Abstract
OBJECTIVE
To determine if early pregnancy serum biomarkers in high-risk women who develop preeclampsia vary according to risk factor.
STUDY DESIGN
We performed a secondary analysis of the Maternal-Fetal Medicine Units Network randomized controlled trial of low-dose aspirin for the prevention of preeclampsia in high-risk women. Serum biomarker levels at enrollment (before initiation of aspirin or placebo) were compared between women who did and did not develop preeclampsia, both for the group as a whole and within each of 4 high-risk groups (insulin-dependent diabetes, hypertension, multiple gestation, and previous preeclampsia) using a regression model adjusting for gestational age at collection and prepregnancy body mass index.
RESULTS
1258 women were included (233 with insulin-dependent diabetes, 387 with chronic hypertension, 315 with a multiple gestation, 323 with previous preeclampsia). Multiple early pregnancy serum biomarkers differed between women who did and did not develop preeclampsia. Each high-risk group had a unique and largely nonoverlapping pattern of biomarker abnormality. Differences between those who did and did not develop preeclampsia were noted in vascular cell adhesion molecule in the diabetes group; human chorionic gonadotropin, soluble tumor necrosis factor receptor-2, tumor necrosis factor-alpha, selectin and angiogenin in the chronic hypertension group; interleukin-6, placental growth factor, soluble fms-like tyrosine kinase plus endoglin to placental growth factor ratio in the multiple gestation group; and angiogenin in the previous preeclampsia group.
CONCLUSION
Patterns of serum biomarkers vary by high-risk group. These data support the hypothesis that multiple pathogenic pathways lead to the disease recognized clinically as preeclampsia.
Keywords: biomarkers, diabetes, hypertension, multiple gestation, preeclampsia
Hypertensive disorders of pregnancy, including preeclampsia and chronic hypertension, continue to be a significant source of maternal and fetal morbidity and mortality.1 Although the ability to identify biomarkers or clinical attributes to predict the development of preeclampsia has had some success, translating these findings into effective preventative or treatment modalities to reduce the burden of disease has been decidedly mixed.2
The Maternal-Fetal Medicine Units (MFMU) Network randomized controlled trial to assess the ability of low-dose aspirin (LDA) to prevent preeclampsia in women at high-risk for developing preeclampsia (MFMU High Risk Aspirin trial) tested the explicit assumption that a prostacyclin/thromboxane (TXA) imbalance is pathogenic in preeclampsia. This study enrolled specifically women with risk factors for preeclampsia including: insulin-dependent diabetes, chronic hypertension, multiple gestation or previous preeclampsia.3 This study demonstrated that LDA was ineffective in the prevention of preeclampsia in the study population as a whole as well as in each high-risk group. In an ancillary study, neither TXA levels at enrollment nor reductions in TXA levels in women receiving LDA were predictive of preeclampsia4 suggesting that preeclampsia occurs in a TXA-independent fashion at least in some women.
It is increasingly becoming recognized that preeclampsia may be a clinical syndrome resulting from many possible antecedents.5,6 We therefore hypothesized that the ability of biomarkers to predict the occurrence of preeclampsia would vary among high-risk subgroups (insulin-dependent diabetes, hypertension, multiple gestation. or previous preeclampsia). As an initial test of this hypothesis, we undertook a secondary analysis of the MFMU High Risk Aspirin dataset to determine whether the levels of multiple serum biomarkers before the initiation of LDA or development of preeclampsia differed in high-risk women who subsequently did vs did not develop preeclampsia. Further, we asked whether such differences varied by high-risk subgroup.
Materials and Methods
Our study is a secondary analysis of the MFMU High Risk Aspirin trial of LDA to prevent preeclampsia.3 Patients were enrolled between 1991 and 1995 at 13 centers, with collection of serum samples starting in 1992. Included patients were at least 12 weeks’ gestational age with at least one risk factor for preeclampsia; namely, insulin-dependent diabetes, chronic hypertension, multiple gestation, or preeclampsia in a previous pregnancy. Full diagnostic criteria for the high-risk subsets and the clinical criteria to define hypertension, proteinuria, and the diagnosis of preeclampsia have been previously published.3 The MFMU study was approved by institutional review boards at each of the study sites with each subject providing written informed consent. This secondary analysis was considered exempt by the Colorado Multiple Institutional Review Board.
