Abstract
Melanomas are comprised of multiple biologically distinct categories, which differ in cell of origin, age of onset, clinical and histologic presentation, pattern of metastasis, ethnic distribution, causative role of UV radiation, predisposing germ line alterations, mutational processes, and patterns of somatic mutations. Neoplasms are initiated by gain of function mutations in one of several primary oncogenes, typically leading to benign melanocytic nevi with characteristic histologic features. The progression of nevi is restrained by multiple tumor suppressive mechanisms. Secondary genetic alterations override these barriers and promote intermediate or overtly malignant tumors along distinct progression trajectories. The current knowledge about pathogenesis, clinical, histological and genetic features of primary melanocytic neoplasms is reviewed and integrated into a taxonomic framework.
Keywords: Genetics, Pathogenesis, Classification, Mutation, Nevi
Classification of melanocytic neoplasms
Melanocytes can give rise to a diverse set of neoplasms with varying anatomic distribution, clinical features, histopathological appearance, and biologic behavior. While melanocytes are most abundant in the skin where they play a critical role in skin pigmentation and sun protection, they are present in other locations throughout the body where they have additional, less well understood functions. While melanocytic neoplasms most frequently arise from melanocytes in the skin they can also arise from autochthonous melanocytes from numerous internal organs including the central nervous system (CNS).
As a rule, benign neoplasms of melanocytic lineage are termed melanocytic nevi (the plural of nevus) and malignant ones are termed melanomas; the often-used term “malignant melanoma” thus represents a tautology. While melanomas can arise from nevi, as can be inferred from the presence of an adjacent nevus remnant that is contiguous with a melanoma, most primary melanomas do not show such an associated precursor nevus. In part this is because the precursor nevus was overgrown by the melanoma during its progression, but in many instances there most likely was no detectable benign precursor state.
It has long been noted that melanoma is comprised of different subtypes, depending on where they arise and that their pathogenesis varies. The current WHO classification (1) is based on the melanoma classification proposed by Clark and colleagues (2) more than 30 years ago, which uses morphologic aspects of the early (radial) growth phase and the body site of the primary melanoma to distinguish four main types: superficial spreading melanoma (SSM), lentigo maligna melanoma (LMM), nodular melanoma (NM) and acral-lentiginous melanoma (ALM). The system captures archetypical patterns of clinical and histological presentation, however a portion of melanomas cannot be unequivocally categorized in any of the categories (3) and its impact on clinical care, in particular that of advanced disease has been limited.
Over the last two decades tremendous progress has been made in uncovering genetic alterations in melanocytic neoplasia and an expanding panel or recurrent driver mutations that activate specific oncogenes or inactivate tumor suppressor genes is emerging. Remarkably, many of the mutations are found in association with specific clinical or histopathological subsets of lesions, strongly supporting the notion of biologically distinct types of melanocytic neoplasms. This is true for melanomas as well as for melanocytic nevi, which express a similar diversity in clinical appearance and histomorphology, age of onset, anatomic site of the tumor, and the genetic alterations present.
A desirable classification system separates individual disease states by considering differences in cell of origin, pathogenesis, clinical and histologic aspects, genetic alterations etc. It can serve as a framework to develop refined approaches for primary prevention, objective diagnostic and staging algorithms, and tailored therapeutic strategies. Such a taxonomy differs from current approaches which rely primarily on one of these dimensions, histology or mutation status, and instead integrates multiple aspects to capture individual disease subtypes.
The organizing principle for this overview is the current, but still incomplete, clinical and genetic data that is available in varying detail across the broad phenotypic spectrum of melanomagenesis. The proposed taxa are separated in one dimension. For some taxa distinct evolutionary stages - reaching from benign, intermediate, to malignant – are separated in the second dimension (figure 1). The guiding principles for distinguishing taxa will be genetic alterations that arise early during progression, clinical or histologic features of the primary tumor, and characteristics of the host such as age of onset, ethnicity, skin type, and as the role of environmental factors such a the role UV radiation.
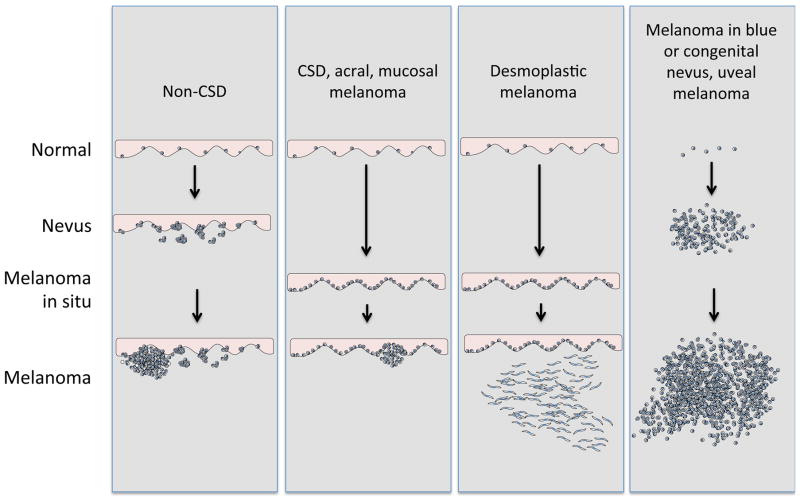
Figure 1. Taxonomy of melanocytic neoplasia.
A) Melanocytic neoplasms arising from epithelium-associated melanocytes. Where applicable, benign or intermediate progression stages are noted. The different classes have different relationship to UV radiation and age distributions, shown at the bottom. B) Melanocytic neoplasms arising from non-epithelium associated melanocytes. The categories have no relationship to UV radiation and, with the exception of congenital nevus-associated melanomas, which occur primarily in prepubertal children, a wide age distribution.
Mutations common to all progression steps within a taxon will be considered probable initiating oncogenic events. They are gain of function mutations in oncogenes that tend to occur in a mutually exclusive pattern. The known initiating oncogenic events in melanocytic neoplasia are listed in table 1. By contrast, oncogenic events that mark the transition to the next progression stage within a given taxon will be considered secondary (or tertiary) oncogenic events. These are commonly loss of function alterations of tumor suppressor genes such as CDNK2A, TP53, PTEN, or BAP1, some of which are also encountered as germ line alterations predisposing to syndromes with various types of melanocytic neoplasms (table 2). Somatic alterations considered progression events are listed in table 3. As a consequence, initiating oncogenic events will be most valuable for separating classes, whereas later events serve to separate progression steps within classes. Primary and secondary oncogenic events will not necessarily have a one-to-one relationship to either taxa or the progression steps within a taxon. Some classes may have identical primary oncogenic events and still deserve to be separated, if there are systematic differences in other relevant parameters. For example, acral melanomas and CSD melanomas both can have KIT mutations but differ sufficiently in other parameters (type of genomic instability, pathogenic role of UV radiation, etc.) and therefore are separated. Similarly, stage-defining secondary oncogenic events may be shared among several classes.
Table 1.
Primary oncogenic events in melanocytic neoplasia
| Type of mutation | Effector pathways | Neoplasms affected | |
|---|---|---|---|
| Primary oncogenic driver mutations | |||
| NRAS | Point mutation | MAP-kinase, PI3-kinase | Congenital nevi, CSD, non, CSD, acral, mucosal melanomas |
| HRAS | Point mutation | MAP-kinase, PI3-kinase | Spitz tumors |
| BRAF | Point mutation, kinase fusions | MAP-kinase | Acquired nevi, non-CSD, CSD (V600K), acral melanomas, Spitz tumors |
| KIT | Point mutation, amplification | PI3-kinase, MAP-kinase, STAT3 | Acral, mucosal melanomas, CSD |
| GNAQ | Point mutation | Protein kinase C, MAP-kinase | Blue nevi, blue nevus-like melanomas, uveal melanomas |
| GNA11 | Point mutation | Protein kinase C, MAP-kinase | Blue nevi, blue nevus-like melanomas, uveal melanomas |
| ALK | Kinase fusions | MAP-kinase, PI3-kinase, STAT3 | Spitz tumors and yet to be defined melanoma subtypes |
| ROS1 | Kinase fusions | MAP-kinase, PI3-kinase, STAT3 | Spitz tumors and yet to be defined melanoma subtypes |
| RET | Kinase fusions | MAP-kinase, PI3-kinase, STAT3 | Spitz tumors and yet to be defined melanoma subtypes |
| NTRK1 | Kinase fusions | MAP-kinase, PI3-kinase, STAT3 | Spitz tumors and yet to be defined melanoma subtypes |
Table 2.
Known germline mutations associated with melanocytic neoplasia
| Gene (OMIM#) | Function | Melanocytic lesions | Other disease associations |
|---|---|---|---|
| CDKN2A (600160) | Encodes two separate proteins: p16 is an inhibitor cyclin-dependent kinases 4 and 6 p14 is an inhibitor of the MDM2 an ubitquitinase that degrades p53 |
Melanoma, dysplastic nevi, | Pancreatic cancer |
| CKD4 (609048) | Active at the G1-S transition, phosphorylates Rb | Melanoma | |
| BAP1 (614327) | Deubiquitinase involved in chromatin modification | Uveal and cutaneous melanoma, atypical Spitz tumors | Mesothelioma, meningioma |
| PRKAR1A (188830) | Regulatory subunit of protein kinase A | Lentigines, blue nevi | Carney complex |
| PTPN11 (602216) | Protein-tyrosine phosphatases that can activate the MAP-kinase pathway | LEOPARD syndrome, Noonan syndrome, juvenile myelomonocytic leukemia, metachondromatosis | |
| STK11 (602216) | Serine/threonine-protein kinase that controls the activity of AMP-activated protein kinase family members | Melanocytic macules of the lips, buccal mucosa, and digits | Peutz-Jeghers syndrome |
| TERT (615134) | Catalytic subunit of telomerase | Melanoma | Other cancers |
| PTEN (601728) | Lipid phosphatase opposing the action of PI3-kinase | Pigmented lesions of the genitalia | Bannayan-Riley-Ruvalcaba syndrome, Cowden syndrome |
| XPA (278700), XPB (610651), XPC (278720), XPD (278730), XPE (278740), XPF (133520), XPG (133530), XPV (278750) | Nucleotide excision DNA repair | Melanoma, lentigines | Xeroderma pigmentosum |
| WRN (277700) | DNA helicase | Acral and mucosal melanoma | Werner syndrome |
| MITF (614456) | Helix-loop-helix-leucine zipper protein important for melanocyte development and differentiation | Melanoma | Renal cell cancer |
| MC1R (613099) | Melanocortin receptor 1, binds melanocyte stimulatory hormone | Freckles, melanoma | Red hair |
Table 3.
Secondary oncogenic events in melanocytic neoplasia
| Secondary oncogenic driver mutations | |||
|---|---|---|---|
| Gain of function alterations | |||
| CCND1 | Amplification | RB pathway | Acral, mucosal melanomas, CSD |
| CDK4 | Amplification | RB pathway | Mucosal, acral melanomas |
| MITF | Amplification | Upregulation of transcriptional targets including MET, BCL-2, CDK2 | Acquired nevi, non-CSD, CSD (V600K), acral melanomas, Spitz tumors |
| TERT | Promoter mutation, amplification | Telomere elongation | Subtype variation for mutations TBD, amplification in acral melanomas |
| CDK4 | Amplification | RB pathway | Mucosal, acral melanomas |
| MEK1,2 | Mutation | MAP-kinase | Subtype spectrum TBD |
| MITF | Amplification | Upregulation of transcriptional targets including MET, BCL-2, CDK2 | Acquired nevi, non-CSD, CSD (V600K), acral melanomas, Spitz tumors |
| CTNNB1 | Mutation | WNT signaling | Subtype spectrum TBD |
| EZH2 | Mutation | Chromatin remodeling | |
| RAC1 | Point mutation | Adhesion, migration, invasion | Subtype spectrum TBD |
| Loss of function alterations | |||
| CDKN2A | Deletion, mutation | RB pathway (via p16), p53 pathway (via p14/ARF) | All melanoma types, dysplastic nevi, atypical Spitz tumors |
| PTEN | Deletion, mutation | PI3-kinase | non-CSD, acral melanomas, melanomas |
| BAP1 | Deletion, mutation | Chromatin remodeling | Uveal melanoma |
| TP53 | Mutation | P53 pathway | |
| ARID1A, 1B and 2 | Mutation, deletion | Chromatin remodeling | Subtype spectrum TBD |
| NF1 | Mutation, deletion | MAP-kinase pathway | CSD, desmoplastic melanoma |
| SMARCA4 | Deletion, mutation | Chromatin remodeling | non-CSD, acral melanomas, melanomas |
| BAP1 | Deletion, mutation | Chromatin remodeling | Uveal melanoma |
| ARID2 | Mutation, deletion | Chromatin remodeling | Subtype spectrum TBD |
| Unknown function alterations | |||
| PPP6C | Mutation | Cell cycle regulation | Subtype spectrum TBD |
| SF3B1 | Mutation | RNA splicing | Uveal melanoma |
| STK19 | Mutation | Serine/threonine protein kinase | Subtype spectrum TBD |
The proposed taxa are summarized in table 4, and the basis for their distinction is detailed in the following paragraphs. While remarkable progress has been made in bringing to light critical genetic alterations, the progress is far from completed. This review attempts to capture the salient aspects of insight into the molecular pathology of melanocytic neoplasia and to present an organizational taxonomic framework that can continue to be filled in and refined, as additional defining features continue to emerge.
Table 4.
