Abstract
Fatigue, a common symptom of many acute and chronic medical conditions, reduces both quality of life and workplace productivity and can be disabling. However, the pathophysiologic mechanisms that underlie fatigue can be difficult to study in human populations due to the patient heterogeneity, the variety of underlying causes and potential triggering events, and inability to collect samples that may be essential to elucidation of mechanisms (e.g., brain). Although the etiology of chronic fatigue syndrome (CFS) remains elusive, some studies have implicated viral infections, including Epstein-Barr virus (EBV), a human gamma herpes virus, as a potential factor in the pathogenesis of CFS. Murine gamma herpes virus 68 (γHV68) is a mouse pathogen that shares many similarities with human γHVs, including EBV. In this study, we use γHV68-infected C57BL/6J mice as a model system for studying the impact of chronic viral infection on sleep-wake behavior, activity patterns, and body temperature profiles. Our data show that γHV68 alters sleep, activity, and temperature in a manner suggestive of fatigue. In mice infected with the highest dose used in this study (40,000 plaque forming units), food intake, body weight, wheel running, body temperature, and sleep were normal until approximately 7 days after infection. These parameters were significantly altered during days 7 through 11, returned to baseline levels at day 12 after infection, and remained within the normal range for the remainder of the 30-day period after inoculation. At that time, both infected and uninfected mice were injected with lipopolysaccahride (LPS), and their responses monitored. Uninfected mice given LPS developed a modest and transient febrile response during the initial light phase (hours 12 through 24) after injection. In contrast, infected mice developed changes in core body temperatures that persisted for at least 5 days. Infected mice showed an initial hypothermia that lasted for approximately 12 h, followed by a modest fever that persisted for several hours. For the remainder of the 5-day recording period, they showed mild hypothermia during the dark phase. Running wheel activity of infected mice was reduced for at least 5 days after injection of LPS, but for only 12 h in uninfected mice. Collectively, these observations indicate that1) physiologic and behavioral processes in mice are altered and recover during an early phase of infection, and 2) mice with latent γHV68 infection have an exacerbated response to challenge with LPS. These findings indicate that laboratory mice with γHV68 infections may provide a useful model for the study of fatigue and other physiologic and behavioral perturbations that may occur during acute and chronic infection with gamma herpes viruses.
Introduction
Fatigue, a common symptom of many acute and chronic medical conditions, reduces both quality of life and workplace productivity and can be disabling (Bombardier and Buchwald, 1996).However, the pathophysiologic mechanisms that underlie fatigue can be difficult to study in human populations due to the patient heterogeneity, the variety of underlying causes and potential triggering events, and inability to collect some samples that may be essential to elucidation of mechanisms (e.g., brains) (Afari and Buchwald, 2003; Evans, 1991; Glaser et al., 2005; Krupp et al., 1991; Kyle and DeShazo, 1992). In addition, the assessment of fatigue based on self-report introduces considerable subjectivity into the results (Hossain et al., 2005). Although many studies have linked fatigue to facets of the immune response, most have emphasized documentation of alterations in immune system status or responses in patients with fatigue, rather than focusing on the relationships between immune responses and behavior.
Chronic, unremitting, disabling fatigue is the key symptom of the condition known as chronic fatigue syndrome (CFS). Although the etiology of CFS remains elusive, some studies have implicated viral infections, including Epstein-Barr virus (EBV), a human gamma herpes virus (γHV), as a potential factor in the pathogenesis of CFS (Glaser and Kiecolt-Glaser, 1998; Jones et al., 1988; Natelson et al., 1994). EBV is a ubiquitous human γHV that can be transmitted via exposure to saliva or blood products, sexual activity and organ transplantation. EBV and other γHVs establish life-long latency in the infected host. The hypothesis that infection with EBV can lead to CFS derives, in part, from reports that patients with CFS have higher antibody titers to EBV than do various control groups (Glaser et al., 2005; Matthews et al., 1991; Swanink et al., 1995). However, although up to 95% of all adults have serologic evidence of previous EBV infection (Rickinson and Kieff, 2001), only a small fraction of adults develop CFS.
Murine gamma herpes virus 68 (γHV68) is a mouse virus that shares many similarities with human γHVs, including EBV (Flano et al., 2002).γHV68 initially causes an acute, lytic infection and then becomes established as a latent infection in laboratory mice. γHV68 pathogenesis has been studied extensively and has proven particularly informative with regard to elucidation of host immune responses during acute infection, latency, and reactivation from latency (Flano et al., 2002; Olivadoti et al., 2007).
In this study, we use γHV68-infected C57BL/6J mice as a model system for studying the impact of chronic viral infection on sleep-wake behavior, activity patterns, and body temperature profiles. We report that acute γHV68 infection alters sleep, activity, and temperature in a manner suggestive of fatigue. In addition, in mice with latent γHV68 infection, the administration of bacterial lipopolysaccharide (LPS) impacts sleep, body temperature, and activity patterns to a greater extent than occurs in non-infected C57BL/6J mice. Collectively, these observations indicate that mice infected with γHV68 may provide a useful model for the study of fatigue and other physiologic and behavioral perturbations during chronic viral infection.
Methods
Mice
Adult male C57BL/6J mice (25–30 g) were purchased from Jackson Laboratory, Bar Harbor, ME and were individually housed in microisolator cages containing running wheels. Mice were maintained on a 12:12 h light: dark cycle at an ambient temperature of 29 ± 1 °C, with water and rodent chow (Lab Diet 5001, PMI Nutrition International, Brentwood, MO) available ad libitum. All procedures were approved by the University of Michigan Committee on Care and Use of Animals in accordance with the US Department of Agriculture Animal Welfare Act and the National Institutes of Health policy on Humane Care and Use of Laboratory Animals.
Surgery
For all surgical procedures, mice were anesthetized with isoflurane (4% induction, 2% maintenance)and sterile technique was used. To permit continuous telemetric monitoring of the electroencephalograph (EEG) and core temperature, mice were implanted with a transmitter [ETA10-F20, Data Sciences International (DSI), St. Paul, MN] in the peritoneal cavity. Insulated biopotential leads from the transmitter were subcutaneously routed to the skull. The biopotential lead wires were then attached to two small stainless steel screws (#80 × 1/8 in., Small Parts, Miami Lakes, FL). These screws were implanted in the skull over the frontal and parietal brain cortices and served as EEG recording electrodes. After surgery mice were placed on a heating pad at 37°C until recovery from anesthesia. The analgesic ibuprofen (0.2 mg/mL) was provided in the drinking water beginning 24 h before surgery and continuing for 48 h after surgery (Hayes et al., 2000). A broad-spectrum antibiotic (Imipenem, 25 mg/kg) and an analgesic (Buprenex, 0.05 mg/kg) were given by subcutaneous injection immediately after surgery. Mice were allowed 21 days to recover from surgery before experiments began, during which time they were housed in the recording room to habituate to the recording environment.
Physiologic and behavioral monitoring
To record the EEG and body temperature, signals from the transmitter were detected by a receiver (RPC-1, DSI) located under each mouse’s cage. The signal was then fed to a DSI analog converter (ART Analog-8 CM, DSI). Frequency signals from the transmitter were converted into EEG and temperature voltages using calibration factors provided by DSI that were specific for each transmitter. The output from the DSI analog converter was then captured by an A/D board (model PCI-3033E, National Instruments, Austin, TX), which digitized the data at 128 Hz with 16-bit precision. The voltage values obtained from the temperature channel of the transmitter were converted to engineering units (°C) by regression using coefficients specific for each transmitter and obtained by calibration with a water bath. This commercially-available telemetry system has previously been used to determine sleep-wake behavior of mice (Morrow and Opp, 2005; Olivadoti and Opp, 2008; Tang and Sanford, 2002), and temperature and activity patterns from other small mammals (Herold et al., 1998).
General activity in the cage was detected using infrared sensors. Movements detected by the sensors were converted to a voltage output, the magnitude of which was directly related to the magnitude of movements detected. Wheel running was determined by detecting TTL pulses triggered by a switch on the axel of a running wheel. All signals (EEG, body temperature, wheel running and cage activity) were stored as binary files until further processing.
