Abstract
Erectile dysfunction (ED) is a serious medical condition in which current treatments are ineffective in prostatectomy and diabetic patients, due to injury to the cavernous nerve (CN), which causes irreversible remodeling of the penis (decreased smooth muscle and increased collagen), through a largely undefined mechanism. We propose that sonic hedgehog (SHH) and neural innervation, are indispensable regulators of collagen in the penis, with decreased SHH protein being an integral component of the fibrotic response to loss of innervation. We examined collagen abundance and morphology in control (Peyronie’s), prostatectomy and diabetic patients, and in rat models of penile development, CN injury, SHH inhibition and under regenerative conditions, utilizing self-assembling peptide amphiphile (PA) nanofiber hydrogels for SHH delivery. Collagen abundance increased in penis of ED patients. In rats, collagen increased with CN injury in a defined time frame independent of injury severity. An inverse relationship between SHH and collagen abundance was identified; SHH inhibition increased and SHH treatment decreased penile collagen. SHH signaling in the pelvic ganglia (PG)/CN is important to maintain CN integrity and when inhibited, down stream collagen induction occurs. Collagen increased throughout penile development and with age, which is important when considering how to treat fibrosis clinically. These studies show that SHH PA treatment reduces collagen under regenerative post-prostatectomy conditions, indicating broad application for ED prevention in prostatectomy, diabetic and aging patients and in other peripheral nerve injuries. The PA nanofiber protein vehicle may be widely applicable as an in vivo delivery tool.
Keywords: self-assembling peptide amphiphile nanofiber hydrogels, Sonic hedgehog, penis, collagen, cavernous nerve injury
Graphical Abstract
Introduction
The cavernous nerve, which provides innervation to the penis, becomes injured during prostatectomy surgery, in diabetic patients, and with aging, resulting in remodeling of penile morphology and erectile dysfunction (ED). The penis is composed of smooth muscle, collagen and elastic fibers. In response to peripheral nerve injury, smooth muscle and elastic fibers decrease while collagen increases in both ED patients [1,2] and animal models [3,4]. This is an irreversible process that underlies ED development. Aging and consumption of high fat diets are also associated with increased collagen abundance and thickening of fibers [5–7]. Current ED treatments are not effective to prevent the penile remodeling, nor long term ED development. The mechanism of how collagen induction occurs in response to CN injury is complex and remains largely undefined.
As is the case with other peripheral nerves, CN regeneration efforts have not translated into improved clinical outcomes, and there is minimal understanding of how neuronal changes impact tissue remodeling. Few factors have been identified in the penis that impact collagen induction. The most widely explored is the TGF-β pathway, which in patients, increases collagen abundance 2.5–4.5 fold [8] and in animal models increases with CN injury [9]. TGF-β-1-induced collagen synthesis is inhibited by cyclic AMP synthesis in human corpora cavernosal smooth muscle cells [10], suggesting a potential mechanism of how TGF-β may be regulated. TGF-β also functions down stream of the adenosine receptor A(2B)R which has been implicated to increase proliferation in corpora cavernosal fibroblast cells [11]. Accumulating evidence suggests that cross talk may occur between the sonic hedgehog (SHH) pathway and TGF-β signaling in several diseases including gastric carcinoma [12], melanoma bone metastasis [13] and pulmonary fibrosis [14]. It is thought that Hedgehog may mediate epithelial-mesenchymal crosstalk in pulmonary fibrosis, with SHH inducing TGF-β in lung fibroblasts while TGF-β induces SHH in cultured alveolar epithelial cells [15]. Thus, we hypothesize that SHH may also serve as an important mediator/regulator of collagen synthesis in normal and injured penis.
We have shown in previous studies that the SHH pathway is critical for the response of the penis and of the CN to denervation, regulating both penile and CN architecture, and smooth muscle apoptosis [16–23]. The SHH pathway has recently been suggested to also play an important role in the pathogenesis of fibrosis [24,14], with activation of the pathway present in fibrotic diseases such as sclerosis, interstitial pneumonitis, injury-related inflammations [25–27] and idiopathic pulmonary fibrosis [28]. In support of this idea, during development, the expression pattern of type XVIII collagen in the ureter bud is responsive to changes in SHH expression in the epithelium [29]. In the adult, hedgehog (Hh) signaling can regulate lung fibrosis [30], and expression of SHH and GLI were correlated with cerulean-induced fibrosis in the pancreas [31], suggesting that SHH may be a regulator of collagen. In this study we will examine if collagen production is responsive to SHH signaling in the penis and in the CN, and to CN regulation, and thus may provide a novel avenue for clinical intervention post prostatectomy and in diabetic patients.
We have described the use of self-assembling peptide amphiphiles (PA) as biological delivery vehicles, to prevent ED-related smooth muscle apoptosis in the penis [20,21]. These versatile hydrogel systems can be molecularly pre-programmed for SHH protein delivery, either from (1) an injectable solution with fast, in situ assembly into a soft hydrogel, or (2) highly aligned monodomain nanofiber bundles with increased mechanical integrity [32, 33]. In both permutations, these PAs offer a customized, biodegradable, solution for delivering proteins in a controlled manner over extended periods, and are easily translatable to patients in the clinic. In this study we will examine a novel neuronal component to collagen regulation and the role of the SHH pathway in the fibrotic response to nerve injury. We will utilize these innovative PA systems for SHH delivery to the luminal surfaces of the corpora cavernosa (via in situ gelation), and SHH delivery to the injured CN from a manipulable supramolecular cable (via monodomain aligned nanofibers). These materials and their technology have potentially broad application to other peripheral nerves and the tissues that they innervate.
Materials and Methods
Animals
199 Sprague-Dawley rats postnatal day 7 (P7) through P300 were obtained from Charles River. The study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal care protocol was approved by the Office of Animal Care and Institutional Biosafety at the University of Illinois at Chicago (OACIB) and by the IACUC committee at Northwestern University and animals were cared for in accordance with institutional approval.