Summary of melanoma classes
| Type of melanoma | Non-CSD | CSD | Acral | Mucosal | Uveal | Desmoplastic | Spitzoid | In congenital nevus |
Blue nevus- like (130, 142) |
|---|---|---|---|---|---|---|---|---|---|
| Main age distribution | 3rd to 6th decade | 7th decade and older | 6th decade and older | 6th decade and older | Wide distribution, avg. age 60 years | 7th decade and older(143) | Mostly children | Mostly children | All age groups |
| Anatomic site | Intermittently sun exposed skin of Trunk, extremities (except glabrous sites and nail apparatus), bulbar conjunctiva | Chronically sun exposed skin of the head, neck, lower arms and lower legs | Palms, soles, nail apparatus | Any mucosal membrane, but primarily nasal cavity and sinuses, oral cavity, ano-rectum, vulva and vagina, tarsal conjunctiva | Posterior and anterior choroid, ciliary body, iris | Mostly head and neck (53%), but also trunk (21%) and extremities (27%) (143) | Head and neck, lower extremities, but can arise anywhere on the skin, including glabrous skin | Primarily the skin but can arise in all tissues involved by congenital nevi | Most common on scalp, trunk, |
| Typical initial clinical presentation | More common in patients with acquired nevi, pigmented. Irregularly pigmented, asymmetrical patch or nodule. | More common in patients with non-melanoma skin cancers. Irregularly pigmented, asymmetrical patch or nodule. | Pigmented macule on glabrous skin or nail apparatus followed nodular growth. | Pigmented macule on mucosa skin or nail apparatus followed nodular growth. | Blurred vision and visual field defects | Unpigmented nodule or plaque often on skin with high cumulative sun exposure. Can have associated brown macule as in LMM | Unpigmented, rapidly growing nodule | Nodular growth within congenital nevus | Black nodular growth |
| Typical histologic features | Early lesions composed of enlarged, round melanocytes with dusty melanin arranged as nests, pagetoid scatter | Pronounced solar elastosis in adjacent skin. Early lesions often show lentiginous growth pattern (LMM) | Early lesions often show extensive lentiginous growth pattern | Early lesions often show extensive lentiginous growth pattern | Solid nodules of spindled or epithelioid melanocytes. | Pauci-cellular proliferation of atypical spindled melanocytes set in a fibrotic dermis. Lentiginous component in 80%, neurotropism in 30–40%. (144) | Compound or intradermal proliferation of large atypical epithelioid or spindled cells with ample cytoplasm, with mitotic figures. | Nodular aggregates of atypical melanocytes with mitotic figures within a congenital nevus | Dermal nodules of large atypical spindled and/or epithelioid melanocytes with mitoses and areas of necrosis. Often adjacent blue nevus of the cellular variant |
| Pattern of metastatization | Frequent involvement of lymph nodes as first manifestation | Equal proportions of satellite or in-transit, lymph node, and distant metastases (145) | Frequent satellite and in-transit and lymph node metastases as first manifestation (145) | Frequent local recurrence, lymph node metastases less frequent than distant metastases | Liver and bone | Primarily to the lung. Infrequent involvement of lymph nodes (7%)(143) | Frequent involvement of lymph nodes as first manifestation | Not enough data | Lymph nodes, liver, lung, bones(130) |
| Female to male incidence ratio | 1 | 0.6 | 1:1(146) | 2:1(110) | 0.76(133) | 0.6(143) | 1.1 | 1.3(118) | 0.53(130) |
| Incidence [per million] (estimated) (108, 110, 133, 143, 147) | 135 | 55 | 2 | 2.2 | 5.1(133) | 5 (144) | Not enough data | Not enough data | Not enough data |
| White to non-White ratio | 6 | 6 | 1.3 | 2 | 5 | Not enough data | 5(148) | Not enough data | Not enough data |
| Related melanoma categories | SSM, NM on skin without CSD | LMM, NM on skin with CSD | ALM, SSM or NM on glabrous skin | Blue nevus-like melanoma | Choroidal, iris and ciliary tract melanoma | Spindle cell melanoma | Atypical Spitz tumor, pediatric melanoma | Not applicable | Uveal melanoma |
| Role of UV | ++ | +++ | (+) | − | (+)? | +++ | (+)? | (+) | (+) |
| Primary oncogenic alterations | BRAF (70%), NRAS (15%) | NRAS (15%), KIT (10%) | KIT (15%), BRAF (15%), NRAS (15%) | KIT 15%, NRAS (15%) | GNAQ and GNA11 (90%) | NF1 (25%) | HRAS, fusions of ROS1, NTRK1, ALK, RET, BRAF, NTRK3 | NRAS (85%), BRAF fusions | GNAQ and GNA11 |
| Secondary genetic alterations | TERT mutations, mutations and deletion of CDKN2A, PTEN, | TERT, TP53 mutations | TERT amplification | TERT amplification | BAP1, | ? | CDKN2A? | ? | ? |
| Benign precursor | Common acquired nevus | ? | ? | Melanotic macule (?) | Uveal nevus | − | Spitz nevus | Congenital nevus | Blue nevus |
| Chromosomal aberrations | Losses of 9, 10, 6q, 8p Gains of 6p, 7, 8q, 1q, 20q, 17q | Losses of 9, 6q, 8p Gains of 6p, 11q13, 8q, 1q, 20q, 17q | Losses of 1p, 3, 6q, 8, and 9p Gains of 1q, 6p, and 8q | ? | ? | ||||
| Mutator mechanism | UV radiation | UV radiation | Amplifications | Amplifications | ? | UV radiation | Rearrangements | ? | ? |
Melanocytic neoplasms originating from epithelial melanocytes
The majority of melanocytic neoplasms show an intraepithelial component, with neoplastic melanocytes present within the epithelium. While this does not necessarily allow the conclusion that the cell of origin of these neoplasms resides within the epithelium, the high mutational burden and UV signature in melanomas originating from the non-glabrous skin strongly suggests that many originate from epidermal melanocytes. Lesions of intraepithelial origin are distinct from melanocytic neoplasms, which consistently lack an epithelial involvement such as uveal melanoma and intradermal melanocytic proliferations, both of which share common mutations in the two G-protein alpha subunits GNAQ and GNA11. They are separated here as a distinct clade and discussed at the end. The other clade of epithelia-associated melanocytic neoplasms is divided further into several classes based on anatomic site and the degree of sun exposure. In Caucasians, which have the highest burden of melanocytic neoplasms, the overwhelming majority of lesions presents on skin that bears hair follicles and is subject to UV radiation, with only a small fraction presenting on hairless (glabrous) skin of the palms and soles or sites covered with mucosa. As discussed below, there are major phenotypic and molecular differences between melanocytic neoplasms on sun-exposed skin depending on the degree of sun-exposure and anatomic site, so that these classes are presented separately. Furthermore, melanomas on glabrous skin and mucosa also have multiple distinctive features, and will be discussed separately. These considerations and those detailed below are the background for the classification schema outlined in figure 1.
Melanocytic neoplasms on hair-bearing skin
Melanomas on sun-exposed skin without cumulative sun-induced damage (non-CSD)
Shortly after the discovery of frequent BRAF mutations in melanoma (4) it became apparent that the mutations were unequally distributed across the phenotypic spectrum of melanocytic neoplasms. Mutations were more common in younger patients whose melanomas arose on skin that were sun-exposed, but not heavily sun-damaged as evidenced by the presence of marked solar elastosis, an accumulation of degenerated elastic fibers (5, 6). Follow-up studies confirmed these associations and added additional distinguishing features of what emerges as a melanoma type that is characterized by a high frequency of specific BRAF mutations with associated clinical and histologic features (6–10) such as increased pigmentation of the primary melanoma by clinical examination, and histopathological features such as a predominance of enlarged, hyperpigmented tumors cells of round rather than spindled shape, increased upward intraepidermal scatter, predominance of tumor cells nests over single cells, and thickening of the involved epidermis. Other genetic alterations associated with this melanoma type include copy number increases of chromosome 7, favoring the chromosome harboring the mutant BRAF allele (5), and loss of chromosome 10, primarily driven by PTEN (11, 6, 12).
Melanomas of this category were also found more frequently to show an associated nevus remnant (9). Independent studies had shown that melanomas on the trunk were associated with increased nevus count and lower self-reported cumulative sun exposure and fewer non-melanoma skin cancers as melanomas on the head and neck (13). These studies also suggested that there were differences among the melanomas arising on the sun-exposed skin. which as a group represent the most common form of melanoma and primarily affect Caucasians. The melanomas on skin without chronic sun-induced damage (non-CSD melanomas) were most commonly of the SSM type according to the WHO classification, but the features described above showed a stronger association with the BRAF mutation status (7) than the WHO classification of SSM.
Common acquired nevi
Studies by Pollock et al. and others showed that BRAF mutations were also present in melanocytic nevi, with common acquired nevi being the type of nevus with the highest BRAF mutation frequency (14). These nevi arise mostly during the first two decades of life, primarily affecting the trunk and extremities, mostly sparing sun-protected sites, and thereby implicating UV radiation as a contributing pathogenic factor. Histologically they are separated into three types, depending on whether they are confined to the epidermis (junctional nevi), or the dermis (dermal nevi), or show both a junctional and a dermal component (compound nevi). There is an unresolved debate among pathologists whether the nevi form at the dermo-epidermal junction with some melanocytes subsequently “dropping” into the subjacent dermis, or whether they originate in deeper structures with subsequent ascent of melanocytes into the overlying epidermis.
The symmetric distribution of the neoplastic cells in most nevi, the monotony of their constituent melanocytes, and the presence of identifiable mutations in bulk populations of nevus cells suggest that they result from the clonal expansion of a single cell. While some studies have found that not all melanocytes within an acquired nevus may have detectable BRAF mutations and concluded that nevi may not be clonal or that BRAF mutations are not an initiating event, this finding may have been due to technical difficulties of quantifying mutant alleles in small numbers of cells (15, 16). Recent immunohistochemistry studies using a BRAF V600E specific antibody show labeling of the majority of neoplastic cells in melanocytic nevi harboring BRAF mutations but no labeling in nevi without BRAF mutations. BRAF V600E mutations were also found to be fully clonal in neoplastic population of melanocytes using digital droplet PCR to quantify the mutant to wild type allelic ratios (17).
Tumor suppressive mechanisms at the junction between nevi and melanomas
The finding of mutations in potent oncogenes in benign nevi such as NRAS in congenital nevi (18), HRAS in Spitz nevi (19), and later BRAF in acquired nevi (14), GNAQ or GNA11 in blue nevi (20, 21) originally came as a surprise, and the mechanisms suppressing the expansion of partially transformed melanocytes in nevi became of significant interest. Many of the studies have been performed in the context of BRAF mutations and therefore are discussed in this section.
It has been posited that the melanocytes constituting a nevus are senescent, based on the expression of β-galactosidase and other senescence-associated markers whereas melanomas have acquired ways to bypass this arrest (22). However the situation is more complex, because nevi routinely contain cells labeling with proliferation markers. Moreover, melanomas and even melanoma metastases, can also have significant numbers of β-galactosidase positive cells (23).
The complexity can in part be attributed to the fact that senescence summarizes a growth-arrested cellular state, which can be reached by a range of different mechanisms (24). One, oncogene-induced senescence has been proposed as an immediate reaction in which a mutation within a critical signaling pathway such as the MAP-kinase pathway leads to non-physiologically high activation, which triggers a stress response leading to the induction of cyclin-dependent kinase inhibitors such as p21 and p16, leading to permanent G1 arrest (25, 26). The critical role of p16 in melanoma is demonstrated by the fact that p16 is disabled by deletion, mutation, or silencing of CDKN2A in the majority of melanomas, and germline mutations in CDKN2A predispose to melanoma with high penetrance(27, 28). Ten percent of melanoma patients have a family history of melanoma and of these 20–40% carry germline mutations of CDKN2A, with mutations mostly in the exons or reading frame that affects p16 and not p14, which is also transcribed from this same gene (29, 30). Approximately 2–3% of melanoma families have CDK4 mutations in the p16 binding domain (31), and a similar percentage has mutations that specifically disable p14/ARF(30, 32), which acts upstream of p53, by inhibiting its ubiquitinase mdm2.
However, nevi are composed of several tens of thousands of cells or more, indicating that the senescence mechanisms at work are not immediate in nature, but engage with some latency. It is conceivable, but difficult to prove, that individuals without nevi are able to effectively induce such an immediate senescence response and thereby subdue any oncogene-induced melanocytic proliferation before they can form a clinically detectable lesion. By contrast, individuals with constitutional defects in immediate-type senescence may engage secondary mechanisms that operate with a longer latency and thus lead to the formation of populations of neoplastic melanocytes large enough to constitute a nevus. Patients with inherited CDKN2A mutations have more and larger nevi than their wild type relatives (33, 34), indicating that p16 exerts its tumor suppressive function from nevus initiation to later phases of nevus growth.
Replicative senescence induced by progressive telomere shortening is a mechanism that is inherently latent in nature. However, critical telomere shortening occurs after approximately 60 population doublings, far more that would be expected to occur in a nevus, if all progeny from the initial founder cell were proliferating equally. Other latent mechanisms that have been linked to senescence include stochastic events triggered by DNA replication stress. Constitutive proliferative signals from activated oncogenes can lead to DNA hyperreplication, resulting in genomic instability in the form of prematurely terminated DNA replication forks and subsequent double stranded DNA breaks. Markers of DNA damage co-segregate with markers of senescence in pre-cancerous lesions, including acquired and dysplastic nevi, implicating oncogene mediated replication stress as an additional barrier towards transformation (35). This mechanism is expected to operate stochastically and could explain why cell proliferation is exhausted in nevi following a period of latency. In support of this model, random DNA copy number changes can be observed within the melanocytes of a nevus by fluorescence in situ hybridization, while clonal chromosomal aberrations are typically only present at the melanoma stage (36). Exhaustion of nucleotides by oncogene-induced suppression of their synthesis has been demonstrated to precede and directly contribute to the DNA damage response (37). Additional mechanisms that have been implicated in melanocyte senescence include paracrine factors such as interleukin-6 and -8 (38). In summary, these findings indicate the existence of multiple, independent barriers that restrain the proliferation of partially transformed melanocytes. A nevus that has reached a stable size thus might represent a population of cells that have been growth-arrested by different mechanisms. This model would explain the mosaic expression of individual senescence markers among the cells of a given nevus (23, 39). It would also explain why the removal of individual mechanism, even critical ones such as p16, is not sufficient to bypass the senescence process of melanocytes (22), as other senescence mechanisms remain intact. The more such barriers become disabled by inherited or by somatic mutations the larger the nevus will grow, and with it the probability of full transformation to melanoma.
