Editor
Mycosis fungoides (MF) and Sézary syndrome (SS), major variants of cutaneous T-cell lymphoma (CTCL), are malignancies of clonal T-lymphocytes, and advanced MF/SS are associated with a 40–47% 5-year survival.1–4 Extracorporeal photopheresis (ECP), a therapy for CTCL, has been demonstrated to have a mean time to clinical response of 22.4±9.6 weeks, which indicates patients should be treated with ECP for up to 8 months before effectiveness can be accurately assessed.5 Currently we cannot predict who will respond to ECP.
MicroRNA (miRNA) are small, non-coding RNA that can inhibit protein translation.6 CTCL has been reported to have altered miRNA expression, including reduced miR-191, miR-223, miR-342, and increased miR-155.7–9 The primary goal of this study was to determine if a select miRNA profile in peripheral blood mononuclear cells (PBMC) obtained at baseline and 3 months of ECP monotherapy could predict clinical response to ECP.
This retrospective study utilized the CTCL tissue repository at Johns Hopkins and was approved by the Johns Hopkins Institutional Review Board. Thirteen subjects were selected with biopsy-proven MF or SS and use of monotherapy ECP. A single Dermatologist oversaw the administration of and response to ECP, as described previously.10
Immediately before the start of ECP and 3 months later, blood was taken and PBMC were isolated and cryopreserved in liquid nitrogen. Total RNA was extracted and TaqMan MicroRNA Assays were run in triplicate, and expression was compared with RNU24. The Student’s t-test and Fisher’s Exact test were used to evaluate for significant differences.
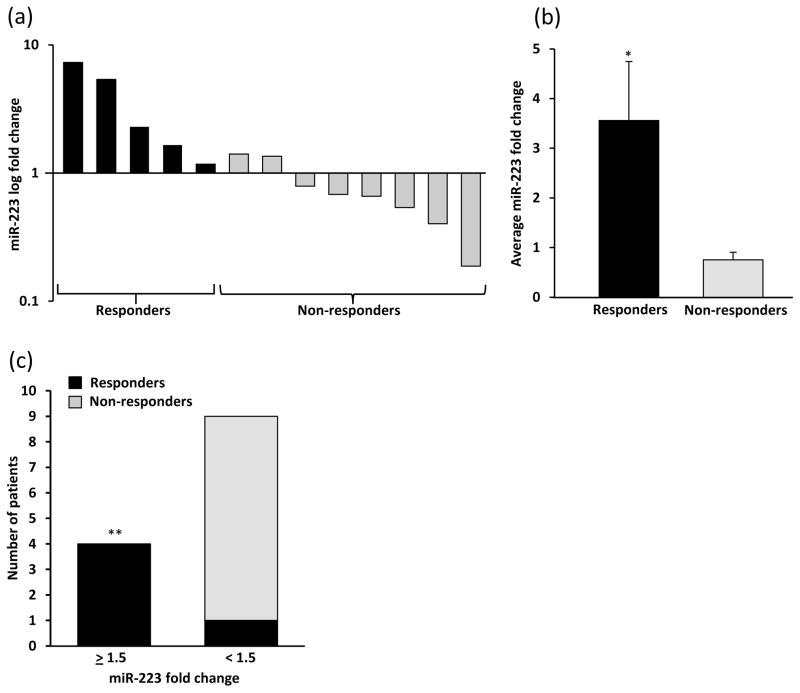
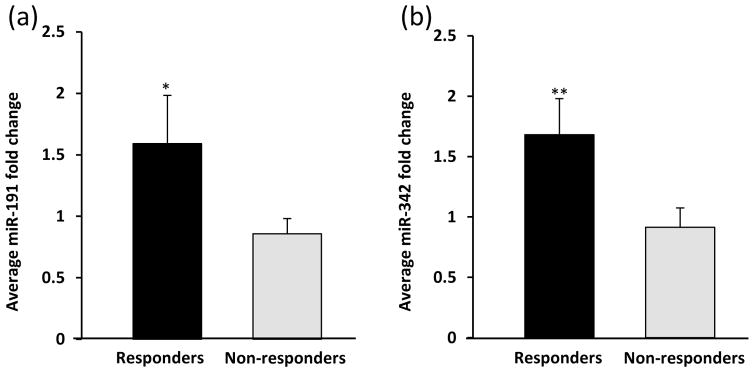
Of the 13 subjects, 5 (38%) achieved an overall clinical response (minimal and partial responses; MR/PR) at the final evaluation (12 months ECP monotherapy or at addition of new systemic therapy). Evaluation of the fold change in miR-223 from baseline to 3 months post-ECP therapy identified a significant increase in miR-223 in those who had a final response (n=5) versus those with no response or disease progression (NR/DP; n=8) [3.56 (SEM±1.19) vs. 0.75 (SEM±0.15) *p=0.01; Fig. 1a, b]. Those whose miR-223 increased over 1.5 fold from baseline to 3 months were more likely to respond to ECP (**p<0.01; Fig. 1c). We also identified an increase in the mean fold change of both miR-191 and miR-342 in MR/PR (n=3) versus NR/DP (n=6) after 3 months of ECP treatment [miR-191: 1.59 (SEM±0.39) vs. 0.86 (SEM±0.13) *p=0.05; miR-342: 1.68 (SEM±0.29) vs. 0.91 (SEM±0.16) **p=0.04; Fig. 2a, b]. We did not identify a significant difference in 3-month change of miR-423-5p (expression not altered in CTCL)8 or miR-155, and there was no difference in baseline expression of any miRNA between responders and non-responders.