The original study investigators selected a panel of serum biomarkers that have been associated with preeclampsia. These biomarkers were measured shortly after study enrollment at 13–26 weeks (mean, 19.6 weeks) and serially during the pregnancy in 2000 of the 2503 patients who agreed to additional blood draws using methods that have been previously published.4,7,8 Specimens were collected at each of the sites, divided into aliquots and frozen to −80 °C for transport to a central laboratory for analysis. For our data analysis, we focused only on the initial serum biomarker levels, before initiation of LDA or the development of preeclampsia, as our primary interest is in early preeclampsia pathogenesis and not abnormalities that may occur with disease development or with LDA treatment. To this end, we compared levels of biomarkers between women who did and did not develop preeclampsia for the overall group and within each high-risk subset. We did not compare biomarker levels between women who did and did not receive LDA within each high-risk subgroup as there was no difference in the outcome of preeclampsia between groups with or without this intervention in the primary MFMU trial. Specifically, the following biomarkers were analyzed: angiogenin, cotinine, endoglin, estriol, estriol/progesterone ratio, human chorionic gonadotropin (hCG), interleukin-2 (IL-2), interleukin-6 (IL-6), placental growth factor (PlGF), progesterone, selectin, soluble fms-like tyrosine kinase (sFlt-1), soluble tumor necrosis factor receptor-1 (sTNFr-1), soluble tumor necrosis factor receptor 2 (sTNFr-2), tumor necrosis factor-alpha, thrombin/antithrombin III complex, TXA, vascular cell adhesion molecule, and the ratio of sFlt-1+endoglin/PlGF. Women were included if they had at least one of these biomarkers available, as the number of women with available results varied and is indicated in the footnotes for each table.
The original trial contains details of the patients included and the study endpoint definitions.3 Briefly, in the original trial, and for our analysis, preeclampsia was defined as hypertension with a systolic and/or diastolic blood pressure greater than 140/90 in association with proteinuria (≥300 mg in 24 hours, or 2 urine dipstick results of 2+ or greater at least 4 hours apart). In women with chronic hypertension, the development of preeclampsia was established with new-onset proteinuria or thrombocytopenia (platelet count <100,000). In women who had documented proteinuria at the time of enrollment, proteinuria needed to worsen to at least 5 times the baseline value to be considered worsening and indicative of preeclampsia. Charts were reviewed prospectively by at least three physicians to ensure that women met these strict diagnostic criteria for preeclampsia.
Comparison of baseline demographics between groups were made using analysis of variance for continuous variables and χ2 for dichotomous variables. Comparisons of levels of serum biomarkers were made between groups using a multiple regression model that included gestational age at the time of study enrollment and maternal body mass index (BMI) as covariates. For continuous measures better summarized by a measure less sensitive to a skewed distribution (such as BMI), analysis was performed on the log-scale, back transformed and presented as geometric means with 95% CI. A P value < .05 was considered statistically significant. All analyses were performed in SAS (SAS Institute, Cary, NC).
Results
A total of 1258 women were included in the analysis: 233 women with insulin-dependent diabetes, 387 with chronic hypertension, 315 with a multiple gestation, and 323 with previous preeclampsia. Demographic characteristics of the study population are detailed in Table 1. Women with diabetes were more often white and had the lowest parity. The chronic hypertension group tended to be older, have a higher BMI and be African American. Women with multiple gestations were enrolled in the study at a slightly later gestational age and delivered at an earlier gestational age. Higher parity and African American race were more common in the group with previous preeclampsia.
TABLE 1.