Activation of telomerase is emerging as a critical barrier to full transformation of melanomas. While telomerase activity is low or absent in nevi, melanoma metastases typically show significantly increased activity, with primary melanomas somewhere in between (40, 41). Amplification of the TERT gene has been reported as a common event in acral melanoma (6, 42) and observed to coincide with the transition to more advanced primaries (43). Recently, frequent mutations in the telomerase promoter have been found in melanoma (44, 45). Two mutational hotspots were found in a mutually exclusive pattern in over 85% of melanoma metastases. The frequency was significantly lower in primary melanomas (33%) and mutations were not found in 25 nevi examined, consistent with the pattern of telomerase activation reported previously. These findings implicate telomere length as a factor limiting net cell expansion somewhere during the evolution of the primary melanoma. Considering that there is a selective advantage for activating telomerase in primary melanomas, in which the numbers of neoplastic melanocytes is significantly below the theoretical Hayflick limit, indicates stochastic attrition of melanocyte either by growth arrest or cell death. It is noteworthy that primary melanomas frequently show areas of regression, i.e. areas in which neoplastic melanocytes have vanished but left behind a telltale sign of their former presence in the form of melanophages, i.e. macrophages that have ingested leftover melanin pigment. While it has been proposed that immune cell mediated killing of melanoma cells is the main mechanism leading to melanoma regression, telomere crisis has been proposed as an alternative mechanism (46).
However, immune-mediated mechanisms undoubtedly play an important role in addition to the cell-autonomous mechanisms outlined above in restraining the expansion of the partially transformed melanocytes of nevi and early melanomas. Nevi often show infiltrates of lymphocytes, and are known to acutely regress when the inflammation is more pronounced. The latter scenario is often accompanied by a clinically visible de-pigmented halo surrounding the nevus. This phenomenon can affect multiple nevi simultaneously, and can even be accompanied by vitiligo, suggesting that immune responses against one nevus can extend to others. It is also well documented that nevi can enlarge or erupt de novo in large numbers under immunosuppresion. Such eruptive nevi also frequently have BRAF mutations and favor sun-exposed sites, indicating that the initiating mechanisms are identical to other acquired nevi (47). In a liver model, hepatocytes that became senescent due to oncogenic RAS were effectively eliminated by immune cells (48). The phenomenon of eruptive nevi in immunosuppressed patients may indicate a similar immune surveillance that restricts the number neoplastic melanocytes in melanocytic nevi. However, it does not happen in all individuals, indicating that other factors are involved.
Epigenetic alterations are emerging as additional tumor suppressive mechanisms in melanoma as evidenced by recurrent mutations in genes involved in chromatin remodeling and dramatic changes in the chromatin state and DNA modifications in melanoma. Sequencing studies have revealed recurrent mutations in components of the SWI/SNF complex including ARID1A, ARID1B, and ARID2 as well as SMARCA4 (12, 49). SETDB1, a histone-methylating enzyme, is amplified in melanoma and its expression is increased compared to melanocytic nevi (50). SETDB1 resides within a narrow locus on chromosome 1q21.3 that has also been implicated in melanoma susceptibility by genome-wide association studies (51). The histone methyl transferase EZH2, a member of the polycomb complex, is overexpressed in melanomas compared to nevi (52) and mutations have been found in a small proportion of melanomas (49). Several studies have reported marked alteration of the chromatin landscape and DNA methylation status during the progression to melanoma. The histone variant macro-2HA is markedly diminished in melanomas compared to nevi due to transcriptional downregulation (53). Genome-wide hypomethylation and hypermethylation of certain promoter regions has been observed in melanoma compared to normal melanocytes or neoplastic melanocytes of nevi (54–56). 5-methyl hydroxylation of cytosine is abundant in melanocytic nevi, but there is a genome-wide loss of 5-hydroxymethyl cytosine (5-hmC) at some point in the transition to melanoma (57). The genetic basis underlying this dramatic change in 5-hmC is currently not clear. In myelodysplastic syndrome and acute myeloid leukemia loss of 5-hmC is caused by loss of function of the 5-methyl-cytosine hydroxylating enzymes of the TET family, which can occur by inactivating mutations or functional inhibition via the production of 2-ketoglutarate caused by mutations in IDH1 or -2 (58, 59). While mutations in TET and IDH appear to be infrequent in melanoma, decreased expression levels of these proteins have been implicated as a mechanism underlying the loss of 5-hmC in melanoma progression (57). Inactivating mutations in the deubiquitinase BAP1 have been found as frequent somatic events during the progression of uveal melanoma, as discussed below (60). Germline mutations in BAP1 were independently discovered in two families with uveal and cutaneous melanoma and atypical Spitz tumors. In this setting, somatic loss of the remaining wild type allele in acquired nevi with BRAF V600E led to clonal expansion of enlarged epithelioid melanocytes with nuclear pleomorphism, but not clear-cut melanoma (61, 62). The resulting lesions show a bi-phasic morphology and represents an example of step-wise tumor progression, with a common acquired nevi initiated by BRAF mutation and promoted by loss of function of BAP1. BAP1 interacts with the polycomb factors ASXL1 and 2 and its substrates HCF-1 and OGT, but the precise mechanism how its loss of function bypasses growth arrest in BRAF mutated nevi remains to be elucidated.
Dysplastic nevi
The concept of dysplastic nevus was introduced in 1978 to identify an indicator of melanoma risk and potential melanoma precursor (63). In a series of melanoma families, family members were noted to have increased numbers of acquired nevi that were unusually large, often exceeding 10 mms, and showed irregular pigmentation. The nevi observed in patients with this syndrome were proposed to have specific histopathological features such as nuclear pleomorphism, bridging of rete ridges, and lamellar fibrosis of the papillary dermis. It later became clear that the histopathological features are very common in acquired nevi in general and are not specifically associated with the syndrome or with the size of the nevi (64, 65). It also became clear that in the general population the risk of progression to melanoma is very low. Dysplastic nevi remain stable for decades and tend to fade away later in life and removal of clinically dysplastic nevi is not recommended as long as a lesion is not suspicious for melanoma.
Despite the changed perceptions, the term ‘dysplastic’ remains widely used among clinicians and pathologists today, but with varying connotations. Clinicians use it to describe acquired nevi measuring 7 mm or more that are flat, brown to orange-brown, do not show the elongated hair of congenital nevi, and can have a darker or lighter center and fuzzy borders. However, similar features are frequently present in smaller nevi as well. Pathologists use it to describe nevi that have irregular architectural and cytological features. Some schools grade lesions from having mild, moderate, to severe ‘dysplasia’ in an attempt to position the lesion on a progression scale between a benign nevus and a melanoma (66). This view contradicts the notion that they are entirely benign lesions, but implies that they represent a spectrum. Another school uses the term to convey histopathological correlation to the clinician’s perception of “dysplasia”, but considers them entirely benign. The latter approach is based on the proposal of Ackerman to term flat nevi with bridging of nests, papillary dermal fibroplasia Clark’s nevi (67). The inconsistent usage and different meanings of the term “dysplastic nevus” has generated significant and unresolved controversy and confusion about the treatment, in particular of incompletely excised lesions.
What remains uncontroversial is that the presence of multiple enlarged acquired nevi is associated with an increased melanoma risk. For this assessment clinical examination is sufficient, and histological examination does not provide additional information. It is also firmly established that nevus size is a heritable trait (68). The higher the count of large nevi, the higher the melanoma risk (69). The presence of clinically dysplastic nevi is thus a symptom of systemic melanoma susceptibility and reflects the inherited loss of genes functionally involved in restraining the proliferation of melanocytes that have acquired oncogenic mutations in genes like BRAF. The phenotype thus is useful to identify patients with increased melanoma risk. By contrast, the degree of histopathologic dysplasia, if used to determine how closely a given lesion comes to bona-fide melanoma, provides information only about the lesion at hand, not about the patient’s overall melanoma risk. Nevi with histopathological dysplasia have been analyzed for allelic imbalance and found to have recurrent losses of heterozygosity of the CDKN2A and TP53 loci (70) and BRAF V600E mutations (14).
In summary the collective findings outlined above indicate that the neoplastic proliferation initiated by mutations in oncogenes is restrained by a multitude of independent mechanisms, which can be overridden by additional mutations that lead to loss of function in multiple tumor suppressor genes. These mutations can be inherited or somatically acquired. The sizes, shapes, and colorations of acquired nevi are more homogeneous within an individual than among different individuals. In twin studies the average nevus size is more tightly correlated among monozygotic compared to dizygotic twins (71). This fact is used clinically to identify pigmented lesions outside of the range defined by the majority of an individual’s nevi as suspicious for melanoma (“ugly duckling” sign). In this light, the phenotype of dysplastic nevus syndrome, i.e. the presence of multiple enlarged acquired nevi, indicates the inherited loss or impairment of senescence barriers. By contrast, the “ugly duckling” lesion arose through the somatic loss of a barrier.
Spitz tumors
The term Spitz nevus makes reference to Sophie Spitz’s description in 1948 of a form of melanoma in children that expresses a benign behavior. This contradiction was resolved later by renaming lesions with these features as Spitz nevi. However, this type of melanocytic neoplasm characterized by a predominance of large polygonal (epithelioid) or spindled melanocytes with enlarged nuclei and often multinucleated cells has retained considerable notoriety, because some cases diagnosed as such by even expert consultants have metastasized widely. Here the term Spitz tumor is used to capture a range of melanocytic neoplasms with overlapping histomorphology that includes Spitz nevi on the benign end of the spectrum and spitzoid melanomas with lethal potential on the other. The term atypical Spitz tumor captures borderline lesions with intermediate histological features. Spitz tumors occur more commonly in children and young adults but can occur in all age groups. Most neoplasms diagnosed as melanoma of childhood fall in the category of Spitz tumors and generally have a lower mortality than melanomas of similar thickness in older patients. Paradoxically they often grow rapidly, show numerous mitotic figures, and commonly have lymph node involvement (72, 73). In one study, 47% of 52 cases showed lymph node metastases, but only a single patient died of metastatic melanoma (73). In conventional melanoma less than 20% of patients have a positive sentinel lymph node, and those that do, have an increased risk for distant metastasis and death (74).
It is becoming increasingly clear that Spitz tumors are a heterogeneous group of genetically and biologically distinct categories. Approximately 20% of cases show oncogenic mutations of HRAS, typically accompanied by copy number increases of the entire short arm of chromosome 11 as the only chromosomal aberration (19). This type of Spitz nevus often presents as a predominantly intradermal horizontally oriented proliferation of large epithelioid melanocytes widely dispersed singly between thickened collagen bundles in the deep reticular dermis. Another variant has a combination of BRAF V600E mutations accompanied by bi-allelic loss of the tumor suppressor BAP1 on chromosome 3p21 (62). These lesions present as intradermal, often dome-shaped or polypoid, vertically oriented proliferations of large epithelioid melanocytes arranged in large cellular aggregates, often in continuity with a conventional acquired nevus. In these combined lesions, the transition to the epithelioid phenotype coincides with the loss of BAP1, demonstrating that this variant of Spitz tumor represents a progression from acquired nevus (61).
The driving oncogenic alterations of the remaining Spitz tumors are beginning to emerge rapidly. Recent studies have revealed a high frequency of rearrangements of kinases in the remainder of Spitz tumors. Rearrangements resulting in fusion kinases of ROS1, ALK, RET, NTRK1, and BRAF were observed in 60% of cases (Wiesner T, He J., Yelensky R, Esteve-Puig R., Botton T., Yeh I, Garrido M, et al., submitted). The rearrangements fuse the intact kinase domains in frame to a wide range of 5′ fusion partners that includes genes involved in similar rearrangements in lung, colon, thyroid, and lymphoma, as well as several novel fusion partners. The 5′ fusion partners in the majority have coiled-coil domains suggesting that they allow the kinase domains to dimerize and autophosphorylate, resulting in ligand-independent constitutive activation of multiple oncogenic signaling pathways including the MAP-kinase, PI3-kinase, STAT pathways with potent induction of proliferation. Many of the kinases involved in the rearrangements are only expressed during development, including neural crest development, but are silenced in adult tissues. The kinase fusions thus simultaneously lead to expression and kinase activation, explaining why rearrangements rather than point mutations are the predominant mode of oncogenic activation.
It is unclear how the specific nature of these oncogenic alterations is related to the unique biological behavior of atypical Spitz tumors and spitzoid melanomas of childhood, with their rapid growth and frequent metastasis but rare lethal outcomes. The clinical behavior indicates that progression is not constrained by the nature of the oncogenic signaling as it can potently drive tumor growth and metastasis. It is conceivable that Spitz tumors are not immortal and lack the high mutation burden that can make mutations in the telomerase promoter likely, as occurs in most lethal melanomas. They also lack the chromosomal-level genomic instability required to amplify the TERT locus, as occurs in acral melanoma. The immune system is another possible mechanism in restraining the proliferation of fusion driven melanocytic neoplasms. Fusion kinases are chimeric proteins with neoantigenic properties, which could render them an easier target for the immune system than oncogenes activated by point mutations.
Pediatric melanoma
Pediatric melanomas represent a heterogeneous group comprised of lesions related to atypical Spitz tumors, melanomas arising in congenital nevi occurring primarily in younger children and non-CSD melanomas in older, post-pubertal children. The latter have similar clinical features and outcomes as those in adults. Pre-pubertal melanomas also occur in non-Whites, but the overwhelming majority of melanomas in post-pubertal affects Whites (75). They are discussed separately under Spitz tumors and non-CSD melanomas. Not all melanomas in pre-pubertal children are spitzoid or arise in congenital nevi, raising the possibility of the existence of additional subtypes.
