google Scholar]
- McDonell MG, Hsiao RC, Russo J, Pasic J, Ries RK. Clinical prevalence and correlates of substance use in adolescent psychiatric emergency patients. Pediatric Emergency Care. 2011;27:384–389. doi: 10.1097/PEC.0b013e318216b248. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swendsen J, Avenevoli S, Case B, Olfson M. Service utilization for lifetime mental disorders in U.S. adolescents: results of the National Comorbidity Survey-Adolescent Supplement (NCS-A). Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(1):32–45. doi: 10.1016/j.jaac.2010.10.006. doi: 10.1016/j.jaac.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people to change addictive behaviors. Guilford; New York: 1991. [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. 2nd edition Guilford Press; New York: 2002. [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Helping people change. 3rd edition Guilford Press; New York: 2013. [Google Scholar]
- Moyers TB, Martin T, Manual JK, Miller WR. Revised global scales: Motivational Interviewing Treatment Integrity 3.1.1 (MITI 3.1.1) 2010 unpublished manual. [Google Scholar]
- Myers MG, Stewart DG, Brown SA. Progression from conduct disorder to antisocial personality disorder following treatment for adolescent substance abuse. American Journal of Psychiatry. 1998;155(4):479–485. doi: 10.1176/ajp.155.4.479. [DOI] [PubMed] [Google Scholar]
- Naar-King S. Motivational interviewing in adolescent treatment. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie. 2011;56(11):651–657. doi: 10.1177/070674371105601103. [DOI] [PubMed] [Google Scholar]
- Nock MK, Green JG, Hwang I, McLaughlin KA, Sampson NA, Zaslavsky AM, Kessler RC. Prevalence, Correlates, and Treatment of Lifetime Suicidal Behavior Among Adolescents: Results From the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatry. 2013:1–11. doi: 10.1001/2013.jamapsychiatry.55. doi: 10.1001/2013.jamapsychiatry.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Chase SK, Svikis DS, Schuster CR. Computer-based brief motivational intervention for perinatal drug use. Journal of Substance Abuse Treatment. 2005;28(4):305–312. doi: 10.1016/j.jsat.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S, Weisner C. Health care services use by adolescents with intakes into an outpatient alcohol and drug treatment program. American Journal on Addictions. 2006;15(Suppl 1):113–121. doi: 10.1080/10550490601006097. doi: 10.1080/10550490601006097. [DOI] [PubMed] [Google Scholar]
- Ramchand R, Griffin BA, Harris KM, McCaffrey DF, Morral AR. A prospective investigation of suicide ideation, attempts, and use of mental health service among adolescents in substance abuse treatment. Psychology of Addictive Behaviors. 2008;22(4):524–532. doi: 10.1037/a0012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley W, Obermayer J, Jean-Mary J. Internet and mobile phone text messaging intervention for college smokers. Journal of American College Health. 2008;57(2):245–248. doi: 10.3200/JACH.57.2.245-248. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Roberts CR, Xing Y. Comorbidity of substance use disorders and other psychiatric disorders among adolescents: Evidence from an epidemiologic survey. Drug and Alcohol Dependence. 2007;88S:S4–S13. doi: 10.1016/j.drugalcdep.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CL, Liddle HA, Greenbaum PE, Henderson CE. Impact of psychiatric comorbidity on treatment of adolescent drug abusers. Journal of Substance Abuse Treatment. 2004;26(2):129–140. doi: 10.1016/S0740-5472(03)00166-1. doi: 10.1016/S0740-5472(03)00166-1. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Results from the 2011 National Survey on Drug use and Health: Summary of national findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2012. NSDUH Series H-44, HHS Publication No. (SMA) 12-4713. [Google Scholar]