Patient tissues
Corpora cavernosal tissue was obtained from 21 patients who underwent penile prosthesis implantation at Northwestern Memorial Hospital. Eight patients had a previous prostatectomy, five had diabetes, and eight control patients underwent corrective surgery for Peyronie’s disease. Peyronie’s disease is a condition of the penis characterized by the alteration in the appearance and cellularity of collagen within the tunica albuginea, which becomes fibrotic with disease progression. While Peyronie’s patients have intromission difficulty, the underlying defect is tunical so the lacunar tissue remains fundamentally normal [34]. Peyronie’s tissue is the best available control other than cadaver tissue and corporal tissue was obtained from a region away from the involved tunica. For the penile prostheses cases, the corporal tissue at the site of corporotomy was identified, minimally handled and a small wedge of lacunar tissue excised prior to any dilation or other corporal manipulation was performed. For the control Peyronie’s cases, the corporal tissue at the site of tunical defect and the surrounding corpora in the vicinity of the planned grafting site were identified. Similarly these normal tissues were minimally handled and a small wedge of lacunar tissue excised prior to any other corporal manipulation or grafting was performed. The tissue was immediately handed to a lab technician present in the operating room and the tissue was snap frozen in liquid nitrogen or fixed in 4% paraformaldehyde overnight at 4°C prior to paraffin embedding. Exclusion criteria included patients under 18 years of age. The complete study protocol was approved by the institutional review board of Northwestern University and written informed consent was obtained from all patients. For the prostatectomy patients it had been 1–17 years since surgery with an average of 6 years. For the diabetic patients, the onset of diabetes was between 7 and 24 years with an average of 12 years.
Hydroxyproline assay
Collagen abundance was quantified on frozen corpora cavernosal tissue using a modified hydroxyproline assay [35]. Each sample was examined in duplicate. The absorbance was read at 550 nm using a plate reader and the collagen content was calculated as μg collagen/mg tissue.
Trichrome stain
Trichrome stain was performed as described previously [36] on penis tissue that was fixed in Bouin’s fixative for 48 hours at 4°C prior to staining and was quantified by Image J analysis (Image J version 1.45 s, down load date 5/22/2012). After background subtraction, the total area of blue (collagen) was selected and quantified in trichrome photos independently of smooth muscle (red), which decreases with nerve injury. Total area of blue/collagen was measured in 25 photos (200X magnification) taken randomly in each tissue (5 photos per section and 5 sections per tissue).
Timecourse of postnatal collagen development
Postnatal day 7, P13, P22, P40, P62, P120 and P200–300 Sprague Dawley rat penis (n=3 for each time point) were fixed in Bouin’s fixative for 48 hours at 4°C prior to examination by trichrome stain to evaluate collagen morphology and abundance with age.
Timecourse of collagen induction after CN injury
Rats were anesthetized and the pelvic ganglia (PG)/CN were surgically exposed and microforceps (size 0.02 × 0.06mm) were used to crush the CN bilaterally for 30 seconds (constant pressure with closed forceps), greater than 5 mm from the PG. This method of CN crush has commonly been used [37,38] and the extent and reproducibility of crush injury were previously verified [21]. Sham surgery (control) was performed by exposing but not crushing the CN (n=6). Rats were sacrificed 4, 7 and 14 days after injury (n=16). For severe crush injury, three consecutive 30 second crushes were performed in the same region of the CN (n=4) and rats were sacrificed at 4 days after injury. For resection injury, a 5mm portion of the CN was removed 5mm from the PG (n=4) and rats were sacrificed at 4 days after injury.
SHH inhibited penis
Affi-Gel beads (100–200 mesh, Bio-Rad, Hercules, CA) were equilibrated with 100μl of 5E1 SHH inhibitor (378 μg/ml, Jessel, Hybridoma Bank University of Iowa) or mouse IgG in 1XPBS (control) overnight at 4°C. Approximately 100μl beads were injected into the corpora cavernosa of the penis of each rat. SHH inhibited rats were sacrificed after 1–2 (n=6), 4 (n=3) and 7 days (n=3) in comparison to mouse IgG/PBS controls (n=6, 6 and 3, respectively).
CN injury with SHH treatment of the penis by peptide amphiphile (PA)
We have previously employed self-assembling peptide amphiphiles as hydrogel carriers for controlled delivery of SHH to relevant tissues in the penis. We have used these carriers in two variants: either through injection and subsequent gelation of V3A3E3-COOH PAs directly within the corpora cavernosa (as a soft gel, coating the interior corpora cavernosal surfaces), or external preparation of V2A2E2-NH2 PAs to form highly aligned, monodomain hydrogels with sufficient mechanical integrity that they can be manipulated and placed on top of an exposed CN. In both cases, SHH is entrapped within the PA hydrogels during cation-based assembly and crosslinking. Both systems were employed in the current work, to assess SHH impact on collagen abundance in penile tissues.
Bilateral CN cut with either SHH (n=12) or BSA (n=12) treatment was performed by injection of a self-assembling peptide amphiphile (PA) hydrogel (V3A3E3-COOH) into the corpora cavernosa of the penis as described previously [20]. A 5-mm section of the CN was removed bilaterally, 5-mm from the PG. A 20-mM solution of PA (50 μl) was added to 5 μl of a solution of SHH protein in water (1.25 μg/μl, R&D Systems, Minneapolis, MN, USA). A 40 mM CaCl2 solution (50 μL) was added to the SHH-PA solution. The skin covering the penis was then retracted and the PA was immediately injected directly into the corpora cavernosa with a 26-gauge needle (105 μl volume) where the PA formed a loose gel lining the sinuses within 30 seconds to 1 minute. The final concentration of PA was 10 mM and CaCl2 was 20 mM. The final amount of SHH protein injected was 6.25 μg per rat. Penises were harvested from euthanized males by sharp dissection 2, 4 and 7 days after SHH protein/PA/CaCl2 injection and were frozen in liquid nitrogen.
SHH protein was also delivered to the penis of CN injured rats by injection of Affi-Gel beads (100–200 mesh, Bio-Rad Laboratories, Hercules, CA) equilibrated with either 1X SHH peptide (n=10, 7.5μg per animal, R & D Systems), 2XSHH peptide (n=9, 15 μg per animal, R&D Systems), or PBS (control, n=9) overnight at 4°C. Approximately 30–40 beads were injected directly into the corpora cavernosa. CN injury only rats served as a positive control (n=4). Collagen was quantified by hydroxyproline assay 4 days after CN injury.
SHH inhibition in the PG
Affi-Gel beads were equilibrated with 5E1 SHH inhibitor (378 μg/ml, n=12) or mouse IgG in PBS (control, n=8) overnight at 4°C. Approximately 10–20 beads were injected directly under the PG bilaterally in adult Sprague-Dawley rats. Injection was not made into the ganglia itself since this would likely destroy the ganglia. Penis tissue was dissected after 2 days of SHH inhibition in the PG.
A second SHH inhibitor, cyclopamine (10μM, n=4) was administered to Sprague Dawley rats by Affi-Gel beads under the PG for 7 days in comparison to PBS treated controls (n=4) and penis tissue was assayed for collagen by hydroxyproline.
