Abstract
Recent models of interval timing have emphasized local, modality-specific processes or a core network centered on a cortico-thalamic-striatal circuit, leaving the role of the cerebellum unclear. We examine this issue, using current taxonomies of timing as a guide to review the association of the cerebellum in motor and perceptual tasks in which timing information is explicit or implicit. Evidence from neuropsychological, neurophysiological, and neuroimaging studies indicates that the involvement of the cerebellum in timing is not restricted to any subdomain of this taxonomy. However, an emerging pattern is that tasks in which timing is done in cyclic continuous contexts do not rely on the cerebellum. In such scenarios, timing may be an emergent property of system dynamics, and especially oscillatory entrainment. The cerebellum may be necessary to time discrete intervals in the absence of continuous cyclic dynamics.
Introduction
While time is a central organizing dimension of experience and interaction with the world [1], the absence of a sensory pathway to directly transduce temporal quantity has made it difficult to understand the neural mechanisms by which we represent time. With the emergence of cognitive neuroscience tools to map function and structure, research on timing focused on identifying the contribution of neuroanatomical structures to tasks involving temporal processing. Inspired by ‘internal clock’ models of timing [2, 3], as well as behavioral work suggesting that timing was supramodal [4, 5], early research of timing attempted to isolate dedicated and centralized timing systems that operate across tasks parameters [6]. Following observations that cerebellar lesions lead to behavioral deficits on a range of timing tasks [7-9], it was hypothesized that the cerebellum played a critical role in the precise representation of temporal information.
Subsequent work, some of it using identical tasks as those used in the cerebellar studies, pointed to the involvement of other neural structures in temporal processing, including the basal ganglia (BG), supplementary motor area (SMA), right inferior frontal cortex, and left inferior parietal cortex [10, 11, 12*, 13]. This body of work has motivated current influential models [14, 15, 16*], including the idea that the core implementation of duration representation centers on a cortico-thalamic-striatal network comprised of the SMA, BG and thalamus, as well as models in which timing is a ubiquitous property of neural function, not dependent on specialized, amodal mechanisms [17].
The role of the cerebellum in this picture is fuzzy. In humans, the cerebellum has generally been less accessible to study with some of the tools of cognitive neuroscience: Few EEG studies have attempted to focus on signal sources attributed to the cerebellum, many fMRI studies employ slice angles that provide minimal cerebellar coverage, and, unlike Parkinson's disease, the treatment of cerebellar disorders has not yet led to the development of pharmacological and physiological interventions that can be exploited to test functional hypotheses [18-19]. Theoretically, different hypotheses have been offered to recast the role of the cerebellum in timing. At one extreme is the view that the cerebellum serves as a compensatory route to support temporal processing, and this becomes especially apparent when the cortico-striatal route is malfunctioning [16]. An alternative is that that the cerebellum is recruited by the cortico-striatal network in a context-dependent manner, for example when timing intervals in the sub-second range [20] (but see [21]).
In evaluating functional hypotheses, it is important to recognize that the scope of timing research has become much broader over the past decades and, as such, the number of tasks falling under this rubric has become much larger. As evident in this volume, “timing tasks” come in many different flavors. The challenge is to identify the computational principles and neural mechanisms that allow us to perceive temporal quantities, exploit temporal regularities, and produce actions that exhibit consistent temporal features. A useful approach is to consider current taxonomic classifications in the timing literature.
In a seminal review, Coull and Nobre [22**] argued that the representation of time may be implemented in different neuroanatomical networks depending on whether timing is explicit or implicit to the task at hand. They suggested that timing is explicit in scenarios in which an overt report of a temporal quantity is required, such as when judging which of two events is longer. Conversely, timing is implicit when an overt report is not required, but rather the temporal information can optimize performance on a non-temporal task. A typical example is when a target in a speeded detection task is likely to appear at a specified point in time following a warning signal. Performance is facilitated when the target appears at the expected time compared to when the timing is unexpectedly changed or when the timing is random. This behavioral change indicates that the interval duration was measured at some level and used to form predictions that guide attention in time [11, 23]. Coull and Nobre [22**] also emphasized that the explicit/implicit dimension is orthogonal to a common distinction that is made in timing literature between motor timing, in which timing is part of the planning or execution of an action, and perceptual timing, tasks in which no movement is required [24]. For example, judging which of two intervals is longer is a case of explicit perceptual timing, while producing a motor act whose length matches that of a previously learned interval is a case of explicit motor timing.
The combination of these two dimensions creates four sub-domains of timing. We examine here if there are particular sub-domains that rely on or involve the cerebellum. Our analysis is limited to tasks in the subsecond range given that most of the work on cerebellar timing has been limited to tasks in this range [20].