Demographic characteristics of study population
| Characteristic | Diabetes n = 233 |
Chronic HTN n = 387 |
Multiple gestation n = 315 |
Previous preeclampsia n = 323 |
P valuea |
|---|---|---|---|---|---|
| Maternal age at randomization, mean ± SD |
26.0 (6.04) | 29.5 (6.48) | 25.0 (5.88) | 24.4 (5.40) | <.001 |
| Race, n (%) | |||||
| White | 139 (59.66) | 96 (24.81) | 109 (34.60) | 71 (21.98) | <.001 |
| Hispanic | 15 (6.44) | 36 (9.30) | 37 (11.75) | 8 (2.48) | |
| African American | 77 (33.05) | 254 (65.63) | 168 (53.33) | 244 (75.54) | |
| Other | 2 (0.86) | 1 (0.26) | 1 (0.32) | 0 (0.00) | |
| Parity (deliveries >20 wks), mean ± SD |
0.82 (1.20) | 1.62 (1.60) | 1.11 (1.21) | 1.77 (1.14) | <.001 |
| Body mass index (kg/m2), geometric mean (95% CI) |
27.1 (26.23–27.96) | 31.9 (31.08–32.78) | 25.7 (24.99–26.36) | 27.4 (26.57–28.16) | <.001 |
| Gestational age at enrollment (wks), mean ± SD |
18.0 (3.69) | 19.8 (3.79) | 21.1 (3.60) | 20.0 (3.96) | <.001 |
| Gestational age at delivery (wks), mean ± SD |
36.0 (4.10) | 36.9 (3.97) | 34.4 (3.59) | 37.5 (3.63) | <.001 |
| Infant death, n (%) | 1 (0.43) | 3 (0.78) | 9 (2.86) | 3 (0.93) | .06 |
| Maternal death, n (%) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.17) | .69 |
CI, confidence interval; HTN, hypertension.
Comparisons between groups were made using analysis of variance for continuous variables, χ2 tests for categorical measures, and Fisher exact test for infant and maternal death.
Metz. Preeclampsia biomarkers in high-risk groups. Am J Obstet Gynecol 2014.
Table 2 shows the comparison of biomarkers for all patients with and without the diagnosis of preeclampsia. The levels of cotinine, endoglin, estriol, estriol/progesterone, IL-2, IL-6, PlGF, selectin, sFlt-1, sTNF-1, thrombin/antithrombin III complex, TXA, and vascular cell adhesion molecule did not differ between women who did vs did not develop preeclampsia. However, angiogenin, hCG, progesterone, sTNFr-2, and tumor necrosis factor-alpha levels were greater in the women who developed preeclampsia than those without preeclampsia with a trend existing for the (sFlt-1+endoglin)/PlGF ratio to be elevated as well (Table 2).
TABLE 2.
Comparisons of baseline values of serum biomarkers for the whole cohort based on preeclampsia status
| Serum biomarkera | Preeclampsia n = 263c |
No preeclampsia n = 995c |
P valueb |
|---|---|---|---|
| Angiogenin, pg/mL | 265 (253.3–276.9) | 240 (234.3–246.6) | <.001 |
| hCG, IU/mLd | 23,952 (20,993–27,328) | 18,141 (17,052–19,299) | <.001 |
| Progesterone, ng/mLd | 48.92 (45.41–52.71) | 44.65 (43.12–46.24) | .03 |
| sTNFr-2, pg/mL | 2707 (2595–2823) | 2572 (2517–2629) | .035 |
| TNF-α, pg/mL | 3.03 (2.87–3.19) | 2.74 (2.60–2.89) | .008 |
| (sFlt-1+endoglin)/PlGF | 42.95 (33.25–55.47) | 32.50 (28.49–37.08) | .06 |
BMI, body mass index; CI, confidence interval; hCG, human chorionic gonadotropin; PlGF, placental growth factor; sFlt-1, soluble fms-like tyrosine kinase; sTNFr-2, soluble tumor necrosis factor receptor 2; TNF-α, tumor necrosis factor-alpha.