Figure 1. Increased miR-223 at 3 months ECP therapy is associated with overall clinical response.
(a) Fold change of miR-223 from baseline to 3 months of ECP monotherapy in individual patients. (b) Mean fold change in miR-223 at 3 months of those with a clinical response between 6 and 12 months of ECP and those that did not respond to ECP (*p=0.01). (c) Fold change of miR-223 from baseline to 3 months post-ECP therapy that was greater than or equal to 1.5 was predictive of clinical response to ECP between 6 and 12 months (**p<0.01).
Figure 2. miR-191 and miR-342 increase at 3 months in those with clinical response to ECP.
Mean fold change of (a) miR-191 (*p=0.05) and (b) miR-342 (**p=0.04) in patients who responded to ECP compared to those who did not respond.
We demonstrate that an early increase of PBMC miR-191, miR-223, and miR-342 at 3 months post-therapy is predictive of a clinical response to ECP between 6 and 12 months. It is not clear if ECP directly increases levels of these miRNA in the malignant T-cell, or if the increase is due to an influx of normal PBMC. Measurement of these miRNA levels is nonetheless useful to help predict those who will respond to ECP. Notably, the miR-423-5p and miR-155 expression did not differ, indicating that our results represent a selective upregulation and not a global miRNA increase.
In advanced MF/SS, we are encountering a dire clinical scenario, as patients face high mortality, limited therapeutic options, and often a long median time to response with current therapies. Therefore, it is imperative we identify predictors of response for ECP in order to reduce time spent on futile therapies with an overall goal of enhancing patient survival. Larger studies are warranted to further evaluate the role of miRNA as predictors of response to therapy in MF/SS.
Acknowledgments
Funding Sources: L.Y.M. is supported by the Dermatology Foundation, and NIH/NCI (K12CA090625), American Cancer Society (IRG-58-009-51), NIH (UL1TR000445), and the Vanderbilt Department of Medicine/Dermatology. C.M.E. is supported by R01CA148950 and the Vanderbilt Department of Pathology, Microbiology and Immunology.
Footnotes
Conflicts of Interest: None to disclose
References
- 1.Lansigan F, Choi J, Foss FM. Cutaneous T-cell lymphoma. Hematology/oncology clinics of North America. 2008;22:979–96. x. doi: 10.1016/j.hoc.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Zackheim HS, Amin S, Kashani-Sabet M, et al. Prognosis in cutaneous T-cell lymphoma by skin stage: long-term survival in 489 patients. Journal of the American Academy of Dermatology. 1999;40:418–25. doi: 10.1016/s0190-9622(99)70491-3. [DOI] [PubMed] [Google Scholar]
- 3.Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides. Critical reviews in oncology/hematology. 2008;65:172–82. doi: 10.1016/j.critrevonc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:4730–9. doi: 10.1200/JCO.2009.27.7665. [DOI] [PubMed] [Google Scholar]
- 5.Edelson R, Berger C, Gasparro F, et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. Preliminary results. The New England journal of medicine. 1987;316:297–303. doi: 10.1056/NEJM198702053160603. [DOI] [PubMed] [Google Scholar]
- 6.Lagos-Quintana M, Rauhut R, Lendeckel W, et al. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–8. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 7.Ballabio E, Mitchell T, van Kester MS, et al. MicroRNA expression in Sezary syndrome: identification, function, and diagnostic potential. Blood. 2010;116:1105–13. doi: 10.1182/blood-2009-12-256719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ralfkiaer U, Hagedorn PH, Bangsgaard N, et al. Diagnostic microRNA profiling in cutaneous T-cell lymphoma (CTCL) Blood. 2011;118:5891–900. doi: 10.1182/blood-2011-06-358382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGirt LY, Adams CM, Baerenwald DA, Zic JA, Eischen CM. miR-223 regulates proliferation and targets proto-oncogenes in mycosis fungoides/cutaneous T-cell lymphoma. Journal of Investigative Dermatology. 2014;134:1101–7. doi: 10.1038/jid.2013.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGirt LY, Thoburn C, Hess A, et al. Predictors of response to extracorporeal photopheresis in advanced mycosis fungoides and Sezary syndrome. Photodermatology, photoimmunology & photomedicine. 2010;26:182–91. doi: 10.1111/j.1600-0781.2010.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]