- Shope JT, Waller PF, Raghunathan TE, Patil SM. Adolescent antecedents of high-risk driving behavior into young adulthood: substance use and parental influences. Accident Analysis and Prevention. 2001;33(5):649–658. doi: 10.1016/s0001-4575(00)00079-8. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A calendar method for assessing alcohol and drug use. Addiction Research Foundation; Toronto, Canada: 1996. [Google Scholar]
- Stein LA, Monti PM, Colby SM, Barnett NP, Golembeske C, Lebeau-Craven R, Miranda R. Enhancing Substance Abuse Treatment Engagement in Incarcerated Adolescents. Psychological Services. 2006;3(1):25–34. doi: 10.1037/1541-1559.3.1.0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling S, Weisner C, Hinman A, Parthasarathy S. Access to treatment for adolescents with substance use and co-occurring disorders: challenges and opportunities. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(7):637–646. doi: 10.1016/j.jaac.2010.03.019. quiz 725-636. doi: 10.1016/j.jaac.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam GA, Stitzer MA, Clemmey P, Kolodner K, Fishman MJ. Baseline depressive symptoms predict poor substance use outcome following adolescent residential treatment. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(8):1062–1069. doi: 10.1097/chi.0b013e31806c7ad0. doi: 10.1097/chi.0b013e31806c7ad0. [DOI] [PubMed] [Google Scholar]
- Suffoletto B, Callaway C, Kristan J, Kraemer K, Clark DB. Text-message-based drinking assessments and brief interventions for young adults discharged from the emergency department. Alcoholism, Clinical and Experimental Research. 2012;36(3):552–560. doi: 10.1111/j.1530-0277.2011.01646.x. doi: 10.1111/j.1530-0277.2011.01646.x. [DOI] [PubMed] [Google Scholar]
- Swanson AJ, Pantalon MV, Cohen KR. Motivational interviewing and treatment adherence among psychiatric and dually diagnosed patients. Journal of Nervous and Mental Disease. 1999;187(10):630–635. doi: 10.1097/00005053-199910000-00007. [DOI] [PubMed] [Google Scholar]
- Tevyaw TO, Monti PM. Motivational enhancement and other brief interventions for adolescent substance abuse: foundations, applications and evaluations. Addiction. 2004;99(Suppl 2):63–75. doi: 10.1111/j.1360-0443.2004.00855.x. [DOI] [PubMed] [Google Scholar]
- Thurstone C, Riggs PD, Salomonsen-Sautel S, Mikulich-Gilbertson SK. Randomized, controlled trial of atomoxetine for attention-deficit/hyperactivity disorder in adolescents with substance use disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(6):573–582. doi: 10.1016/j.jaac.2010.02.013. doi: 10.1016/j.jaac.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson KL, Brown SA, Abrantes A. Psychiatric comorbidity and substance use treatment outcomes of adolescents. Psychology of Addictive Behaviors. 2004;18(2):160–169. doi: 10.1037/0893-164X.18.2.160. doi: 10.1037/0893-164X.18.2.160. [DOI] [PubMed] [Google Scholar]
- Vourakis C. Admission variables as predictors of completion in an adolescent residential drug treatment program. Journal of Child and Adolescent Psychiatric Nursing. 2005;18(4):161–170. doi: 10.1111/j.1744-6171.2005.00031.x. doi: 10.1111/j.1744-6171.2005.00031.x. [DOI] [PubMed] [Google Scholar]
- Weaver MF, Dupre MA, Cropsey KL, Koch JR, Sood BA, Wiley JL, Balster RL. Addiction epidemiology in adolescents receiving inpatient psychiatric treatment. Addictive Behaviors. 2007;32(12):3107–3113. doi: 10.1016/j.addbeh.2007.06.008. doi: 10.1016/j.addbeh.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitbeck EA, Hoyt DR, Yoder KA. A risk-amplification model of victimization and depressive symptoms among runaway and homeless adolescents. American Journal of Community Psychology. 1999;27:273–296. doi: 10.1023/A:1022891802943. [DOI] [PubMed] [Google Scholar]
- Wutzke SE, Conigrave KM, Saunders JB, Hall WD. The long-term effectiveness of brief interventions for unsafe alcohol consumption: a 10-year follow-up. Addiction. 2002;97(6):665–675. doi: 10.1046/j.1360-0443.2002.00080.x. [DOI] [PubMed] [Google Scholar]