The role of UV radiation and skin pigmentation
People living in geographic regions with increased UV exposure have an increased melanoma risk (76). However, the relationship between melanoma risk and degree of exposure to UV radiation is complex. Paradoxically, melanomas on intermittently exposed skin such as the trunk and proximal extremities develop at a younger age, whereas melanomas on sites that are more frequently exposed such as the face, ears, and neck develop significantly later in life (10, 77). Whole genome and exome sequencing studies have revealed a high burden of mutations in the genomes of melanomas originating from sun-exposed sites, with cytosine to thymidine transitions prevailing, thereby providing compelling genetic validation for UV radiation as a major mutagenic factor (12, 49, 78). Consistent with the finding of melanomas with BRAF mutations primarily occurring on the intermittently exposed skin, the genomes of BRAF-mutant melanomas have mutation burdens intermediate (ca. 30,000) between chronically (ca. 100,000) and unexposed sites (<1,000) (12). Approximately 80% of mutations are compatible with UV-related mutagenesis confirming that UV radiation is the predominant mutagen involved in the pathogenesis. The exceptionally high mutation rates in melanomas originating from sun-exposed skin raise the questions whether inherited defects in DNA repair contribute to the mutation burden and melanoma susceptibility (12, 49). Some studies have found polymorphisms in DNA repair genes such as ERCC2 to be associated with melanoma risk (79) and others have found high frequencies of somatic loss of function mutations of genes in the DNA damage response pathways including ERCC2 (80).
While the above associations identify UV radiation as a pathogenetic factor for melanocytic neoplasia, the most common mutation in BRAF at codon V600 is a thymidine to adenine transversion and thus not a classic UV-signature mutation. BRAF mutations also arise in thyroid or colorectal cancer, indicating that UV is not required for their formation. This however does not rule out a causal role of UV radiation in melanocytic neoplasia. While UV radiation primarily causes mutations at pyrimidine dimers it causes a broad range of other mutations as well (81). Indirect effects of UV radiation have also to be considered, because interaction of UV radiation with melanins generates free radicals. Data from genetically engineered mouse models have shown that UVA radiation requires melanin to be present to induce melanoma formation (82). While the specific mutations that induced melanoma formation in this model are not yet known, the results clearly establish an interaction between UV radiation and melanin as a causative mechanism. Additional studies have confirmed and further refined the role of melanin. Using a mouse model in which mutant BRAF V600E can be conditionally activated in melanocytes, melanomas only developed if melanin, pheomelanin specifically, was present (Sidebar). Significant oxidative DNA and lipid damage occurred in the normal skin of “red head” mice that due to a loss of MC1R function produced only pheomelanin, even in the absence of UV radiation. In this model, the BRAF mutations were already pre-engineered so that melanoma formation depended on additional, yet unknown mutations to occur. However, the experiment provided additional evidence of the mutagenic effect of pheomelanin, or an increased pheomelanin to eumelanin ratio. How the balance of pheomelanin and eumelanin affects mutagenesis is not known, but as the melanosome ultrastructure differs between light- and dark-skinned individuals, it has been proposed that eumelanin encases pheomelanin as an antioxidative coating in dark skin melanosomes and that this function is impaired in patients with light skin (83, 84). There also is emerging evidence that induction of the DNA damage response may be hard-wired into the tanning response. Stimulation of MC1R with melanocyte stimulatory hormone (MSH) not only is the main initiator of pigment synthesis in melanocytes but may also directly activate scavenging of reactive oxygen species (85).
Sidebar.
Melanocyte development and the tanning response
Melanocytes are derived from the neural crest. There are two developmental pathways that give rise to melanocyte progenitor cells (140). The dorso-lateral pathway generates melanoblasts that travel through the mesoderm to colonize epidermis, mucosa, and hair follicles. A second population of neural crest cells gives rise to bipotent precursors of Schwann cells and melanocytes, migrating ventromedially to reach the skin. There are differences in the signals involved in fate determination and migration of the two developmental pathways, with END3 signaling playing a major role in the ventromedial pathway.
After migration is completed, the majority of differentiated melanocytes reside within epithelia, mostly the epidermis and hair follicles of the skin, but also in mucosal epithelium. They are also found in significant numbers in the leptomeninges, the inner ear, and at lower density in many internal organs. The lifespan and –cycle of melanocytes is not fully understood. In hair follicles, the number of melanocytes waxes and wanes with the cycling of the follicle, and the stem cells to replenish melanocyte populations in early anagen reside in the bulge region of the hair follicle (141). These melanocyte stem cells can also replenish lost epidermal melanocytes in conditions like vitiligo. However, it remains to be determined how the distance and the kinetics with which stem cells within the hair follicle contribute to melanocytes of the interfollicular epidermis under homeostatic conditions.
The main function of differentiated melanocytes is to produce melanin and to distribute it to surrounding epithelial keratinocytes to protect them from UV radiation. There are two chemically distinct types of melanin. One, eumelanin, is black and represents the more abundant form in dark-skinned people. The other, pheomelanin contains benzothiazine and –thiazole and is of more orange coloration. While it is also present in higher concentration in the melanocytes of dark-skinned individuals, its relative abundance is much higher in individuals with light complexion, who have lower amounts of eumelanin. Eumelanin production is subject to dynamic regulation as part of the tanning response, in which activation of the melanocortin receptor 1 (MC1R) by melanoycte stimulating hormone (MSH), synthesized by keratinocytes, stimulates pigment synthesis via the induction of the transcription factor MITF.
The majority of individuals of European descent have inherited variants in MC1R, which blunt the receptor’s ability to activate downstream signaling and to induce tanning in response to binding of MSH. Several loss of function variants of MC1R are highly associated with red hair, poor tanning and freckling of the skin and increased melanoma risk (86). Germline polymorphism in a range of other genes affecting skin pigmentation including ASIP, OCA2, SLC45A2, TYRP1, and TYR have been associated with melanoma risk (51, 87–92), but inherited polymorphisms in MC1R are probably the most important genetic factor among risk alleles that occur at high frequency in populations. The risk conveyed by germline variants of MC1R might differ for the various molecular subtypes of melanoma. In non-CSD melanomas MC1R variants were strongly associated with BRAF mutations in some studies (93, 94), but not in others (95, 96). This may be due different ethnic compositions of the cohorts or differences in the proportion of melanoma types. CSD melanomas have a low frequency of BRAF mutations. Those that occur are primarily of the V600K rather than V600E type (97). In addition, BRAF mutations in CSD melanomas are associated with wild type MC1R alleles (98), indicating that any interactions between BRAF and MC1R may be complex.
The consistent observation that melanocytic neoplasms with BRAF V600E mutations arise early in life and decrease in frequency later, while V600K show an opposite behavior is a puzzle to be solved. The early onset and multiplicity of nevi with BRAF mutations in some individuals suggests a genetically determined susceptibility that manifests itself in a high mutation rate in melanocytes. The odds of mutating one of the single base pairs to generate the C.1799T>A mutation resulting in the V600E allele is exceedingly low so that the presence of multiple independent nevi with these mutations on the skin indicates a high mutation burden in normal melanocytes already at a young age. In addition to sun exposure in early life, neonatal blue light therapy for the management of hyperbilirubinaemia, which is administrated to a significant proportion of newborns and contains some UVA, has been shown to increase the number of nevi (99). Sun protection in children has been demonstrated to reduce nevus formation (100). The link between sun exposure in early life and nevus and melanoma risk has led to increased legislation to restrict access to tanning beds for minors, the use of which has been associated with melanoma risk (101).
In summary, both the number and the size of acquired nevi contributing to melanoma risk can be interpreted in the light of the factors outlined above. The number of nevi in an individual is proportional to the number of initiating events, which reflects the mutational burden in melanocytes of the skin. This mutation burden is a product of cumulative exposure to UV radiation and the host’s defense and repair mechanisms consisting of the production of melanin and detoxifying ROS as well as the ability repair UV-radiation-mediated DNA damage through DNA repair.
Melanomas on skin with cumulative sun-induced damage (CSD melanomas)
The second most common type of melanoma in Caucasians arises in areas chronically exposed to the sun, predominantly the face, ears, neck, and lower extremities. These melanomas tend to arise approximately 2 decades after the peak of non-CSD malanomas, typically in individuals 60 years or older (10). They are not associated with increased number of acquired nevi but instead with signs of high cumulative exposure to UV radiation such as solar elastosis and non-melanoma skin cancers (5, 102). Melanomas on chronic sun-exposed skin differ in the genetic alterations from non-CSD melanomas in that they have infrequent BRAF mutations, with BRAF V600K being more frequent than BRAF V600E mutations (5, 12, 97), inactivating mutations of NF1 in 30% (12), copy number increase of CCND1 in 20% (6, 103), activating mutations of KIT in approximately 10% (104), increased mutational frequencies in TP53, and ARID2 (12) and differences in the pattern of chromosomal aberrations (6) as shown in table 1. Their mutation burden is very high, with the number of somatic mutations in the range of 100,000 and above (49). The most common presentation is that of LMM, in which a pigmented macule comprised of a subtle proliferation of single intraepidermal melanocytes develops, often after many years, a nodular growth with dermal invasion. However, melanomas that present as nodular melanomas without an adjacent in situ component show indistinguishable genetic alterations, indicating that they an accelerated transition from in situ to invasive melanoma rather than being a unique class of melanoma (6).
Desmoplastic melanomas
Desmoplastic melanomas are primarily intradermal proliferations of spindled melanocytes with atypical nuclei that are interspersed at varying density between thickened collagen bundles. They most commonly arise on the chronically sun-exposed skin of the head and neck. They have distinctive clinical and genetic characteristics that indicate that they represent a distinct subtype of melanoma. They lack activating mutations of any of the known melanoma oncogenes BRAF (105), NRAS, KIT, GNAQ, GNA11 but have loss of function mutations in NF1 in approximately 25% of cases (unpublished observation). They show growth along nerves in 30–40% of cases, a feature that is associated with an increased risk of recurrence. While little about their pathogenesis is known, the preference for heavily sun exposed sites indicates UV radiation as a pathogenetic factor. The majority of desmoplastic melanomas show a lentiginous component as in LMM and whole genome sequencing studies have revealed a high mutation burden with a strong UV signature as in CSD melanomas (unpublished observation), indicating a superficial location of their cell of origin.
Acral and mucosal melanomas
Melanomas originating from the glabrous (non-hair-bearing) skin and the nail apparatus have characteristic morphological, epidemiological, and genetic features that set them aside from other subtypes. They share several features with melanomas originating from mucosal epithelia (mucosal melanomas) and therefore are discussed together. Glabrous skin, by definition, lacks hair follicles, but has eccrine glands. Mucosa lacks either structures, but has, depending on the site, mucosal glands. These differences may be relevant for the cell of origin of these melanoma types. While the bulge region of the hair follicle harbors melanocyte stem cells in the non-glabrous skin, the existence of an equivalent melanocyte stem cell reservoir in glabrous skin is unclear. Acral melanomas frequently involve the eccrine sweat ducts, and early acral melanoma in situ shows a characteristic preference to grow around the openings of eccrine sweat ducts along the ridges of the dermatoglyphs, a pattern that provides diagnostic information (106). It remains to be determined whether the apparent tropism of acral melanoma to eccrine glands is indicative of their harboring the cell of origin or playing other important roles during the early phases of the disease.
Both acral melanoma and mucosal melanoma often show a lentiginous component, in which melanocytes are found predominantly as single units in the basal layer of the epithelium. For this reason, the term acral lentiginous melanoma (ALM) has been employed (107). However, the characteristic genetic alterations detailed below are found in virtually all melanomas presenting on glabrous skin, so that the term acral melanoma is used here to encompass all traditional melanoma types, ALM, NM and SSM that can present on glabrous skin.
As shown in table 4, acral and mucosal melanomas do not show the dramatically increased frequency in Caucasians as do melanomas on sun-exposed skin (108, 109). Acral melanomas arise on relatively sun-protected sites, and additionally are shielded by a thickened cornified layer or a nail plate. Epidemiologic studies do not indicate an increased in incidence with latitude or degree of sun exposure. These findings make UV radiation an unlikely pathogenetic factor, which is supported by whole genome sequencing studies, which do not reveal the high degree of UV signature mutations as found in melanomas originating from the non-glabrous skin (12, 49). Mucosal melanomas primarily involve the mucosa of the anogenital region, nasal cavity, and paranasal sinuses, but can occur in all mucosal sites (110).
The spectrum of somatic mutations in acral and mucosal melanomas differs from melanomas on sun-exposed sites. In acral melanoma, mutations in BRAF occur in 15%, much less frequent than in non-CSD melanomas. They are absent in mucosal melanoma. Activating mutations or amplifications of wild type KIT are found in 15–40% of acral melanoma and mucosal melanoma, and approximately 15% have NRAS mutations (49, 104, 111). A unique feature of acral and mucosal melanomas is the high frequency of gene amplifications throughout the genome (42). The majority of acral melanomas have multiple (5 on average) focused gene amplifications, most commonly involving the loci that include CCND1 (11q13), hTERT (5p15), CDK4 (12q14), RICTOR (5p13), and KIT and PDGFRA (4q12)(6, 12, 42). Mucosal melanomas share the high frequency of amplifications but the frequency at individual loci differs, possibly indicating biological differences between these melanoma types (6, 112). As opposed to other solid tumors, where gene amplification typically arise later during progression, amplifications in acral melanoma are already present in the earliest detectable phases of the disease (42). The nature of the genomic instability that favors gene amplifications over other mutation types remains to be determined.