The methods used to determine arousal states of mice have been previously described (Morrow and Opp, 2005; Olivadoti and Opp, 2008). Briefly, the EEG was digitally filtered during acquisition into delta (0.5 – 4.0 Hz) and theta (6.0 – 9.0 Hz) frequency bands. These filtered EEG signals were integrated over 1-s periods and stored as part of the binary file structure. Arousal state[wakefulness (W), slow-wave sleep (SWS), or paradoxical sleep (PS)]was determined for each 10-s epoch of the recording period based on the EEG, integrated delta and theta frequency components of the EEG, and activity (wheel running, general cage activity). Wakefulness (W) was defined based on a low amplitude, mixed frequency (delta and theta frequency components generally equivalent) EEG accompanied by wheel running or general cage activity, with activity-dependent increases in core body temperature. Slow-wave sleep (SWS) was identified by increased absolute EEG amplitude, integrated values for the delta frequency band greater than those for theta, lack of wheel running or general cage activity, and a reduction in core body temperature upon entry into SWS. Paradoxical sleep (PS) was identified as a low amplitude EEG, with integrated values for the delta frequency band less than those for the theta frequency band and lack of wheel running or general cage activity. Any epoch containing either movement artifacts or electrical noise was tagged and excluded from subsequent spectral analyses. The raw, non-integrated EEG signals were processed offline using fast Fourier transforms (FFT) to yield power spectra between 0.5 and 40 Hz in 0.5 Hz frequency bins. These spectra were computed by averaging the five consecutive 2-s EEG segments comprising each 10-s epoch. The resulting spectrum was matched to state to provide state-specific spectra. Our primary focus was on the power in the delta frequency band during SWS. These values for delta power during SWS were obtained by summing the values of all 0.5 Hz frequency bins from 0.5 – 4.0 Hz.
The extent to which sleep was consolidated or fragmented was determined by evaluating the number of transitions from one arousal state to the next. These determinations were made irrespective of arousal state designation and without the use of arbitrary criteria for sleep architecture parameters. For example, a series of 10-s epochs designated as W-W-W-SWS-SWS-SWS-W-SWS-PS would be considered to include four state transitions.
Virus and inoculation procedures
γHV68 stocks were generously supplied by Dr. Marcia A. Blackman (Trudeau Institute, Saranac Lake, NY). For inoculation, mice were lightly anesthetized with isoflurane and either 400 or 40,000 plaque forming units (pfu) in 30 to 50 µl Hank’s Balanced Salt Solution (HBSS; vehicle) were deposited into the nares using a pipettor. Approximately half of the volume was deposited into each nostril. Mice were observed until ambulatory after sedation.
Experimental design
The experimental protocol used in this study is depicted schematically in Fig. 1. Mice were assessed for the effects of γHV68 infection on patterns of activity, core body temperature, food consumption, body weight and sleep-wake behavior. To permit assessment of “voluntary” locomotor activity, mice were housed in cages equipped with running wheels for two weeks prior to data collection to permit adaptation to the wheel. After adaptation, vehicle (HBSS, 30 to 50 µL) was administered intranasally at dark onset, and recordings of the EEG, core body temperature, wheel running and general cage activity were obtained for four consecutive days. Mice were then inoculated intranasally at dark onset with either 400 pfu (n=13) or 40,000 pfu (n=8) of γHV68. Recording continued for 30 consecutive days after γHV68 inoculation. Body weight and food consumption were measured daily at dark-onset.
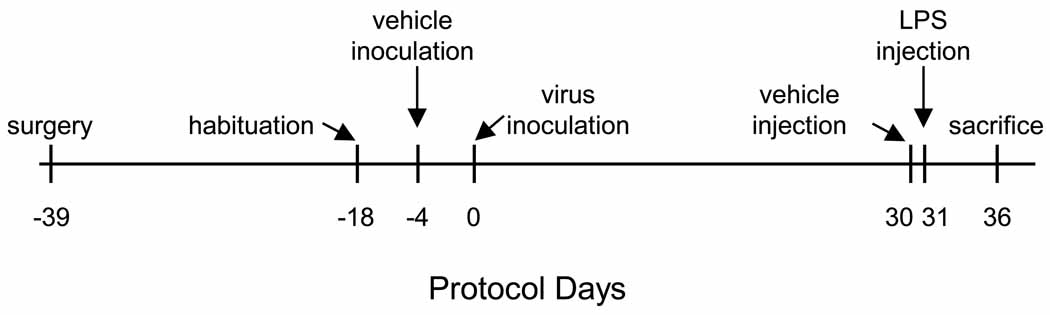
Fig. 1.
This figure is a schematic representation of the protocol used in this study. Male C57BL/6J mice were implanted with telemeters and allowed 21 days for recovery from surgery. These animals were then placed individually in shoeboxes containing running wheels and allowed two weeks for habituation. All mice were then inoculated intranasally with vehicle (Hank’s balanced salt solution) and baseline recordings were obtained for 4 consecutive days. All mice were then inoculated intranasally with vehicle containing one of two doses of murine gamma herpes virus 68 (γHV68; 400 or 40,000 plaque forming units) and recordings continued for 30 days. After this 30 day recording period, all mice were injected intraperitoneally with pyrogen-free saline (vehicle) and 24 h recordings obtained. Mice were then injected intraperitoneally with lipopolysaccharide (LPS) and recordings continued for an additional 5 days. In the text of this paper, all protocol days are referenced to Day 0, when animals were inoculated with virus.
Effects of LPS on sleep, activity patterns and core body temperature mice infected with γHV68
At dark-onset on day 30 after viral inoculation (Fig. 1), all mice were injected intraperitoneally with 0.2 mL of a vehicle solution [pyrogen-free saline (PFS); Abbott Laboratories, North Chicago, IL] and recordings were obtained for 24 h. Mice were then injected intraperitoneally at dark onset with 10 µg of LPS (Escherichia coli serotype O111:B4; Sigma-Aldrich, St. Louis, MO) in 0.2 ml of PFS and recording continued for 5 days.
Verification of viral infection
Mice were sacrificed by under deep isoflurane anesthesia at the end of the protocol. Spleens were removed, flash-frozen in liquid nitrogen, and stored at −80 °C. To verify that mice had been infected with γHV68, DNA was extracted from the spleen samples and used to determine viral load by quantitative real-time PCR as previously described (Nguyen et al., 2008). Three mice that had been inoculated with γHV68 did not have detectable viral DNA in spleen. Data from these mice were excluded from subsequent analyses.
Statistical analyses
Statistical analyses were performed using SPSS (SPSS, Inc., Chicago, IL). Data were evaluated in 6 and 12 h time blocks using linear mixed models analyses with Bonferroni correction. The amount of time spent in vigilance states (W, SWS, PS), core body temperature, wheel running, general cage activity, transitions from one state to another, delta power during SWS, food consumption and body weight were the dependent variables and manipulation (vehicle, γHV68 dose, LPS) was the independent variable. An alpha level of p< 0.05 was accepted for all statistical tests as indicating significant departures from control values.