The role of the cerebellum in explicit and implicit motor timing
The cerebellum has traditionally been associated with motor timing. Cerebellar ataxia includes, among others, difficulties in the precise temporal control of voluntary action. Arm and eye movements are dysmetric, under- or overshooting the target [25, 26], and speech becomes dysarthric, with a loss of clarity of individual phonemes and abnormalities in rate and modulation [27]. Direct tests of explicit motor timing have involved tasks such as the interval reproduction task in which the onset or duration of a movement must match a previously learned, discrete interval. Cerebellar patients exhibit larger variability when in reproducing the intervals [20]. Correspondingly, activation increases in the cerebellum are observed during interval reproduction [24], and applying transcranial magnetic stimulation (TMS) to the cerebellum can produce temporal distortions [28].
Individuals with cerebellar lesions are also impaired on the synchronization-continuation tapping task (SCT). In this task, traditionally viewed as the task of choice for explicit sensorimotor timing, repetitive movements (e.g. tapping) are first synchronized to an external metronome and then continued at the same pace after the metronome is terminated. Again, the primary deficit is an increase in variability, observed during both the paced and unpaced phases [7, 9, 29]. Moreover, imaging studies in healthy individuals have reported increased activation in the cerebellum during both stages of the SCT [30-33]. Recent modeling work confirms that the deficit in this task in cerebellar patients is related to variability of a timekeeping component [34].
The cerebellum is implicated not only in explicit, but also in implicit motor timing. Perhaps the best evidence for this comes from the vast literature on eyeblink conditioning. Learning in this task not only requires forming an association between the conditioned (e.g., tone) and unconditioned stimuli (e.g., airpuff), but also learning the precise temporal relationship between these events. This allows for the execution of the conditioned response (CR) at the expected time of the unconditioned stimulus. Timing is implicit, not only in the sense that participants are unaware of the temporal regularity, but also in that timing here entails a form of prediction that allows the formation of a novel association. Lesions to the cerebellum impair this learning, and specifically the ability to accurately time the CR [35-36]. Indeed, in trained animals, lesions of the cerebellar cortex abolish the precise timing; the CR is now time-locked to the CS rather than the US [35]. Eyeblink conditioning has been perhaps the most sophisticated model system for studying the cellular and molecular mechanisms of timing [37, 38*, 39].
However, the cerebellum is not necessary in all implicit motor timing contexts. Spencer and colleagues [40, 41**, but see 42] found that individuals with cerebellar degeneration exhibit minimal impairment when producing circles at a constant rate. This performance stands in contrast to the increased variability observed when the periodic movements are produced by finger tapping or alternating phases of circle drawing and pauses. The authors proposed that the cerebellum is not essential when timing is emergent, reflecting the operation of a control variable associated with regulating dynamics in a continuous manner. This hypothesis is consistent with the finding that discontinuous rhythmic movements activate the cerebellum more compared to continuous rhythmic movements [43].
The cerebellum in explicit and implicit perceptual timing
The functional domain of the cerebellum is not limited to sensorimotor control, with neuroimaging and neuropsychological studies suggesting a role in domains as diverse as attention, affect, and language. As part of the interest in non-motor functions of the cerebellum, there has been considerable study of explicit and implicit timing tasks. Duration estimation/discrimination tasks have been used to study explicit timing, as the response requirements are minimal and control tasks can involve similar stimuli but require perceptual judgments of non-temporal properties (e.g., position, loudness). Several lines of evidence indicate that the cerebellum is essential for duration estimation. Patients with cerebellar degeneration exhibit elevated discrimination thresholds on duration discrimination tasks (e.g., require greater difference to determine if a test interval is longer or shorter than a standard interval) [7, 44], and imaging studies in healthy individual have revealed task-specific activations within the cerebellum [45-46] or changes in cerebro-cerebellar interactions, especially with the SMA and premotor cortex [47-48].
Interestingly, as shown in a series of studies using an extensive battery of tests, not all forms of explicit perceptual timing depend on the cerebellum [49*, 50, 51]. While confirming earlier reports of increased perceptual thresholds in judging the duration of single intervals, the results revealed an interesting dissociation. The patients did not show an impairment when the temporal judgments were conducted in the context of rhythmic streams; for example, when the task required deciding which of two streams was more isochronous, or which contained a temporal deviance from isochronism. Thus, the performance of cerebellar patients was impaired for single intervals but not for beat-based judgments [49*]. A similar dissociation was observed following continuous transcranial magnetic theta-burst stimulation to the cerebellum in healthy individuals [50] and in fMRI [51].
