Abstract
Background
To assess the cardiovascular (CV) risk associated with the use of incretin-based therapy in adult patients with type 2 diabetes mellitus (T2DM) primary prevention group with low CV risks.
Methods
The clinical studies on incretin-based therapy published in medical journals until August 2014 were comprehensively searched using MEDLINE, EMBASE and CENTRAL with no language restriction. The studies were systemically reviewed and evaluated for CV risks using a meta-analysis approach and where they meet the following criteria: clinical trial, incidence of predefined CV disease, T2DM with no comorbidities, age > 18 years old, duration of at least 12 weeks, incretin-based therapy compared with other antihyperglycaemic agents or placebo. Statistical analyses were performed using a Mantel-Haenszel (M-H) test. The odds ratios (OR) and their 95% confidence interval (CI) were estimated and displayed for comparison.
Results
A total of 75 studies comprising 45,648 patients with T2DM were selected. The pooled estimate demonstrated no significance in decreased CV risk with incretin-based therapy versus control (M-H OR, 0.90; 95% CI, 0.81–1.00).
Conclusions
This meta-analysis suggests that incretin-based therapy show no significant protective effect on CV events in T2DM primary prevention group with low CV risks. Prospective randomized controlled trials are required to confirm the results of this analysis.
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic and progressive disease associated with both microvascular and macrovascular complications [1]. The risk of cardiovascular (CV) disease is known to be higher in people with diabetes compared to those without diabetes [2] and CV disease accounts for excess mortality in T2DM [3].
In the assessment of CV risks, glycated hemoglobin control was conventionally thought as related to CV risk owing to the United Kingdom Prospective Diabetes Study (UKPDS) 10-year follow-up study. The study demonstrated a significant reduction in myocardial infarction (MI) and all-cause mortality in overweight newly diagnosed patients with T2DM in intensive glycemic control with metformin [4]. Stemming from these results, improved glycemic control has been traditionally thought to reduce the risk of the microvascular complications of diabetes.
However, more recently, the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) and the Veterans Affairs Diabetes Trial (VADT) did not find significant beneficial effects of intensive glucose control in nonfatal MI, nonfatal stroke, and overall CV mortality [5, 6]. Taken together, the results from clinical trials introduced controversy about the effect of glycemic control on CV disease risk, and uncertainty remains regarding whether any particular glucose lowering strategy actually lowers CV risk.
A recent perspective article published in New England Journal of Medicine by the US Food and Drug Administration (FDA) advisory committee members stated that the optimal approach to the reduction of cardiovascular risk in diabetes patients should focus on the management of standard cardiovascular risk factors rather than intensive glycemic control.[7]
From a drug safety perspective, there has been increasing concern and need of assurance regarding antihyperglycemic agents’ cardiovascular safety. After the concerns raised in 2008 about the cardiac safety of rosiglitazone, the FDA issued an updated Guidance for Industry that required pre and post approval studies to rule out excess cardiovascular risk of any new antidiabetic drug. [8].
In four previous CV trials on incretins [9–12], there was no evidence of an increase or decrease in the number of major adverse cardiovascular events but there were safety concerns regarding a possible elevated risk in hospitalization for heart failure.
Hence, there is a need for a rigorous evaluation of the cardiovascular safety of GLP-1 receptor agonists and DPP-4 inhibitors. In the absence of head-to-head trials, this analysis may provide valuable insight into the comparative outcomes of incretin overall class versus placebo or active control.
As a part of this study, we conducted a systematic review of randomized and controlled studies to provide a comprehensive assessment regarding the risk of cardiovascular diseases associated with DPP-4 inhibitors and GLP-1 receptor agonists compared to placebo or other antihyperglycaemic agents.
Materials and Methods
Data sources and searches
We conducted a search in MEDLINE (via PubMed), EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) up to August 2014. We developed a search strategy using MeSH and free text terms. Study type was restricted to randomized controlled trials, controlled trials, clinical trial, controlled clinical trial, controlled studies and clinical studies in humans.
Study selection
We included studies that (1) enrolled adult patients (of at least 18 years of age) with T2DM with no other complications, (2) compared DPP-4 inhibitors or GLP-1 receptor agonists against placebo (placebo-controlled) or other antihyperglycemic agents (active-controlled), (3) duration of at least 12 weeks, and (4) had explicit reported events of predefined CV outcomes. Trials with shorter duration were excluded because of inadequate time to assess changes in glycemic efficacy, since hemoglobin A1c reflects glycemia during previous 3 months [13].
We followed systematic approach to only include studies with patients who have no other complications at baseline in order to target the study group as primary prevention population and compare the CV effect of incretin in this patient group who are low CV risk patients without significant cardiovascular disease comorbidities or significant laboratory changes. To be classified as T2DM with no other complications, we ensured that the patients included had no underlying diseases at baseline. We also collected information on CV and renal biomarkers such as systolic blood pressure (SBP), diastolic blood pressure (DBP), HDL (high density lipoprotein) cholesterol, LDL (low density lipoprotein) cholesterol, total cholesterol (TC), triglycerides (TG), creatinine clearance (CrCl), serum creatinine (SCr), and glomerular filtration rate (GFR); we reviewed baseline level of each biomarker to exclude any above normal results. We excluded patients with baseline hypertension (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg [14]) or history of hypertension or an antihypertensive treatment, dyslipidemia (HDL < 40 mg/dL, LDL > 130 mg/dL, TC > 200 mg/dL, TG > 150 mg/dL [15]), impaired renal function (CrCl < 30 mL/min, SCr > 1.2 mg/dL, GFR < 30 mL/min[16]) or history of renal disease of disease treatment, and history of other vascular diseases or disease treatment.
The predefined CV outcomes were classified as described below. This classification was reviewed by a cardiologist.
Death: cardiac death, sudden death, all causes of death.
Heart failure: heart failure, cardiac failure, cardiac myopathy.
Hypertension: hypertension, blood pressure change, hypertensive crisis.
Vascular disorders: dyslipidemia, hyperlipidemia, stroke, thrombosis, deep vein thrombosis, arteriosclerosis, raised triglycerides, raised LDL, decreased HDL, lipidemia, hypercholesterolemia, aortic valve sclerosis.
Coronary artery disease: angina, myocardial infarction, ischemia, revascularization, acute coronary syndrome, coronary artery blockage, ST elevation myocardial infarction, non-ST elevation myocardial infarction coronary artery stenosis, coronary artery disease.
Arrhythmia: arrhythmia, tachycardia, bradycardia, atrial fibrillation, atrial flutter, ventricular fibrillation, ventricular flutter, cardio-respiratory arrest, palpitation, ventricular extra systoles, supraventricular extra systoles, left bundle branch block.
Other/ Non-specified: chest pain, hypotension, cardiomegaly, cerebral infarction, syncope.
Data extraction and quality assessment
Publications retrieved from three search engines were imported into the reference management software (Endnote® X6, X7; Thomson Reuters, New York, NY). After removing duplicate results, two reviewers (KJY, CMJ) independently screened all titles, abstracts, and full texts according to the study process. Any discrepancies were resolved by discussion and adjudication by the third reviewer (YSW).
For quality assessment, we used Cochrane Collaboration’s tool [17] to assess the risk of bias of the included trials. We considered random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and incomplete outcome data and evaluated whether the adjudication of CV events was carried out. Risk of bias was evaluated at three levels: ‘low (low risk of bias)’, ‘high (high risk of bias)’ and ‘unclear’. The result was not used as a criterion for the selection of trials, but only for descriptive purposes.
To assess possible publication or disclosure bias we used funnel plots, the Begg adjusted rank correlation test [18]. Asymmetry in a funnel plot (also known as small study effects [19]) is potentially indicative of publication biases.
All analyses were performed using Review Manager 5.3 software (RevMan version 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration).
Data synthesis
We further collected information from the selected trials on study characteristics (study design, study duration, total study population, total safety population), baseline patient characteristics (mean age, percentage of male, mean duration of diabetes, mean body mass index [BMI], mean fasting plasma glucose [FPG]), interventions (incretin treatment, control treatment, dose, drug used across groups, mode of therapy), duration of treatment and number of CV events reported.
For extension trials, we used data from the longest follow-up. If treatment assignments were exchanged or both arms were assigned to incretin therapy in the extension period, we collected data before that point, if provided.
Trials were excluded if intervention and comparator groups were both based on incretin therapy with no placebo or other antihyperglycemic group (e.g. sitagliptin vs. exenatide trial). If placebo and other antihyperglycemic agent group were both included or multiple incretin arms were included in the study, we reconstructed the study arms into incretin vs. comparator arm.
Add-on therapies included other oral antihyperglycemic agents (i.e., sulphonylureas, thiazolidinediones and biguanides) and injectable therapies (i.e., insulin) co-administered with the incretin-based therapies or the comparator arm (combination therapy). If there was at least one arm treated with a combination therapy, trial was classified as add-on therapy trial.
Active controlled studies were those compared with other antihyperglycaemic agents and placebo controlled studies were trials where comparator arm was placebo.
Data analysis
We assessed heterogeneity between studies using the χ2 test and I2 statistic with a significance threshold for α of 0.10 [20]. We report the results of fixed-effect model because generally the heterogeneity data was not present in the studies included. We conducted a sensitivity analysis using an alternative heterogeneity consideration model on those subgroup analyses with high heterogeneity. We pooled trials using the Mantel-Haenszel method, since the number of CV events is dichotomous variables, Mantel-Haenszel odds ratio (MH-OR) with 95% confidence interval was calculated for all CV events defined. We performed a primary analysis to find out the overall incretin effect on predefined general CV risk. Furthermore, diverse subgroup analyses were performed: type of incretin (DPP-4 inhibitors vs. control, GLP-1 receptor agonist vs. control); type of control with mode of therapy (placebo controlled in mono therapy, placebo controlled in add-on therapy, active controlled in mono therapy, active controlled in add-on therapy); classified CV outcomes; and individual incretin agents.