While melanocytic nevi are not infrequent in acral sites, and can also affect the nail apparatus, they do not appear to play a significant role as precursors, as they are infrequently found contiguously with acral melanomas. Limited information is available on the somatic mutations in acral nevi, but KIT mutations have not been found (113). Similarly, nevi are not found in association with mucosal melanomas. Instead the earliest detectable manifestation of acral and mucosal melanoma is melanoma in situ, in which a subtle intraepithelial proliferation of single melanocytes with enlarged nuclei and uneven and thickened dendrites are present, often extending over considerable distances. Studies in acral melanoma using fluorescence in situ hybridization revealed individual, evenly spaced, morphologically normal appearing but genetically aberrant melanocytes, colonizing stretches of ‘normal’ skin up to 1 cm wide immediately adjacent to obvious melanoma in situ. These “field cells” share the same amplifications as the histopathologically apparent melanoma cells, albeit often at lower copy number, indicating that they are genetically not as evolved and represent an earlier progression phase (42, 43). Preliminary studies suggest that KIT mutations may arise early and are followed by amplification of CCND1, and subsequently hTERT, which coincides with the development of substantial increase in the neoplastic cell population (43). This is consistent with in experimental studies, in which mutant KIT primarily induces migration and survival rather than significant cell proliferation (114, 115). Accordingly, KIT mutations may differ from BRAF mutations in that they are not sufficient to allow any significant proliferation that results in an equivalent of a nevus as seen with BRAF mutations. The relatively high frequency of CCND1 amplifications in these melanoma types may indicate that additional genetic alterations that drive proliferation may be required. A low to moderate frequency of KIT mutations is common to acral, mucosal, and CSD melanomas, which all tend to share an often extensive lentiginous growth.
The role of KIT in melanoma is complex, as it has been shown to inhibit proliferation or cell survival in certain settings and therefore was originally considered to play a tumor suppressive role in melanoma. This was based on the observation that KIT protein levels decline from the in situ to invasive components and forced expression of KIT in melanoma cell lines decreasing their ability to proliferate and/or survive (116). However, in certain settings, KIT acts as an oncogene as patients whose melanomas harbor activating mutations in KIT can show dramatic responses to KIT inhibitors (117).
Melanocytic neoplasms originating from melanocytes not associated with epithelia
Congenital nevi
Nevi that develop in utero are termed congenital melanocytic nevi. These, by definition, develop in the absence of UV radiation. However, the definition is often extended to include nevi that arose shortly after birth. Congenital nevi have several distinctive features that set them apart from acquired nevi. They tend to be significantly larger and can cover extensive parts of the body surface. During childhood and adolescence they increase in size proportionally with the body area and are termed either large or giant congenital melanocytic nevi, when their predicted size exceeds 20 or 60 cm of an adult, respectively. In particular these larger congenital nevi can also involve extracutaneous sites including the CNS. This condition, neuro-cutaneous melanosis, can develop severe or even lethal compression symptoms of vital structures of the CNS. On the skin, congenital nevi often show marked hypertrichosis with hyperpigmented terminal hairs, which acquired nevi do not. Their constituent melanocytes are also found in deeper structures of the skin and beyond, occasionally involving deep soft tissue and muscle. Large and giant congenital nevi frequently present with satellite lesions, with discrete, morphologically similar, but smaller nevi present elsewhere on the skin. 2–3% of patients with large or giant congenital nevi develop melanomas, with 70% of melanomas developing by puberty. Melanomas can develop within any tissue involved by the nevus and risk increases with the size and cellularity of the nevus (118). Primarily in neonates and mostly in giant congenital nevi, rapidly proliferating nodules can develop, which can reach several centimeters in diameter, show an increased mitotic rate and be difficult to histologically distinguish from melanoma (119). These proliferating nodules have characteristic chromosomal aberrations with gains and losses of multiple entire chromosomes rather copy number changes involving chromosome fragments as seen commonly in melanomas (120). This pattern of aberrations is suggestive of a chromosome segregation defect in the constituent melanocytes.
In contrast to acquired nevi in which BRAF mutations predominate, large and giant congenital nevi carry NRAS mutations in over 80% of cases (121). In patients with satellite lesions the same NRAS mutations are shared between anatomically separate lesions, indicating that they originate from a common ancestor (122). The clonal relationship between discrete lesions indicates that the mutated progenitor cells have the ability to move apart and form separate. While satellites are typically present at birth they continue to develop until age 5 and their continued emergence is linked to the risk of developing neuro-cutaneous melanosis (123). These findings indicate that satellites are formed from individual mutated progenitors that migrated along the axis of the neural crest and obtained access to different segments from which they migrate and clonally expand. The founding NRAS mutation results in an expanded progenitor pool that exceeds the receiving capacity of the destination epithelia, with excess cells piling up in the dermis and other tissues along the way. A similar phenomenon has been observed with hypermorphic mutations in GNAQ and GNA11 in mouse models (124). Other oncogenic alterations reported in congenital nevi involve kinase fusions of BRAF, which have been reported in individual cases of congenital nevi (125). Patients with larger congenital nevi are also at increased risk for other neoplasms including lipo- and rhabdomyosarcomas, indicating that the cell of origin may retain pluripotency.
Blue nevi and related neoplasms
Blue nevi comprise a group of neoplasms characterized by the proliferation of dendritic, spindled, ovoid, or epithelioid melanocytes in tissues without any significant epithelial involvement (126). While the majority involve the skin, they can be encountered in many other organs such as the lung, intestinal tract, prostate, and the central nervous system (CNS), where there are called melanocytomas. Blue nevi can be acquired or congenital. The latter category includes a range of conditions including Mongolian spot, nevus of Ito, and nevus of Ota, which all present as hyperpigmented patches comprised of paucicellular proliferations of pigmented melanocytes in the dermis. As they spare the epidermis they are also referred to as dermal melanocytoses. Nevi of Ota and Ito have a segmental pattern involving branches of the trigeminal nerve or cervical nerves, respectively. The distribution along innervation segments and cell morphology gives testimony to their close relationship to Schwann cells with which they share a common progenitor cell (127). Lesions with prominent areas of Schwann cell differentiation are termed neurocristic hamartomas.
The term blue nevus, in the strict sense, refers to discrete, non-segmental lesions that can vary in size from typically under a centimeter to several centimeters in congenital lesions. Blue nevi express a range of cellularity that reaches from paucicellular lesions similar to the dermal melanocytoses to highly cellular nodules refered to as cellular blue nevi.
The molecular characteristics that are shared between neoplasms of this family are somatic mutations of GNAQ or GNA11, two close related α-subunits of the Gαq family (20, 21). Mutations in GNAQ and GNA11 occur at the glutamine at position 209 or, in approximately 5% of cases, at the arginine at position 183. The Q209 mutations complete abrogate the GTPase activity, locking them in a GTP-bound, constitutively activated state. The R183 mutations are thought to maintain residual enzymatic activity and to be weakly activating. Mutations in these genes occur in a mutually exclusive pattern and are found in the majority of blue nevi including the segmental dermal melanocytoses. GNAQ and GNA11 convey signals from a broad range of G-protein coupled receptors. In melanocytes endothelin receptors utilize Gαq family members. Because endothelin signaling is critical for melanocyte development, in particular for melanocytes developing through the ventromedial pathway, it is likely that the mutations simulate sustained endothelin signaling. To date recurrent mutations in these genes have not been found in other neoplastic condition. Their downstream effectors include phospholipase C, which releases two potent second messengers, diacylglycerol and inositol-3-phosphate from membrane phospholipids, which activate protein kinases C the MAP-kinase pathway and other signaling cascades.
Blue nevi can also arise as part of Carney’s complex, a tumor predisposition syndrome caused by germline loss of function mutations in PRKAR1A, one of the two regulatory subunits of protein kinase A, resulting in increased PKA activity (128). Blue nevi in individuals with Carney’s complex often are comprised of epithelioid melanocytes. The spectrum of somatic mutations in blue nevi arising in the context of Carney’s complex is not known.
As with other melanocytic nevi, there is a malignant counterpart, which is referred to as blue nevus-like melanoma, or sometimes self-contradictorily as ‘malignant blue nevus’. These melanomas can arise within blue nevi as evidenced by a contiguous, morphologically benign lesion, or can occur de novo. While these blue nevus-like melanomas are rare, blue nevi are also much less frequent than acquired nevi, and the relative ratio of benign to malignant lesions may be within the range to that of other categories. The genetic alterations in blue nevus-like melanoma have not been systematically studied but have similarities with blue nevi and uveal melanoma with frequent mutations in GNAQ or GNA11 and losses of chromosome 3 (21, 129). Blue nevus-like melanomas further share with uveal melanoma a propensity to metastasize to the liver and bone (130).
Melanocytoma of the central nervous system
Melanocytomas of the CNS are primary melanocytic neoplasms that arise form autochtonous melanocytes of the leptomeninges and typically are discovered when they cause focal neurological symptoms by impinging on critical structures. They have strong morphological similarities to the spectrum of blue nevi described above and also harbor frequent mutations of GNAQ or GNA11 (131). Similar to blue nevi there is malignant counterpart to melanocytomas that has morphological features similar to that of blue nevus-like melanoma (132).
Uveal melanoma
Uveal melanoma is the most common intraocular cancer and accounts for approximately 5% of all melanomas in the US. As opposed to melanomas on the skin, its incidence has been stable around 5 per million (133). With 10-year survival rates at 50% it ranks among the most lethal presentations of melanoma. The cells of origin are interstitial melanocytes that are found abundantly in the choroid, ciliary body, and iris of the eye, which together comprise the uveal tract. They are neural crest derived, as opposed to the pigmented cells of the retinal pigment epithelium, which are derived from the anterior neural plate. Different from melanocytes of the skin, uveal melanocytes do not reside within an epithelium.
Clinical and histopathological evidence suggests that most uveal melanomas arise from uveal nevi. The prevalence of uveal nevi ranges between 4.6 and 7.9% in Caucasians and based on the number of reported uveal melanoma the risk of transformation has been estimated to be one in 8,845 per year (134). Mutations in GNAQ or GNA11 occur in approximately 85% of uveal melanoma and are mutually exclusive. They are considered an early event as they are not associated with outcome and can already be detected in uveal nevi (21). Loss of function mutations in the deubiquitinase BAP1 are found in 49% of uveal melanoma, with a significant higher frequency (85%) in tumors that become metastatic (60). A major selective force for the frequent loss of chromosome 3, a major negative prognostic indicator in uveal melanoma (135), is the elimination of the remaining wild type allele of BAP1. The precise mechanisms underlying how elimination of BAP1 function promotes uveal melanoma growth are not fully understood. BAP1 is a nuclear deubiquitinase, which interacts with several proteins involved in chromatin modification including host cell factor 1 (HCF-1), UDP-glucose-dependent O-glucosyltransferase (OGT), and the polycomb group proteins ASXL1 and 2. The disruption of this complex by loss of BAP1 is thought to result in altered histone modifications and a deregulated gene expression patter (136). Gene expression profiling of uveal melanomas has identified two separate classes. Class 1 tumors have a better prognosis, while class 2 tumors have a significantly increased risk of metastasis. The latter pattern is closely associated with BAP1 loss and may be a direct consequence of the ensuing epigenetic deregulation (137).
Another gene found recurrently mutated in uveal melanoma is the splicing factor SF3B1. Mutations are found in 19% of uveal melanomas, primarily in class 1 tumors, and thus primarily affect melanomas without BAP1 mutations (138). The mutations affect codon 625 and are typically heterozygous. It remains to be determined whether their effect is caused through gained or altered function or by a dominant negative effect. In zebra fish, germline loss of function mutations of SF3B1 results in a pigmentation phenotype caused by a failure of neural crest development. Loss of SF3B1 in this model leads to mis-splicing of critical neural crest transcription factors such as sox10 and tfab2a. Re-expression of properly spliced versions of these proteins can rescue the neural crest defect (139). While the link between SF3B1 function and neural crest development is intriguing, the specific SF3B1 mutations found in uveal melanoma do not appear to be associated with mis-splicing of any of these specific transcription factors (138)
Figure 2.
Histologic patterns of archetypical progression patterns for the classes depicted in figure 2
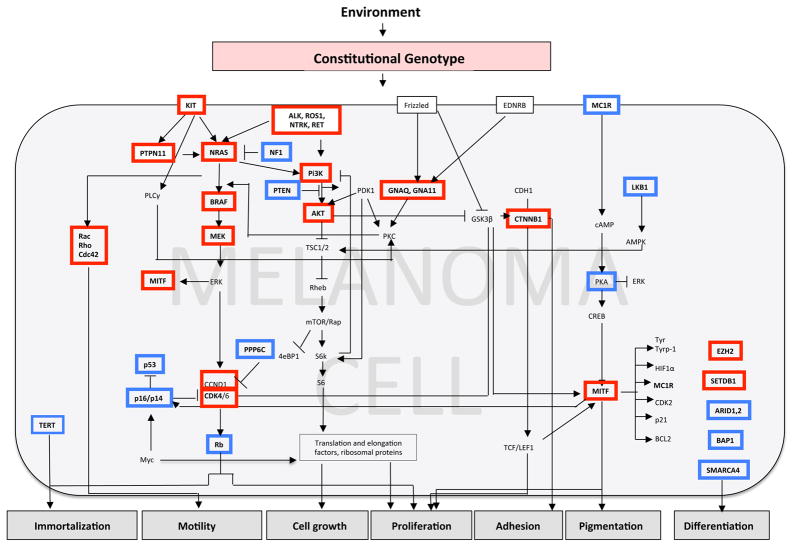
Figure 3. Signaling pathways disrupted by genetic alterations and their relationship to the hallmarks of melanoma.
Genes boxed in red are affected by gain of function mutations, those in blue by loss of function mutations.
Summary points.