A representative task of implicit perceptual timing is the temporal orienting task in which participants use trial-by-trial cues to temporally anticipate a target stimulus that requires a discriminative response [11, 52, 53]. Performance benefits are observed when the interval between the cue and target is fixed. Imaging studies have revealed increased activations in the cerebellum in this task relative to conditions in which prediction is purely spatial [11] or in conditions in which the target is temporally unpredictable [52]. However, when speeded responses are required, the temporal cue may facilitate an anticipatory perceptual process or motor preparation, or both [53]. Furthermore, the fact that the duration is not overtly reported does not rule out the possibility that it is covertly tracked, perhaps even in an explicit manner, similar to that in duration estimation tasks. Thus it is problematic to refer to timing as purely ‘implicit’ when the interval between the cue and target is fixed. In an interesting variation, the cue-target interval is not specified on each trial, but preparation can be adjusted according to the probability distribution of intervals in previous trials [54]. Thus, while the motor component remains, timing would now seem to be more implicit given that there is no single interval to be timed. Patients with lesions to the cerebellum show difficulties in performing this adjustment [55].
In another task used to study perceptual timing, a moving object disappears behind an occluder and then reappears after a short interval. In the timing condition, the judgment is based on whether the object reappeared at the expected or unexpected time; in the spatial condition, the judgment is based on whether the object appeared at an expected or unexpected location. Timing here may be implicit, in that the judgment is assumed to be based on an inference about the velocity of the stimulus. Cerebellar activation is greater in the timing condition compared to the spatial condition [56]. Similarly, patients with cerebellar degeneration have difficulty on interception tasks [57].
Timing is also considered implicit when the stimulation is rhythmic such as in music, speech, and biological motion. In a representative task, participants are presented with a rhythmic stream of stimuli prior to the appearance of a target that requires a non-speeded response concerning a non-temporal property (58, 59). Performance benefits are observed when the target appears on the beat relative to when the target is off the beat (or, in other control conditions, when the stream is non-isochronous). This implies the operation of a predictive temporal adjustment, similar to that in temporal cueing tasks. This form of anticipation appears to be implicit, as it occurs even when explicit timing is engaged by a secondary task [60*]. In contrast to the implicit perceptual timing tasks reviewed above, imaging studies involving rhythmic predictions fail to find activations in the cerebellum [61, 62*]. Indeed, a contrast of isochronous versus non-isochronous streams often finds relatively greater cerebellar activation in the non-isochronous condition [62*].
Absence of cerebellar involvement in temporal representation arising from rhythmic dynamics
An interesting pattern emerges when considering those tasks, shown in red in Figure 1, that do not appear to involve the cerebellum. A common denominator to these tasks is that timing can be based on some form of continuous dynamics. One example from the motor domain is circle drawing in which it has been proposed that the continuous nature of the movements allows timing to become implicit [41**]. In the perceptual domain, representative explicit and implicit tasks include beat-based temporal discriminations and rhythmic orientation, respectively.
Figure 1.
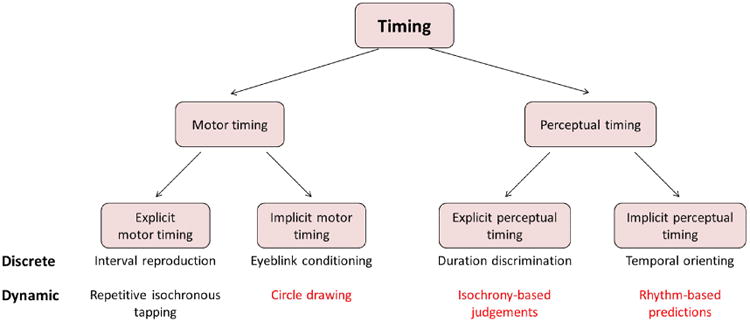
The involvement of the cerebellum in subdomains of timing. As suggested by Coull and Nobre [22**], timing can be conducted explicitly or implicitly, in the motor or perceptual domains. Representative tasks are listed for each subdomain, some of which involve the cerebellum (black) and some that do not (red). The cerebellum is associated with all tasks that are based on temporal contexts defined by discrete intervals. In contrast, tasks that do not involve the cerebellum are those in which timing is established or emerges from a continuous cyclic context.