Results
Effects of γHV68 infection on wheel running, core body temperature, food consumption, body weight, and sleep
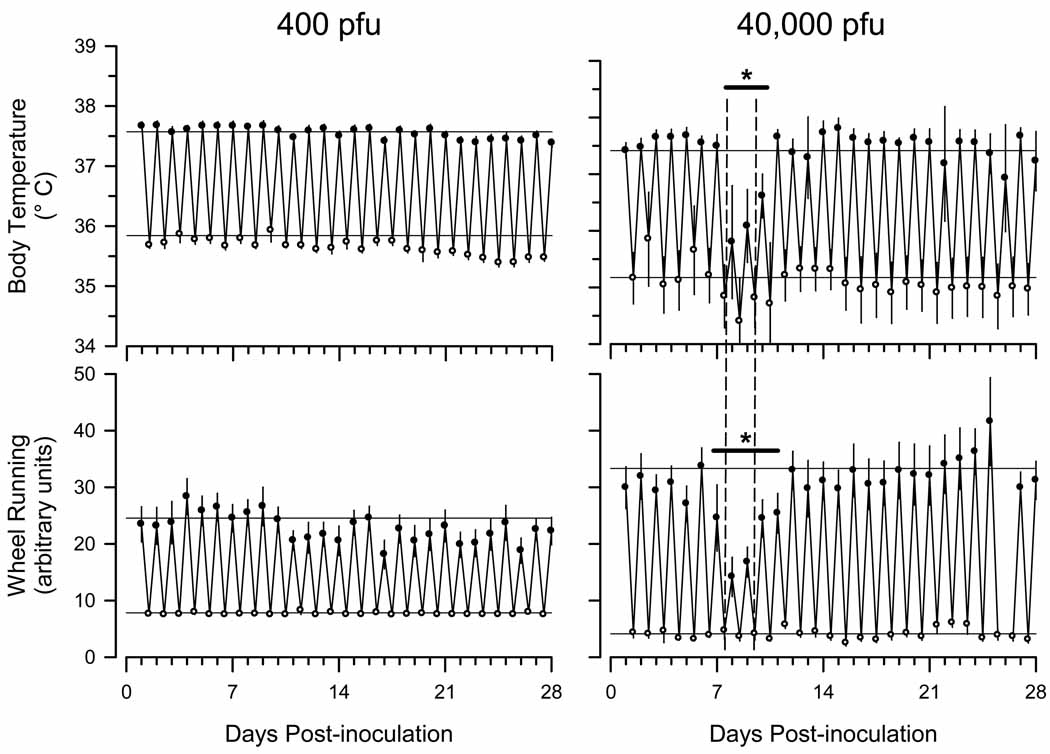
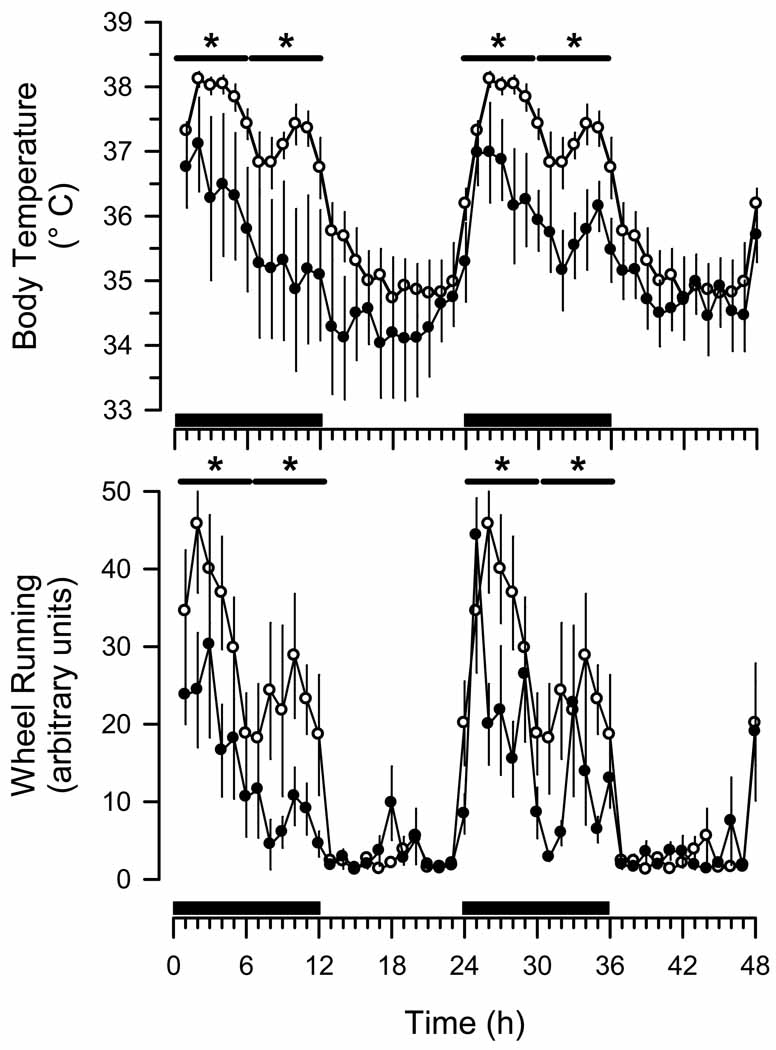
Wheel running (Fig. 2) and general cage activity (data not shown) were not significantly altered relative to baseline values in mice infected with 400 pfuγHV68. In contrast, wheel running of mice infected with 40,000 pfu of γHV68 was significantly reduced during days 7 to 11 after inoculation (Fig. 2). This effect was most pronounced during the dark phase of the diurnal cycle (Figs. 2, 3).Core body temperature of mice infected with 40,000 pfu of γHV68 was reduced during days 7 to 11 after infection(Fig. 2), particularly during the dark phase of the diurnal cycle (Figs. 2, 3). Body temperature did not differ significantly from pre-infection baseline in mice infected with 400 pfu of γHV68 over the course of the experiment (Fig. 2).
Fig. 2.
Murine gamma herpes virus 68 (γHV68) transiently alters core body temperature and wheel running activity of C57BL/6J mice. Mice were inoculated intranasally with Hank’s balanced salt solution containing either 400 plaque forming units (pfu) of γHV68 (n = 13) or 40,000 pfu (n = 8) according to the protocol detailed in Fig. 1. Values (mean ± SEM) are depicted for the 12 h dark period (filled circles) and the 12 h light period (open circles) for 28 consecutive days after intranasal inoculation with virus. The horizontal lines on each panel represent the mean dark or light phase values obtained during the 4 day baseline recording period (protocol days −4 to 0). Data for wheel running activity from the 40,000 pfu group are missing from the dark period of day 26 due to computer failure. The vertical dashed lines on the panels depicting data from the 40,000 pfu group demarcate post-inoculation days 8 and 9. Responses to γHV68 on post-inoculation days 8 and 9 are presented in detail in Fig. 3. Horizontal bars with asterisks indicate statistically significant differences from control values (p < 0.05).
Fig. 3.
Murine gamma herpes virus 68 (γHV68) alters the magnitude, but not the timing, of diurnal patterns of core body temperature and wheel running activity of C57BL/6J mice. Mice (n = 8) were inoculated intranasally with Hank’s balanced salt solution and baseline recordings obtained for 4 days according to the protocol detailed in Fig. 1. Hourly values obtained for core body temperature and wheel running activity during these baseline conditions were averaged across this 4 day recording period to produce one 24 h profile. The 24 h profile of core body temperature rhythms and wheel running activity is double plotted (open circles; mean ± SEM). These same mice were subsequently inoculated intranasally with Hank’s balanced salt solution containing 40,000 pfu of γHV68 according to the protocol detailed in Fig. 1. Hourly values (mean ± SEM) are depicted for the 48 h period that constitute post-inoculation protocol days 8 and 9 (filled circles). Black bars on the x-axis denote the dark period of the light: dark cycle. Horizontal bars with asterisks indicate time blocks during which statistically significant differences from control values were revealed (p < 0.05).