All reported means are geometric means with 95% CI with the exception of angiogenin, which is reported as arithmetic means with 95% CI;
Comparisons between groups were made using a regression model adjusting for gestational age at the time the serum biomarker was drawn and prepregnancy maternal BMI;
Available sample size for each biomarker ranged as follows: angiogenin (214–790), hCG (108–491), progesterone (108–494), sTNFr-2 (214–790), TNF-α (261–59), (sFlt-1+endoglin)/PlGF (38–138) for preeclampsia and no preeclampsia groups, respectively;
Multiple gestation group excluded for this comparison.
Metz. Preeclampsia biomarkers in high-risk groups. Am J Obstet Gynecol 2014.
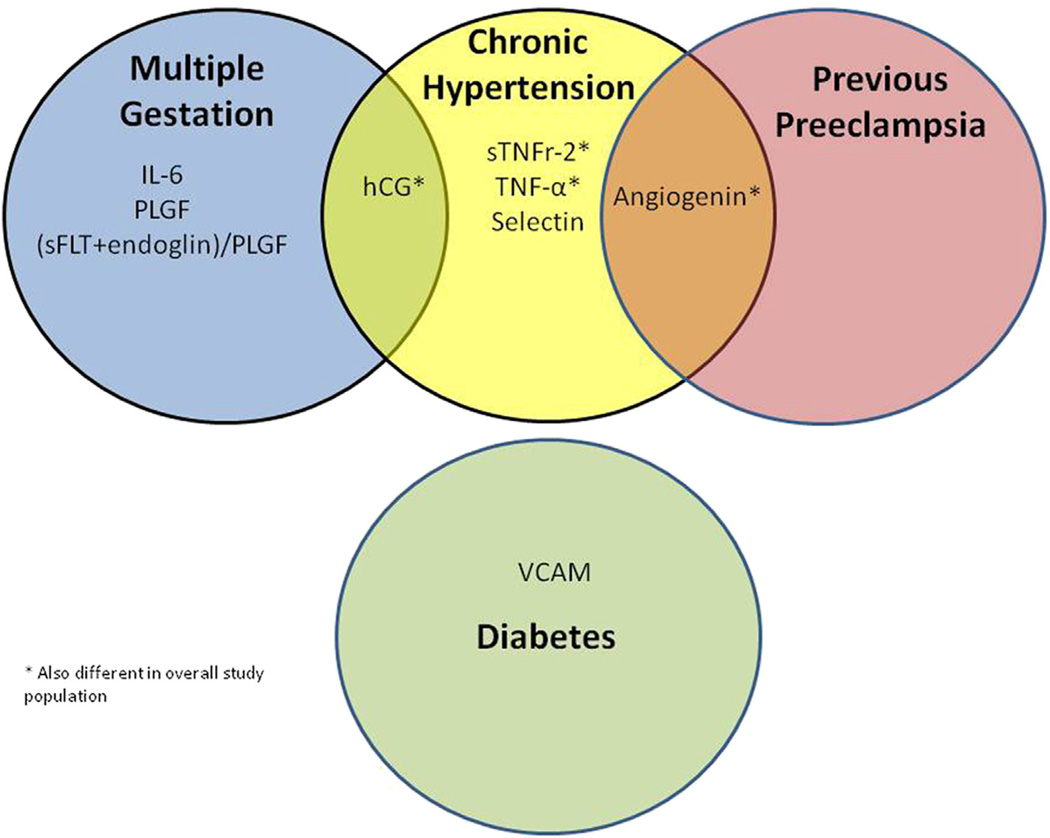
Comparisons of biomarkers between women who did vs those who did not develop preeclampsia within each high-risk subgroup showed considerable heterogeneity (Table 3). Angiogenin levels were higher in women with chronic hypertension and previous preeclampsia who developed preeclampsia than in those who did not (Table 3). Women with multiple gestation or chronic hypertension who developed preeclampsia had higher levels of hCG than those who did not develop preeclampsia (Table 3). There were no biomarkers that were associated with the development of preeclampsia across all high-risk subgroups (Figure).
TABLE 3.