Presumably, non-cerebellar circuits such as the basal ganglia or SMA are essential for rhythmic processing [62-64]. Alternatively, in continuous cyclic contexts, timing may be an emergent property of system dynamics. For example, temporal regularities in circle drawing can emerge from a control parameter such as the maintenance of a constant angular velocity [40, 41**]. In rhythm-based perceptual timing, a similar indirect representation of time could come about from the exploitation of neural oscillations in sensory circuits. While oscillatory mechanisms are central in some models of dedicated timing (e.g., pacemaker-accumulator models [2, 3]), exposure to rhythmic stimuli may induce oscillations in sensory circuits or entrain pre-existing oscillations in a stimulus-driven manner [65, 66]. Recent evidence indicates that these oscillations can support temporal predictions [58, 59, 67**, 68]. Oscillations can also inform explicit temporal judgments. For example, a deviation in a rhythmic sequence can be expressed as a phase shift of a stimulus relative to an entrained oscillator.
Notably, Coull and Nobre have suggested that, for implicit perceptual timing, a distinction should be made between predictions that emerge as a by-product of temporal regularities in the stimulus (e.g., motion, rhythm or the passage of time itself) and endogenous predictions that are based on an internal comparison of elapsed time to a memorized interval (e.g., as in single-interval temporal orientating tasks) [22**]. With respect to the cerebellum, the relevant functional distinction is between timing that is performed discretely between defined events and timing that emerges from the continuous dynamics, with the cerebellum critical for the former. This distinction is not only relevant for implicit perceptual timing, but applies generally.
One branch that fails to follow the interval/rhythmic taxonomic dissociation is the finding that cerebellar pathology does disrupt performance in rhythmic tapping tasks. Ironically, this is the task that provided the first direct tests of the cerebellar timing hypothesis [7]. It is important to consider two issues here. First, repetitive tapping relies on the prediction of forthcoming events such as a metronome signal during a pacing phase or the sensory events of the tap during both paced and unpaced phases. These predictions, or rather violations of such predictions may trigger corrective processes. The cerebellum is strongly linked, even in non-temporal domains, to the generation of sensorimotor predictions and use of this information for error-based adjustments [69]. Thus, cerebellar involvement in rhythmic tapping may be related to other functions of this structure (see also [70]).
Second, while repetitive tapping would appear to be a rhythmic task, formal models suggest that the series of events are really the result of a concatenation process of successive samples from a single-interval control process [71]. Consistent with this hypothesis, performance in repetitive tapping, but not circle drawing, is correlated with explicit duration discrimination [72]. These two hypotheses linking the cerebellum to repetitive tapping, one based on error correction and the other on a concatenation process of discrete intervals/events, would suggest that this task is misclassified as representative of continuous, explicit motor timing.
Conclusions
We have examined three prominent dimensions in the timing literature in search of general principles that can help define the functional domain of the cerebellum in temporal processing. As summarized in this brief review, the evidence indicates that the domain encompasses both motor and perceptual timing, and is observed in both explicit and implicit tasks. We have highlighted one important constraint, namely that the cerebellum may be required when tasks require timing single intervals, or what we have called event timing [38, 39], but not when timing is inherent to rhythmic or continuous dynamics.
Importantly, we do not claim that this pattern is unique to the cerebellum, or that other brain regions are not essential for event timing as defined here. Certainly the neuroimaging evidence suggests that cerebellar activation in such tasks is accompanied by activation in a wide network of timing-related structures, including the BG and SMA [15, 30-33, 45-46, 52], as well as modulation of cerebro-cerebellar interactions [47-48]. The cerebellum may contribute to this network through its capability to precisely time isolated intervals in the absence of a temporal context.
It should also be noted that there is considerable unevenness in terms of the amount and variety of evidence associated with the different branches in Figure 1, including some conditions that have yet to be tested in lesion studies. We hope that this review will help inspire new tests to fill in these gaps, as well as motivate experimental designs that can provide direct tests of the value of this sort of taxonomic classification.
Highlights.