Results
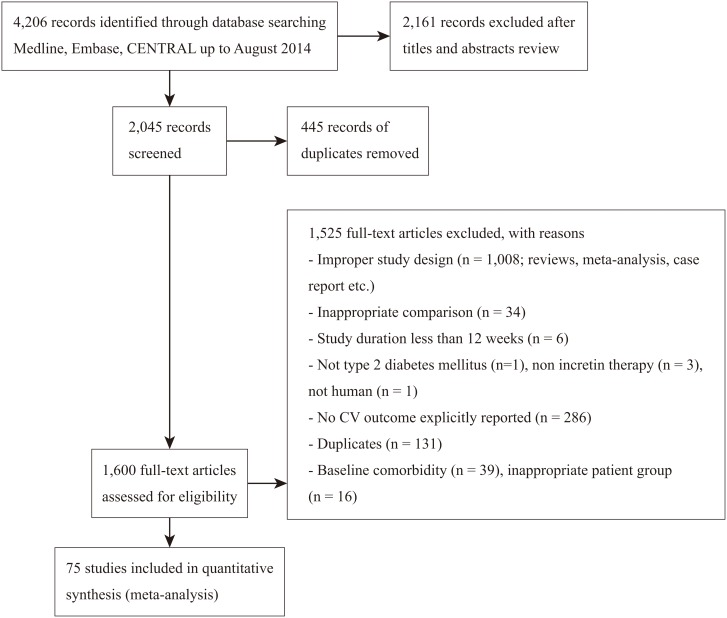
Our search yielded 4,206 potentially relevant reports. After screening titles and abstracts, we retrieved 1,600 reports for full text screening. A total of 75 studies were eligible for final inclusion comprising 45,648 patients (Fig 1). The median duration of the 75 trials—all industry funded—was 35 weeks (ranging from: 12 to 112 weeks). The trials enrolled a mean of 608 (ranging from 36 to 3,118 patients), and the population for safety analysis accounted for a mean of 610 patients (ranging from 36 to 3,099). The mean age was 56.1 years old and 54.1% were males. The mean BMI was 30.3 kg/m2 (ranging from 24.1 to 33.9 kg/m2), the mean baseline HbA1c was 8.3% (ranging from 6.6 to 11.4%), the mean FPG was 9.5 mmol/L (ranging from 6.9 to 12.2 mmol/L), and the mean duration of diabetes was 6.5 years (ranging from 1.3 to 12.6 years). The average value was calculated excluding those not reported (Table 1).The intervention characteristics and number of CV events reported in each trial are summarized in Table 2. Fifty-eight trials tested DPP-4 inhibitors, 16 tested GLP-1 receptor agonists, and one tested both agents (Table 2).
Fig 1. Flow chart of article selection (PRISMA flow diagram).
Table 1. Characteristics of included trials of incretin treatment in patients with type 2 diabetes mellitus.
| Author (year) | Total population | Safety population | Study duration (weeks) | Mean Age (years) | Male (%) | Mean diabetes duration (years) | Mean BMI (kg/m2) | Mean HbA1C (%) | MeanFPG (mmol/L) |
|---|---|---|---|---|---|---|---|---|---|
| Aschner 2006[37] | 741 | 741 | 24 | 54.2 | 51.7 | 4.4 | 30.5 | 8.0 | 9.7 |
| Aschner 2010[38] | 1,050 | 1,050 | 24 | 56.0 | 46.0 | 2.4 | 30.8 | 7.2 | 7.9 |
| Barnett 2012[39] | 455 | 455 | 24 | 57.2 | 41.7 | 11.9 | 32.3 | 8.7 | 9.6 |
| Bergenstal 2009[40] | 372 | 372 | 24 | 52.6 | 48.1 | 9.0 | 33.8 | 10.2 | 11.3 |
| Blonde 2009[41] | 2,664 | 2,627 | 12 | 55.6 | 51.8 | 5.1 | 32.4 | 8.0 | 9.3 |
| Bolli 2009[42] | 576 | 576 | 52 | 56.6 | 62.9 | 6.4 | 32.2 | 8.4 | 10.9 |
| Bosi 2007[43] | 416 | 541 | 24 | 54.2 | 57.4 | 6.3 | 32.7 | 8.4 | 9.9 |
| Bosi 2009[22] | 1,179 | 1,171 | 24 | 52.8 | 58 | 2.0 | 31.2 | 8.7 | 10.4 |
| Chacra 2011[44] | 768 | 768 | 76 | 55.1 | 45.1 | 6.9 | 29 | 8.4 | 9.6 |
| Del 2011[45] | 503 | 503 | 24 | 55.7 | 48.3 | NR | 29.1 | 8 | 8.9 |
| Dobs 2013[46] | 262 | 260 | 54 | 54.5 | 54.8 | 9.3 | 30.3 | 8.8 | 10.1 |
| Filozof 2010[47] | 1,007 | 1,007 | 52 | 59.5 | 52 | 6.6 | 31 | 8.5 | 10.7 |
| Fonesca 2012(2)[48] | 361 | 361 | 12 | 53.7 | 51.5 | 1.3 | 31.9 | 8.0 | 9.0 |
| Fonseca 2012[49] | 282 | 282 | 18 | 55.4 | 46.1 | 6.2 | 30.9 | 8.3 | 9.0 |
| Fonseca 2013[50] | 313 | 313 | 26 | 56.0 | 62.3 | 9.8 | 29.9 | 8.8 | 9.8 |
| Forst 2010[51] | 333 | 333 | 12 | 60.0 | 58 | 7 | 31.9 | 8.3 | 10.3 |
| Gallwitz 2012[52] | 1,029 | 1019 | 48 | 56.0 | 54.0 | 5.7 | 32.5 | 7.5 | 8.8 |
| Gallwitz 2012(2)[23] | 1,551 | 1551 | 104 | 59.8 | 60.5 | 715(47.1%)* | 30.2 | 7.7 | 9.1 |
| Garber 2007[53] | 398 | 462 | 24 | 54.3 | 50.0 | 4.7 | 32.4 | 8.7 | 10.1 |
| Garber 2008[54] | 408 | 515 | 24 | 58.2 | 59.0 | 7.2 | 31.3 | 8.5 | 10.4 |
| Garber 2011[55] | 745 | 745 | 104 | 53.0 | 50.0 | 5.4 | 33.1 | 8.3 | 9.4 |
| Goke 2013[56] | 858 | 858 | 104 | 57.6 | 51.8 | 5.4 | 31.4 | 7.7 | 9.0 |
| Goodman 2009[57] | 370 | 370 | 24 | 54.8 | 57.5 | NR | 31.5 | 8.6 | 10.9 |
| Grunberge 2012[58] | 164 | 164 | 12 | 56.6 | 45.1 | 3.9 | 32.1 | 7.2 | NR |
| Haak 2012[59] | 791 | 791 | 24 | 55.3 | 53.8 | 562(74.3%)* | 29.1 | 8.7 | 10.8 |
| Henry 2011[21] | 36 | 36 | 12 | 55.6 | 38.9 | 3.1 | 32.9 | 6.8 | 7.1 |
| Hollander 2011[60] | 565 | 565 | 76 | 54.0 | 49.6 | 5.2 | 30.0 | 8.3 | 9.0 |
| Inagaki 2012[61] | 427 | 427 | 26 | 56.8 | 67.9 | 9 | 26.2 | 8.5 | 9.0 |
| Inagaki 2014[62] | 322 | 322 | 12 | 59.8 | 60.3 | 6.4 | 25.3 | 8.1 | 9.1 |
| Iwamoto 2010[63] | 363 | 363 | 12 | 59.8 | 61.7 | 5.4 | 24.5 | 7.6 | 8.2 |
| Kaku 2011[64] | 339 | 339 | 52 | 60.1 | 62.8 | 6.7 | 26.1 | 7.9 | NR |
| Kaku 2011(2)[65] | 400 | 400 | 52 | 58.3 | 67.3 | 8.3 | 24.8 | 9.3 | NR |
| Matthews 2010[66] | 3,118 | 3,099 | 104 | 57.5 | 53.5 | 5.7 | 31.8 | 7.3 | 9.2 |
| Matyjaszek-Matuszek 2013[67] | 80 | 80 | 26 | 60 | 43.8 | 8.4 | 32.1 | 7.9 | 9.6 |
| Mohan 2009[68] | 530 | 530 | 18 | 50.9 | 58.0 | 2.0 | 25.0 | 8.7 | 10.5 |
| Moses 2014[69] | 257 | 257 | 24 | 57.0 | 59.9 | NR | 29.3 | 8.3 | 8.8 |
| Nauck 2007[70] | 1,172 | 1,172 | 52 | 56.7 | 59.2 | 6.4 | 31.2 | 7.7 | 9.2 |
| Nauck 2009[71] | 527 | 527 | 26 | 54.8 | 50.3 | 6 | 32 | 302(57.3%)** | 9.5 |
| Nauck 2013[24] | 1,091 | 1,087 | 104 | 56.7 | 58.2 | 7.6 | 31 | 8.4 | 10 |
| Nonaka 2008[72] | 151 | 151 | 12 | 55.3 | 63 | 4 | 25.2 | 7.6 | 9.1 |
| Olansky 2011[73] | 1,246 | 1,246 | 44 | 49.7 | 56.5 | 3.4 | 33.3 | 9.9 | 10.3 |
| Perez-Monteverde 2011[74] | 492 | 452 | 40 | 51.1 | 61.0 | 3.2 | 29.8 | 9.1 | 10.3 |
| Pfutzner 2011[75] | 1,306 | 1,306 | 76 | 52.0 | 49.2 | 1.7 | 30.4 | 9.5 | 11.1 |
| Phillis-Tsimikas 2013[76] | 447 | 454 | 26 | 40.8 | 58.6 | 7.8 | 30.4 | 8.9 | 9.6 |
| Pinget 2013[77] | 484 | 484 | 24 | 55.8 | 52.3 | 8.1 | 33.9 | 8.1 | 9.1 |
| Pratley 2006[78] | 98 | 98 | 12 | 55.7 | 42.9 | 4.3 | 30.0 | 8.0 | 9.6 |
| Pratley 2009[79] | 500 | 500 | 26 | 56.6 | 52.2 | 7.7 | 30.1 | 8.1 | NR |
| Pratley 2009(2)[80] | 493 | 493 | 24 | 55.4 | 58.2 | 7.6 | 32.8 | 8.0 | NR |
| Pratley 2013[81] | 760 | 751 | 24 | 56.4 | 49 | 8.8 | 32.7 | 8.3 | 10.0 |
| Prato 2011[82] | 503 | 503 | 24 | 55.7 | 48.3 | NR | 29.1 | 8 | 7.1 |
| Ratner 2010[83] | 129 | 129 | 12 | 57 | 14.0 | 7.0 | 32.4 | 7.9 | 9.1 |
| Raz 2008[84] | 190 | 190 | 30 | 54.8 | 46.3 | 7.9 | 30.2 | 9.2 | 11.1 |
| Reasner 2011[85] | 1,246 | 1,246 | 18 | 49.7 | 56.5 | 3.4 | 33.3 | 9.9 | 12.2 |
| Riddle 2013[86] | 495 | 495 | 24 | 57 | 46 | 12.5 | 32.1 | 8.4 | 8.0 |
| Rosenstock 2006[87] | 353 | 353 | 24 | 56.3 | 55.5 | 6.1 | 31.5 | 8.0 | 9.3 |
| Rosenstock 2009[88] | 598 | 591 | 80 | 54.3 | 56.6 | 2.2 | 32.6 | 8.6 | 9.9 |
| Rosenstock 2009(2)[89] | 390 | 390 | 26 | 56.8 | 41.4 | 12.6 | 32.5 | 9.3 | 10.6 |
| Rosenstock 2011[90] | 859 | 859 | 24 | 57.3 | 50.5 | 9.3 | 30.2 | 8.3 | 9.5 |
| Ross 2012[91] | 491 | 491 | 12 | 58.6 | 57 | 227(46.2%)* | 29.6 | 7.9 | 9.2 |
| Russel-Jones 2012[92] | 820 | 820 | 26 | 53.8 | 59.0 | 2.7 | 31.2 | 8.5 | 9.9 |
| Scherbaum 2008[93] | 131 | 131 | 112 | 63.1 | 59.5 | 2.3 | 30.3 | 6.6 | 6.9 |
| Schernthaner 2013[94] | 755 | 755 | 52 | 56.7 | 55.9 | 9.6 | 31.6 | 8.1 | 9.3 |
| Seino 2012[95] | 312 | 312 | 64 | 60.2 | 66.7 | 9.8 | 24.7 | 8.6 | NR |
| Seino 2012(2)[96] | 288 | 288 | 12 | 52.6 | 68.8 | 6.3 | 25.9 | 8 | NR |
| Seino 2014[97] | 215 | 212 | 16 | 57 | 69.8 | 7 | 25.1 | 8.6 | NR |
| Strain 2013[98] | 278 | 278 | 24 | 74.8 | 45.3 | 11.4 | 29.8 | 11.4 | 9.8 |
| Tajima 2011[99] | 138 | 138 | 12 | 60.8 | 58 | 9.1 | 24.6 | 8.4 | 8.6 |
| Tajima 2013[100] | 133 | 133 | 12 | 60.5 | 65.4 | 7.2 | 24.1 | 7.9 | 8.4 |
| Taskinen 2011[101] | 700 | 700 | 24 | 56.5 | 54 | 310(44.3%)* | 29.9 | 8.1 | 9.4 |
| Terra 2011[102] | 301 | 301 | 12 | 56.2 | 66.4 | 7.1 | 32.0 | 8.3 | 9.5 |
| Vilsboll 2010[103] | 641 | 641 | 24 | 57.8 | 51.0 | 12.5 | 31.0 | 8.7 | 9.8 |