SHH treatment of uninjured PG/CN
Affi-Gel beads (100–200 mesh) were equilibrated with SHH or BSA (control) protein (0.25μg/μl) overnight at 4°C. Approximately 10–20 beads were injected under the PG bilaterally and rats were sacrificed after 2 days. Penis from SHH (n=4) and BSA treated (n=3) rats were frozen for hydroxyproline analysis.
CN crush with either SHH or BSA/MSA protein treatment of the CN by linear monodomain PA
(C16)-V2A2E2-(NH2) PA was prepared as previously described [21]. Briefly, a 100mM solution of (C16)-V2A2E2-(NH2) in water was heated to 80°C, held for 30 minutes, and slowly cooled to room temperature. 200mM CaCl2 was added to a glass slide and 8μl of the heat-cycled PA plus either 2.27μg SHH or bovine serum albumin/mouse serum albumin (BSA/MSA, control) proteins were extruded with a pipet tip onto the slide to form a linear monodomain PA hydrogel. PG/CN were crushed bilaterally for 30 seconds as described above. Linear PA with the protein was transferred with forceps on top of the crushed CNs bilaterally so that each rat received 4.54μg SHH or BSA/MSA protein. The release rate of SHH protein from the PA was previously determined to be 90% by 75 hours [21]. Rats were sacrificed after 1–2 and 4 days. Penis from SHH (n=14) and BSA/MSA treated (n=10) rats were frozen for hydroxyproline assay.
Immunohistochemical analysis (IHC)
IHC was performed on control (n=3) and CN injured (n=3) frozen penis sections which were cut 14μm and were fixed in acetone at 4°C for 15 minutes. OCT was removed with two washes of 1X PBS prior to blocking with 3% milk in PBS for one hour at 4°C. Sections were incubated overnight at 4°C with rabbit polyclonal antibodies against collagen type I and type IV (Ab34710 and Ab6586 respectively, 1μg/μl, Abcam) and goat polyclonal antibodies against collagen type II alpha 1 (C-19, SC-7763, and N-19, SC-7764) and collagen type III alpha 1 C-15 (SC-8781, Santa Cruz). Secondary antibodies were 1/150 diluted chicken anti-goat and chicken anti-rabbit (Molecular Probes). Sections were mounted using DPX Mounting media (Electron Microscopy Sciences, Hatfield, PA) and microscopy performed using a Leica DM2500 Microscope.
Statistical Analysis
Statistics were performed where appropriate using the Excel program (Microsoft) and the results were reported ± the standard error of the mean. A t-test was performed to determine significant differences (p≤0.05) between two groups. For comparisons across three groups, statistics were performed by ANOVA with a Scheffe’s posthoc or Dunnett’s test using the SSPS statistical program.
Results
Collagen quantification in corpora cavernosal tissue of Peyronie’s, prostatectomy and diabetic patients
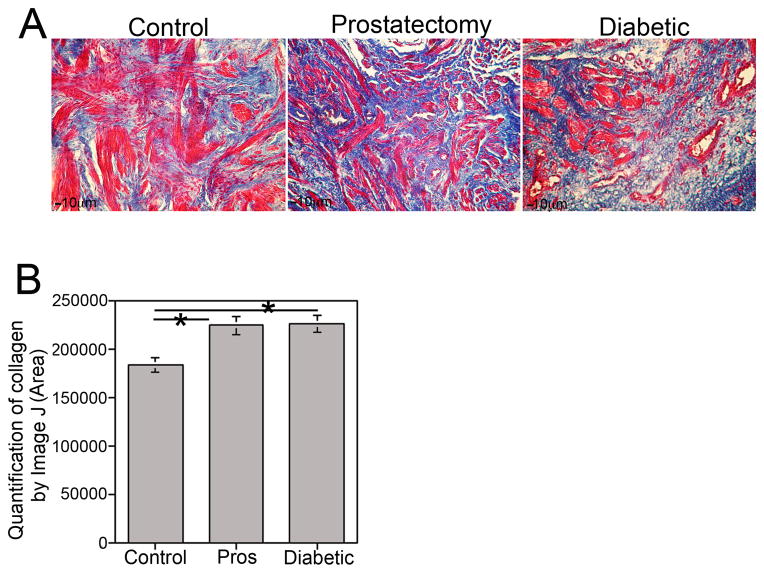
Trichrome stain was performed on corpora cavernosal tissue of Peyronie’s (control), diabetic and prostatectomy patients (Figure 1A) who had ED. Smooth muscle (red) and collagen (blue) were abundant in control Peyronie’s tissue. In comparison to controls, collagen was significantly increased 19% in diabetic (p=0.015) and 18% in the corpora cavernosa of prostatectomy patients (p=0.009, Figure 1B).
Figure 1.
(A) Trichrome stain of corpora cavernosal tissue from control Peyronie’s, prostatectomy and diabetic patients (100X magnification). (B) Quantification of collagen was performed from trichrome stained tissues using Image J with background subtraction. Collagen levels were significantly higher (19%, p=0.015) in diabetic and in prostatectomy (18%, p=0.009) patients in comparison to control Peyronie’s patients.
Time course of collagen development in the penis
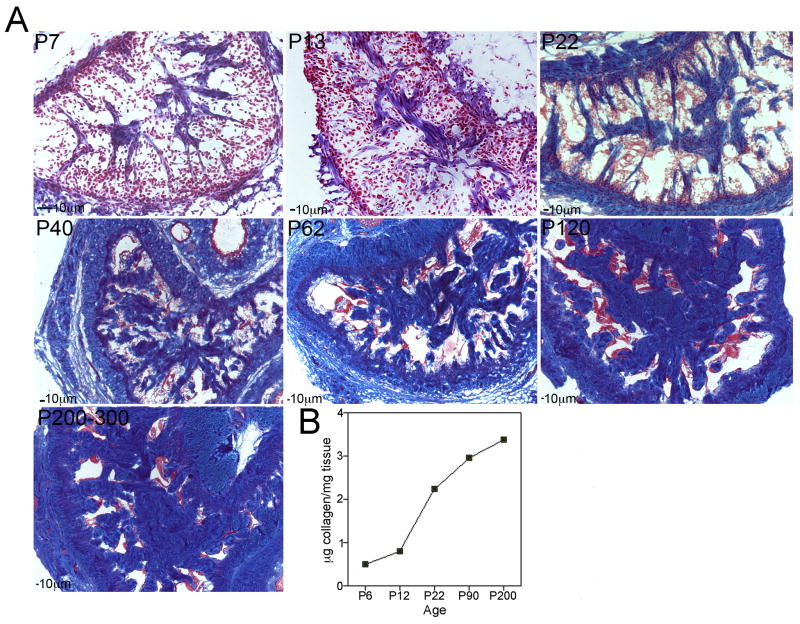
In the rat, differentiation and growth of penile tissues occurs primarily in the postnatal period after birth [16]. We examined collagen morphology and abundance in the rat penis with increasing postnatal age (Figure 2). At P7, collagen was identifiable within the corpora cavernosa (Figure 2A). At P13, collagen fibers were more abundant and thicker in appearance (Figure 2A). By P22, a network of collagen fibers was more clearly defined (Figure 2A). Cavernae and trabeculae resemble the adult configuration at P40 and are exclusively of the adult type by P60 [39]. Between P60 and P120, substantial collagen growth occurs. Collagen appears even more abundant in the aged penis (P200–300, Figure 2A). Collagen abundance was assayed in one set of corpora cavernosal tissue by hydroxyproline assay. Collagen increased with age in the penis (Figure 2B).