Examine the involvement of the cerebellum in timing using current timing taxonomies
Cerebellar involved in explicit and implicit timing in motor and perceptual tasks
The cerebellum is not required when timing emerges from dynamic, rhythmic contexts
Acknowledgments
This work was supported by National Institute of Health NS092079 grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kant I. Critique of pure reason. Cambridge: Cambridge University Press; 1781/1999. [Google Scholar]
- 2.Treisman M. Temporal discrimination and the indifference interval: Implications for a model of the “internal clock”. Psychological Monographs. 1963;77:1–31. doi: 10.1037/h0093864. [DOI] [PubMed] [Google Scholar]
- 3.Gibbon J, Church RM, Meck WH. Scalar timing in memory. Ann NY Acad Sci. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- 4.Keele SW, Pokorny RA, Corcos DM, Ivry RB. Do perception and motor production share common timing mechanisms: a correctional analysis. Acta Psychol (Amst) 1985;60(2–3):173–191. doi: 10.1016/0001-6918(85)90054-x. [DOI] [PubMed] [Google Scholar]
- 5.Ivry RB, Hazeltine RE. The perception and production of temporal intervals across a range of durations: evidence for a common timing mechanism. J Exp Psycho Hum Percept Perform. 1995;21:1–12. doi: 10.1037//0096-1523.21.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Ivry RB, Schlerf JE. Dedicated and intrinsic models of time perception. Trends Cog Sci. 2008;12:273–280. doi: 10.1016/j.tics.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivry RB, Keele SW. Timing functions of the cerebellum. J Cogn Neurosci. 1989;1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- 8.Ivry RB, Keele SW, Diener HC. Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Exp Brain Res. 1988;73:167–180. doi: 10.1007/BF00279670. [DOI] [PubMed] [Google Scholar]
- 9.Franz EA, Ivry RB, Helmuth LL. Reduced timing variability in patients with unilateral cerebellar lesions during bimanual movements. J Cogn Neurosci. 1996;8:107–118. doi: 10.1162/jocn.1996.8.2.107. [DOI] [PubMed] [Google Scholar]
- 10.Bengtsson SL, Ehrsson HH, Forssberg H, Ullen F. Effector-independent voluntary timing: behavioural and neuroimaging evidence. Eur J Neurosci. 2005;22:3255–3265. doi: 10.1111/j.1460-9568.2005.04517.x. [DOI] [PubMed] [Google Scholar]
- 11.Coull JT, Nobre AC. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J Neurosci. 1998;18:7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Mello GB, Soares S, Paton JJ. A scalable population code for time in the striatum. Curr Biol. 2015;25(9):1113–22. doi: 10.1016/j.cub.2015.02.036. Performance on an interval timing task can be decoded from the temporal pattern of population activity in the striatum of the rat. The temporal pattern is rescaled when the interval changes, supporting a representation of relative time. [DOI] [PubMed] [Google Scholar]
- 13.Merchant H, Perez O, Zarco W, Gamez J. Interval tuning in the primate medial premotor cortex as a general timing mechanism. J Neurosci. 2013;33(21):9082–96. doi: 10.1523/JNEUROSCI.5513-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matell MS, Meck WH. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Cogn Brain Res. 2004;21:139–70. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Coull JT, Cheng RK, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011;36:3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Merchant H, Harrington DL, Meck WH. Neural basis of the perception and estimation of time. Annu Rev Neurosci. 2013;36:313–36. doi: 10.1146/annurev-neuro-062012-170349. An extensive review paper of the neural mechanisms of interval timing that promotes the idea of a core cortico-thalamic-striatal network. Based on findings from different methods and paradigms, the authors suggest that other structures related to timing, such as the cerebellum, interact with the core network in a context-dependent manner. [DOI] [PubMed] [Google Scholar]
- 17.Finnerty GT, Shadlen MN, Jazayeri M, Nobre AC, Buonomano DV. Time in cortical circuits. J Neurosci. 2015;35(41):13912–6. doi: 10.1523/JNEUROSCI.2654-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulberti A, Moll CK, Hamel W, Buhmann C, Koeppen JA, Boelmans K, Zittel S, Gerloff C, Westphal M, Schneider TR, Engel AK. Predictive timing functions of cortical beta oscillations are impaired in Parkinson's disease and influenced by L-DOPA and deep brain stimulation of the subthalamic nucleus. Neuroimage Clin. 2015;9:436–49. doi: 10.1016/j.nicl.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cope TE, Grube M, Mandal A, Cooper FE, Brechany U, Burn DJ, Griffiths TD. Subthalamic deep brain stimulation in Parkinson's disease has no significant effect on perceptual timing in the hundreds of milliseconds range. Neuropsychologia. 2014;57:29–37. doi: 10.1016/j.neuropsychologia.