Serum biomarkers for high-risk groups that differed based on preeclampsia status
| Serum biomarkera | Preeclampsia | No preeclampsia | P valueb |
|---|---|---|---|
| Diabetes | n = 50c | n = 183c | |
| VCAM result, ng/mL | 1328 (1171–1507) | 1134 (1053–1221) | .035 |
| Chronic hypertension | n = 103c | n = 284c | |
| Angiogenin, pg/mL | 289 (267.3–309.8) | 261 (248.5–273.3) | .028 |
| hCG, IU/mL | 24,360 (20,279–29,262) | 16,724 (15,295–18,287) | <.001 |
| Selectin, ng/mL | 59.77 (53.67–66.57) | 52.11 (48.94–55.48) | .031 |
| sTNFr-2, pg/mL | 2718 (2527–2923) | 2477 (2374–2585) | .032 |
| TNF-α, pg/mL | 3.09 (2.82–3.38) | 2.62 (2.40–2.87) | .012 |
| Multiple gestation | n = 47c | n = 268c | |
| hCG, IU/mL | 39,117 (31,057–49,269) | 27,124 (24,994–29,436) | .004 |
| IL-6, pg/mL | 2.52 (2.17–2.93) | 1.90 (1.63–2.21) | .013 |
| PlGF, pg/mL | 334.8 (275.6–406.6) | 453.6 (417.0–493.5) | .005 |
| sFlt-1+endoglin/PlGF | 43.13 (28.60–65.03) | 20.19 (17.57–23.20) | <.001 |
| Previous preeclampsia | n = 63c | n = 260c | |
| Angiogenin, pg/mL | 264 (241.0–286.4) | 232 (221.1–243.1) | .015 |
BMI, body mass index; CI, confidence interval; hCG, human chorionic gonadotropin; IL-6, interleukin-6; PlGF, placental growth factor; sFlt-1, soluble fms-like tyrosine kinase; STNFr-2, soluble tumor necrosis factor receptor 2; TNF-α, tumor necrosis factor-alpha; VCAM, vascular cell adhesion molecule.
All reported means are geometric means with 95% CI with the exception of angiogenin, which is reported as arithmetic means with 95% CI;
Comparisons between groups were made using a regression model adjusting for gestational age at the time the serum biomarker was drawn and maternal BMI;
Available sample size for each subgroup-biomarker varied as follows for preeclampsia and no preeclampsia groups, respectively: diabetes: VCAM (30–88); Chronic hypertension: angiogenin, selectin, sTNFr-2 (79–231), hCG (53–221), TNF-α (102–101); Multiple gestation: hCG (26–209), IL-6 (47–47), PlGF (37–197), (sFlt-1+endoglin)/PlGF (9–78); Previous preeclampsia: angiogenin (50–212).
Metz. Preeclampsia biomarkers in high-risk groups. Am J Obstet Gynecol 2014.
FIGURE. Overlap in biomarkers associated with preeclampsia among high-risk women.
hCG, human chorionic gonadotropin; IL-6, interleukin-6; PlGF, placental growth factor; sFlt-1, soluble fms-like tyrosine kinase-1; sTNFr-2, soluble tumor necrosis factor receptor 2; TNF-α, tumor necrosis factor-alpha; VCAM, vascular cell adhesion molecule.
Metz. Preeclampsia biomarkers in high-risk groups. Am J Obstet Gynecol 2014.
Comment
We found that serum biomarkers vary among women at risk for preeclampsia. This nonoverlapping pattern of biomarkers for high-risk subgroups of women (insulin-dependent diabetes, chronic hypertension, multiple gestation, and previous preeclampsia) suggests that preeclampsia is a heterogeneous disease with multiple physiologic pathways. Sibai et al8 previously reported the different levels of sTNFr-2 in women who develop preeclampsia. Here we extend these findings to show that this difference arises primarily in the group with chronic hypertension.