Figure 2.
Trichrome stain of penis tissue was performed at several key time points during postnatal development (P7–P300) to show collagen morphology and development (A, 50–200X magnification). Collagen was quantified in one set of corpora cavernosal tissue from each age of penis (B). Collagen abundance increased with age.
Quantification of collagen with CN injury
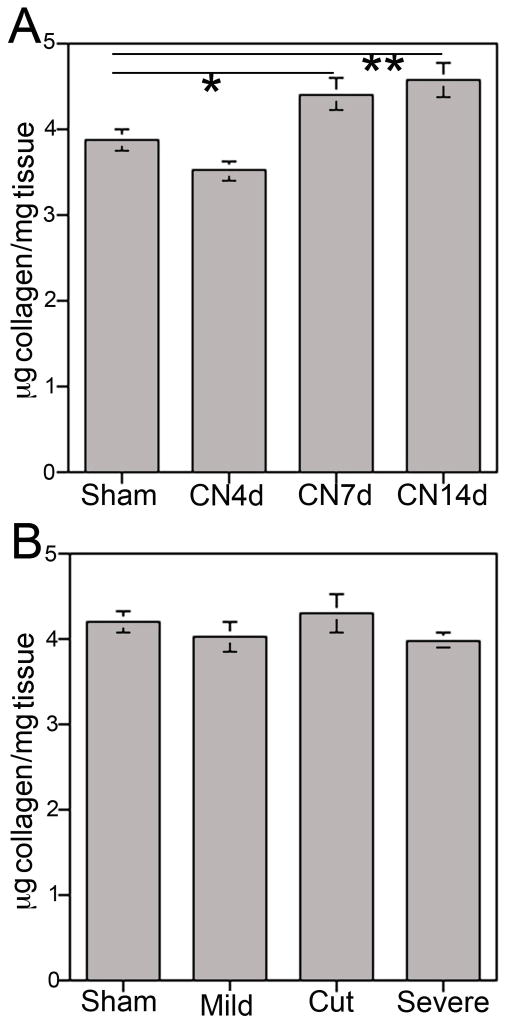
Total collagen abundance was quantified by hydroxyproline assay in corpora cavernosal tissue of rats that underwent bilateral CN crush for 30 seconds. Collagen was not increased at four days after injury (p=0.992, Figure 3A) however collagen significantly increased 12% 7 days after injury (p=0.038), and 16% at 14 days after injury (p=0.010, Figure 3A). No change in collagen was observed at 4 days after injury when a more severe CN crush injury was performed (3 times 30 seconds) or CN resection (p=0.560, Figure 3B).
Figure 3.
(A) Quantification of collagen by hydroxyproline assay of corpora cavernosal tissue from rats that underwent sham surgery or CN crush showed significantly increased collagen at 7 (p=0.0.038) and 14 (p=0.010) days after injury. (B) Quantification of collagen by hydroxyproline assay of corpora cavernosal tissue from rats that underwent sham surgery, mild or severe crush injury, or CN resection showed no change in collagen at 4 days after injury (p=0.560).
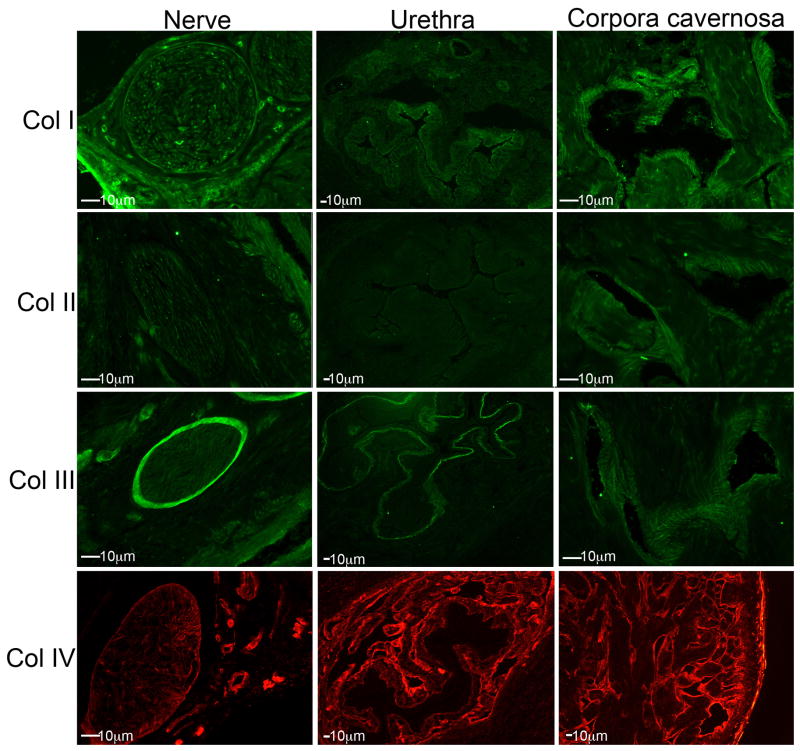
Localization of collagen subtypes in sham and CN injured rat penes
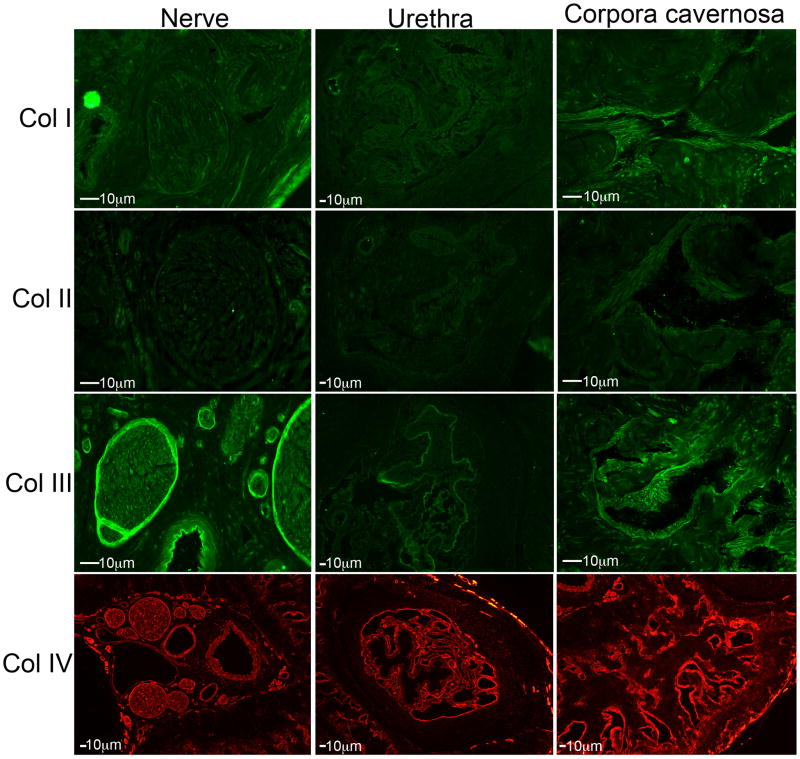
IHC analysis was performed on frozen penis sections assayed for collagen I–IV in sham (Figure 4) and CN injured 4day (Figure 5) rat penis. Collagen IV had the most pronounced staining through out the corpora cavernosa and in the perineurium of the nerves in the nerve bundle. Collagen IV was also abundantly expressed in concentric rings surrounding the urethra, but was not identified within the urethral epithelium (Figure 4). Collagen III was mildly expressed in the corpora cavernosa surrounding the sinusoidal spaces. It was also highly abundant in the perineurium of nerves in the nerve bundle and in the basement membrane of the urethra (Figure 4). Collagen II was faintly expressed in the corpora cavernosa in the area surrounding the sinusoidal spaces but was not identified in the nerve bundle or in the urethra (Figure 4). This was verified using two different collagen II antibodies. Collagen I was moderately expressed in the corpora cavernosa in the region surrounding the sinusoidal spaces and also in between the sinuses. Collagen I was only faintly present in the nerves of the nerve bundle and moderately in the epithelium of the urethra (Figure 4). No change in collagen localization was observed 4 days after CN injury (Figure 5). Collagen II was identifiable in the urethra after CN injury, but not present in normal tissue. The staining intensity appeared more intense for collagen III and IV after CN injury (Figure 5). No change in localization of collagen I–IV were observed 14 days after injury (Data not shown).
Figure 4.