There are multiple distinct categories of melanocytic neoplasms that differ in clinical and histologic presentation, cell of origin, age of onset, ethnic variation, pathogenetic role of UV radiation, predisposing germ line alterations, patterns of somatic mutations, type of genomic instability, and preferential sites of metastasis.
Melanocytic neoplasms are initiated by somatic mutations that activate oncogenes. In some categories of melanocytic neoplasia they induce benign neoplasms, termed melanocytic nevi. The proliferation of melanocytes within nevi is constrained by a multiplicity of factors, including cell cycle checkpoints, telomere length, secreted factors, and the immune system. Some melanoma categories do not have a distinctively recognizable benign precursor stage.
Mutations in initiating oncogenes are not sufficient to form melanoma. Subsequent genetic alterations override the tumor suppressive mechanisms operative in melanocytic nevi and lead to the progressive evolution of cells with an increasingly malignant phenotype. The subsequent genetic alterations differ among categories of melanocytic neoplasms.
In concert, the combination of somatic mutations disrupt essential signaling pathways controlling cell proliferation, growth, motility, stromal interactions, differentiation status and interaction with the immune system, giving rise to distinct phenotypic presentations of neoplasms.
Melanomas on the sun-exposed skin harbor a high burden of somatic mutations with the signature of UV radiation and begin as an intraepidermal growth, indicating that they arise from melanocytes within the epidermis.
Melanomas of the uveal tract of the eye and intradermal melanocytic proliferations such a blue nevi and related neoplasms arise from melanocytes not associated with epithelia and have distinct sets of genetic alterations.
UV radiation can cause mutations by direct interaction with DNA or indirectly through the generation of ROS. An increased ratio of pheomelanin to eumelanin may increase the mutation rate via ROS and thereby the melanoma risk.
Separating categories of melanocytic neoplasms based on reproducible associations between pathogenetically relevant genetic alterations and clinical phenotypes leads to an improved disease classification, which will facilitate future research and clinical management.
Acknowledgments
This work was supported by NIH grants R01- CA131524, R01 - CA142873, P01 CA025874. I would like to thank Daniel Pinkel, Philip LeBoit, Iwei Yeh, Hunter Shain, and Thomas Botton, and Scott Dalton for critically reading the manuscript and provide helpful suggestions. The amount of literature on this topic is enormous and I apologize to my colleagues whose works I did not cite because of the limited number of allowed references.
Abbreviations
- SSM
Superficial spreading melanoma
- LMM
Lentigo maligna melanoma
- ALM
Acral lentiginous melanoma
- NM
Nodular melanoma
- ROS
Reactive oxygen species
- CSD
cumulative sun-induced damage
- CNS
central nervous system
- 5-hmC
5-hydroxymethyl cytosine
- WHO
World Health Organization
- UV
Ultraviolet
References
- 1.Cancer IA for R on. Pathology And Genetics of Skin Tumours. World Health Organization; 2006. [Google Scholar]
- 2.Clark WH, Elder DE, Van Horn M. The biologic forms of malignant melanoma. Hum Pathol. 1986;17(5):443–50. doi: 10.1016/s0046-8177(86)80032-6. [DOI] [PubMed] [Google Scholar]
- 3.Weyers W, Euler M, Diaz-Cascajo C, Schill WB, Bonczkowitz M. Classification of cutaneous malignant melanoma: a reassessment of histopathologic criteria for the distinction of different types. Cancer. 1999;86(2):288–99. doi: 10.1002/(sici)1097-0142(19990715)86:2<288::aid-cncr13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 5.Maldonado JL, Fridlyand J, Patel H, Jain AN, Busam K, Kageshita T, Ono T, Albertson D, Pinkel D, Bastian BC. Determinants of BRAF mutations in primary melanoma. J Natl Cancer Inst. 2003;95(24):1878–90. doi: 10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- 6.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 7.Viros A, Fridlyand J, Bauer J, Lasithiotakis K, Garbe C, Pinkel D, Bastian BC. Improving Melanoma Classification by Integrating Genetic and Morphologic Features. PLoS Med. 2008;5(6):e120. doi: 10.1371/journal.pmed.0050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Kelly JW, Trivett M, Murray WK, Dowling JP, Wolfe R, Mason G, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol. 2007;127(4):900–905. doi: 10.1038/sj.jid.5700632. [DOI] [PubMed] [Google Scholar]
- 9.Purdue MP, From L, Armstrong BK, Kricker A, Gallagher RP, McLaughlin JR, Klar NS, Marrett LD. Etiologic and Other Factors Predicting Nevus-Associated Cutaneous Malignant Melanoma. Cancer Epidemiol Biomarkers Prev. 2005;14(8):2015–2022. doi: 10.1158/1055-9965.EPI-05-0097. [DOI] [PubMed] [Google Scholar]
- 10.Lachiewicz AM, Berwick M, Wiggins CL, Thomas NE. Epidemiologic Support for Melanoma Heterogeneity Using the Surveillance, Epidemiology, and End Results Program. Journal of Investigative Dermatology. 2008;128(1):243–245. doi: 10.1038/sj.jid.5701028. [DOI] [PubMed] [Google Scholar]
- 11.Tsao H, Goel V, Wu H, Yang G, Haluska FG. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. Journal of Investigative Dermatology. 2004;122(2):337–341. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, Cheng E, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nature Genetics. 2012;44(9):1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiteman DC, Watt P, Purdie DM, Hughes MC, Hayward NK, Green AC. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95(11):806–12. doi: 10.1093/jnci/95.11.806. [DOI] [PubMed] [Google Scholar]
- 14.Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33(1):19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 15.Lin J, Takata M, Murata H, Goto Y, Kido K, Ferrone S, Saida T. Polyclonality of BRAF mutations in acquired melanocytic nevi. J Natl Cancer Inst. 2009;101(20):1423–1427. doi: 10.1093/jnci/djp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yancovitz M, Litterman A, Yoon J, Ng E, Shapiro RL, Berman RS, Pavlick AC, et al. Intra- and Inter-Tumor Heterogeneity of BRAFV600EMutations in Primary and Metastatic Melanoma. PLoS ONE. 2012;7(1):e29336. doi: 10.1371/journal.pone.0029336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeh I, von Deimling A, Bastian BC. Clonal BRAFV600E mutations in melanocytic nevi imply an initiating role of BRAFV600E in melanocytic neoplasia. J Natl Cancer Inst. doi: 10.1093/jnci/djt119. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carr J, Mackie RM. Point mutations in the N-ras oncogene in malignant melanoma and congenital naevi. Br J Dermatol. 1994;131(1):72–7. doi: 10.1111/j.1365-2133.1994.tb08460.x. [DOI] [PubMed] [Google Scholar]
- 19.Bastian BC, LeBoit PE, Pinkel D. Mutations and Copy Number Increase of HRAS in Spitz Nevi with Distinctive Histopathological Features. The American Journal of Pathology. 2000;157(3):967–972. doi: 10.1016/S0002-9440(10)64609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O’Brien JM, Simpson EM, Barsh GS, Bastian BC. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457(7229):599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, Obenauf AC, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363(23):2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michaloglou C, Vredeveld LCW, Soengas MS, Denoyelle C, Kuilman T, van der Horst CMAM, Majoor DM, Shay JW, Mooi WJ, Peeper DS. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436(7051):720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 23.Tran SL, Haferkamp S, Scurr LL, Gowrishankar K, Becker TM, Desilva C, Thompson JF, Scolyer RA, Kefford RF, Rizos H. Absence of Distinguishing Senescence Traits in Human Melanocytic Nevi. Journal of Investigative Dermatology. 2012;132(9):2226–2234. doi: 10.1038/jid.2012.126. [DOI] [PubMed] [Google Scholar]
- 24.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes & Development. 2010;24(22):2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA, Grosveld G, Sherr CJ. Tumor Suppression at the Mouse INK4a Locus Mediated by the Alternative Reading Frame Product p19 ARF. Cell. 1997;91(5):649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 26.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88(5):593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 27.Kamb A. Role of a cell cycle regulator in hereditary and sporadic cancer. COLD SPRING HARBOR SYMPOSIA ON QUANTITATIVE BIOLOGY. 1994;59:39–47. doi: 10.1101/sqb.1994.059.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Hussussian CJ, Struewing JP, Goldstein AM, Higgins PA, Ally DS, Sheahan MD, Clark WH, Tucker MA, Dracopoli NC. Germline p16 mutations in familial melanoma. Nat Genet. 1994;8(1):15–21. doi: 10.1038/ng0994-15. [DOI] [PubMed] [Google Scholar]
- 29.FitzGerald MG, Harkin DP, Silva-Arrieta S, MacDonald DJ, Lucchina LC, Unsal H, O’Neill E, et al. Prevalence of germ-line mutations in p16, p19ARF, and CDK4 in familial melanoma: analysis of a clinic-based population. PNAS. 1996;93(16):8541–8545. doi: 10.1073/pnas.93.16.8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein AM, Chan M, Harland M, Gillanders EM, Hayward NK, Avril M-F, Azizi E, et al. High-risk Melanoma Susceptibility Genes and Pancreatic Cancer, Neural System Tumors, and Uveal Melanoma across GenoMEL. Cancer Res. 2006;66(20):9818–9828. doi: 10.1158/0008-5472.CAN-06-0494. [DOI] [PubMed] [Google Scholar]
- 31.Zuo L, Weger J, Yang Q, Goldstein AM, Tucker MA, Walker GJ, Hayward N, Dracopoli NC. Germline mutations in the p16INK4a binding domain of CDK4 in familial melanoma. Nat Genet. 1996;12(1):97–9. doi: 10.1038/ng0196-97. [DOI] [PubMed] [Google Scholar]
- 32.Randerson-Moor JA, Harland M, Williams S, Cuthbert-Heavens D, Sheridan E, Aveyard J, Sibley K, et al. A germline deletion of p14(ARF) but not CDKN2A in a melanoma-neural system tumour syndrome family. Hum Mol Genet. 2001;10(1):55–62. doi: 10.1093/hmg/10.1.55. [DOI] [PubMed] [Google Scholar]
- 33.Florell SR, Meyer LJ, Boucher KM, Porter-Gill PA, Hart M, Erickson J, Cannon-Albright LA, et al. Longitudinal Assessment of the Nevus Phenotype in a Melanoma Kindred. Journal of Investigative Dermatology. 2004;123(3):576–582. doi: 10.1111/j.0022-202X.2004.23312.x. [DOI] [PubMed] [Google Scholar]
- 34.Bishop JAN, Wachsmuth RC, Harland M, Bataille V, Pinney E, Mack P, Baglietto L, Cuzick J, Bishop DT. Genotype/Phenotype and Penetrance Studies in Melanoma Families with Germline CDKN2A Mutations. Journal of Investigative Dermatology. 2000;114(1):28–33. doi: 10.1046/j.1523-1747.2000.00823.x. [DOI] [PubMed] [Google Scholar]
- 35.Gorgoulis VG, Vassiliou L-VF, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434(7035):907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 36.Bastian BC, Olshen AB, LeBoit PE, Pinkel D. Classifying melanocytic tumors based on DNA copy number changes. Am J Pathol. 2003;163(5):1765–70. doi: 10.1016/S0002-9440(10)63536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aird KM, Zhang G, Li H, Tu Z, Bitler BG, Garipov A, Wu H, et al. Suppression of Nucleotide Metabolism Underlies the Establishment and Maintenance of Oncogene-Induced Senescence. Cell Reports. doi: 10.1016/j.celrep.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuilman T, Michaloglou C, Vredeveld LCW, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133(6):1019–31. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 39.Maldonado JL, Timmerman L, Fridlyand J, Bastian BC. Mechanisms of Cell-Cycle Arrest in Spitz Nevi with Constitutive Activation of the MAP-Kinase Pathway. The American Journal of Pathology. 2004;164(5):1783–1787. doi: 10.1016/S0002-9440(10)63736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramirez RD, D’Atri S, Pagani E, Faraggiana T, Lacal PM, Taylor RS, Shay JW. Progressive increase in telomerase activity from benign melanocytic conditions to malignant melanoma. Neoplasia. 1999;1(1):42–9. doi: 10.1038/sj.neo.7900004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudolph P, Schubert C, Tamm S, Heidorn K, Hauschild A, Michalska I, Majewski S, Krupp G, Jablonska S, Parwaresch R. Telomerase activity in melanocytic lesions : A potential marker of tumor biology [In Process Citation] Am J Pathol. 2000;156(4):1425–32. doi: 10.1016/S0002-9440(10)65011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bastian BC, Kashani-Sabet M, Hamm H, Godfrey T, Moore DH, Brocker EB, LeBoit PE, Pinkel D. Gene amplifications characterize acral melanoma and permit the detection of occult tumor cells in the surrounding skin. Cancer Research. 2000;60(7):1968–1973. [PubMed] [Google Scholar]