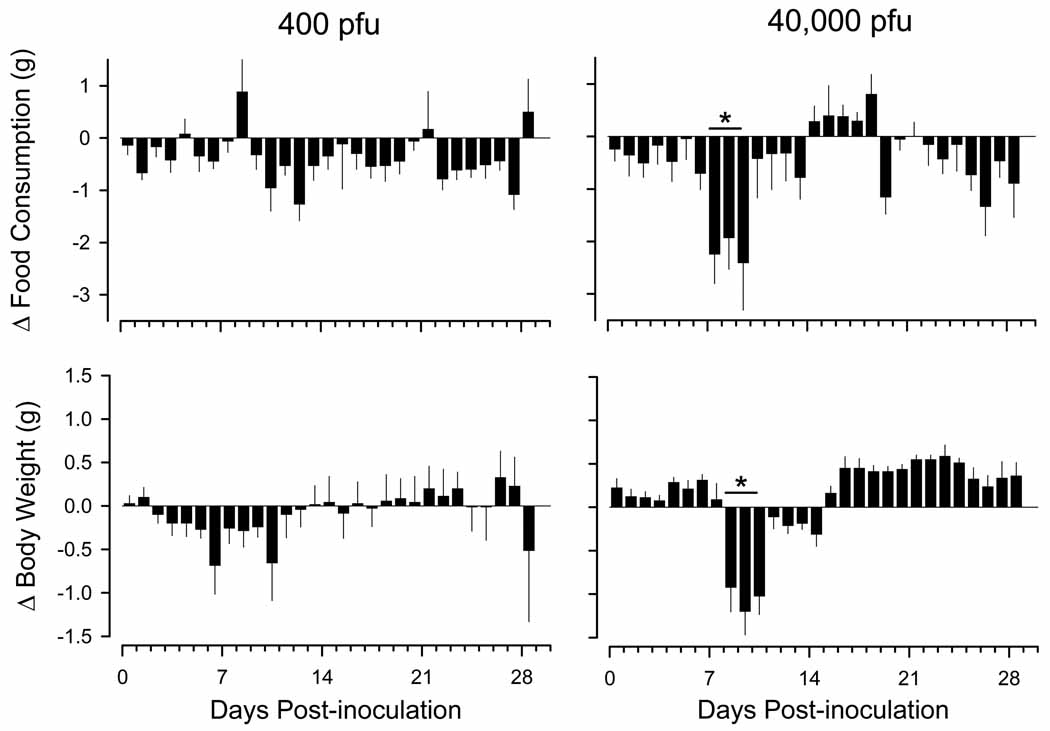
Compared to values obtained prior to infection, food intake and body weight decreased transiently during days 7 to 10 after infection with 40,000 pfu of γHV68(Fig. 4). In contrast, food intake and body weight did not change significantly after infection with 400 pfu of γHV68 (Fig. 4).
Fig. 4.
Infection with murine gamma herpes virus 68 (γHV68) reduces food consumption and body weights of C57BL/6J mice. Mice were inoculated intranasally with Hank’s balanced salt solution containing either 400 plaque forming units (pfu) of γHV68 (n = 13) or 40,000 pfu (n = 8) according to the protocol detailed in Figure 1. Daily values (mean ± SEM) for food consumption and body weights are expressed as absolute differences from average values obtained during the 4 day period of baseline recordings (protocol days −4 to 0), which is depicted by the zero line. Horizontal lines with asterisks denote statistically significant differences from control values (p < 0.05). Note that a statistically significant reduction in food consumption preceded by one day the loss of body weight.
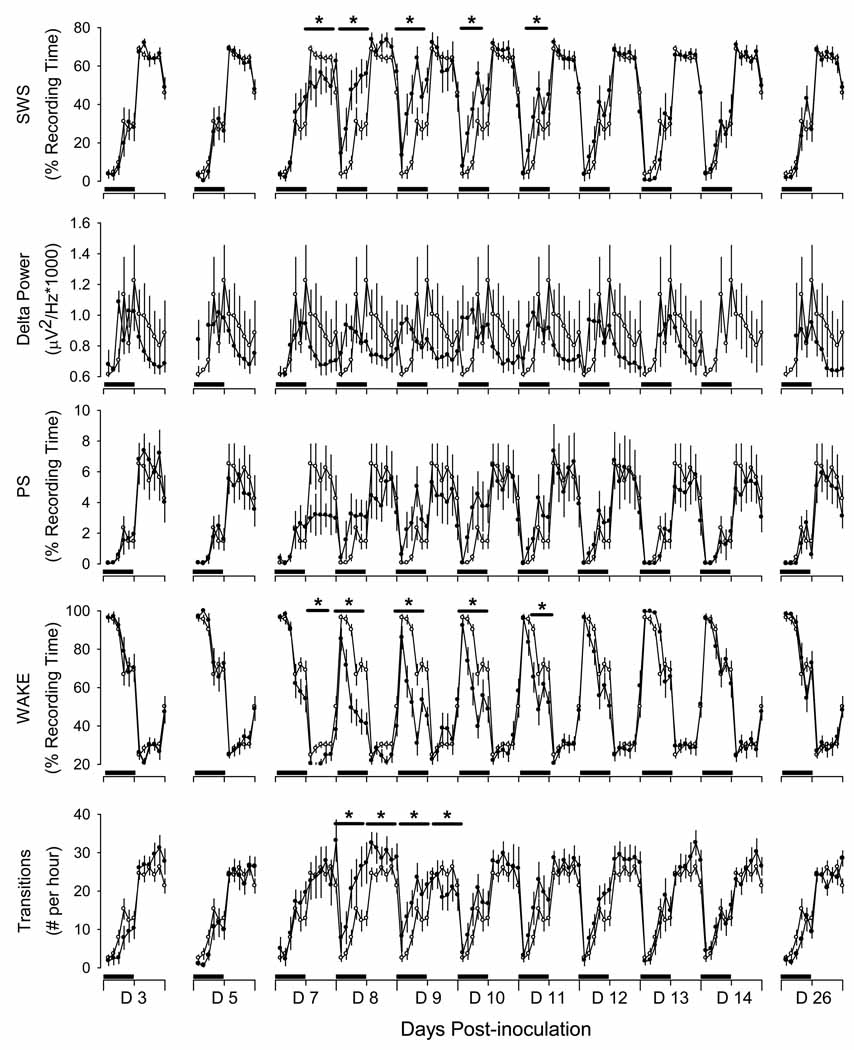
Infection with 400 pfu of γHV68 did not significantly alter sleep-wake behavior in mice (data not shown). In contrast, infection with 40,000 pfu of γHV68 induced transient changes in sleep-wake behavior, with a general pattern of increased SWS and PS and reduced W during the dark phase. These changes in sleep-wake behavior occurred during days 7 through 11 after infection (Fig. 5). Relative to pre-infection control values, sleep-wake behavior was not altered on days 3 or 5 after infection. Mice spent less time in SWS during the light phase of day post-infection day 7. Subsequently, however, mice developed an increase in time spent in SWS that was apparent during the dark periods on days 8 through 11 after infection (Fig. 5). Changes in PS were generally of the same pattern as those of SWS; time spent in PS was reduced during the light phase on days 7 and 8 after infection, but increased during the dark phase on post-infection days 8 through 12. Changes in time spent awake generally were opposite those of time spent in SWS and PS, with consistent reductions in time awake during the dark phase on days 7 through 11 after infection (Fig. 5). In addition, sleep was more fragmented on days 8 and 9 after infection, with no significant change in delta power(Fig. 5).
Figure 5.
Sleep of male C57BL/6J mice infected with murine gamma herpes virus 68 (γHV68) is altered during the period of transition from active infection of the upper respiratory tract to chronic infection of the spleen. Baseline recordings were obtained from instrumented mice (n = 8) after for 4 days after intranasal inoculation with Hank’s balanced salt solution as detailed in the protocol depicted in Fig. 1. Values obtained during these baseline conditions were averaged across this 4 day recording period to produce one 24 h profile. The 24 h profiles for these parameters are presented in this figure as open circles (mean ± SEM). Recordings were obtained for 30 days after intranasal inoculation with 40,000 pfu HV68, as depicted in protocol Fig. 1 and as detailed in the methods section (filled symbols). Data from selected post-inoculation days are presented in this figure as 2-h averages for visual clarity. Black bars on the x-axis denote the dark portion of the light: dark cycle. Horizontal lines with asterisks identify periods during which statistically significant differences between control and experimental conditions were revealed (p < 0.05; see methods). SWS: Slow wave sleep; PS: Paradoxical sleep; WAKE: wakefulness.
To summarize, in mice infected with 40,000 pfu of γHV68, food intake, body weight, wheel running, body temperature, and sleep were normal until approximately 7 days after infection, were significantly altered during days 7 through 11, and returned to control values at day 12 after infection. For the remainder of the 30 day post-inoculation protocol values for these parameters were within the normal range.
Effects of LPS on sleep, activity, and core body temperature of mice infected with γHV68
To determine whether latent γHV68 infection modulates behavioral changes induced by subsequent immune challenge, γHV68-infected mice were injected intraperitoneally at dark onset with PFS (vehicle) on day 30 after γHV68 infection, and with LPS on day 31. As compared with the 24 h period after vehicle injection, mice previously infected with 40,000 pfu of γHV68 reduced their food intake through day 4 after LPS administration (data not shown), with an associated significant reduction in body weight during the first two days after LPS administration (data not shown). These reductions were significantly greater than those occurring in mice inoculated with 400 pfu of γHV68 and subsequently injected with LPS (data not shown).