| White 2014[104] | 160 | 160 | 12 | 55.4 | 53.1 | 6.0 | 33.1 | 7.9 | 9.1 |
| Williams-Herman 2009[105] | 1,091 | 1,091 | 54 | 53.5 | 50.6 | 4.5 | 32.1 | 8.8 | 11.1 |
| Wysham 2014[106] | 976 | 976 | 26 | 55.6 | 58.4 | 9.0 | 33.3 | 8.1 | 9.0 |
| Yang 2011[107] | 570 | 570 | 24 | 54.1 | 48.3 | 5.1 | 26.2 | 7.9 | 8.8 |
| Average value across included trials | 608 | 610 | 35.1 | 56.1 | 54.1 | 6.5 | 30.3 | 8.3 | 9.5 |
BMI = body mass index; FPG = fasting plasma glucose; NR = not reported
* No (%) of patients with no more than 5 years’ diabetes duration
** No (%) of patients with HbA1c < 8%
Table 2. Intervention characteristics of included trials of incretin treatment in patients with type 2 diabetes mellitus.
| Author (year) | Incretin | Control | Drugs used across groups | Mode of therapy | ||
|---|---|---|---|---|---|---|
| type | CV event | type | CV event | |||
| Aschner 2006 | sitagliptin | 14/488 | placebo | 6/253 | none | mono |
| Aschner 2010 | sitagliptin | 12/528 | metformin | 4/522 | none | mono |
| Barnett 2012 | saxagliptin | 1/304 | placebo | 0/12 | insulin ±metformin | add on |
| Bergenstal 2009 | exenatide | 0/124 | biphasic insulin aspart | 1/248 | none | mono |
| Blonde 2009 | vildagliptin | 5/1756 | thiazolidinediones | 1/871 | metformin | add on |
| Bolli 2009 | vildagliptin | 2/296 | pioglitazone | 6/280 | metformin | add on |
| Bosi 2007 | vildagliptin | 6/360 | placebo | 3/181 | metformin | add on |
| Bosi 2009* | vildagliptin | 17/879 | metformin | 12/292 | metformin | add on |
| Chacra 2011 | saxagliptin | 92/501 | placebo | 45/267 | glyburide | add on |
| Del 2011 | linagliptin | 21/336 | placebo | 6/167 | none | mono |
| Dobs 2013 | sitagliptin | 2/170 | placebo | 0/90 | metformin +rosiglitazone | add on |
| Filozof 2010 | vildagliptin | 36/510 | gliclazide | 43/493 | metformin | add on |
| Fonesca 2012(2) | lixisenatide | 1/239 | placebo | 0/122 | none | mono |
| Fonseca 2012† | saxagliptin | 1/238 | metformin | 2/144 | metformin | add on |
| Fonseca 2013 | sitagliptin | 0/157 | placebo | 1/156 | metformin +pioglitazone | add on |
| Forst 2010 | linagliptin | 3/197 | placebo, glimepiride | 0/136 | metformin | add on |
| Gallwitz 2012 | exenatide | 0/511 | glimepiride | 4/508 | metformin | add on |
| Gallwitz 2012(2) | linagliptin | 62/776 | glimepiride | 86/775 | metformin | add on |
| Garber 2007 | vildagliptin | 1/304 | placebo | 1/158 | pioglitazone | add on |
| Garber 2008 | vildagliptin | 6/339 | placebo | 1/176 | glimepiride | add on |
| Garber 2011 | liraglutide | 59/497 | glimepiride | 34/248 | none | mono |
| Goke 2013 | saxagliptin | 20/428 | glipizide | 32/430 | metformin | add on |
| Goodman 2009 | vildagliptin | 5/248 | placebo | 3/122 | metformin | add on |
| Grunberge 2012 | dulaglutide | 1/132 | placebo | 0/32 | none | mono |
| Haak 2012* | linagliptin | 0/428 | placebo/metformin | 1/429 | metformin | add on |
| Henry 2011 | saxagliptin | 0/20 | placebo | 0/16 | none | mono |
| Hollander 2011 | saxagliptin | 36/381 | placebo | 14/184 | thiazolidinediones | add on |
| Inagaki 2012 | exenatide | 1/215 | insulin glargine | 0/212 | biguanide ±thiazolidinedione | add on |
| Inagaki 2014 | SYR-472 (DPP-4 inhibitor) | 10/266 | placebo | 3/55 | none | mono |
| Iwamoto 2010 | sitagliptin | 3/290 | placebo | 0/73 | none | mono |
| Kaku 2011 | alogliptin | 2/224 | placebo | 1/115 | pioglitazone | add on |
| Kaku 2011(2) | liraglutide | 34/268 | glibendamide | 24/132 | none | mono |
| Matthews 2010 | vildagliptin | 104/ 1553 | glimepiride | 125/ 1546 | metformin | add on |
| Matyjaszek-Matuszek 2013 | exenatide | 2/40 | insulin glargine | 0/40 | metformin +sulfonylurea | add on |
| Mohan 2009 | sitagliptin | 1/352 | placebo | 0/178 | none | mono |
| Moses 2014 | saxagliptin | 12/129 | placebo | 9/128 | metformin +sulfonylurea | add on |
| Nauck 2007 | sitagliptin | 0/588 | glipizide | 2/584 | metformin | add on |
| Nauck 2009 | alogliptin | 14/423 | placebo | 7/104 | metformin | add on |
| Nauck 2013 | liraglutide | 68/724 | glimepiride/ placebo | 14/363 | metformin | add on |
| Nonaka 2008 | sitagliptin | 0/75 | placebo | 2/76 | none | mono |
| Olansky 2011¥ | sitagliptin | 0/625 | placebo | 1/621 | metformin other OHA | add on |
| Perez-Monteverde 2011** | sitagliptin | 1/222 | pioglitazone | 0/230 | metformin | add on |
| Pfutzner 2011* | saxagliptin | 71/978 | metformin | 27/328 | metformin | add on |
| Phillis-Tsimikas 2013 | sitagliptin | 0/228 | insulin degludec | 1/226 | metformin | add on |
| Pinget 2013 | lixisenatide | 0/323 | placebo | 1/161 | pioglitazone± metformin | add on |
| Pratley 2006 | vildagliptin | 6/70 | placebo | 6/28 | none | mono |
| Pratley 2009 | alogliptin | 18/401 | placebo | 2/99 | glyburide | add on |
| Pratley 2009(2) | alogliptin | 19/397 | placebo | 1/97 | pioglitazone | add on |
| Pratley 2013 | taspoglutide | 4/494 | pioglitazone | 2/257 | sulphonylurea ±metformin | add on |
| Prato 2011 | linagliptin | 21/336 | placebo | 6/167 | none | mono |
| Ratner 2010 | taspoglutide | 0/97 | placebo | 1/32 | metformin | add on |
| Raz 2008 | sitagliptin | 5/96 | placebo | 5/94 | metformin | add on |
| Reasner 2011¥ | Sitagliptin | 6/625 | placebo | 10/621 | metformin | add on |
| Riddle 2013 | lixisenatide | 1/328 | placebo | 0/167 | basal insulin ± metformin | add on |
| Rosenstock 2006 | sitagliptin | 1/175 | placebo | 0/178 | pioglitazone | add on |
| Rosenstock 2009 | vildagliptin | 46/393 | rosiglitazone | 27/198 | none | mono |
| Rosenstock 2009(2) | alogliptin | 1/260 | placebo | 0/129 | insulin | add on |
| Rosenstock 2011 | lixisenatide | 1/574 | placebo | 0/285 | sulfonulurea ± metformin | add on |
| Ross 2012 | linagliptin | 6/447 | placebo | 0/44 | metformin | add on |
| Russel-Jones 2012ǂ | exenatide/ sitagliptin | 6/411 | pioglitazone/ metformin | 15/409 | none | mono |
| Scherbaum 2008 | vildagliptin | 6/68 | placebo | 2/63 | none | mono |
| Schernthaner 2013 | sitagliptin | 0/378 | canagliflozin | 2/377 | metformin +sulfonylurea | add on |
| Seino 2012 | alogliptin | 2/209 | placebo | 0/103 | glimepiride | add on |
| Seino 2012(2) | alogliptin | 3/188 | placebo | 2/100 | metformin | add on |
| Seino 2014 | albiglutide | 4/159 | placebo | 1/53 | none | mono |
| Strain 2013* | vildagliptin | 6/139 | placebo | 3/139 | sulphonylurea | add on |
| Tajima 2011 | sitagliptin | 0/67 | placebo | 2/71 | glimepiride | add on |
| Tajima 2013 | sitagliptin | 1/70 | placebo | 0/63 | voglibose | add on |
| Taskinen 2011 | linagliptin | 17/523 | placebo | 6/177 | metformin | add on |
| Terra 2011 | PF-734200 (DPP-4 inhibitor) | 2/225 | placebo | 1/76 | metformin | add on |
| Vilsboll 2010 | sitagliptin | 0/322 | placebo | 2/319 | insulin ± metformin | add on |
| White 2014 | saxagliptin | 3/74 | placebo | 2/86 | metformin | add on |
| Williams-Herman 2009* | sitagliptin | 1/551 | Placebo, metformin | 1/540 | metformin | add on |
| Wysham 2014 | dulaglutide/exenatide | 1/835 | placebo | 0/141 | metformin + pioglitazone | add on |
| Yang 2011 | saxagliptin | 11/283 | placebo | 19/287 | metformin | add on |
*: Not all but some treatment groups were co-administered with metformin or sulfonylurea.