Immunohistochemical analysis showing the localization of collagen I, II, III and IV in the nerve bundle, urethra and corpora cavernosa of normal Sprague Dawley rat penis. (100–200X magnification).
Figure 5.
Immunohistochemical analysis showing the localization of collagen I–IV in rat penis tissue four days after CN crush (100–200X magnification).
SHH inhibition in the penis increases collagen abundance in the corpora cavernosa
Trichrome stain and hydroxyproline assay were used to quantify collagen in penis tissue that was treated with 5E1 SHH inhibitor via Affi-Gel beads injected into the corpora cavernosa for 1–2, 4 and 7 days in comparison to control rats that were treated with mouse IgG in 1XPBS. Trichrome stain showed abundant collagen (blue) and smooth muscle (red) in penis tissue of the control group (Figure 6A). There was a progressive loss of smooth muscle, as documented previously [19] with SHH inhibition, and collagen appeared increased by visual observation (Figure 6A). Quantification of collagen in the corpora cavernosa by hydroxyproline assay showed that collagen abundance increased in response to SHH inhibition by 17% (p=0.045) at 1–2 days of inhibition and by 21% at 4 days of inhibition (p=0.030, Figure 6B), indicating that collagen induction is responsive to changes in SHH signaling.
Figure 6.
(A) Trichrome stain of penis tissue that was treated by Affi-Gel beads with either 5E1 SHH inhibitor or mouse IgG in 1X PBS for 2, 4 and 7 days (400X magnification). Smooth muscle and collagen were abundant in control penis. There was a progressive loss of smooth muscle and collagen appeared elevated with SHH inhibition. (B) Quantification of collagen by hydroxyproline assay showed a 17% increase after 1–2 days (p=0.045) and a 21% increase (p=0.030) after 4 days of SHH inhibition.
CN injury with SHH treatment of the penis by peptide amphiphile (PA) or Affi-Gel beads
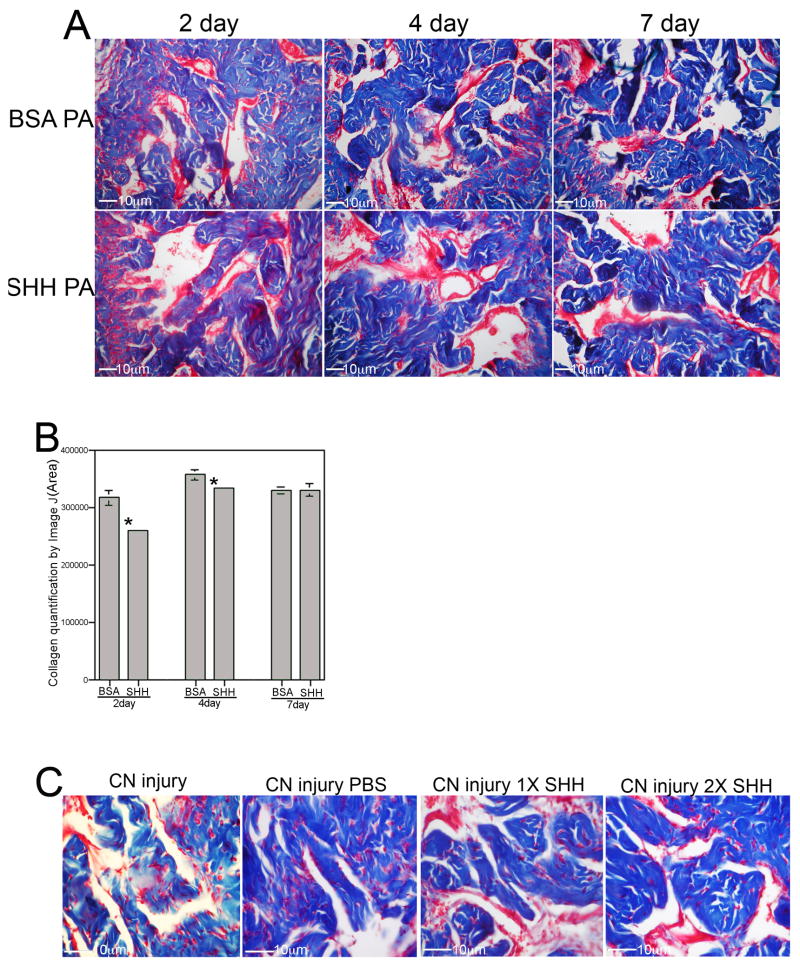
The response of the penis to SHH signaling under regenerative conditions was examined in CN resected rats. A CN resection model was used to assay response of the penis to SHH treatment since it is the most severe form of CN injury. If a response is seen in this model than treatment is likely more effective in a CN crush model. Trichrome stain was performed on penis tissue from rats that underwent bilateral CN resection and were treated with either SHH or BSA (control) protein by PA injected into the corpora cavernosa of the penis. Smooth muscle and collagen were abundant in the SHH PA treated rats (Figure 7A). Smooth muscle appeared less abundant and collagen more abundant after injury in the BSA treated group. When quantified by Image J analysis, collagen was 19% lower in the SHH treated group than in the BSA treated group (p=0.002) at 2 days after injury and 7% lower (p=0.039) at 4 days after injury (Figure 7B). By 7 days after injury, no difference in collagen abundance was apparent with SHH treatment (p=0.485, Figure 7B). SHH protein would be largely expended from the PA by 7 days as previously shown [20], while collagen levels increased 12% 7 days after CN injury.