Although numerous studies and metaanalyses have shown a beneficial effect of LDA in specific populations of women,9–14 the results are far from uniform.2 Importantly, the MFMU High-Risk Aspirin trial of LDA to prevent preeclampsia in women at high risk of the disease based on chronic hypertension, insulin-dependent diabetes, multiple gestations, or a previous pregnancy complicated by preeclampsia showed no benefit of LDA.3
One possible explanation for the inconsistent effect of LDA is that the prostaglandin/TXA imbalance that LDA targets is not present in all cases of preeclampsia. In fact, specific data support this hypothesis. In the trial of low-risk nulliparas by Hauth et al,15 LDA was effective in reducing the incidence of preeclampsia, and this effect was directly correlated with a 2-fold or greater reduction in TXA during pregnancy. In contrast, in the MFMU High-Risk Aspirin Trial, neither initial TXA levels nor a decrease in TXA during pregnancy were associated with the occurrence of preeclampsia.4 These observations support the hypothesis that there are TXA-dependent and TXA-independent variants of preeclampsia.
Although standard diagnostic criteria for preeclampsia exist, clinicians recognize preeclampsia as an extremely heterogeneous disease. It is possible that this heterogeneity is a reflection of the interaction of multiple underlying processes that vary from patient to patient and, in turn, vary by kind of preexisting condition(s) thought to increase the risk of preeclampsia. If so, the likelihood that a therapeutic intervention such as LDA would reduce the frequency of its development might also vary by type of preexisting condition. There have been efforts to improve on the efficacy of therapeutic interventions for reducing the occurrence of preeclampsia. For example, researchers have suggested that the gestational age at which LDA is initiated is the limiting factor in determining LDA effectiveness.10 Sixteen weeks gestation, the point in pregnancy by which trophoblast invasion of the maternal spiral arteries is generally considered to be complete, has been a popular break point. However, no plausible mechanism by which LDA promotes placental invasion has been offered to support this hypothesis, and numerous trials where LDA was started well after 16 weeks and was very effective exist.9
Stratifying patients at risk for preeclampsia by specific risk factors may be a productive strategy for untangling different pathophysiologic processes and improving therapeutic effectiveness of various interventions.6,79,9
Our study showed that each high-risk subgroup was characterized by a different pattern of altered biologic mediators at study enrollment. This supports the hypothesis that subset-specific disease pathways result in the final common symptom complex or disease recognized as preeclampsia. In keeping with the present results are those of Maynard et al6 who found that 3 angiogenic markers (PlGF, sFlt-1, endoglin) differed in a prospectively-collected high-risk cohort of women with chronic hypertension, multiple gestation, insulin-dependent diabetes, or previous preeclampsia compared with low-risk controls. Their study focused on changes in angiogenic markers over time, and complements our results by demonstrating different longitudinal patterns of angiogenic biomarkers among high-risk groups.
Powers et al7 performed a secondary analysis of the same MFMU High Risk Aspirin Trial that we used for our analysis, and demonstrated that elevated concentrations of sFlt-1 and soluble endoglin, and decreased levels of PlGF were associated with the development of preeclampsia, especially in the third trimester. However, they noted that these differences were small and should not be used in clinical practice to predict preeclampsia. They did not assess other markers to look at differences in patterns by high-risk group as we have done.
The strengths of this study are the prospective data collection that occurred at the time of the original trial by trained research nurses. We corrected for gestational age at time of blood draw and maternal BMI, a correction that other investigators have not performed and one that did affect the significance of some of our results (data not shown). The cohort is large and diverse, and includes many of the population groups at increased risk of preeclampsia. Preeclampsia was the primary outcome of the original trial and allowed us to make rigorous comparisons between women who did and did not develop preeclampsia during the original trial.
As this is a hypothesis-generating study, we chose not to correct for multiple comparisons. In addition, we compared only previously selected biomarkers, and made comparisons only between the originally identified high-risk groups. This avoids the typical problems associated with post hoc definition of subgroups. That being said, there is still a possibility that some of these associations are a result of chance alone.
We do not claim from these data to have definitively established that certain biomarkers are linked with the development of preeclampsia in a specific high-risk group. Such a claim could only be made by confirmation in independent data sets or other prospective studies. Neither is this study intended to advocate for checking these biomarker levels as a means of identifying women who are likely to develop preeclampsia. Rather, our data lend important evidence to the developing hypothesis that preeclampsia is multifactorial and likely results from different pathophysiologic pathways related to different risk factors. This study may be useful in motivating research directed at preeclampsia prevention in specific subgroups. Our findings also provide additional explanation for why previous attempts to prevent preeclampsia with a single agent such as LDA have been unsuccessful. We are hopeful that this view of preeclampsia will help open the way to better understanding, prevention, and treatment.