- 43.North JP, Kageshita T, Pinkel D, Leboit PE, Bastian BC. Distribution and Significance of Occult Intraepidermal Tumor Cells Surrounding Primary Melanoma. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, et al. TERT Promoter Mutations in Familial and Sporadic Melanoma. Science. 2013 doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 45.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly Recurrent TERT Promoter Mutations in Human Melanoma. Science. 2013 doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bastian BC. Hypothesis: a role for telomere crisis in spontaneous regression of melanoma. Arch Dermatol. 2003;139(5):667–8. doi: 10.1001/archderm.139.5.667. [DOI] [PubMed] [Google Scholar]
- 47.Sekulic A, Colgan Mb, Davis Mdp, DiCaudo Dj, Pittelkow Mr. Activating BRAF mutations in eruptive melanocytic naevi. British Journal of Dermatology. 2010;163(5):1095–1098. doi: 10.1111/j.1365-2133.2010.09989.x. [DOI] [PubMed] [Google Scholar]
- 48.Kang T-W, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479(7374):547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 49.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat J-P, Nickerson E, et al. A Landscape of Driver Mutations in Melanoma. Cell. 2012;150(2):251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ceol CJ, Houvras Y, Jane-Valbuena J, Bilodeau S, Orlando DA, Battisti V, Fritsch L, et al. The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset. Nature. 2011;471(7339):513–517. doi: 10.1038/nature09806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MacGregor S, Montgomery GW, Liu JZ, Zhao ZZ, Henders AK, Stark M, Schmid H, et al. Genome-wide association study identifies a new melanoma susceptibility locus at 1q21.3. Nature Genetics. 2011;43(11):1114–1118. doi: 10.1038/ng.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McHugh JB, Fullen DR, Ma L, Kleer CG, Su LD. Expression of polycomb group protein EZH2 in nevi and melanoma. Journal of Cutaneous Pathology. 2007;34(8):597–600. doi: 10.1111/j.1600-0560.2006.00678.x. [DOI] [PubMed] [Google Scholar]
- 53.Kapoor A, Goldberg MS, Cumberland LK, Ratnakumar K, Segura MF, Emanuel PO, Menendez S, et al. The histone variant macroH2A suppresses melanoma progression through regulation of CDK8. Nature. 2010;468(7327):1105–1109. doi: 10.1038/nature09590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoon DSB, Spugnardi M, Kuo C, Huang SK, Morton DL, Taback B. Profiling epigenetic inactivation of tumor suppressor genes in tumors and plasma from cutaneous melanoma patients. Oncogene. 2004;23(22):4014–4022. doi: 10.1038/sj.onc.1207505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu S, Ren S, Howell P, Fodstad O, Riker AI. Identification of novel epigenetically modified genes in human melanoma via promoter methylation gene profiling. Pigment Cell & Melanoma Research. 2008;21(5):545–558. doi: 10.1111/j.1755-148X.2008.00484.x. [DOI] [PubMed] [Google Scholar]
- 56.Shen L, Kondo Y, Guo Y, Zhang J, Zhang L, Ahmed S, Shu J, Chen X, Waterland RA, Issa J-PJ. Genome-Wide Profiling of DNA Methylation Reveals a Class of Normally Methylated CpG Island Promoters. PLoS Genet. 2007;3(10):e181. doi: 10.1371/journal.pgen.0030181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lian CG, Xu Y, Ceol C, Wu F, Larson A, Dresser K, Xu W, et al. Loss of 5-Hydroxymethylcytosine Is an Epigenetic Hallmark of Melanoma. Cell. 2012;150(6):1135–1146. doi: 10.1016/j.cell.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ko M, Huang Y, Jankowska AM, Pape UJ, Tahiliani M, Bandukwala HS, An J, et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature. 2010;468(7325):839–843. doi: 10.1038/nature09586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, Li Y, et al. Leukemic IDH1 and IDH2 Mutations Result in a Hypermethylation Phenotype, Disrupt TET2 Function, and Impair Hematopoietic Differentiation. Cancer Cell. 2010;18(6):553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harbour JW, Onken MD, Roberson EDO, Duan S, Cao L, Worley LA, Council ML, Matatall KA, Helms C, Bowcock AM. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330(6009):1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wiesner T, Obenauf AC, Murali R, Fried I, Griewank KG, Ulz P, Windpassinger C, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nature Genetics. 2011 doi: 10.1038/ng.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wiesner T, Murali R, Fried I, Cerroni L, Busam K, Kutzner H, Bastian BC. A Distinct Subset of Atypical Spitz Tumors is Characterized by BRAF Mutation and Loss of BAP1 Expression. Am J Surg Pathol. 2012 doi: 10.1097/PAS.0b013e3182498be5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reimer RRCW. Precursor lesions in familial melanoma: A new genetic preneoplastic syndrome. JAMA. 1978;239(8):744–746. [PubMed] [Google Scholar]
- 64.Piepkorn M, Meyer LJ, Goldgar D, Seuchter SA, Cannon-Albright LA, Skolnick MH, Zone JJ. The dysplastic melanocytic nevus: a prevalent lesion that correlates poorly with clinical phenotype. J Am Acad Dermatol. 1989;20(3):407–415. doi: 10.1016/s0190-9622(89)70050-5. [DOI] [PubMed] [Google Scholar]
- 65.Ackerman AB. What naevus is dysplastic, a syndrome and the commonest precursor of malignant melanoma? A riddle and an answer. Histopathology. 1988;13(3):241–256. doi: 10.1111/j.1365-2559.1988.tb02036.x. [DOI] [PubMed] [Google Scholar]
- 66.Duncan LM, Berwick M, Bruijn JA, Byers HR, Mihm MC, Barnhill RL. Histopathologic recognition and grading of dysplastic melanocytic nevi: an interobserver agreement study. J Invest Dermatol. 1993;100(3):318S–321S. doi: 10.1111/1523-1747.ep12470215. [DOI] [PubMed] [Google Scholar]
- 67.Ackerman AB, Milde P. Naming acquired melanocytic nevi. Common and dysplastic, normal and atypical, or Unna, Miescher, Spitz, and Clark? Am J Dermatopathol. 1992;14(5):447–453. doi: 10.1097/00000372-199210000-00013. [DOI] [PubMed] [Google Scholar]
- 68.Goldgar DE, Cannon-Albright LA, Meyer LJ, Pipekorn MW, Zone JJ, Skolnick MH. Inheritance of Nevus Number and Size in Melanoma and Dysplastic Nevus Syndrome Kindreds. JNCI J Natl Cancer Inst. 1991;83(23):1726–1733. doi: 10.1093/jnci/83.23.1726. [DOI] [PubMed] [Google Scholar]
- 69.Tucker MA, Halpern A, Holly EA, Hartge P, Elder DE, Sagebiel RW, Guerry Dth, Clark WH. Clinically recognized dysplastic nevi. A central risk factor for cutaneous melanoma [see comments] Jama. 1997;277(18):1439–44. [PubMed] [Google Scholar]
- 70.Hussein MRA-E, Wood GS. Molecular Aspects of Melanocytic Dysplastic Nevi. The Journal of Molecular Diagnostics. 2002;4(2):71–80. doi: 10.1016/S1525-1578(10)60684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Whiteman DC, Pavan WJ, Bastian BC. The melanomas: a synthesis of epidemiological, clinical, histopathological, genetic, and biological aspects, supporting distinct subtypes, causal pathways, and cells of origin. Pigment Cell & Melanoma Research. 2011;24(5):879–897. doi: 10.1111/j.1755-148X.2011.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Busam KJ, Murali R, Pulitzer M, McCarthy SW, Thompson JF, Shaw HM, Brady MS, et al. Atypical Spitzoid Melanocytic Tumors With Positive Sentinel Lymph Nodes in Children and Teenagers, and Comparison With Histologically Unambiguous and Lethal Melanomas. The American Journal of Surgical Pathology. 2009;33(9):1386–1395. doi: 10.1097/PAS.0b013e3181ac1927. [DOI] [PubMed] [Google Scholar]
- 73.Ludgate MW, Fullen DR, Lee J, Lowe L, Bradford C, Geiger J, Schwartz J, Johnson TM. The atypical Spitz tumor of uncertain biologic potential. Cancer. 2009;115(3):631–641. doi: 10.1002/cncr.24047. [DOI] [PubMed] [Google Scholar]
- 74.Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, Nieweg OE, et al. Sentinel-Node Biopsy or Nodal Observation in Melanoma. New England Journal of Medicine. 2006;355(13):1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 75.Lange JR, Palis BE, Chang DC, Soong S-J, Balch CM. Melanoma in Children and Teenagers: An Analysis of Patients From the National Cancer Data Base. JCO. 2007;25(11):1363–1368. doi: 10.1200/JCO.2006.08.8310. [DOI] [PubMed] [Google Scholar]
- 76.Crombie IK. Variation of melanoma incidence with latitude in North America and Europe. British Journal of Cancer. 1979;40:774–781. doi: 10.1038/bjc.1979.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elwood JM, Gallagher RP. Body site distribution of cutaneous malignant melanoma in relationship to patterns of sun exposure. Int J Cancer. 1998;78(3):276–80. doi: 10.1002/(SICI)1097-0215(19981029)78:3<276::AID-IJC2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 78.Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, Varela I, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2009;463(7278):191–196. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mocellin S, Verdi D, Nitti D. DNA repair gene polymorphisms and risk of cutaneous melanoma: a systematic review and meta-analysis. Carcinogenesis. 2009;30(10):1735–1743. doi: 10.1093/carcin/bgp207. [DOI] [PubMed] [Google Scholar]
- 80.Nikolaev SI, Rimoldi D, Iseli C, Valsesia A, Robyr D, Gehrig C, Harshman K, et al. Exome sequencing identifies recurrent somatic MAP2K1 and MAP2K2 mutations in melanoma. Nature Genetics. 2012;44(2):133–139. doi: 10.1038/ng.1026. [DOI] [PubMed] [Google Scholar]
- 81.Besaratinia A, Pfeifer GP. Sunlight ultraviolet irradiation and BRAF V600 mutagenesis in human melanoma. Human Mutation. 2008;29(8):983–991. doi: 10.1002/humu.20802. [DOI] [PubMed] [Google Scholar]
- 82.Noonan FP, Zaidi MR, Wolnicka-Glubisz A, Anver MR, Bahn J, Wielgus A, Cadet J, et al. Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment. Nature Communications. 2012;3:884. doi: 10.1038/ncomms1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Agrup G, Hansson C, Rorsman H, Rosengren E. The effect of cysteine on oxidation of tyrosine, dopa, and cysteinyldopas. Arch Dermatol Res. 1982;272(1–2):103–115. doi: 10.1007/BF00510400. [DOI] [PubMed] [Google Scholar]
- 84.Simon JD, Peles DN. The Red and the Black. Acc Chem Res. 2010;43(11):1452–1460. doi: 10.1021/ar100079y. [DOI] [PubMed] [Google Scholar]
- 85.Song X, Mosby N, Yang J, Xu A, Abdel-Malek Z, Kadekaro AL. alpha-MSH activates immediate defense responses to UV-induced oxidative stress in human melanocytes. Pigment Cell Melanoma Res. 2009;22(6):809–818. doi: 10.1111/j.1755-148X.2009.00615.x. [DOI] [PubMed] [Google Scholar]
- 86.Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11(3):328–330. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- 87.Brown KM, Macgregor S, Montgomery GW, Craig DW, Zhao ZZ, Iyadurai K, Henders AK, et al. Common sequence variants on 20q11.22 confer melanoma susceptibility. Nat Genet. 2008;40(7):838–40. doi: 10.1038/ng.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han J, Kraft P, Nan H, Guo Q, Chen C, Qureshi A, Hankinson SE, et al. A genome-wide association study identifies novel alleles associated with hair color and skin pigmentation. PLoS Genet. 2008;4(5):e1000074. doi: 10.1371/journal.pgen.1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bishop DT, Demenais F, Iles MM, Harland M, Taylor JC, Corda E, Randerson-Moor J, et al. Genome-wide association study identifies three loci associated with melanoma risk. Nature Genetics. 2009;41(8):920–925. doi: 10.1038/ng.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Falchi M, Bataille V, Hayward NK, Duffy DL, Bishop JAN, Pastinen T, Cervino A, et al. Genome-wide association study identifies variants at 9p21 and 22q13 associated with development of cutaneous nevi. Nat Genet. 2009;41(8):915–919. doi: 10.1038/ng.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duffy DL, Zhao ZZ, Sturm RA, Hayward NK, Martin NG, Montgomery GW. Multiple Pigmentation Gene Polymorphisms Account for a Substantial Proportion of Risk of Cutaneous Malignant Melanoma. Journal of Investigative Dermatology. 2010;130(2):520–528. doi: 10.1038/jid.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barrett JH, Iles MM, Harland M, Taylor JC, Aitken JF, Andresen PA, Akslen LA, et al. Genome-wide association study identifies three new melanoma susceptibility loci. Nature Genetics. 2011;43(11):1108–1113. doi: 10.1038/ng.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Landi MT, Bauer j, Pfeiffer RM, Elder DE, Hulley B, Minghetti P, Calista D, Pinkel D, Kanetsky PA, Bastian BC. MC1R Germline Variants Confer Risk for BRAF-Mutant Melanoma. Science. 2006;313(5786):512–2. doi: 10.1126/science.1127515. [DOI] [PubMed] [Google Scholar]
- 94.Fargnoli MC, Fargnoli MC, Pike K, Pfeiffer RM, Tsang S, Rozenblum E, Munroe DJ, et al. MC1R variants increase risk of melanomas harboring BRAF mutations. J Invest Dermatol. 2008;128(10):2485–2490. doi: 10.1038/jid.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thomas NE, Kanetsky PA, Edmiston SN, Alexander A, Begg CB, Groben PA, Hao H, et al. Relationship between Germline MC1R Variants and BRAF-Mutant Melanoma in a North Carolina Population-Based Study. Journal of Investigative Dermatology. 2010;130(5):1463–1465. doi: 10.1038/jid.2009.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hacker E, Hayward NK, Dumenil T, James MR, Whiteman DC. The Association between MC1R Genotype and BRAF Mutation Status in Cutaneous Melanoma: Findings from an Australian Population. Journal of Investigative Dermatology. 2010;130(1):241–248. doi: 10.1038/jid.2009.182. [DOI] [PubMed] [Google Scholar]