**: metformin was co-administered from the start of phase B
†: metformin was uptitrated in control group where we defined these treatment arms as add-on therapy, and mentioned metformin as drug used across groups
ǂ: Among 4 study groups in this study, we reconstructed the study population and lumped exenatide and sitagliptin group as ‘incretin’ and pioglitazone and metformin group as ‘non-incretin comparator’.
¥: incretin groups consisted fixed dose combination formulation with metformin
FDC: fixed-dose combination, OHA: oral hyperglycaemic agent
+: both A and B, -: either A or B, ±: A with or without B, /: A,B consists each separate arm
The heterogeneity between included trials was low according to both of the statistics tests we used (I2 = 2%, χ2 test = 74.12 [p = 0.44]). Seven studies had a prospective independent clinical event committee (CEC), which reviewed and adjudicated events suspected to be CV outcomes.
The shape of Begg’s funnel plot (S2 Fig) showed only minor asymmetry (with or without inclusion of the studies lacking individual participant data), and Egger’s test for asymmetry was not significant (P = 0.14). Thus a publication bias mechanism was not considered a major cause for concern in our study.
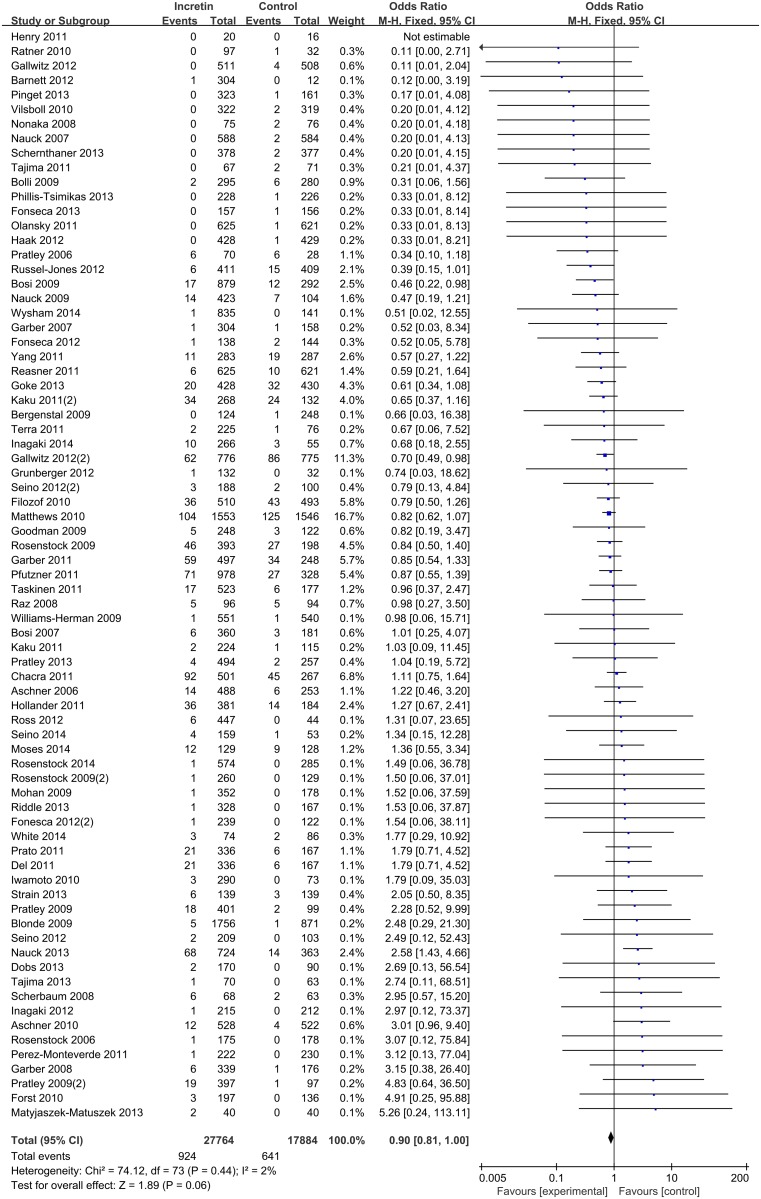
Of the 75 randomized controlled trials reporting predefined CV outcomes, only one study stated that no events of CV disease occurred during the course of study [21]. In total, 27,764 patients were recruited in intervention groups reporting 924 CV events (3.3%), and 17,884 patients were recruited in control group reporting 641 CV events (3.6%). Two out of 75 included trials, both with DPP-4 inhibitors, independently showed statistical significance in lowering CV risk [22, 23], and one GLP-1 receptor agonist study, in which liraglutide was added to metformin, showed statistical significance in increased CV risk [24].
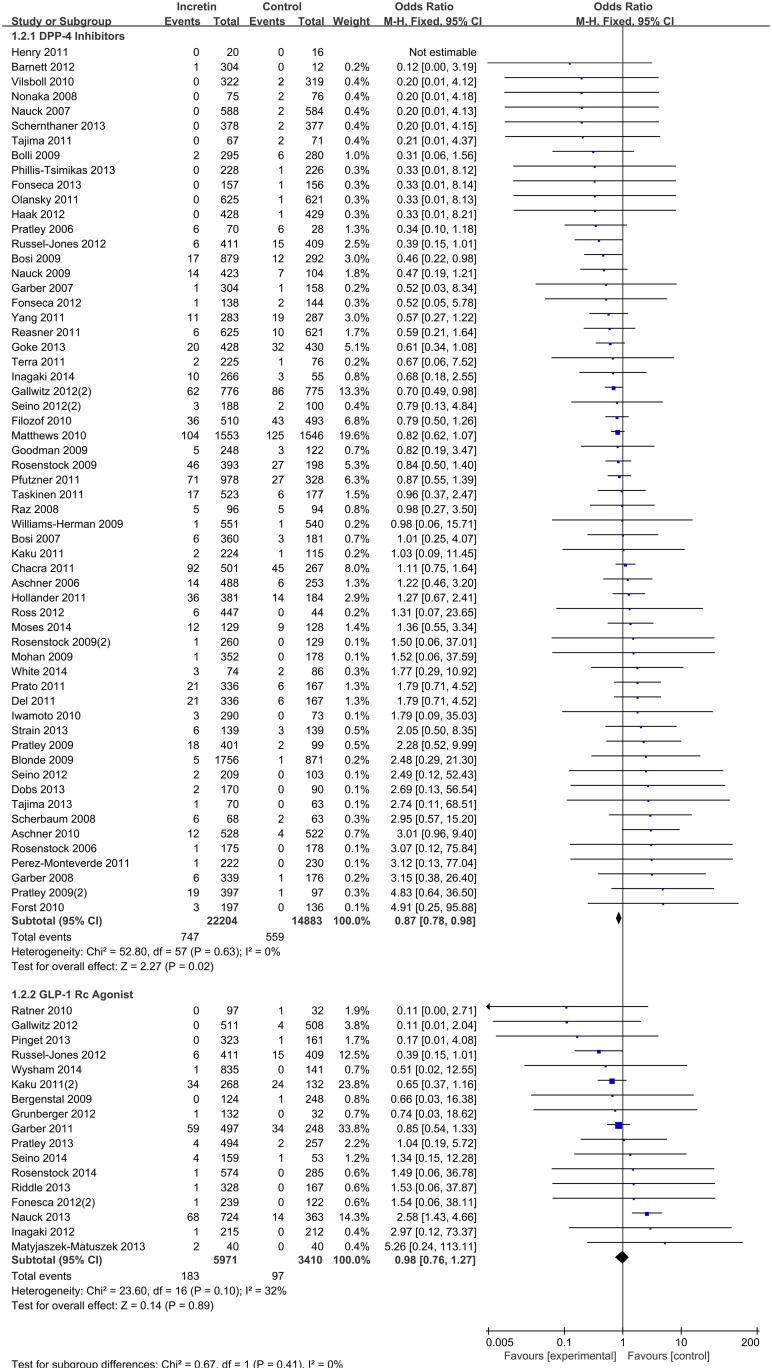
In our primary analysis, pooled estimates of 75 studies showed no significance in beneficial effect of all incretin versus control (M-H OR 0.90, 95% CI 0.81–1.00) on CV risk (Fig 2). Evaluated as a subgroup, DPP-4 inhibitors alone were mildly protective compared to control (M-H OR 0.87, 95% CI 0.78–0.98), whereas the subgroup including only GLP-1 agonist showed no evidence of protection (M-H OR 0.98, 95% CI 0.76–1.27) (Fig 3) (χ2 test for subgroup differences, p-value = 0.41).
Fig 2. Risk of cardiovascular events between patients with type 2 diabetes mellitus with no other complications treated with incretin or control.
Fig 3. Subgroup analysis by types of incretin therapy.
Also when we explored the sources of heterogeneity by type of control and mode of therapy, incretin therapy showed beneficial effect in comparison to an active comparator in add-on therapy (M-H OR 0.83, 95% CI 0.71–0.96). Whereas no such effect was observed in placebo-controlled trials and active-controlled mono therapy trials (S3 Fig) (χ2 test for subgroup differences, p-value = 0.10).
In subgroup analysis by type of 8 predefined CV outcome, no significant effect of incretin was observed in any. In case of death and heart failure, there were many cells with zero events and the maximum number of events per arm was 3, resulting in a small number of total reports and a wide confidence interval (S4 Fig) (χ2 test for subgroup differences, p-value = 0.35).
The subgroup analysis by type of individual incretins did not show difference among those agents except for vildagliptin, of which showed CV protective effect (M-H OR 0.82,95% CI 0.68–0.99) (S5 Fig) (χ2 test for subgroup differences, p-value = 0.72).