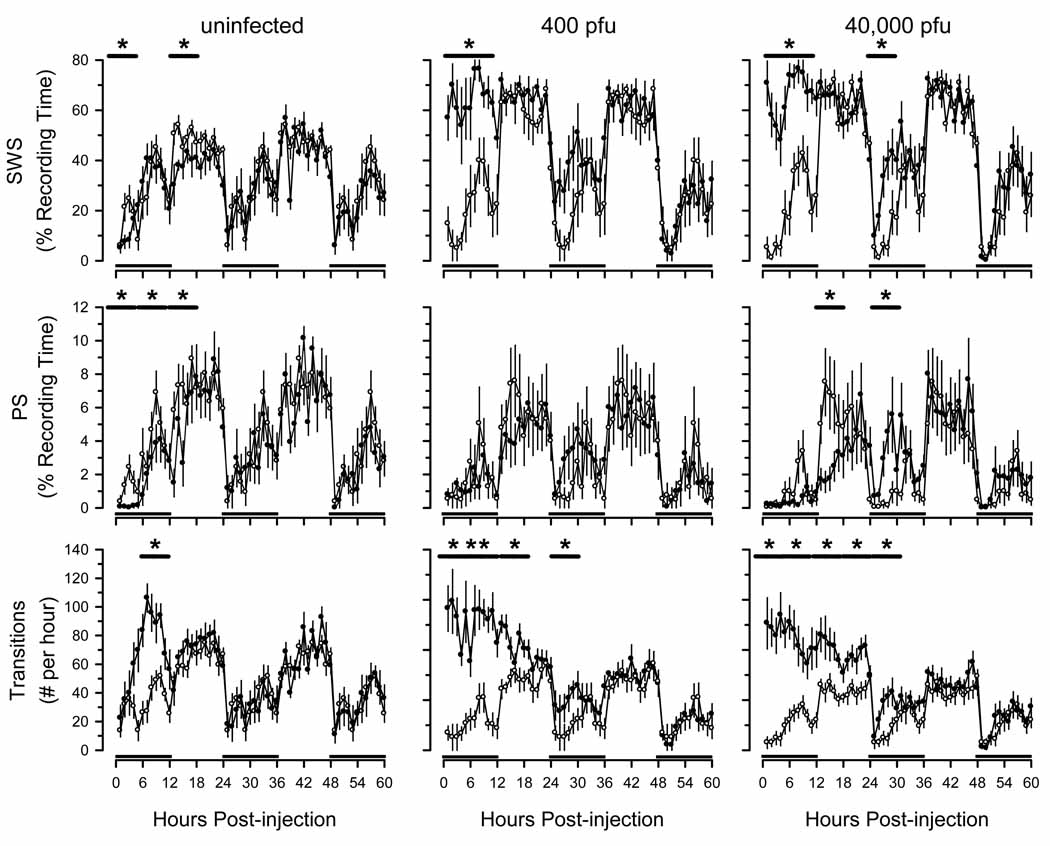
Injection of LPS into uninfected mice at dark onset was associated with an increase in time spent in SWS and a reduction in PS during hours 1 through 6 after injection (Fig. 6). This initial reduction in SWS was followed by an increase in SWS for the remainder of the 12 h dark period. In uninfected mice, PS sleep was suppressed for approximately 18 h after administration of LPS (Fig. 6). Sleep was fragmented in these mice during hours 6 through 12 after injection, as evidenced by a significant increase in the number of transitions from one arousal state to another (Fig. 6). In summary, facets of sleep in uninfected mice injected with LPS were altered, but under the conditions of this study sleep-wake behavior was normal by 18 h after injection.
Fig. 6.
Effects of lipopolysaccharide (LPS) on sleep-wake behavior are exacerbated in C57BL/6J mice previously inoculated with murine gamma herpes virus 68 (γHV68). Thirty days after intranasal inoculation with γHV68 (400 pfu: n = 13; 40,000 pfu: n = 8), mice were injected intraperitoneally with pyrogen-free saline as detailed in protocol Fig. 1 and in the methods section. Recordings were obtained for 24 h after these control injections, and are triple plotted (open symbols). These same animals were then injected intraperitoneally with 10 µg LPS and recordings continued for 5 days (closed symbols). A separate group of animals (n = 6) that had not been inoculated with γHV68 (ie., uninfected) were injected intraperitoneally with pyrogen-free saline and control recordings obtained (open symbols). These control animals were then injected with LPS, as in the other groups (closed symbols). On this figure, values are plotted for the first 60 h after injection of LPS. All values are hourly means (±SEM). Black bars on the x-axis denote the dark portion of the light: dark cycle. Horizontal lines with asterisks indicate statistically significant differences between control and experimental values (p < 0.05).
In mice previously infected with 400 pfu of γHV68, LPS administration was associated with a significant increase in time spent in SWS during hours 1 through 12 and 18 through 36 after LPS administration(Fig. 6). On days 3 through 5 after LPS administration, these mice spent less time in SWS during the light phase (data not shown). Despite their greater amount of time asleep, the sleep of these mice was fragmented for 36 hours after LPS administration (Fig. 6) and during the light phase on days 3, 4 and 5 (data not shown). The time spent awake was reduced during the first 12 h after LPS injection but was higher during the light phase on days 3 through 5 after injection of LPS (data not shown).
Mice previously inoculated with 40,000 pfu of γHV68 spent more time in SWS during hours 1 through 12 and 24 through 30 after injection of LPS, with a reduction in PS time during hours 12 through 18 after injection (Fig. 6). These mice also showed fragmentation of sleep during the first 36hours after injection (Fig. 6). The reduction in PS was greater in magnitude in these mice than in mice inoculated with the lower dose of γHV68. However, other effects were not markedly influenced by infectious dose.
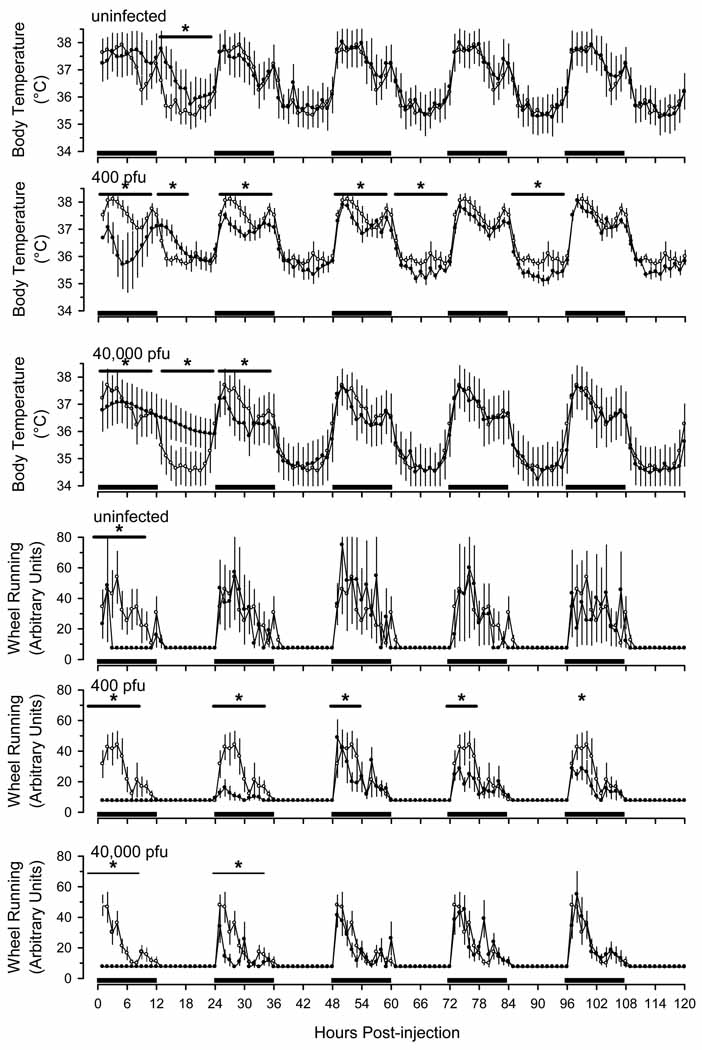
Uninfected mice given LPS developed a modest and transient febrile response during the initial light phase after injection (Fig. 7). In contrast, core body temperatures of mice that had previously been infected with 400 pfu of γHV68 were altered for at least 5 days after injection of LPS (Fig. 7). These mice showed an initial hypothermia that lasted for approximately 12 h, followed by a modest fever that persisted for several hours. For the remainder of the 5-day recording period, they showed mild hypothermia during the dark phase. Mice inoculated with 40,000 pfu of γHV68 showed a similar but less pronounced pattern of changes after LPS administration (Fig. 7). The hypothermic responses of mice inoculated with either 400 pfu or 40,000 pfu of γHV68 were of greater magnitude and of longer duration than those that occurred in uninfected mice.