Figure 7.
Trichrome stain of penis tissue of rats that under went CN resection and SHH or BSA treatment by peptide amphiphile delivery to the penis (250X magnification). (B) Quantification of collagen by Image J analysis showed decreased collagen in CN injured penis with SHH treatment at 2 days (19% decrease, p=0.002) and 4 days (7% decrease, p=0.039) after injury/treatment. By 7 days SHH protein had been expelled from the PA and there was no difference in collagen abundance (p=0.485). (C) SHH protein delivery by Affi-Gel bead injection to the penis showed comparable results to peptide amphiphile delivery with unchanged corpora cavernosal morphology 4 days after CN resection in the presence of exogenous SHH protein.
These results were replicated using Affi-gel bead delivery of SHH protein to the penis at the time of CN resection, indicating that corpora cavernosal morphology (smooth muscle and collagen) appeared normal four days after CN injury in the presence of SHH protein (Figure 7C).
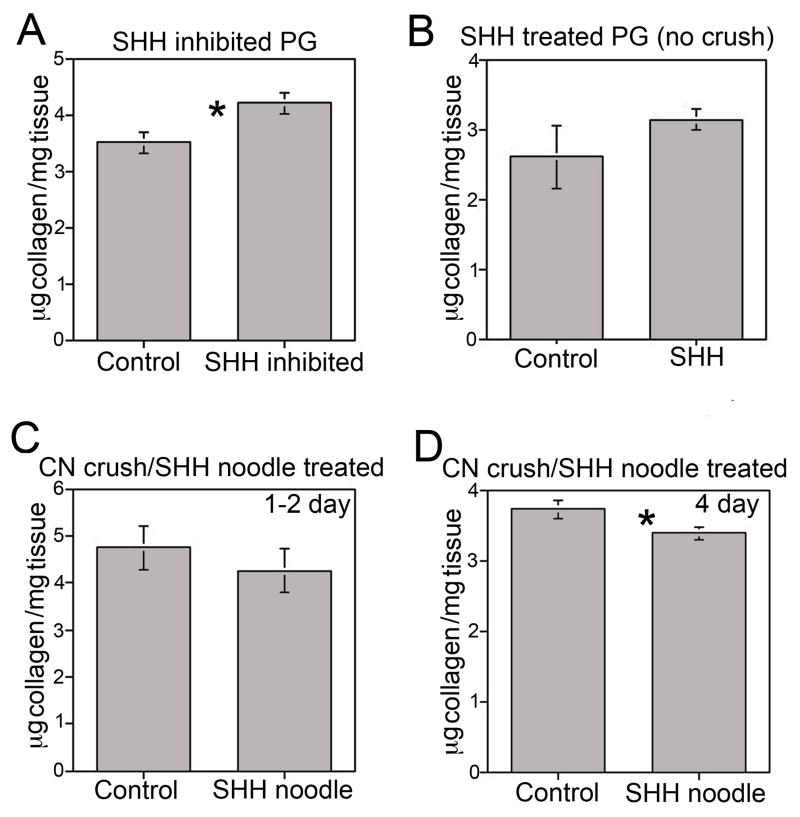
Collagen abundance in the penis after SHH inhibition in the PG
Since SHH signaling in the PG/CN impacts penile smooth muscle, we examined if it also impacts collagen. Total collagen was assayed in the corpora cavernosa by hydroxyproline quantification after SHH inhibition in the PG using the 5E1 SHH inhibitor. Collagen increased 17% in the penis when SHH was inhibited in the PG for 2 days (p=0.011, Figure 8A). These results were verified using a second SHH inhibitor, cyclopamine, administered for 7 days to the PG, which showed a 12% increase in collagen (Cyclopamine:3.99±0.251, Control:3.51±0.094, p=0.05).
Figure 8.
(A) Quantification of collagen by hydroxyproline assay of penis tissue from Sprague Dawley rats that underwent SHH inhibition in the PG with the 5E1 inhibitor or mouse IgG in PBS control. SHH inhibition in the PG for two days resulted in a 17% increase in collagen in the penis (p=0.011). (B) Quantification of collagen by hydroxyproline assay of penis tissue from rats in which uninjured/normal PG were treated with SHH protein or BSA protein (Control) for 2 days (without crush injury), showed no change in collagen (p=0.131). Quantification of collagen in penis tissue of rats that under went CN crush and SHH or BSA/MSA treatment by linear peptide amphiphile treatment for 1–2 (C) and 4 (D) days showed no difference in collagen at 1–2 days (p=0.240), but a 9% lower collagen abundance at 4 days of SHH treatment (p=0.025).
Collagen abundance in the penis after SHH treatment of uninjured PG
Collagen was quantified by hydroxyproline assay in the corpora cavernosa of rats treated with either SHH or BSA (control) protein by Affi-Gel beads under the PG. Collagen abundance in the penis was unchanged with SHH treatment of normal/uninjured PG for 2 days (p=0.132, Figure 8B).