After performing the subgroup analyses to detect heterogeneity, we found that only two variables in all separate analyses displayed high heterogeneity (p-value lower than 0.1): the ‘other/non-specified’ subgroup by classification of predefined CV outcome, and the ‘liraglutide’ subgroup by individual incretin agent analysis. We performed a sensitivity analysis on these two subgroups using random-effect model and the results were consistent.
Discussion
Main findings
This meta-analysis included a comprehensive search for all trials with incretin-based therapies (GLP-1 receptor agonists and DPP-4 inhibitors) for type 2 diabetes treatment. The wide-ranging search allowed separate analyses by type of incretin, type of control and mode of therapy, classification of CV outcome and individual incretin agent.
The overall heterogeneity was low, including all subgroup analyses performed in the present study, which allowed us to apply a fixed-effect model rather than a random-effect model for data pooling.
Overall, incretin-based therapies showed a trend towards lower risk of cardiovascular disease compared to placebo or other antihyperglycemic agents, although the difference was not statistically significant.
The subgroup analysis by type of incretin showed statistical significance between groups; the effect of DPP-4 inhibitors on overall reduction of CV risk was greater than that of GLP-1 receptor agonists. These results suggest that, although drugs may share the pharmacological mechanism of increasing incretin activity, they may have different effects on CV risks. Contrary to our results, a retrospective analysis of the insurance claims database showed lower risk of CV events and hospitalizations in treatment with exenatide twice daily therapies than other-glucose lowering therapies [25].
Interestingly, incretin-based therapies were associated with a reduction of CV risk when compared to active antihyperglycemic agents treated in add-on therapies, whereas this effect disappeared when including placebo-comparator trials. Most of the trials in this group (79%) were DPP-4 inhibitors in combination with metformin compared to thiazolidinediones and sulfonylureas. It suggests incretin-based therapies added to metformin or other antihyperglycemic agents (as it is frequently used as second line therapy in treatment of T2DM) have beneficial effects on decreasing CV risks.
No additional effect was found in the subtypes of CV outcomes. Considering that we excluded a number of trials with baseline CV risks in patient characteristics, we infer that incretin-based therapies are associated with lower risk of major CV events, such as cardiac death and heart failure, in primary prevention group patients with low CV risk. Yet, as we collected safety outcomes reported from each study, which incorporates signs and symptoms that are not always a specific diagnosis, definitions of specific major CV events may have varied across studies.
In addition, three individual incretin agents, alogliptin, albiglutide, and liraglutide had higher odds of overall CV risk than comparators, but this difference was not statistically significant. However, vildagliptin was suggested to have a lower risk of CV disease with borderline significance (OR 0.82, 95% CI 0.68 to 0.99), which is consistent with the results from pooled data of 25 phase 3 trials assessing cardio-cerebrovascular safety of vildagliptin [26].
Comparison with other studies
The available evidence regarding incretin-based therapies association with CV risk is currently contradictory. The reports indicate either a detrimental or a beneficial effect. Here we review several studies according to their study design.
Preclinical data indicated a potential cardio protective effect of DPP-4 inhibitors by increasing the concentration not only of GLP-1, but of other vasoactive peptides as well [27]. Some evidence shows that GLP-1 might have beneficial effects on the myocardium and on endothelial function [28] and GLP-1 has been found to be cardio protective in experimental models of heart failure and myocardial infarction [29].
Epidemiologic study data have shown the opposite results of our study, suggesting that exenatide reduces CV disease events (myocardial infarction, ischemic stroke, or coronary revascularization procedure) [25] and sitagliptin increases the risk of CV disease related hospital admissions and deaths [30]. But in both studies, when history of CV disease was measured, 50 to 60% of included patients with hypertension and dyslipidemia apart from our included patient group. And also both these studies were retrospective database analyses performed using insurance claim data, which are known to have substantial limitations such as misclassification of exposure and outcome using ICD codes mapping.
Patient level results from all completed phase 2/ 3 studies of liraglutide, alogliptin and vildaglipitin respectively showed no relevant significant effect on CV events [26, 31, 32].
Since US FDA now requires all new antidiabetic agents to undergo a thorough long-term CV risk assessment[8], recently four large-scale trials designed for this purpose in incretin have been carried out and currently there are many trials still ongoing expected to be published in forthcoming years (i.e CAROLINA, EXSCEL, LEADER and et cet.).
SAVOR TMI-53 included 16,492 patients with a history of, or at risk of CV events and they were randomly assigned to receive saxagliptin or placebo for an average of 2.1 years. The overall hazard ratio was 1.00 (95% CI 0.89–1.12), but the risk of hospitalization for heart failure was significant (hazard ratio 1.27, 95% CI 1.07–1.51) [9].
In the alogliptin trial (Examination of Cardiovascular Outcomes with Alogliptin vs. Standard of Care in Patients with Type 2 Diabetes Mellitus and Acute Coronary Syndrome, EXAMINE), where 5,380 patients were randomly assigned to receive alogliptin or placebo for a median of 18 months after an episode of acute myocardial infarction or unstable angina. The hazard ratio was 0.96 (upper boundary of the one-sided repeated confidence interval, 1.16) [33].
In the TECOS (cardiovascular outcomes trial of sitagliptin in T2DM), 14,671 patients were assigned to a group where either sitagliptin or placebo were added to their existing therapy, the median follow-up was 3 years. The trial achieved its primary endpoint of noninferiority for the composite CV endpoint of CV-related death, non-fatal myocardial infarction, non-fatal stroke, or unstable angina requiring hospitalization.—[11]. The observation that sitagliptin therapy was not associated with a change in long-term rates of cardiovascular events is consistent with the findings from shorter-term outcome trials of other DPP-4 inhibitors, including the above mentioned saxagliptin and alogliptin.
Last, but most recently, first GLP-1 receptor agonist CV outcome trial result was published which met the pre-specified criterion of non-inferiority versus placebo for the composite primary endpoint of CV death, non-fatal MI, non-fatal stroke and hospitalization for unstable angina (HR 1.02, 0.89–1.17) [12] (Lixisenatide CV outcome trial was published in December 2015. As our search includes records up to August 2014, this was not included in current meta-analysis. Only included in the discussion upon reviewer’s request).
In randomized controlled CV outcome trials, the patient inclusion criteria were history of established CV disease or multiple CV risk factors. All asserted such criteria because it is known that the risk of CV disease is 2 to 4 times higher in people with diabetes[34]. However, it is also known that diabetes substantially increases the risk of major CV complications with and without an established CV disease. Our study focused on the primary prevention patient group with low CV risk. This may explain the differences in results.
Two other meta-analyses have assessed the risk of CV disease among patients using incretins, both examining DPP-4 inhibitors. In the first meta-analysis, overall risk of acute heart failure was higher in patients treated with DPP-4 inhibitors compared to placebo or active comparators (M-H OR: 1.19 (95% CI 1.03–1.37) [35]. However, SAVOR TIMI-53 (Saxagliptin Assessment of Vascular Outcomes Recorded in patients with diabetes mellitus- Thrombolysis in Myocardial Infarction trial) trial [9] accounted for almost two thirds of all included events in this meta-analysis for heart failure, which raises question because SAVOR was large CV outcome trial resulting in safety concerns regarding possible elevated risk in hospitalization for heart failure. Therefore, this might have affected the results of our study suggesting a higher OR in the DPP-4 group.
Another DPP-4 inhibitor meta-analysis included 70 trials, enrolling 41,959 patients with a mean follow-up of 44.1 weeks. The MH-OR was 0.71 (95% CI 0.59–0.86), 0.64 (95% CI 0.44–0.94), 0.77 (95% CI 0.48–1.24) and 0.60 (95% CI 0.41–0.88) for major adverse cardiovascular events (MACE), myocardial infarction, stroke and mortality, respectively. Treatment with DPP4-inhibitors was suggested to reduce the risk of CV events (particularly myocardial infarction) and all-cause mortality in patients with T2DM [36]. The result showed a similar trend of lowering CV risk in DPP-4 inhibitors as our study but had statistical significance. This study had no further restrictions in baseline CV risk, and primary endpoint was only MACE and it also used clinicaltrials.gov as data source. These may have affected difference in included studies and difference in statistical significance.
The heterogeneity of results across meta-analyses or pooled analyses could depend on differences across studies in trial inclusion criteria, definition of events and event adjudication. With respect to pooled analyses of patient-level data, which are available only for each compound separately, the present meta-analysis has the advantage of integrating results for the whole class, thus increasing sample size and statistical power.
Limitations
The primary limitation of this study is that the analysis was executed on summaries of trial results because original source data at patient-level were not available. This prevented the use of potentially more informative descriptions of events. Some of the adverse events reported were aggregated in the study result, for example, reported as non-specified CV disorder, vascular disease, etc. This may had effect in underestimating subgroup analysis of classified CV outcomes. Also, when screening patients with other comorbidities at baseline, we used patient baseline characteristics described in main result only. This practice may not have sufficiently ruled out trials with CV risk patients in the baseline. Similarly, we only included trials explicitly reporting a number of CV adverse events. Thus studies suggesting safety results without specific numbers for each treatment arm, such as ‘cardiovascular event was similar in both groups’ were omitted. In addition, we only gathered information on reported adverse events and we did not focus on the change of cardiovascular markers.
A further limitation is that it is possible that a publication bias affected this analysis, although the funnel plot did not show = significant asymmetry. We did not look for unpublished studies through other sources, so publication bias remains a relevant issue in this review.
Long term safety is of particular concern, the need for long term data on CV outcomes is especially important given the concerns with thiazolidinediones, but trials included had relatively short durations. Mean duration of included studies were 35 weeks, ranging from 12 to 112 weeks, but 70% of the trials included lasted for less than 30 weeks.
Most of the studies included were not primarily aimed at CV end-points. In addition, most trials did not centrally adjudicate CV outcomes. For this reason, a method for assessing CV events was not clearly specified in most instances, and definitions of specific CV events may have differed across studies. In many cases, events were not described in published reports or only available in online supplements. However, we made a persistent effort to collect CV outcomes and excluding patients with underlying disease or any other complications.
Conclusion
In conclusion, the results of this meta-analysis suggest that incretin-based therapy show no significant protective effect on CV events in T2DM primary prevention group with low CV risks.
Current evidence is however, not definitive and associations in prospective long-term safety controlled trials are required to clearly determine the risk/benefit ratio for incretin-based therapies.