Fig. 7.
Lipopolysaccharide (LPS)-induced alterations in core body temperature and wheel running are exacerbated in C57BL/6J mice previously inoculated with murine gamma herpes virus 68 (γHV68). Thirty days after intranasal inoculation with γHV68 (400 pfu: n = 13; 40,000 pfu: n = 8), mice were injected intraperitoneally with pyrogen-free saline as detailed in protocol Fig. 1 and in the methods section. Recordings were obtained for 24 h after these control injections, and are triple plotted (open symbols). These same animals were then injected intraperitoneally with 10 µg LPS and recordings continued for 5 days (closed symbols). A separate group of animals (n = 6) that had not been inoculated with γHV68 (ie., uninfected) were injected intraperitoneally with pyrogen-free saline and control recordings obtained (open symbols). These control animals were then injected with LPS, as in the other groups (closed symbols). All values are hourly means (±SEM). Black bars on the x-axis denote the dark portion of the light: dark cycle. Horizontal lines with asterisks indicate statistically significant differences between control and experimental values (p < 0.05).
Uninfected mice demonstrated a marked reduction in wheel running activity during the initial 12 h after LPS injection and returned to normal thereafter (Fig. 7). In contrast, mice that had previously been infected with 400 pfu of γHV68 reduced their wheel running for at least 5 days after injection of LPS (Fig. 7). Mice inoculated with 40,000 pfu of γHV68 showed a similar but less pronounced pattern of changes after LPS administration (Fig. 7). Thus, the responses of mice inoculated with either 400 pfu or 40,000 pfu of γHV68 were of greater magnitude and of longer duration than those that occurred in uninfected mice.
Discussion
Consistent with past reports using other infectious challenges [reviewed in (Toth and Verhulst, 2003)], the current study demonstrates that mice develop behavioral and physiologic signs of illness after inoculation with γHV68. More specifically, our data indicate that mice respond to infection with 40,000 pfu of γHV68 with significant reductions in food intake, body weight, wheel running, and core temperature, as well as changes in sleep, during the 7 to 11 day period after infection, as compared with baseline values and values obtained before and after this interval throughout 30 days after inoculation. The period of 7 to 11 days after intranasal inoculation with γHV68 coincides with the reported transition from the active, lytic infection in lung to latent infection in the spleen and other sites (Doherty et al., 2001; Flano et al., 2005; Hwang et al., 2008). This transition from active infection in the lung to latent infection in the spleen and other organs is also the time during which expression of several inflammatory chemokines and cytokines is most pronounced in the lungs after intranasal infection with γHV68 (Weinberg et al., 2002). In addition, we show that mice with latent γHV68 infection are more sensitive than uninfected mice to the development of behavioral and physiologic signs of illness when exposed to a subsequent immune challenge (LPS).γHV68 infection is associated with a variety of behavioral and physiologic effects that depend on the predominant state of the virus (lytic versus latent infection) and on the presence of a secondary stimulus.
In our study, behavioral and physiologic changes in mice occurred consistently during acute infection with 40,000 pfu of γHV68, but were generally absent in mice inoculated with the 400 pfu dose. A previous study found that latent viral loads in lung and spleen are not dependent on the infectious dose of γHV68 administered via either an intranasal or intraperitoneal route, although peak titers in the lung are higher in magnitude and occur earlier in mice inoculated with a high dose compared to mice inoculated with a low dose (Tibbetts et al., 2003).Our data suggest that the larger infectious dose triggers a more severe clinical illness than does the lower dose in that mice infected with the high dose of γHV68 developed transient anorexia, weight loss and hypothermia during the time period corresponding to acute infection.
A primary goal of our study was to assess sleep and fatigue during both lytic and latent γHV68 infection. With regard to sleep, we observed significant changes in normal patterns and architecture of SWS, PS, and wakefulness at times that correspond to peak lytic replication in the lungs after inoculation with a high dose of virus. This relationship suggests that changes in sleep-wake behavior during infection with γHV68 may be mediated by infection-related pulmonary damage and/or by the host immune response, as has been proposed previously for other infectious conditions (Imeri and Opp, 2009; Krueger and Toth, 1994; Toth and Verhulst, 2003).
In our study, we operationally defined fatigue as a reduction in voluntary activity(i.e., wheel running) as opposed to essential activity (i.e., locomotion in the cage, which is necessary for feeding and drinking). This definition is supported by a recent evaluation of fatigue in mice treated with chemotherapeutic drugs (Ray et al., 2011). Similarly, others have used reductions in locomotor activity of mice as an indicator of fatigue in response to challenge with viral proteins (Padgett et al., 2004). Human fatigue can similarly be expressed as continued performance of essential activities (e.g., employment, care-giving)with curtailment of non-essential, voluntary or recreational activities (Rakib et al., 2005). A number of chronic viral diseases are associated with fatigue. For example, chronic infection with hepatitis C virus is associated with fatigue that adversely affects quality of life, and up to 65% of patients chronically infected with hepatitis C virus report problems with sleep that occur independent of antiviral therapy with interferon-alpha and prior to advanced stages of liver disease (Carlson et al., 2010). Debilitating fatigue and poor sleep quality are also common symptoms among individuals infected with human immunodeficiency virus (HIV) (Davis, 2004).In HIV-infected patients, poor nighttime sleep and daytime sleepiness both correlate significantly with fatigue intensity (Salahuddin et al., 2009).
Chronic fatigue syndrome (CFS) is a specific clinical condition characterized by disabling fatigue and various non-specific accompanying symptoms that persist for at least 6 months in the absence of an explanatory medical diagnosis. Various studies have sought to identify immunologic disturbances in patients with CFS, generating reports of altered levels of cytokines that could contribute to fatigue and flu-like symptoms (Broderick et al., 2010; Lorusso et al., 2009; Nakamura et al., 2010; ter Wolbeek et al., 2007).Studies of patients developing CFS after acute viral infection indicate that initial infection severity was the single best predictor of persistent fatigue (Klimas and Koneru, 2007). Investigations to identify the underlying cause of CFS have used genetic signatures to describe biologic subgroups (Klimas and Koneru, 2007; Zhang et al., 2010). Many persistent cases have been linked to expression of genes related to Epstein- Barr virus (EBV) (Klimas and Koneru, 2007), which has many similarities to γHV68. Other viruses that have been implicated in CFS include varicella-zoster virus, human herpes virus-6 and -7, parvovirus B19, and enterovirus (Fremont et al., 2009; Shapiro, 2009; Zhang et al., 2010). However, the association of CFS with viral infection is tenuous, as many conflicting reports appear in the literature. For example, a recent study reported detection of the human gamma-retrovirus known as xenotropic murine leukemia virus-related virus (XMRV) in 67% of CFS patients as compared with 4% of healthy controls and suggested that XMRV may contribute to the pathogenesis of CFS (Lombardi et al., 2009). However, separate studies of European cohorts of CFS patients have not confirmed these results (Erlwein et al., 2010; Groom et al., 2010; van Kuppeveld et al., 2010).Such discrepancies could be due to the multifactorial and poorly defined nature of CFS.