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: evidence from neuroimaging. Curr Opin Neurobiol. 2003;13:250–255. doi: 10.1016/s0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 21.Gooch CM, Wiener M, Wencil EB, Coslett HB. Interval timing disruptions in subjects with cerebellar lesions. Neuropsychologia. 2010;48:1022–31. doi: 10.1016/j.neuropsychologia.2009.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Coull JT, Nobre AC. Dissociating explicit timing from temporal expectations with fMRI. Curr opin Neurobiol. 2008;18:137–144. doi: 10.1016/j.conb.2008.07.011. A seminal paper in which the authors highlight a taxonomy of timing centered on two dimensions. One dimension relates to whether timing is explicit (“how long”) or implicit (“when”). The other dimension relates to whether that information is used in perception or in the control of movement. In a review focused on the neuroimaging literature, the authors suggest that explicit and implicit timing are mediated by distinct neural circuits. [DOI] [PubMed] [Google Scholar]
- 23.Miniussi C, Wilding EL, Coull JT, Nobre AC. Orienting attention in time: Modulation of brain potentials. Brain. 1999;122:1507–1518. doi: 10.1093/brain/122.8.1507. [DOI] [PubMed] [Google Scholar]
- 24.Bueti D, Walsh V, Frith C, Rees G. Different brain circuits underlie motor and perceptual representations of temporal intervals. J Cog Neurosci. 2008;20(2):204–214. doi: 10.1162/jocn.2008.20017. [DOI] [PubMed] [Google Scholar]
- 25.Flament D, Hore J. Movement and electromyographic disorders associated with cerebellar dysmetria. J Neurophysiol. 1986;55(6):1221–1233. doi: 10.1152/jn.1986.55.6.1221. [DOI] [PubMed] [Google Scholar]
- 26.Bhanpuri NH, Okamura AM, Bastian AJ. Predicting and correcting ataxia using a model of cerebellar function. Brain. 2014;137(7):1931–44. doi: 10.1093/brain/awu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ackermann H, Hertrich I. Speech rate and rhythm in cerebellar dysarthria: an acoustic analysis of syllabic timing. Folia Phoniatr Logop. 1994;46(2):70–8. doi: 10.1159/000266295. [DOI] [PubMed] [Google Scholar]
- 28.Koch G, Oliveri M, Caltagirone C. Neural networks engaged in milliseconds and seconds time processing: evidence from transcranial magnetic stimulation and patients with cortical or subcortical dysfunction. Philos Trans R Soc Lond B Biol Sci. 2009;364:1907–18. doi: 10.1098/rstb.2009.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diedrichsen J, Ivry RB, Pressing J. Cerebellar and basal ganglia contributions to interval timing. In: Meck WH, editor. Functional and Neural Mechanisms of Interval Timing. Boca Raton, FL: CRC Press; 2003. pp. 457–481. [Google Scholar]
- 30.Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR. Distributed neural systems underlying the timing of movements. J Neurosci. 1997;17(14):5528–5535. doi: 10.1523/JNEUROSCI.17-14-05528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hove MJ, Fairhurst MT, Kotz SA, Keller PE. Synchronizing with auditory and visual rhythms: an fMRI assessment of modality differences and modality appropriateness. Neuroimage. 2013;67:313–21. doi: 10.1016/j.neuroimage.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Pecenka N, Engel A, Keller PE. Neural correlates of auditory temporal predictions during sensorimotor synchronization. Front Hum Neurosci. 2013;7:380. doi: 10.3389/fnhum.2013.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chauvigné LAS, Gitau KM, Brown S. The neural basis of audiomotor entrainment: an ALE meta-analysis. Front Hum Neurosci. 2014;8:776. doi: 10.3389/fnhum.2014.00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Steen MC, Schwartze M, Kotz SA, Keller PE. Modeling effects of cerebellar and basal ganglia lesions on adaptation and anticipation during sensorimotor synchronization. Ann NY Acad Sci. 2015;1337:101–110. doi: 10.1111/nyas.12628. [DOI] [PubMed] [Google Scholar]
- 35.Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci. 1993;13:1708–18. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerwig M, Kolb FP, Timmann D. The involvement of the human cerebellum in eyeblink conditioning. Cerebellum. 2007;6(1):38–57. doi: 10.1080/14734220701225904. [DOI] [PubMed] [Google Scholar]