Supporting Information
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(PDF)
(DOC)
Acknowledgments
Authors would like to thank Mr. Byunghak Jin, Mr. Jaeyul Yoo for their contributions to this research. This study was supported by Global Center of Excellence in Clinical Trials (HI14C1234), Yonsei University Health System funded by Ministry of Health & Welfare, Republic of Korea.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by Global Center of Excellence in Clinical Trials (HI14C1234), Yonsei University Health System funded by Ministry of Health & Welfare, Republic of Korea.
References
- 1.Meigs JB. Epidemiology of cardiovascular complications in type 2 diabetes mellitus. Acta diabetologica. 2003;40 Suppl 2:S358–61. Epub 2004/01/06. 10.1007/s00592-003-0120-0 . [DOI] [PubMed] [Google Scholar]
- 2.Manuel DG, Schultz SE. Health-related quality of life and health-adjusted life expectancy of people with diabetes in Ontario, Canada, 1996–1997. Diabetes Care. 2004;27(2):407–14. Epub 2004/01/30. . [DOI] [PubMed] [Google Scholar]
- 3.Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care. 1998;21(7):1138–45. Epub 1998/07/08. . [DOI] [PubMed] [Google Scholar]
- 4.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53. Epub 1998/09/22. . [PubMed] [Google Scholar]
- 5.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. The New England journal of medicine. 2008;358(24):2560–72. Epub 2008/06/10. 10.1056/NEJMoa0802987 . [DOI] [PubMed] [Google Scholar]
- 6.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. The New England journal of medicine. 2009;360(2):129–39. Epub 2008/12/19. 10.1056/NEJMoa0808431 . [DOI] [PubMed] [Google Scholar]
- 7.Hiatt WR, Kaul S, Smith RJ. The cardiovascular safety of diabetes drugs—insights from the rosiglitazone experience. The New England journal of medicine. 2013;369(14):1285–7. Epub 2013/09/03. 10.1056/NEJMp1309610 . [DOI] [PubMed] [Google Scholar]
- 8.Guidance for industry: diabetes melitus-evaluating cardiovascular risk in new antidiabetic therpies to treat type 2 diabetes. 2008.
- 9.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. The New England journal of medicine [Internet]. 2013; 369(14):[1317–26 pp.]. [DOI] [PubMed] [Google Scholar]
- 10.White WB, Bakris GL, Bergenstal RM, Cannon CP, Cushman WC, Fleck P, et al. EXamination of CArdiovascular OutcoMes with AlogliptIN versus Standard of CarE in Patients with Type 2 Diabetes Mellitus and Acute Coronary Syndrome (EXAMINE): A cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. American Heart Journal. 2011;162(4):620–6. e1 10.1016/j.ahj.2011.08.004 [DOI] [PubMed] [Google Scholar]
- 11.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. The New England journal of medicine. 2015;373(3):232–42. 10.1056/NEJMoa1501352 . [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. The New England journal of medicine. 2015;373(23):2247–57. 10.1056/NEJMoa1509225 . [DOI] [PubMed] [Google Scholar]
- 13.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27(7):1761–73. Epub 2004/06/29. . [DOI] [PubMed] [Google Scholar]
- 14.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–20. Epub 2013/12/20. 10.1001/jama.2013.284427 . [DOI] [PubMed] [Google Scholar]
- 15.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–45. Epub 2013/11/14. 10.1161/01.cir.0000437738.63853.7a . [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Annals of internal medicine. 2003;139(2):137–47. Epub 2003/07/16. . [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT A D S J. Assessing risk of bias in included studies: Cochrane Collaboration; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315(7109):629–34. Epub 1997/10/06. ; PubMed Central PMCID: PMCPmc2127453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. Journal of clinical epidemiology. 2000;53(11):1119–29. Epub 2000/12/07. . [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327(7414):557–60. Epub 2003/09/06. 10.1136/bmj.327.7414.557 ; PubMed Central PMCID: PMCPmc192859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henry RR, Smith SR, Schwartz SL, Mudaliar SR, Deacon CF, Holst JJ, et al. Effects of saxagliptin on beta-cell stimulation and insulin secretion in patients with type 2 diabetes. Diabetes, obesity & metabolism. 2011;13(9):850–8. Epub 2011/05/11. 10.1111/j.1463-1326.2011.01417.x . [DOI] [PubMed] [Google Scholar]
- 22.Bosi E, Dotta F, Jia Y, Goodman M. Vildagliptin plus metformin combination therapy provides superior glycaemic control to individual monotherapy in treatment-naive patients with type 2 diabetes mellitus. Diabetes, obesity & metabolism [Internet]. 2009; 11(5):[506–15 pp.]. [DOI] [PubMed] [Google Scholar]
- 23.Gallwitz B, Rosenstock J, Rauch T, Bhattacharya S, Patel S, Eynatten M, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet [Internet]. 2012; 380(9840):[475–83 pp.]. [DOI] [PubMed] [Google Scholar]
- 24.Nauck M, Frid A, Hermansen K, Thomsen AB, During M, Shah N, et al. Long-term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes: 2-year results from the LEAD-2 study. Diabetes, Obesity and Metabolism. 2013;15(3):204–12. 10.1111/dom.12012 [DOI] [PubMed] [Google Scholar]
- 25.Best JH, Hoogwerf BJ, Herman WH, Pelletier EM, Smith DB, Wenten M, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care. 2011;34(1):90–5. Epub 2010/10/12. 10.2337/dc10-1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schweizer A, Dejager S, Foley JE, Couturier A, Ligueros-Saylan M, Kothny W. Assessing the cardio-cerebrovascular safety of vildagliptin: Meta-analysis of adjudicated events from a large Phase III type 2 diabetes population. Diabetes, Obesity and Metabolism. 2010;12(6):485–94. 10.1111/j.1463-1326.2010.01215.x [DOI] [PubMed] [Google Scholar]
- 27.Scheen AJ. Cardiovascular effects of gliptins. Nature reviews Cardiology. 2013;10(2):73–84. Epub 2013/01/09. 10.1038/nrcardio.2012.183 . [DOI] [PubMed] [Google Scholar]
- 28.Courreges JP, Vilsboll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, et al. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analogue, on cardiovascular risk biomarkers in patients with Type 2 diabetes. Diabetic medicine: a journal of the British Diabetic Association. 2008;25(9):1129–31. Epub 2009/02/03. 10.1111/j.1464-5491.2008.02484.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu-Hamdah R, Rabiee A, Meneilly GS, Shannon RP, Andersen DK, Elahi D. Clinical review: The extrapancreatic effects of glucagon-like peptide-1 and related peptides. The Journal of clinical endocrinology and metabolism. 2009;94(6):1843–52. Epub 2009/04/02. 10.1210/jc.2008-1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eurich DT, Simpson S, Senthilselvan A, Asche CV, Sandhu-Minhas JK, McAlister FA. Comparative safety and effectiveness of sitagliptin in patients with type 2 diabetes: retrospective population based cohort study. BMJ (Clinical research ed). 2013;346:f2267 Epub 2013/04/27. 10.1136/bmj.f2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marso SP, Lindsey JB, Stolker JM, House JA, Martinez Ravn G, Kennedy KF, et al. Cardiovascular safety of liraglutide assessed in a patient-level pooled analysis of phase 2: 3 liraglutide clinical development studies. Diabetes & vascular disease research: official journal of the International Society of Diabetes and Vascular Disease. 2011;8(3):237–40. Epub 2011/06/10. 10.1177/1479164111408937 . [DOI] [PubMed] [Google Scholar]
- 32.White WB, Pratley R, Fleck P, Munsaka M, Hisada M, Wilson C, et al. Cardiovascular safety of the dipetidyl peptidase-4 inhibitor alogliptin in type 2 diabetes mellitus. Diabetes, obesity & metabolism. 2013;15(7):668–73. Epub 2013/03/16. 10.1111/dom.12093 . [DOI] [PubMed] [Google Scholar]
- 33.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. The New England journal of medicine [Internet]. 2013; 369(14):[1327–35 pp.]. [DOI] [PubMed] [Google Scholar]
- 34.National diabetes fact sheet; general information and national estimates on diabetes in the United States. 2007.