- 97.Menzies AM, Haydu LE, Visintin L, Carlino MS, Howle JR, Thompson JF, Kefford RF, Scolyer RA, Long GV. Distinguishing Clinicopathologic Features of Patients with V600E and V600K BRAF-Mutant Metastatic Melanoma. Clin Cancer Res. 2012;18(12):3242–3249. doi: 10.1158/1078-0432.CCR-12-0052. [DOI] [PubMed] [Google Scholar]
- 98.Hacker E, Nagore E, Cerroni L, Woods SL, Hayward NK, Chapman B, Montgomery GW, Soyer HP, Whiteman DC. NRAS and BRAF Mutations in Cutaneous Melanoma and the Association with MC1R Genotype: Findings from Spanish and Austrian Populations. Journal of Investigative Dermatology. 2012 doi: 10.1038/jid.2012.385. [DOI] [PubMed] [Google Scholar]
- 99.Olah J, Toth-Molnar E, Kemeny L, Csoma Z. Long-term hazards of neonatal blue light phototherapy. British Journal of Dermatology. 2013 doi: 10.1111/bjd.12335. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 100.Bauer J, Buttner P, Wiecker TS, Luther H, Garbe C. Effect of sunscreen and clothing on the number of melanocytic nevi in 1,812 German children attending day care. Am J Epidemiol. 2005;161(7):620–7. doi: 10.1093/aje/kwi086. [DOI] [PubMed] [Google Scholar]
- 101.Light TIA for R on CWG on artificial ultraviolet (UV) and Cancer S. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: A systematic review. International Journal of Cancer. 2007;120(5):1116–1122. doi: 10.1002/ijc.22453. [DOI] [PubMed] [Google Scholar]
- 102.Whiteman DC, Watt P, Purdie DM, Hughes MC, Hayward NK, Green AC. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95(11):806–12. doi: 10.1093/jnci/95.11.806. [DOI] [PubMed] [Google Scholar]
- 103.Glatz-Krieger K, Pache M, Tapia C, Fuchs A, Savic S, Glatz D, Mihatsch M, Meyer P. Anatomic site-specific patterns of gene copy number gains in skin, mucosal, and uveal melanomas detected by fluorescence in situ hybridization. Virchows Arch. 2006;449(3):328–333. doi: 10.1007/s00428-006-0167-8. [DOI] [PubMed] [Google Scholar]
- 104.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic Activation of KIT in Distinct Subtypes of Melanoma. J Clin Oncol. 2006;24(26):4340–6. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 105.Davison JM, Rosenbaum E, Barrett TL, Goldenberg D, Hoque MO, Sidransky D, Westra WH. Absence of V599E BRAF mutations in desmoplastic melanomas. Cancer. 2005;103(4):788–92. doi: 10.1002/cncr.20861. [DOI] [PubMed] [Google Scholar]
- 106.Saida T. Malignant Melanoma on the Sole: How to Detect the Early Lesions Efficiently. Pigment Cell Research. 2000;13:135–139. doi: 10.1034/j.1600-0749.13.s8.24.x. [DOI] [PubMed] [Google Scholar]
- 107.Arrington JHd, Reed RJ, Ichinose H, Krementz ET. Plantar lentiginous melanoma: a distinctive variant of human cutaneous malignant melanoma. Am J Surg Pathol. 1977;1(2):131–43. [PubMed] [Google Scholar]
- 108.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83(8):1664–1678. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 109.Kuchelmeister C, Schaumburg-Lever G, Garbe C. Acral cutaneous melanoma in caucasians: clinical features, histopathology and prognosis in 112 patients. Br J Dermatol. 2000;143(2):275–80. doi: 10.1046/j.1365-2133.2000.03651.x. [DOI] [PubMed] [Google Scholar]
- 110.McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW. Incidence of noncutaneous melanomas in the U.S. Cancer. 2005;103(5):1000–7. doi: 10.1002/cncr.20866. [DOI] [PubMed] [Google Scholar]
- 111.Beadling C, Jacobson-Dunlop E, Hodi FS, Le C, Warrick A, Patterson J, Town A, et al. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res. 2008;14(21):6821–8. doi: 10.1158/1078-0432.CCR-08-0575. [DOI] [PubMed] [Google Scholar]
- 112.Van Dijk M, Sprenger S, Rombout P, Marres H, Kaanders J, Jeuken J, Ruiter D. Distinct chromosomal aberrations in sinonasal mucosal melanoma as detected by comparative genomic hybridization. Genes Chromosomes Cancer. 2003;36(2):151–8. doi: 10.1002/gcc.10156. [DOI] [PubMed] [Google Scholar]
- 113.Park E, Yang S, Emley A, DeCarlo K, Richards J, Mahalingam M. Lack of Correlation Between Immunohistochemical Expression of CKIT and KIT Mutations in Atypical Acral Nevi. The American Journal of Dermatopathology. 2012;34(1):41–46. doi: 10.1097/DAD.0b013e31821ec0ef. [DOI] [PubMed] [Google Scholar]
- 114.Alexeev V, Yoon K. Distinctive role of the cKit receptor tyrosine kinase signaling in mammalian melanocytes. J Invest Dermatol. 2006;126(5):1102–10. doi: 10.1038/sj.jid.5700125. [DOI] [PubMed] [Google Scholar]
- 115.Monsel G, Ortonne N, Bagot M, Bensussan A, Dumaz N. c-Kit mutants require hypoxia-inducible factor 1α to transform melanocytes. Oncogene. 2009;29(2):227–236. doi: 10.1038/onc.2009.320. [DOI] [PubMed] [Google Scholar]
- 116.Huang S, Jean D, Luca M, Tainsky MA, Bar-Eli M. Loss of AP-2 results in downregulation of c-KIT and enhancement of melanoma tumorigenicity and metastasis. Embo Journal. 1998;17(15):4358–69. doi: 10.1093/emboj/17.15.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carvajal RD, Antonescu CR, Wolchok JD, Chapman PB, Roman R-A, Teitcher J, Panageas KS, et al. KIT as a therapeutic target in metastatic melanoma. JAMA. 2011;305(22):2327–2334. doi: 10.1001/jama.2011.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Watt AJ, Kotsis SV, Chung KC. Risk of Melanoma Arising in Large Congenital Melanocytic Nevi: A Systematic Review. Plastic and Reconstructive Surgery. 2004;113(7):1968–1974. doi: 10.1097/01.prs.0000122209.10277.2a. [DOI] [PubMed] [Google Scholar]
- 119.Mancianti ML, Clark WH, Hayes FA, Herlyn M. Malignant melanoma simulants arising in congenital melanocytic nevi do not show experimental evidence for a malignant phenotype. Am J Pathol. 1990;136(4):817–29. [PMC free article] [PubMed] [Google Scholar]
- 120.Bastian BC, Xiong J, Frieden IJ, Williams ML, Chou P, Busam K, Pinkel D, LeBoit PE. Genetic changes in neoplasms arising in congenital melanocytic nevi: Differences between nodular proliferations and melanomas. Am J Pathol. 2002;161(4):1163–9. doi: 10.1016/S0002-9440(10)64393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bauer J, Curtin JA, Pinkel D, Bastian BC. Congenital melanocytic nevi frequently harbor NRAS mutations but no BRAF mutations. J Invest Dermatol. 2007;127(1):179–82. doi: 10.1038/sj.jid.5700490. [DOI] [PubMed] [Google Scholar]
- 122.Kinsler VA, Thomas AC, Ishida M, Bulstrode NW, Loughlin S, Hing S, Chalker J, et al. Multiple Congenital Melanocytic Naevi and Neurocutaneous Melanosis are Caused by Post-Zygotic Mutations in Codon 61 of NRAS. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Slutsky JB, Barr JM, Femia AN, Marghoob AA. Large Congenital Melanocytic Nevi: Associated Risks and Management Considerations. Seminars in Cutaneous Medicine and Surgery. 2010;29(2):79–84. doi: 10.1016/j.sder.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 124.Van Raamsdonk CD, Fitch KR, Fuchs H, de Angelis MH, Barsh GS. Effects of G-protein mutations on skin color. Nat Genet. 2004;36(9):961–8. doi: 10.1038/ng1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dessars B, De Raeve LE, Housni HE, Debouck CJ, Sidon PJ, Morandini R, Roseeuw D, Ghanem GE, Vassart G, Heimann P. Chromosomal Translocations as a Mechanism of BRAF Activation in Two Cases of Large Congenital Melanocytic Nevi. J Invest Dermatol. 2007;127(6):1468–1470. doi: 10.1038/sj.jid.5700725. [DOI] [PubMed] [Google Scholar]
- 126.Murali R, McCarthy SW, Scolyer RA. Blue nevi and related lesions: a review highlighting atypical and newly described variants, distinguishing features and diagnostic pitfalls. Adv Anat Pathol. 2009;16(6):365–382. doi: 10.1097/PAP.0b013e3181bb6b53. [DOI] [PubMed] [Google Scholar]
- 127.Sherman L, Stocker KM, Morrison R, Ciment G. Basic fibroblast growth factor (bFGF) acts intracellularly to cause the transdifferentiation of avian neural crest-derived Schwann cell precursors into melanocytes. Development. 1993;118(4):1313–1326. doi: 10.1242/dev.118.4.1313. [DOI] [PubMed] [Google Scholar]
- 128.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, CYS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26(1):89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 129.Maize JC, McCalmont TH, Carlson JA, Busam KJ, Kutzner H, Bastian BC. Genomic analysis of blue nevi and related dermal melanocytic proliferations. Am J Surg Pathol. 2005;29(9):1214–20. doi: 10.1097/01.pas.0000165527.01816.d1. [DOI] [PubMed] [Google Scholar]
- 130.Martin RCW, Murali R, Scolyer RA, Fitzgerald P, Colman MH, Thompson JF. So-called “malignant blue nevus. Cancer. 2009;115(13):2949–2955. doi: 10.1002/cncr.24319. [DOI] [PubMed] [Google Scholar]
- 131.Murali R, Wiesner T, Rosenblum M, Bastian B. GNAQ and GNA11 mutations in melanocytomas of the central nervous system. Acta Neuropathologica. 2012;123(3):457–459. doi: 10.1007/s00401-012-0948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brat DJ. 16 - Melanocytic Neoplasms of the Central Nervous System. In: Perry Arie, MD, Daniel M, Brat J., editors. Practical Surgical Neuropathology. New York: Churchill Livingstone; 2010. pp. 353–359. [Google Scholar]
- 133.Singh AD, Turell ME, Topham AK. Uveal Melanoma: Trends in Incidence, Treatment, and Survival. Ophthalmology. 2011;118(9):1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 134.Singh AD, Kalyani P, Topham A. Estimating the Risk of Malignant Transformation of a Choroidal Nevus. Ophthalmology. 2005;112(10):1784–1789. doi: 10.1016/j.ophtha.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 135.Prescher G, Bornfeld N, Hirche H, Horsthemke B, Jöckel KH, Becher R. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347(9010):1222–5. doi: 10.1016/s0140-6736(96)90736-9. [DOI] [PubMed] [Google Scholar]
- 136.Dey A, Seshasayee D, Noubade R, French DM, Liu J, Chaurushiya MS, Kirkpatrick DS, et al. Loss of the Tumor Suppressor BAP1 Causes Myeloid Transformation. Science. 2012 doi: 10.1126/science.1221711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Onken MD, Ehlers JP, Worley LA, Makita J, Yokota Y, Harbour JW. Functional gene expression analysis uncovers phenotypic switch in aggressive uveal melanomas. Cancer Res. 2006;66(9):4602–9. doi: 10.1158/0008-5472.CAN-05-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Harbour JW, Roberson EDO, Anbunathan H, Onken MD, Worley LA, Bowcock AM. Recurrent mutations at codon 625 of the splicing factor SF3B1 in uveal melanoma. Nature Genetics. 2013 doi: 10.1038/ng.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.An M, Henion PD. The zebrafish sf3b1 b460 mutant reveals differential requirements for the sf3b1 pre-mRNA processing gene during neural crest development. The International Journal of Developmental Biology. 2012;56(4):223–237. doi: 10.1387/ijdb.113383ma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sommer L. Generation of melanocytes from neural crest cells. Pigment Cell & Melanoma Research. 2011;24(3):411–421. doi: 10.1111/j.1755-148X.2011.00834.x. [DOI] [PubMed] [Google Scholar]
- 141.Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson IJ, Barrandon Y, Miyachi Y, Nishikawa S. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416(6883):854–60. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 142.Granter SR, McKee PH, Calonje E, Mihm MC, Jr, Busam K. Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: a clinicopathologic study of 10 cases on the spectrum of so-called “malignant blue nevus”. Am J Surg Pathol. 2001;25(3):316–323. doi: 10.1097/00000478-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 143.Lens Mb, Newton-Bishop Ja, Boon Ap. Desmoplastic malignant melanoma: a systematic review. British Journal of Dermatology. 2005;152(4):673–678. doi: 10.1111/j.1365-2133.2005.06462.x. [DOI] [PubMed] [Google Scholar]
- 144.Busam KJ. Desmoplastic Melanoma. Clinics in Laboratory Medicine. 2011;31(2):321–330. doi: 10.1016/j.cll.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 145.Meier F, Will S, Ellwanger U, Schlagenhauff B, Schittek B, Rassner G, Garbe C. Metastatic pathways and time courses in the orderly progression of cutaneous melanoma. British Journal of Dermatology. 2002;147(1):62–70. doi: 10.1046/j.1365-2133.2002.04867.x. [DOI] [PubMed] [Google Scholar]
- 146.Bradford PTGA. Acral lentiginous melanoma: Incidence and survival patterns in the united states, 1986–2005. Arch Dermatol. 2009;145(4):427–434. doi: 10.1001/archdermatol.2008.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hu D-N, Yu G-P, McCormick SA, Schneider S, Finger PT. Population-Based Incidence of Uveal Melanoma in Various Races and Ethnic Groups. American Journal of Ophthalmology. 2005;140(4):612e1–612.e8. doi: 10.1016/j.ajo.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 148.Moore-Olufemi S, Herzog C, Warneke C, Gershenwald JE, Mansfield P, Ross M, Prieto V, Lally KP, Hayes-Jordan A. Outcomes in Pediatric Melanoma. Annals of Surgery. 2011;253(6):1211–1215. doi: 10.1097/SLA.0b013e318217e852. [DOI] [PubMed] [Google Scholar]