Acknowledgments
The contents of this report represent the views of the authors and do not represent the views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network or the National Institutes of Health.
Footnotes
The authors report no conflict of interest.
REFERENCES
- 1.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 2.Sibai BM. Prevention of preeclampsia: a big disappointment. Am J Obstet Gynecol. 1998;179:1275–1278. doi: 10.1016/s0002-9378(98)70146-2. [DOI] [PubMed] [Google Scholar]
- 3.Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338:701–705. doi: 10.1056/NEJM199803123381101. [DOI] [PubMed] [Google Scholar]
- 4.Hauth J, Sibai B, Caritis S, et al. Maternal serum thromboxane B2 concentrations do not predict improved outcomes in high-risk pregnancies in a low-dose aspirin trial. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1998;179:1193–1199. doi: 10.1016/s0002-9378(98)70130-9. [DOI] [PubMed] [Google Scholar]
- 5.Hypertension in pregnancy: executive summary. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 6.Maynard SE, Crawford SL, Bathgate S, et al. Gestational angiogenic biomarker patterns in high risk preeclampsia groups. Am J Obstet Gynecol. 2013;209:53.e1–59.e1. doi: 10.1016/j.ajog.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Powers RW, Jeyabalan A, Clifton RG, et al. Soluble fms-like tyrosine kinase 1 (sFlt1), endoglin and placental growth factor (PlGF) in preeclampsia among high risk pregnancies. PloS One. 2010;5:e13263. doi: 10.1371/journal.pone.0013263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sibai B, Romero R, Klebanoff MA, et al. Maternal plasma concentrations of the soluble tumor necrosis factor receptor 2 are increased prior to the diagnosis of preeclampsia. Am J Obstet Gynecol. 2009;200:630.e1–638.e1. doi: 10.1016/j.ajog.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heyborne KD. Preeclampsia prevention: lessons from the low-dose aspirin therapy trials. Am J Obstet Gynecol. 2000;183:523–528. doi: 10.1067/mob.2000.106757. [DOI] [PubMed] [Google Scholar]
- 10.Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116:402–414. doi: 10.1097/AOG.0b013e3181e9322a. [DOI] [PubMed] [Google Scholar]
- 11.Coomarasamy A, Honest H, Papaioannou S, Gee H, Khan KS. Aspirin for prevention of preeclampsia in women with historical risk factors: a systematic review. Obstet Gynecol. 2003;101:1319–1332. doi: 10.1016/s0029-7844(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 12.Coomarasamy A, Papaioannou S, Gee H, Khan KS. Aspirin for the prevention of preeclampsia in women with abnormal uterine artery Doppler: a meta-analysis. Obstet Gynecol. 2001;98:861–866. doi: 10.1016/s0029-7844(01)01569-1. [DOI] [PubMed] [Google Scholar]
- 13.Roberge S, Giguere Y, Villa P, et al. Early administration of low-dose aspirin for the prevention of severe and mild preeclampsia: a systematic review and meta-analysis. Am J Perinatol. 2012;29:551–556. doi: 10.1055/s-0032-1310527. [DOI] [PubMed] [Google Scholar]
- 14.Roberge S, Villa P, Nicolaides K, et al. Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: a systematic review and meta-analysis. Fetal Diagn Ther. 2012;31:141–146. doi: 10.1159/000336662. [DOI] [PubMed] [Google Scholar]
- 15.Hauth JC, Goldenberg RL, Parker CR, Jr, Copper RL, Cutter GR. Maternal serum thromboxane B2 reduction versus pregnancy outcome in low-dose aspirin trial. Am J Obstet Gynecol. 1995;173:578–584. doi: 10.1016/0002-9378(95)90285-6. [DOI] [PubMed] [Google Scholar]