Our results also show that as compared with uninfected mice, mice with latent γHV68 infections display exaggerated and prolonged alterations in sleep and temperature in response to LPS administration. The impact of LPS administration was some what less apparent in mice that had received a higher initial challenge dose of γHV68. This result is contrary to expectations of a positive relationship between infectious dose and severity of clinical signs and may reflect the complexity of host responses to acute challenge in the presence of latent infectious conditions. Moreover, the eventual latent viral load in spleens of mice after γHV68 infectionis independent of the initial challenge dose (Tibbetts et al., 2003).
The heightened sensitivity of mice with latent γHV68 infection to LPS challenge is reminiscent of the severe response to LPS that occurs in mice deficient for IL-6, IL-10, or the p50 sub-unit of NF-κB (Jhaveri et al., 2006; Morrow and Opp, 2005; Toth and Opp, 2001). The robust response of mice with latent infections further suggests that they maintain analtered immune steady-state that is related to enforcing viral latency, reacting to LPS-induced viral reactivation from latency (Gargano et al., 2009)or both. We and others have reported that latent γHV68 infection changes both immune function and susceptibility to other pathogens in latently infected mice (Barton et al., 2007; Nguyen et al., 2008; Yager et al., 2009). These changes appear to be related to persistent up-regulation of IFN-γ in latently infected mice (Barton et al., 2007; Nguyen et al., 2008). In CFS patients, fatigue scores were significantly associated with production of both TNF-α and IL-6 in whole blood incubated with LPS, whereas only TNF-α showed this relationship in control patients (Gaab et al., 2005). Taken together, such findings suggest that virus-related immune perturbations may underlie the immunologic, behavioral, and physiologic changes that occur in disease states such as CFS. γHV68 infection of mice may prove useful in further delineating such interactions because it has the advantage of determining the pathogenesis of a virus in its natural host. Understanding how symptoms relate to the initial and chronic course of an infection is important in developing strategies for improving the quality of life for people with chronic viral infections.
Acknowledgments
The technical assistance of Ms. Jill Priestley, Ms. Amrita George, and Dr. Richard Raymond is acknowledged and greatly appreciated. This work was supported in part by NIH grant F31-MH078682 (MDO, MRO), R01-AI080576 (LAT, MRO), the SIU School of Medicine (LAT), and the UM Department of Anesthesiology (MRO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am.J Psychiatry. 2003;160:221–236. doi: 10.1176/appi.ajp.160.2.221. [DOI] [PubMed] [Google Scholar]
- Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HWt. Herpes virus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- Bombardier CH, Buchwald D. Chronic fatigue, chronic fatigue syndrome, and fibromyalgia. Disability and health-care use. Med Care. 1996;34:924–930. doi: 10.1097/00005650-199609000-00005. [DOI] [PubMed] [Google Scholar]
- Broderick G, Fuite J, Kreitz A, Vernon SD, Klimas N, Fletcher MA. A formal analysis of cytokine networks in chronic fatigue syndrome. Brain Behav Immun. 2010;24:1209–1217. doi: 10.1016/j.bbi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MD, Hilsabeck RC, Barakat F, Perry W. Role of Sleep Disturbance in Chronic Hepatitis C Infection. Curr Hepat Rep. 2010;9:25–29. doi: 10.1007/s11901-010-0030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S. Clinical sequelae affecting quality of life in the HIV-infected patient. J Assoc Nurses AIDS Care. 2004;15:28S–33S. doi: 10.1177/1055329004269478. [DOI] [PubMed] [Google Scholar]
- Doherty PC, Christensen JP, Belz GT, Stevenson PG, Sangster MY. Dissecting the host response to a gamma herpes virus. Philos Trans R Soc Lond B Biol Sci. 2001;356:581–593. doi: 10.1098/rstb.2000.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlwein O, Kaye S, McClure MO, Weber J, Wills G, Collier D, Wessely S, Cleare A. Failure to detect the novel retrovirus XMRV in chronic fatigue syndrome. PLoS One. 2010;5:e8519. doi: 10.1371/journal.pone.0008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AS. Chronic fatigue syndrome: thoughts on pathogenesis. Reviews of Infectious Diseases. 1991;13:S56–S59. doi: 10.1093/clinids/13.supplement_1.s56. [DOI] [PubMed] [Google Scholar]
- Flano E, Jia Q, Moore J, Woodland DL, Sun R, Blackman MA. Early establishment of gamma herpes virus latency: implications for immune control. J Immunol. 2005;174:4972–4978. doi: 10.4049/jimmunol.174.8.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flano E, Woodland DL, Blackman MA. A mouse model for infectious mononucleosis. Immunol.Res. 2002;25:201–217. doi: 10.1385/IR:25:3:201. [DOI] [PubMed] [Google Scholar]
- Fremont M, Metzger K, Rady H, Hulstaert J, De Meirleir K. Detection of herpes viruses and parvovirus B19 in gastric and intestinal mucosa of chronic fatigue syndrome patients. In Vivo. 2009;23:209–213. [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Heitz V, Engert V, Schad T, Schurmeyer TH, Ehlert U. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology. 2005;30:188–198. doi: 10.1016/j.psyneuen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Gargano LM, Forrest JC, Speck SH. Signaling through Toll-like receptors induces murine gamma herpes virus 68 reactivation in vivo. J Virol. 2009;83:1474–1482. doi: 10.1128/JVI.01717-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-associated immune modulation: relevance to viral infections and chronic fatigue syndrome. Am J Med. 1998;105:35S–42S. doi: 10.1016/s0002-9343(98)00160-0. [DOI] [PubMed] [Google Scholar]
- Glaser R, Padgett DA, Litsky ML, Baiocchi RA, Yang EV, Chen M, Yeh PE, Klimas NG, Marshall GD, Whiteside T, Herberman R, Kiecolt-Glaser J, Williams MV. Stress-associated changes in the steady-state expression of latent Epstein-Barr virus: implications for chronic fatigue syndrome and cancer. Brain Behav Immun. 2005;19:91–103. doi: 10.1016/j.bbi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Groom HC, Boucherit VC, Makinson K, Randal E, Baptista S, Hagan S, Gow JW, Mattes FM, Breuer J, Kerr JR, Stoye JP, Bishop KN. Absence of xenotropic murine leukaemia virus-related virus in UK patients with chronic fatigue syndrome. Retrovirology. 2010;7:10. doi: 10.1186/1742-4690-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes KE, Raucci JA, Jr, Gades NM, Toth LA. An evaluation of analgesic regimens for abdominal surgery in mice. Contemp. Top. Lab Anim Sci. 2000;39:18–23. [PubMed] [Google Scholar]
- Herold N, Spray S, Horn T, Henriksen SJ. Measurements of behavior in the naked mole-rat after intraperitoneal implantation of a radio-telemetry system. Journal of Neuroscience Methods. 1998;81:151–158. doi: 10.1016/s0165-0270(98)00028-4. [DOI] [PubMed] [Google Scholar]
- Hossain JL, Ahmad P, Reinish LW, Kayumov L, Hossain NK, Shapiro CM. Subjective fatigue and subjective sleepiness: two independent consequences of sleep disorders? J Sleep Res. 2005;14:245–253. doi: 10.1111/j.1365-2869.2005.00466.x. [DOI] [PubMed] [Google Scholar]
- Hwang S, Wu TT, Tong LM, Kim KS, Martinez-Guzman D, Colantonio AD, Uittenbogaart CH, Sun R. Persistent gamma herpes virus replication and dynamic interaction with the host in vivo. J Virol. 2008;82:12498–12509. doi: 10.1128/JVI.01152-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat. Rev. Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri KA, Ramkumar V, Trammell RA, Toth LA. Spontaneous, homeostatic, and inflammation-induced sleep in NF-kappaB p50 knockout mice. Am. J. Physiol Regul. Integr. Comp Physiol. 2006;291:R1516–R1526. doi: 10.1152/ajpregu.00262.2006. [DOI] [PubMed] [Google Scholar]