- 37.Johansson F, Jirenhed DA, Rasmussen A, Zucca R, Hesslow G. Memory trace and timing mechanism localized to cerebellar Purkinje cells. Proc Natl Acad Sci USA. 2014;111(41):14930–4. doi: 10.1073/pnas.1415371111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Halverson HE, Khilkevich A, Mauk MD. Relating Cerebellar Purkinje cell activity to the timing and amplitude of conditioned eyelid responses. J Neurosci. 2015;35:7813–32. doi: 10.1523/JNEUROSCI.3663-14.2015. A systematic demonstration of the link between adaptive timing in eyeblink conditioning and the cerebellum, using single-cell recordings in the rabbit. The authors use various training protocols to manipulate anticipatory timing of the conditioned response and show that this behavior is related to changes in Purkinje cell activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heiney SA, Wohl MP, Chettih SN, Ruffolo LI, Medina JF. Cerebellar-dependent expression of motor learning during eyeblink conditioning in head-fixed mice. J Neurosci. 2014;34(45):14845–53. doi: 10.1523/JNEUROSCI.2820-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spencer RMC, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous movements by cerebellar lesions. Science. 2003;300:1437–1439. doi: 10.1126/science.1083661. [DOI] [PubMed] [Google Scholar]
- 41**.Spencer RMC, Ivry RB. Cerebellum and timing. In: Manto M, Gruol D, Schmahmann J, Koibuchi N, Rossi F, editors. Handbook of the Cerebellum and Cerebellar Disorders. Springer Press; 2013. A review paper focuses on the hypothesis that, for timing in the subsecond range, the cerebellum is essential for explicit event timing, but not implicit emergent timing. [Google Scholar]
- 42.Bo J, Block HJ, Clark JE, Bastian AJ. A cerebellar deficit in sensorimotor prediction explains movement timing variability. J Neurophysiol. 2008;100(5):2825–32. doi: 10.1152/jn.90221.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spencer RMC, Verstynen T, Brett M, Ivry RB. Cerebellar activation during discrete and not continuous timed movements: an fMRI study. NeuroImage. 2007;36:378–387. doi: 10.1016/j.neuroimage.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ackermann H, Gräber S, Hertrich I, Daum I. Cerebellar contributions to the perception of temporal cues within the speech and nonspeech domain. Brain Lang. 1999;67(3):228–41. doi: 10.1006/brln.1999.2056. [DOI] [PubMed] [Google Scholar]
- 45.Tregellas JR, Davalos DB, Rojas DC. Effect of task difficulty on the functional anatomy of timing. Neuroimage. 2006;32:307–315. doi: 10.1016/j.neuroimage.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 46.Mathiak K, Hertrich I, Grodd W, Ackermann H. Discrimination of temporal information at the cerebellum: functional magnetic resonance imaging of nonverbal auditory memory. Neuroimage. 2004;21(1):154–62. doi: 10.1016/j.neuroimage.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 47.Shih LY, Yeh TC, Kuo WJ, Tzeng OJ, Hsieh JC. Effect of temporal difficulty on cerebrocerebellar interaction during visual duration discrimination. Behav Brain Res. 2010;207(1):155–60. doi: 10.1016/j.bbr.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Aso K, Hanakawa T, Aso T, Fukuyama H. Cerebro-cerebellar interactions underlying temporal information processing. J Cogn Neurosci. 2010;22(12):2913–25. doi: 10.1162/jocn.2010.21429. [DOI] [PubMed] [Google Scholar]
- 49*.Grube M, Cooper FE, Chinnery PF, Griffiths TD. Dissociation of duration-based and beat-based auditory timing in cerebellar degeneration. Proc Natl Acad Sci USA. 2010;107:11597–11601. doi: 10.1073/pnas.0910473107. Using a comprehensive pattern of tasks, this study demonstrates a dissociation in the performance of patients with cerebellar degeneration between timing in discrete and rhythmic contexts for tasks that require explicit perceptual timing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grube M, Lee KH, Griffiths TD, Barker AT, Woodruff PW. Transcranial magnetic theta-burst stimulation of the human cerebellum distinguishes absolute, duration-based from relative, beat-based perception of subsecond time intervals. Front Psychol. 2010;1:171. doi: 10.3389/fpsyg.2010.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teki S, Grube M, Kumar S, Griffiths TD. Distinct neural substrates of duration-based and beat-based auditory timing. J Neurosci. 2011;31:3805–3812. doi: 10.1523/JNEUROSCI.5561-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coull JT, Davranche K, Nazarian B, Vidal F. Functional anatomy of timing differs for production versus prediction of time intervals. Neuropsychologia. 2013;51(2):309–19. doi: 10.1016/j.neuropsychologia.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 53.Davranche K, Nazarian B, Vidal F, Coull JT. Orienting attention in time activates left intraparietal sulcus for perceptual and motor task goals. J Cog Neurosci. 2011;23:3318–3330. doi: 10.1162/jocn_a_00030. [DOI] [PubMed] [Google Scholar]
- 54.Bueti D, Bahrami B, Walsh V, Rees G. Encoding of temporal probabilities in the human brain. J Neurosci. 2010;30(12):4343–52. doi: 10.1523/JNEUROSCI.2254-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trillenberg P, Verleger R, Teetzmann A, Wascher E, Wessel K. On the role of the cerebellum in exploiting temporal contingencies: evidence from response times and preparatory EEG potentials in patients with cerebellar atrophy. Neuropsychologia. 2004;42(6):754–63. doi: 10.1016/j.neuropsychologia.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 56.O'Reilly JX, Mesulam MM, Nobre AC. The cerebellum predicts the timing of perceptual events. J Neurosci. 2008;28(9):2252–60. doi: 10.1523/JNEUROSCI.2742-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bares M, Lungu OV, Liu T, Waechter T, Gomez CM, Ashe J. The neural substrate of predictive motor timing in spinocerebellar ataxia. Cerebellum. 2011;10(2):233–44. doi: 10.1007/s12311-010-0237-y. [DOI] [PubMed] [Google Scholar]