Quantification of collagen in the penis after CN injury and either SHH or BSA/MSA treatment of the PG/CN by linear monodomain PA
The response of the penis to SHH signaling under regenerative conditions was examined by quantifying collagen in the corpora cavernosa after CN crush and SHH or BSA/MSA (control) treatment of the CN via linear monodomain PA for 1–2 and 4 days. Hydroxyproline assay showed that collagen abundance was unaltered after 1–2 days of SHH treatment (p=0.240, Figure 8C). However, at 4 days, collagen in the corpora cavernosa was 9% lower (p=0.025, Figure 8D).
Discussion
Collagen is a major component of the extracellular matrix and is important for development of tissues and adult homeostasis [40]. There are 21 different types of collagen that have been identified in mammals, which provide strong supportive extracellular scaffolds. As the fibrillar procollagen is secreted from cells, proteases cleave the propeptides from the collagenous domains [40]. The fibrillar forms of collagen are types I, II, III, V and XI. Type I collagen is most abundant in the body, present in bones and teeth, while type II collagen composes the major structural protein of cartilage [40]. In rats, collagen is typically transversely oriented in bundles and is the most abundant component of trabeculae with a volumetric density of 62.7% [41]. For comparison, smooth muscle has a volumetric density of 9.1% and elastic fibers of 4.9% [41]. Human corpora cavernosa differs from that of rats by containing smaller amounts of smooth muscle, more collagen, and the presence of fibrillar collagen and smooth muscle cell subendothelial layers [41].
In this study we examined whether collagen synthesis in the corpora cavernosa of the penis is responsive to perturbation of SHH signaling. When SHH was inhibited in normal penis, collagen increased by ~20% at 1–2 days and 4 days of inhibition. This result identifies an inverse relationship between SHH levels and collagen abundance. Collagen increases in the penis with CN injury (Figure 3); since we’ve previously shown that SHH protein decreases in the penis with CN injury [18], this suggests that SHH inhibition may play a role in collagen induction. When SHH was administered to the corpora cavernosa by peptide amphiphile nanofiber hydrogel at the time of CN injury, collagen was lower in the SHH treated rats at 2 and 4 days after injury. We’ve previously quantified the in vitro release rate of SHH protein from the PA [20]. Approximately 50% of the protein is released within 24 hours and an additional ~20% is released over the following 5 days. Since the serum half-life for SHH protein is about an hour, and 50% of the protein was released in the first day, it is not surprising that collagen induction is suppressed more in the first two days when SHH protein abundance is higher, and suppression dwindles as protein levels decline (Figure 7B). TGFβ1 induction by hypoxia, which accompanies CN injury, occurs in in vitro cultured corpora cavernosal smooth muscle cells, with a peak time of 48 hours [42], suggesting the importance of this early induction window to collagen production. These findings indicate that SHH signaling, either inhibition or treatment has a selective impact on collagen abundance in the penis, when administered directly to the corpora cavernosa. These findings suggest that for maximal suppression of collagen induction after nerve injury, such as would be needed for clinical translation to prostatectomy patients, several sequential SHH PA injections might be needed to maintain SHH levels and prevent collagen synthesis.
We examined whether SHH signaling in the PG/CN also impacts collagen abundance in the penis. These studies were performed because SHH is an integral component of normal PG/CN homeostasis and when inhibited, demyelination and axonal degeneration occur, resulting in down stream smooth muscle changes in the penis and initiation of penile remodeling [21]. SHH inhibition in the PG/CN, using two different antibody (5e1) and chemical (cyclopamine) inhibitors, increased collagen in the penis (Figure 8). This finding supports the idea that CN integrity is important to regulation of collagen abundance in the penis and indicates that SHH signaling is integral to maintain CN integrity. Since SHH protein is decreased in the PG/CN with CN injury, with a maximal decrease in the active form apparent at 7 days after injury [22], and collagen increases are first measurable in the penis 7 days after CN injury, this suggests that SHH signaling in the PG/CN may play an important role in regulating collagen abundance in the penis. When the CN was crushed, SHH treatment of the CN had no effect on collagen abundance at 2 days after injury however collagen was decreased in the presence of SHH treatment at 4 days after injury. At 2 days after injury, endogenous SHH protein is reduced ~25%, and it is plausible that added SHH protein by PA may compensate for the drop in endogenous levels, resulting in no change in neural integrity or collagen abundance. At 4 days after injury, endogenous SHH protein levels in the PG/CN are reduced ~50% [22], so addition of SHH by PA would have greater impact on nerve integrity and thus down stream collagen abundance in the penis, even if SHH protein delivered by PA is less at 4 days than 2 days, as previously documented [21]. These results indicate that collagen abundance in the penis is responsive to SHH signaling in the PG/CN, although changes in collagen take longer and are less robust than when SHH is applied directly to the corpora cavernosa. This is in opposition to the response of penile smooth muscle, which shows more pronounced apoptosis suppression when SHH treatment occurs in the CN.
Neural innervation is required to maintain collagen homeostasis in the penis, since CN crush injury causes a progressive increase in collagen abundance with time after injury (Figure 3). This is supported by our findings that SHH inhibition in the PG/CN results in increased collagen abundance in the penis (Figure 8) since SHH inhibition damages the CN. This is a novel finding since potential neural regulation of collagen has not previously been explored. We tested whether the severity of CN injury affects the time line of collagen induction by performing a more severe crush and resection injury (Figure 3B). Neither of the more severe injuries was able to increase collagen earlier than the mild crush. Thus the integrated response of the PG/CN and penis to CN injury, results in a predictable early response of the CN, including demyelination and axonal degeneration of CN fibers. Loss of innervation leads to an early wave of primarily smooth muscle apoptosis in the corpora cavernosa that peaks at 4 days after injury [22], followed by a slower induction of collagen at 7 days after injury (Figure 3). The response of collagen to loss of neural innervation might also explain why collagen appeared more abundant at P200–P300 than P120 (Figure 2), since spontaneous CN injury occurs in ~23% of Sprague Dawley rats as they age [43].
We examined the time course of collagen and smooth muscle development in the penis. In the rat, differentiation of the penile tissues occurs primarily in the postnatal period after birth. At E19, the corpora cavernosa consists of an undifferentiated mesenchyme, cuboidal epithelium and a thin, poorly defined basement membrane that the attenuated endothelium rests on [44]. Differentiation into erectile tissue containing both lacunae and trabeculae occurs during the first week after birth [45]. At P7, collagen is identifiable within the corpora cavernosa as thin fibers. At P13, collagen fibers are more abundant and thicker in appearance with a more clearly defined network. By P14, cavernous spaces appear large, irregularly shaped and are lined by an attenuated endothelium [39]. By P22, smooth muscle has the characteristic appearance and collagen fibers appear as an interconnected mesh within the corpora cavernosa. Cavernae resemble the adult configuration by P40 and are exclusively of the adult type by P60 [39]. Between P60 and the adult (P120) penis, growth of smooth muscle and collagen occur. Once the architecture of the penis is defined in the adult, it has been thought that the morphology does not change. However collagen appears even more abundant in the aged penis (P200–300), with smaller sinusoidal lumens. Collagen growth increases throughout postnatal penile development and may continue to grow/proliferate as a natural part of the aging process as CN degeneration occurs with age. This is important when considering how we think about and treat fibrosis clinically.