- 35.Monami M, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and heart failure: a meta-analysis of randomized clinical trials. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2014;24(7):689–97. Epub 2014/05/06. 10.1016/j.numecd.2014.01.017 . [DOI] [PubMed] [Google Scholar]
- 36.Monami M, Ahren B, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes, obesity & metabolism. 2013;15(2):112–20. Epub 2012/08/29. 10.1111/dom.12000 . [DOI] [PubMed] [Google Scholar]
- 37.Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes care [Internet]. 2006; 29(12):[2632–7 pp.]. [DOI] [PubMed] [Google Scholar]
- 38.Aschner P, Katzeff HL, Guo H, Sunga S, Williams-Herman D, Kaufman KD, et al. Efficacy and safety of monotherapy of sitagliptin compared with metformin in patients with type 2 diabetes. Diabetes, obesity & metabolism [Internet]. 2010; 12(3):[252–61 pp.]. [DOI] [PubMed] [Google Scholar]
- 39.Barnett AH, Charbonnel B, Donovan M, Fleming D, Chen R. Effect of saxagliptin as add-on therapy in patients with poorly controlled type 2 diabetes on insulin alone or insulin combined with metformin. Current medical research and opinion [Internet]. 2012; 28(4):[513–23 pp.]. [DOI] [PubMed] [Google Scholar]
- 40.Bergenstal R, Lewin A, Bailey T, Chang D, Gylvin T, Roberts V. Efficacy and safety of biphasic insulin aspart 70/30 versus exenatide in subjects with type 2 diabetes failing to achieve glycemic control with metformin and a sulfonylurea. Current medical research and opinion [Internet]. 2009; 25(1):[65–75 pp.]. [DOI] [PubMed] [Google Scholar]
- 41.Blonde L, Dagogo-Jack S, Banerji MA, Pratley RE, Marcellari A, Braceras R, et al. Comparison of vildagliptin and thiazolidinedione as add-on therapy in patients inadequately controlled with metformin: Results of the GALIANT trial—A primary care, type 2 diabetes study. Diabetes, Obesity and Metabolism. 2009;11(10):978–86. 10.1111/j.1463-1326.2009.01080.x [DOI] [PubMed] [Google Scholar]
- 42.Bolli G, Dotta F, Colin L, Minic B, Goodman M. Comparison of vildagliptin and pioglitazone in patients with type 2 diabetes inadequately controlled with metformin. Diabetes, obesity & metabolism [Internet]. 2009; 11(6):[589–95 pp.]. [DOI] [PubMed] [Google Scholar]
- 43.Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes care [Internet]. 2007; 30(4):[890–5 pp.]. [DOI] [PubMed] [Google Scholar]
- 44.Chacra AR, Tan GH, Ravichandran S, List J, Chen R. Safety and efficacy of saxagliptin in combination with submaximal sulphonylurea versus up-titrated sulphonylurea over 76 weeks. Diabetes & vascular disease research [Internet]. 2011; 8(2):[150–9 pp.]. [DOI] [PubMed] [Google Scholar]
- 45.Del PS, Barnett AH, Huisman H, Neubacher D, Woerle HJ, Dugi KA. Effect of linagliptin monotherapy on glycaemic control and markers of beta-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes, obesity & metabolism [Internet]. 2011; 13(3):[258–67 pp.]. [DOI] [PubMed] [Google Scholar]
- 46.Dobs AS, Goldstein BJ, Aschner P, Horton ES, Umpierrez GE, Duran L, et al. Efficacy and safety of sitagliptin added to ongoing metformin and rosiglitazone combination therapy in a randomized placebo-controlled 54-week trial in patients with type 2 diabetes. Journal of diabetes [Internet]. 2013; 5(1):[68–79 pp.]. [DOI] [PubMed] [Google Scholar]
- 47.Filozof C, Gautier JF. A comparison of efficacy and safety of vildagliptin and gliclazide in combination with metformin in patients with type 2 diabetes inadequately controlled with metformin alone: A 52-week, randomized study. Diabetic Medicine. 2010;27(3):318–26. 10.1111/j.1464-5491.2010.02938.x [DOI] [PubMed] [Google Scholar]
- 48.Fonseca VA, Alvarado-Ruiz R, Raccah D, Boka G, Miossec P, Gerich JE. Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care. 2012;35(6):1225–31. Epub 2012/03/21. 10.2337/dc11-1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fonseca V, Zhu T, Karyekar C, Hirshberg B. Adding saxagliptin to extended-release metformin vs. uptitrating metformin dosage. Diabetes, Obesity and Metabolism. 2012;14(4):365–71. 10.1111/j.1463-1326.2011.01553.x [DOI] [PubMed] [Google Scholar]
- 50.Fonseca V, Staels B, Morgan JD, Shentu Y, Golm GT, Johnson-Levonas AO, et al. Efficacy and safety of sitagliptin added to ongoing metformin and pioglitazone combination therapy in a randomized, placebo-controlled, 26-week trial in patients with type 2 diabetes. Journal of diabetes and its complications [Internet]. 2013; 27(2):[177–83 pp.]. [DOI] [PubMed] [Google Scholar]
- 51.Forst T, Uhlig-Laske B, Ring A, Graefe-Mody U, Friedrich C, Herbach K, et al. Linagliptin (BI 1356), a potent and selective DPP-4 inhibitor, is safe and efficacious in combination with metformin in patients with inadequately controlled Type 2 diabetes. Diabetic Medicine. 2010;27(12):1409–19. 10.1111/j.1464-5491.2010.03131.x [DOI] [PubMed] [Google Scholar]
- 52.Gallwitz B, Guzman J, Dotta F, Guerci B, Simo R, Basson BR, et al. Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open-label, randomised controlled trial. Lancet. 2012;379(9833):2270–8. Epub 2012/06/12. 10.1016/s0140-6736(12)60479-6 . [DOI] [PubMed] [Google Scholar]
- 53.Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes, obesity & metabolism. 2007;9(2):166–74. Epub 2007/02/16. 10.1111/j.1463-1326.2006.00684.x . [DOI] [PubMed] [Google Scholar]
- 54.Garber AJ, Foley JE, Banerji MA, Ebeling P, Gudbjornsdottir S, Camisasca RP, et al. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes, obesity & metabolism. 2008;10(11):1047–56. Epub 2008/02/21. 10.1111/j.1463-1326.2008.00859.x . [DOI] [PubMed] [Google Scholar]
- 55.Garber A, Henry RR, Ratner R, Hale P, Chang CT, Bode B. Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes, Obesity and Metabolism. 2011;13(4):348–56. 10.1111/j.1463-1326.2010.01356.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goke B, Gallwitz B, Eriksson JG, Hellqvist A, Gause-Nilsson I. Saxagliptin vs. glipizide as add-on therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: long-term (52-week) extension of a 52-week randomised controlled trial. International journal of clinical practice. 2013;67(4):307–16. Epub 2013/05/03. . [DOI] [PubMed] [Google Scholar]
- 57.Goodman M, Thurston H, Penman J. Efficacy and tolerability of vildagliptin in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et métabolisme. 2009;41(5):368–73. 10.1055/s-0028-1104604 [DOI] [PubMed] [Google Scholar]
- 58.Grunberger G, Chang A, Garcia Soria G, Botros FT, Bsharat R, Milicevic Z. Monotherapy with the once-weekly GLP-1 analogue dulaglutide for 12weeks in patients with Type2 diabetes: Dose-dependent effects on glycaemic control in a randomized, double-blind, placebo-controlled study. Diabetic Medicine. 2012;29(10):1260–7. 10.1111/j.1464-5491.2012.03745.x [DOI] [PubMed] [Google Scholar]
- 59.Haak T, Meinicke T, Jones R, Weber S, von Eynatten M, Woerle HJ. Initial combination of linagliptin and metformin improves glycaemic control in type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes, obesity & metabolism. 2012;14(6):565–74. Epub 2012/02/24. 10.1111/j.1463-1326.2012.01590.x . [DOI] [PubMed] [Google Scholar]
- 60.Hollander PL, Li J, Frederich R, Allen E, Chen R. Safety and efficacy of saxagliptin added to thiazolidinedione over 76 weeks in patients with type 2 diabetes mellitus. Diabetes & vascular disease research: official journal of the International Society of Diabetes and Vascular Disease. 2011;8(2):125–35. Epub 2011/05/13. 10.1177/1479164111404575 . [DOI] [PubMed] [Google Scholar]
- 61.Inagaki N, Atsumi Y, Oura T, Saito H, Imaoka T. Efficacy and Safety Profile of Exenatide Once Weekly Compared With Insulin Once Daily in Japanese Patients With Type 2 Diabetes Treated With Oral Antidiabetes Drug(s): Results From a 26-Week, Randomized, Open-Label, Parallel-Group, Multicenter, Noninferiority Study. Clinical Therapeutics. 2012;34(9):1892–908. e1 10.1016/j.clinthera.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 62.Inagaki N, Onouchi H, Sano H, Funao N, Kuroda S, Kaku K. SYR-472, a novel once-weekly dipeptidyl peptidase-4 (DPP-4) inhibitor, in type 2 diabetes mellitus: A phase 2, randomised, double-blind, placebo-controlled trial. The Lancet Diabetes and Endocrinology. 2014;2(2):125–32. 10.1016/S2213-8587(13)70149-9 [DOI] [PubMed] [Google Scholar]
- 63.Iwamoto Y, Taniguchi T, Nonaka K, Okamoto T, Okuyama K, Ferreira JCA, et al. Dose-ranging efficacy of sitagliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Endocrine Journal. 2010;57(5):383–94. [DOI] [PubMed] [Google Scholar]
- 64.Kaku K, Itayasu T, Hiroi S, Hirayama M, Seino Y. Efficacy and safety of alogliptin added to pioglitazone in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label long-term extension study. Diabetes, obesity & metabolism. 2011;13(11):1028–35. Epub 2011/06/21. 10.1111/j.1463-1326.2011.01460.x . [DOI] [PubMed] [Google Scholar]
- 65.Kaku K, Rasmussen MF, Nishida T, Seino Y. Fifty-two-week, randomized, multicenter trial to compare the safety and efficacy of the novel glucagon-like peptide-1 analog liraglutide vs glibenclamide in patients with type2 diabetes. Journal of Diabetes Investigation. 2011;2(6):441–7. 10.1111/j.2040-1124.2011.00128.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matthews DR, Dejager S, Ahren B, Fonseca V, Ferrannini E, Couturier A, et al. Vildagliptin add-on to metformin produces similar efficacy and reduced hypoglycaemic risk compared with glimepiride, with no weight gain: Results from a 2-year study. Diabetes, Obesity and Metabolism. 2010;12(9):780–9. 10.1111/j.1463-1326.2010.01233.x [DOI] [PubMed] [Google Scholar]
- 67.Matyjaszek-Matuszek B, Lenart-Lipinska M, Rogalska D, Nowakowski A. Exenatide twice daily versus insulin glargine for the treatment of type 2 diabetes in Poland—Subgroup data from a randomised multinational trial GWAA. Endokrynologia Polska. 2013;64(5):375–82. 10.5603/EP.2013.0021 [DOI] [PubMed] [Google Scholar]
- 68.Mohan V, Yang W, Son HY, Xu L, Noble L, Langdon RB, et al. Efficacy and safety of sitagliptin in the treatment of patients with type 2 diabetes in China, India, and Korea. Diabetes Research and Clinical Practice. 2009;83(1):106–16. 10.1016/j.diabres.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 69.Moses RG, Kalra S, Brook D, Sockler J, Monyak J, Visvanathan J, et al. A randomized controlled trial of the efficacy and safety of saxagliptin as add-on therapy in patients with type 2 diabetes and inadequate glycaemic control on metformin plus a sulphonylurea. Diabetes, Obesity and Metabolism. 2014;16(5):443–50. 10.1111/dom.12234 [DOI] [PubMed] [Google Scholar]
- 70.Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP, Tesone P, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: A randomized, double-blind, non-inferiority trial. Diabetes, Obesity and Metabolism. 2007;9(2):194–205. [DOI] [PubMed] [Google Scholar]
- 71.Nauck MA, Ellis GC, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double-blind, placebo-controlled study. International journal of clinical practice. 2009;63(1):46–55. Epub 2009/01/08. 10.1111/j.1742-1241.2008.01933.x . [DOI] [PubMed] [Google Scholar]