- Jones JF, Williams M, Schooley RT, Robinson C, Glaser R. Antibodies to Epstein-Barr virus-specific DNase and DNA polymerase in the chronic fatigue syndrome. Arch Intern Med. 1988;148:1957–1960. [PubMed] [Google Scholar]
- Klimas NG, Koneru AO. Chronic fatigue syndrome: inflammation, immune function, and neuroendocrine interactions. Curr Rheumatol Rep. 2007;9:482–487. doi: 10.1007/s11926-007-0078-y. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Toth LA. Cytokines as regulators of sleep. Annals of the New York Acadamy of Sciences. 1994;739:299–310. doi: 10.1111/j.1749-6632.1994.tb19832.x. [DOI] [PubMed] [Google Scholar]
- Krupp LB, Mendelson WB, Friedman R. An overview of chronic fatigue syndrome. Journal of Clinical Psychiatry. 1991;52:403–410. [PubMed] [Google Scholar]
- Kyle DV, DeShazo RD. Chronic fatigue syndrome: a conundrum. American Journal of the Medical Sciences. 1992;303:28–34. doi: 10.1097/00000441-199201000-00007. [DOI] [PubMed] [Google Scholar]
- Lombardi VC, Ruscetti FW, Das Gupta J, Pfost MA, Hagen KS, Peterson DL, Ruscetti SK, Bagni RK, Petrow-Sadowski C, Gold B, Dean M, Silverman RH, Mikovits JA. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science. 2009;326:585–589. doi: 10.1126/science.1179052. [DOI] [PubMed] [Google Scholar]
- Lorusso L, Mikhaylova SV, Capelli E, Ferrari D, Ngonga GK, Ricevuti G. Immunological aspects of chronic fatigue syndrome. Autoimmun Rev. 2009;8:287–291. doi: 10.1016/j.autrev.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Matthews SG, Heavens RP, Sirinathsinghji DJS. Cellular localization of corticotropin releasing factor mRNA in the ovine brain. Molecular Brain Research. 1991;11:171–176. doi: 10.1016/0169-328x(91)90119-i. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Opp MR. Diurnal variation of lipopolysaccharide-induced alterations in sleep and body temperature of interleukin-6-deficient mice. Brain, Behavior and Immunity. 2005;19:40–51. doi: 10.1016/j.bbi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Schwander SK, Donnelly R, Ortega F, Togo F, Broderick G, Yamamoto Y, Cherniack NS, Rapoport D, Natelson BH. Cytokines across the night in chronic fatigue syndrome with and without fibromyalgia. Clin Vaccine Immunol. 2010;17:582–587. doi: 10.1128/CVI.00379-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natelson BH, Ye N, Moul DE, Jenkins FJ, Oren DA, Tapp WN, Cheng YC. High titers of anti-Epstein-Barr virus DNA polymerase are found in patients with severe fatiguing illness. J Med Virol. 1994;42:42–46. doi: 10.1002/jmv.1890420109. [DOI] [PubMed] [Google Scholar]
- Nguyen Y, McGuffie BA, Anderson VE, Weinberg JB. Gamma herpes virus modulation of mouse adenovirus type 1 pathogenesis. Virology. 2008;380:182–190. doi: 10.1016/j.virol.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivadoti M, Toth LA, Weinberg J, Opp MR. Murine gamma herpes virus 68: a model for the study of Epstein-Barr virus infections and related diseases. Comp Med. 2007;57:44–50. [PubMed] [Google Scholar]
- Olivadoti MD, Opp MR. Effects of i.c.v. administration of interleukin-1 on sleep and body temperature of interleukin-6-deficient mice. Neuroscience. 2008;153:338–348. doi: 10.1016/j.neuroscience.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett DA, Hotchkiss AK, Pyter LM, Nelson RJ, Yang E, Yeh PE, Litsky M, Williams M, Glaser R. Epstein-Barr virus-encoded dUTPase modulates immune function and induces sickness behavior in mice. J Med Virol. 2004;74:442–448. doi: 10.1002/jmv.20196. [DOI] [PubMed] [Google Scholar]
- Rakib A, White PD, Pinching AJ, Hedge B, Newbery N, Fakhoury WK, Priebe S. Subjective quality of life in patients with chronic fatigue syndrome. Qual Life Res. 2005;14:11–19. doi: 10.1007/s11136-004-1693-y. [DOI] [PubMed] [Google Scholar]
- Ray MA, Trammell RA, Verhulst SJ, Ran S, Toth LA. Model development for the assessment of fatigue during chemotheraby in mice. Comp Med. 2011 in press. [PMC free article] [PubMed] [Google Scholar]
- Rickinson AB, Kieff E. Epstein-Barr virus. In: Knipe DM, Howley PM, Griffin DE, Martin MA, Lamb RA, Roizman B, editors. Fields Virology. Philadelphia: Lippincott, Williams, & Wilkins; 2001. [Google Scholar]
- Salahuddin N, Barroso J, Leserman J, Harmon JL, Pence BW. Daytime sleepiness, nighttime sleep quality, stressful life events, and HIV-related fatigue. J Assoc Nurses AIDS Care. 2009;20:6–13. doi: 10.1016/j.jana.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro JS. Does varicella-zoster virus infection of the peripheral ganglia cause Chronic Fatigue Syndrome? Med Hypotheses. 2009;73:728–734. doi: 10.1016/j.mehy.2009.04.043. [DOI] [PubMed] [Google Scholar]
- Swanink CM, Vercoulen JH, Bleijenberg G, Fennis JF, Galama JM, van der Meer JW. Chronic fatigue syndrome: a clinical and laboratory study with a well matched control group. J Intern Med. 1995;237:499–506. doi: 10.1111/j.1365-2796.1995.tb00876.x. [DOI] [PubMed] [Google Scholar]
- Tang X, Sanford LD. Telemetric recording of sleep and home cage activity in mice. Sleep. 2002;25:691–699. [PubMed] [Google Scholar]
- ter Wolbeek M, van Doornen LJ, Kavelaars A, van de Putte EM, Schedlowski M, Heijnen CJ. Longitudinal analysis of pro- and anti-inflammatory cytokine production in severely fatigued adolescents. Brain Behav Immun. 2007;21:1063–1074. doi: 10.1016/j.bbi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Tibbetts SA, Loh J, Van Berkel V, McClellan JS, Jacoby MA, Kapadia SB, Speck SH, Virgin HWt. Establishment and maintenance of gamma herpes virus latency are independent of infective dose and route of infection. J Virol. 2003;77:7696–7701. doi: 10.1128/JVI.77.13.7696-7701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth LA, Opp MR. Cytokine- and microbially-induced sleep responses of interleukin-10 deficient mice. American Journal of Physiology. 2001;280:R1806–R1814. doi: 10.1152/ajpregu.2001.280.6.R1806. [DOI] [PubMed] [Google Scholar]
- Toth LA, Verhulst SJ. Strain differences in sleep patterns of healthy and influenza-infected inbred mice. Behav. Genet. 2003;33:325–336. doi: 10.1023/a:1023402709896. [DOI] [PubMed] [Google Scholar]
- van Kuppeveld FJ, de Jong AS, Lanke KH, Verhaegh GW, Melchers WJ, Swanink CM, Bleijenberg G, Netea MG, Galama JM, van der Meer JW. Prevalence of xenotropic murine leukaemia virus-related virus in patients with chronic fatigue syndrome in the Netherlands: retrospective analysis of samples from an established cohort. BMJ. 2010;340:c1018. doi: 10.1136/bmj.c1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JB, Lutzke ML, Efstathiou S, Kunkel SL, Rochford R. Elevated chemokine responses are maintained in lungs after clearance of viral infection. J Virol. 2002;76:10518–10523. doi: 10.1128/JVI.76.20.10518-10523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager EJ, Szaba FM, Kummer LW, Lanzer KG, Burkum CE, Smiley ST, Blackman MA. gamma-Herpes virus-induced protection against bacterial infection is transient. Viral Immunol. 2009;22:67–72. doi: 10.1089/vim.2008.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Gough J, Christmas D, Mattey DL, Richards SC, Main J, Enlander D, Honeybourne D, Ayres JG, Nutt DJ, Kerr JR. Microbial infections in eight genomic subtypes of chronic fatigue syndrome/myalgic encephalomyelitis. J Clin Pathol. 2010;63:156–164. doi: 10.1136/jcp.2009.072561. [DOI] [PMC free article] [PubMed] [Google Scholar]