- 58.Henry MJ, Herrmann B2, Obleser J. Entrained neural oscillations in multiple frequency bands comodulate behavior. Proc Natl Acad Sci USA. 2014;111(41):14935–40. doi: 10.1073/pnas.1408741111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cravo AM, Rohenkohl G, Wyart V, Nobre AC. Temporal expectation enhances contrast sensitivity by phase entrainment of low-frequency oscillations in visual cortex. J Neurosci. 2013;33(9):4002–10. doi: 10.1523/JNEUROSCI.4675-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60*.Breska A, Deouell LY. Automatic bias of temporal expectations following temporally regular input independently of high-level temporal expectation. J Cogn Neurosci. 2014;26(7):1555–71. doi: 10.1162/jocn_a_00564. This study uses a variant of a temporal orienting task in which the temporal cues to guide attention are orthogonal to a task-irrelevant rhythmic stream. Even in these conditions, behavioral and electrophysiological indices of temporal preparation are driven by the rhythm interval. Furthermore, a qualitatively different electrophysiological signature was observed when the rhythm was intentionally used to form predictions. [DOI] [PubMed] [Google Scholar]
- 61.Geiser E, Zaehle T, Jancke L, Meyer M. The neural correlate of speech rhythm as evidenced by metrical speech processing. J Cogn Neurosci. 2008;20(3):541–52. doi: 10.1162/jocn.2008.20029. [DOI] [PubMed] [Google Scholar]
- 62*.Grahn JA, Rowe JB. Finding and feeling the musical beat: Striatal dissociations between detection and prediction of regularity. Cereb Cortex. 2013;23(4):913–921. doi: 10.1093/cercor/bhs083. An fMRI study designed to ask whether activity in neural regions associated with beat processing is related to beat detection, beat monitoring, or beat adjustment. Striatal activity was greatest when a beat was maintained relative to conditions in which the beat varied. The authors hypothesize that this indicates a role in beat-based temporal prediction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grahn JA, Brett M. Impairment of beat-based rhythm discrimination in Parkinson's disease. Cortex. 2009;45(1):54–61. doi: 10.1016/j.cortex.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 64.Schubotz RI, Friederici AD, von Cramon DY. Time perception and motor timing: A common cortical and subcortical basis revealed by fMRI. NeuroImage. 2000;11:1–12. doi: 10.1006/nimg.1999.0514. [DOI] [PubMed] [Google Scholar]
- 65.Jones MR. Attending to sound patterns and the role of entrainment. In: Nobre AC, Coull JT, editors. Attention and Time. New York: Oxford University Press; 2010. [Google Scholar]
- 66.Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32(1):9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67**.Lakatos P, Musacchia G, O'Connel MN, Falchier AY, Javitt DC, Schroeder CE. The spectrotemporal filter mechanism of auditory selective attention. Neuron. 2013;77(4):750–61. doi: 10.1016/j.neuron.2012.11.034. An impressive demonstration of how rhythm-driven neural oscillations, in which time is represented implicitly, can act as a tool for temporal prediction. Laminar profiles recorded from the primary auditory cortex in the monkey revealed that neural oscillations entrain to the one of two interleaved rhythmic streams with the phase dependent on whether the neurons are tuned to the pitch of the attended or unattended stream. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spaak E, de Lange FP, Jensen O. Local Entrainment of Alpha Oscillations by Visual Stimuli Causes Cyclic Modulation of Perception. J Neurosci. 2014;34:3536–3544. doi: 10.1523/JNEUROSCI.4385-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci. 2010;33:89–108. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- 70.Molinari M. Sensorimotor Synchronization. In: Consensus paper: roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2012;11(2):457–87. doi: 10.1007/s12311-011-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wing AM. Voluntary timing and brain function: an information processing approach. Brain Cogn. 2002;48(1):7–30. doi: 10.1006/brcg.2001.1301. [DOI] [PubMed] [Google Scholar]
- 72.Zelaznik HN, Spencer RM, Ivry RB. Dissociation of explicit and implicit timing in repetitive tapping and drawing movements. J Exp Psychol Hum Percept Perform. 2002;28(3):575–88. doi: 10.1037//0096-1523.28.3.575. [DOI] [PubMed] [Google Scholar]