- 72.Nonaka K, Kakikawa T, Sato A, Okuyama K, Fujimoto G, Kato N, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes research and clinical practice. 2008;79(2):291–8. Epub 2007/10/16. 10.1016/j.diabres.2007.08.021 . [DOI] [PubMed] [Google Scholar]
- 73.Olansky L, Reasner C, Seck TL, Williams-Herman DE, Chen M, Terranella L, et al. A treatment strategy implementing combination therapy with sitagliptin and metformin results in superior glycaemic control versus metformin monotherapy due to a low rate of addition of antihyperglycaemic agents. Diabetes, obesity & metabolism. 2011;13(9):841–9. Epub 2011/05/04. 10.1111/j.1463-1326.2011.01416.x . [DOI] [PubMed] [Google Scholar]
- 74.Perez-Monteverde A, Seck T, Xu L, Lee MA, Sisk CM, Williams-Herman DE, et al. Efficacy and safety of sitagliptin and the fixed-dose combination of sitagliptin and metformin vs. pioglitazone in drug-naive patients with type 2 diabetes. International Journal of Clinical Practice. 2011;65(9):930–8. 10.1111/j.1742-1241.2011.02749.x [DOI] [PubMed] [Google Scholar]
- 75.Pfutzner A, Paz-Pacheco E, Allen E, Frederich R, Chen R. Initial combination therapy with saxagliptin and metformin provides sustained glycaemic control and is well tolerated for up to 76 weeks. Diabetes, obesity & metabolism. 2011;13(6):567–76. Epub 2011/02/24. 10.1111/j.1463-1326.2011.01385.x . [DOI] [PubMed] [Google Scholar]
- 76.Philis-Tsimikas A, Prato S, Satman I, Bhargava A, Dharmalingam M, Skjoth TV, et al. Effect of insulin degludec versus sitagliptin in patients with type 2 diabetes uncontrolled on oral antidiabetic agents. Diabetes, obesity & metabolism [Internet]. 2013; 15(8):[760–6 pp.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pinget M, Goldenberg R, Niemoeller E, Muehlen-Bartmer I, Guo H, Aronson R. Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P). Diabetes, obesity & metabolism. 2013;15(11):1000–7. Epub 2013/05/01. 10.1111/dom.12121 . [DOI] [PubMed] [Google Scholar]
- 78.Pratley RE, Jauffret-Kamel S, Galbreath E, Holmes D. Twelve-week monotherapy with the DPP-4 inhibitor vildagliptin improves glycemic control in subjects with type 2 diabetes. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2006;38(6):423–8. Epub 2006/07/11. 10.1055/s-2006-944546 . [DOI] [PubMed] [Google Scholar]
- 79.Pratley RE, Kipnes MS, Fleck PR, Wilson C, Mekki Q, Castano P, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes inadequately controlled by glyburide monotherapy. Diabetes, Obesity and Metabolism. 2009;11(2):167–76. 10.1111/j.1463-1326.2008.01016.x [DOI] [PubMed] [Google Scholar]
- 80.Pratley RE, Reusch JE, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin added to pioglitazone in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Current medical research and opinion. 2009;25(10):2361–71. Epub 2009/08/05. 10.1185/03007990903156111 . [DOI] [PubMed] [Google Scholar]
- 81.Pratley RE, Urosevic D, Boldrin M, Balena R. Efficacy and tolerability of taspoglutide versus pioglitazone in subjects with type 2 diabetes uncontrolled with sulphonylurea or sulphonylurea-metformin therapy: A randomized, double-blind study (T-emerge 6). Diabetes, Obesity and Metabolism. 2013;15(3):234–40. 10.1111/dom.12009 [DOI] [PubMed] [Google Scholar]
- 82.Prato S. Linagliptin for the treatment of type 2 diabetes. Expert opinion on pharmacotherapy [Internet]. 2011; 12(17):[2759–62 pp.]. [DOI] [PubMed] [Google Scholar]
- 83.Ratner R, Nauck M, Kapitza C, Asnaghi V, Boldrin M, Balena R. Safety and tolerability of high doses of taspoglutide, a once-weekly human GLP-1 analogue, in diabetic patients treated with metformin: a randomized double-blind placebo-controlled study. Diabetic medicine: a journal of the British Diabetic Association. 2010;27(5):556–62. Epub 2010/06/12. 10.1111/j.1464-5491.2010.02990.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raz I, Chen Y, Wu M, Hussain S, Kaufman KD, Amatruda JM, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Current Medical Research and Opinion. 2008;24(2):537–50. 10.1185/030079908X260925 [DOI] [PubMed] [Google Scholar]
- 85.Reasner C, Olansky L, Seck TL, Williams-Herman DE, Chen M, Terranella L, et al. The effect of initial therapy with the fixed-dose combination of sitagliptin and metformin compared with metformin monotherapy in patients with type 2 diabetes mellitus. Diabetes, Obesity and Metabolism. 2011;13(7):644–52. 10.1111/j.1463-1326.2011.01390.x [DOI] [PubMed] [Google Scholar]
- 86.Riddle MC, Aronson R, Home P, Marre M, Niemoeller E, Miossec P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes care. 2013;36(9):2489–96. Epub 2013/05/01. 10.2337/dc12-2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: A 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clinical Therapeutics. 2006;28(10):1556–68. [DOI] [PubMed] [Google Scholar]
- 88.Rosenstock J, Niggli M, Maldonado-Lutomirsky M. Long-term 2-year safety and efficacy of vildagliptin compared with rosiglitazone in drug-naive patients with type 2 diabetes mellitus. Diabetes, Obesity and Metabolism. 2009;11(6):571–8. 10.1111/j.1463-1326.2008.01021.x [DOI] [PubMed] [Google Scholar]
- 89.Rosenstock J, Rendell MS, Gross JL, Fleck PR, Wilson CA, Mekki Q. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1C) without causing weight gain or increased hypoglycaemia. Diabetes, obesity & metabolism. 2009;11(12):1145–52. Epub 2009/09/18. 10.1111/j.1463-1326.2009.01124.x . [DOI] [PubMed] [Google Scholar]
- 90.Rosenstock J, Hanefeld M, Shamanna P, Min KW, Boka G, Miossec P, et al. Beneficial effects of once-daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in Type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal-S). Journal of Diabetes and its Complications. 2014;28(3):386–92. 10.1016/j.jdiacomp.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 91.Ross SA, Rafeiro E, Meinicke T, Toorawa R, Weber-Born S, Woerle HJ. Efficacy and safety of linagliptin 2.5 mg twice daily versus 5 mg once daily in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, placebo-controlled trial. Current medical research and opinion. 2012;28(9):1465–74. Epub 2012/07/24. 10.1185/03007995.2012.714360 . [DOI] [PubMed] [Google Scholar]
- 92.Russell-Jones D, Cuddihy RM, Hanefeld M, Kumar A, González JG, Chan M, et al. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study. Diabetes care [Internet]. 2012; 35(2):[252–8 pp.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scherbaum WA, Schweizer A, Mari A, Nilsson PM, Lalanne G, Wang Y, et al. Evidence that vildagliptin attenuates deterioration of glycaemic control during 2-year treatment of patients with type 2 diabetes and mild hyperglycaemia. Diabetes, obesity & metabolism. 2008;10(11):1114–24. Epub 2008/03/22. 10.1111/j.1463-1326.2008.00875.x . [DOI] [PubMed] [Google Scholar]
- 94.Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes care. 2013;36(9):2508–15. Epub 2013/04/09. 10.2337/dc12-2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seino Y, Hiroi S, Hirayama M, Kaku K. Efficacy and safety of alogliptin added to sulfonylurea in Japanese patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial with an open-label, long-term extension study. Journal of Diabetes Investigation. 2012;3(6):517–25. 10.1111/j.2040-1124.2012.00226.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seino Y, Miyata Y, Hiroi S, Hirayama M, Kaku K. Efficacy and safety of alogliptin added to metformin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label, long-term extension study. Diabetes, obesity & metabolism. 2012;14(10):927–36. Epub 2012/05/16. 10.1111/j.1463-1326.2012.01620.x . [DOI] [PubMed] [Google Scholar]
- 97.Seino Y, Inagaki N, Miyahara H, Okuda I, Bush M, Ye J, et al. A randomized dose-finding study demonstrating the efficacy and tolerability of albiglutide in Japanese patients with type 2 diabetes mellitus. Current Medical Research and Opinion. 2014;30(6):1095–106. 10.1185/03007995.2014.896327 [DOI] [PubMed] [Google Scholar]
- 98.Strain WD, Lukashevich V, Kothny W, Hoellinger MJ, Paldanius PM. Individualised treatment targets for elderly patients with type 2 diabetes using vildagliptin add-on or lone therapy (INTERVAL): a 24 week, randomised, double-blind, placebo-controlled study. Lancet. 2013;382(9890):409–16. Epub 2013/05/28. 10.1016/s0140-6736(13)60995-2 . [DOI] [PubMed] [Google Scholar]
- 99.Tajima N, Kadowaki T, Odawara M, Nishii M, Taniguchi T, Ferreira JCA. Addition of sitagliptin to ongoing glimepiride therapy in Japanese patients with type 2 diabetes over 52 weeks leads to improved glycemic control. Diabetology International. 2011;2(1):32–44. [Google Scholar]
- 100.Tajima N, Kadowaki T, Okamoto T, Sato A, Okuyama K, Minamide T, et al. Sitagliptin added to voglibose monotherapy improves glycemic control in patients with type 2 diabetes. Journal of Diabetes Investigation. 2013;4(6):595–604. 10.1111/jdi.12116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Taskinen M, Rosenstock J, Tamminen I, Kubiak R, Patel S, Dugi KA, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled study. Diabetes, Obesity and Metabolism. 2011;13(1):65–74. 10.1111/j.1463-1326.2010.01326.x [DOI] [PubMed] [Google Scholar]
- 102.Terra SG, Somayaji V, Schwartz S, Lewin AJ, Teeter JG, Dai H, et al. A Dose-Ranging Study of the DPP-IV Inhibitor PF-734200 Added to Metformin in Subjects With Type 2 Diabetes*. Experimental and clinical endocrinology & diabetes [Internet]. 2011; 119(7):[401–7 pp.]. [DOI] [PubMed] [Google Scholar]
- 103.Vilsboll T, Rosenstock J, Yki-Jarvinen H, Cefalu WT, Chen Y, Luo E, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes, Obesity and Metabolism. 2010;12(2):167–77. 10.1111/j.1463-1326.2009.01173.x [DOI] [PubMed] [Google Scholar]
- 104.White JL, Buchanan P, Li J, Frederich R. A randomized controlled trial of the efficacy and safety of twice-daily saxagliptin plus metformin combination therapy in patients with type 2 diabetes and inadequate glycemic control on metformin monotherapy. BMC Endocrine Disorders. 2014;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Williams-Herman D, Johnson J, Teng R, Luo E, Davies MJ, Kaufman KD, et al. Efficacy and safety of initial combination therapy with sitagliptin and metformin in patients with type 2 diabetes: A 54-week study. Current Medical Research and Opinion. 2009;25(3):569–83. 10.1185/03007990802705679 [DOI] [PubMed] [Google Scholar]
- 106.Wysham C, Blevins T, Arakaki R, Colon G, Garcia P, Atisso C, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care. 2014;37(8):2159–67. 10.2337/dc13-2760 [DOI] [PubMed] [Google Scholar]
- 107.Yang W, Pan CY, Tou C, Zhao J, Gause-Nilsson I. Efficacy and safety of saxagliptin added to metformin in Asian people with type 2 diabetes mellitus: a randomized controlled trial. Diabetes research and clinical practice. 2011;94(2):217–24. Epub 2011/08/30. 10.1016/j.diabres.2011.07.035 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(PDF)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.