In an effort to understand how collagen changes in response to nerve injury, we examined the localization of collagen sub-types I, II, III and IV in normal and CN injured rat penis. Collagen IV appeared most abundant, with staining present in the corpora cavernosa, nerve bundle (including the perineurium) and in concentric layers surrounding the urethra. Collagen III, I and II were also present in the corpora cavernosa in descending order of observed intensity. Collagen III had pronounced staining in the perineurium, while collagen I was faint. Collagen III was localized in the basement membrane of the urethra, while collagen I was present in the columnar epithelium. Collagen II was not detectable in the urethral tissue. It is interesting that the different collagen sub-types were identified in adjacent layers of the urethra and may suggest cooperative function. The perineurium and urethral staining appeared less intense after CN injury for collagen I. Collagen II was identifiable in the urethra after CN injury but not in normal tissue, and it’s intensity was also increased in the corpora cavernosa with injury. The localization of collagen III and IV did not change with CN injury, however the intensity of the staining appeared higher. This is consistent with a previous report of increased collagen III after CN injury [9].
In order to understand the clinical significance of changes observed in our animal models, we quantified collagen in corpora cavernosal tissue from Peyronie’s (control), prostatectomy and diabetic patients. Collagen increased ~20% in both prostatectomy and diabetic patients relative to Peyronie’s controls. This is an important observation since collagen synthesis and remodeling appears to be an ongoing process that occurs with age and is also responsive to changes in neuronal input/signaling that may occur with injury and disease. We have shown previously in these patient tissues that SHH protein is decreased in the corpora cavernosa [23], consistent with our observations that SHH inhibition causes increased collagen abundance in the penis. While it is clear that perturbation of SHH signaling either directly in the penis or indirectly in the PG/CN, impact collagen production, further study is required to understand the mechanism of how this occurs. In previous animal studies we’ve shown that SHH may have translational potential to prevent smooth muscle changes that occur with prostatectomy [20]. The results presented here suggest that SHH treatment using monodomain gels containing PA nanofibers may have the added benefit of reducing collagen under regenerative post-prostatectomy conditions. This multi-pronged approach, which impacts smooth muscle degeneration, collagen synthesis and CN regeneration, has wide implications for ED prevention in prostatectomy, diabetic and aging patients and in other peripheral nerve injuries.
Acknowledgments
Grant support: DK101536, DK079184
This project was supported by NIH/NIDDK Award numbers R01DK101536 and R01DK079184. Peptide synthesis, purification, and characterization were performed by staff in the Peptide Synthesis Core Facility of the Simpson Querrey Institute at Northwestern University. The U.S. Army Research Office, the U.S. Army Medical Research and Material Command, and Northwestern University provided funding to develop this facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ryu J-K, Han J-Y, Chu Y-C, Song SU, Lee K-H, Yoon S-M, Suh J-K, Kim S-J. Expression of cavernous transforming growth factor-β1 and its Type II receptor in patients with erectile dysfunction. Int J Androl. 2004;27:42–49. doi: 10.1046/j.0105-6263.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- 2.Iacono F, Giannella R, Somma P, Manno G, Fussco F, Mirone V. Histological alterations in cavernous tissue after radical prostatectomy. J Urol. 2005;173:1673–1676. doi: 10.1097/01.ju.0000154356.76027.4f. [DOI] [PubMed] [Google Scholar]
- 3.Hu W-L, Hu L-Q, Li S-W, Zheng X-M, Tian B-C. Expression of transforming growth factor-β1 in penile tissue from rats with bilateral cavernosal nerve ablation. BJU International. 2004;94:424–428. doi: 10.1111/j.1464-410X.2004.04969.x. [DOI] [PubMed] [Google Scholar]
- 4.Atilgan D, Parlaktas BS, Uluocak N, Erdemir F, Markoc F, Saylan O, Erkorkmaz U. The effects of trimetazidine and sildenafil on bilateral cavernosal nerve injury induced oxidative damage and cavernosal fibrosis in rats. Scientific World Journal. 2014 Mar 18;2014:970363. doi: 10.1155/2014/970363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrer JE, Velez JD, Herrera AM. Age-related morphological changes in smooth muscle and collagen content in human corpus cavernosum. J Sex Med. 2010;7:2723–2728. doi: 10.1111/j.1743-6109.2009.01508.x. [DOI] [PubMed] [Google Scholar]
- 6.Klimachev VV, Neimark AI, Gerval’d Via, Bobrov IP, Avdalian AM, Muzalevskaia NI, Gerval’d IV, Liev ART, Cherdantseva TM. Age changes of the connective tissue structures of human penis. Morfologiia. 2011;140:42–45. [PubMed] [Google Scholar]
- 7.Junior MJL, Oliveira FA, Silva PC, Furriel A, Sampaio FJ, Gregorio BM. Lard and/or canola oil-rich diets induce penile morphological alterations in a rat model. Acta Cir Bras. 2014;29(Suppl 1):39–44. doi: 10.1590/s0102-86502014001300008. [DOI] [PubMed] [Google Scholar]
- 8.Moreland RB, Traish AM, McMillin MA, Smith B, Goldstein I, Saenz de Tejada I. PGE1 suppresses the induction of collagen synthesis by transforming growth factor-β1 in human corpua cavernosum smooth muscle. J Urol. 1995;153:826–834. [PubMed] [Google Scholar]
- 9.Leungwattanakij S, Bivalacqua TJ, Usta MF, Yang D-Y, Hyun J-S, Champion HC, Abdel-Mageed AB, Hellstrom WJG. Cavernous neurotomy causes hypoxia and fibrosis in rat corpus cavernous. J Androl. 2003;24:239–245. doi: 10.1002/j.1939-4640.2003.tb02668.x. [DOI] [PubMed] [Google Scholar]
- 10.Moreland RB, Gupta S, Goldstein I, Traish A. Cyclic AMP modulates TGF-beta 1-induced fibrillar collagen synthesis in cultured human corpus cavernosum smooth muscle cells. Int J Impot Res. 1998;10:159–163. doi: 10.1038/sj.ijir.3900323. [DOI] [PubMed] [Google Scholar]
- 11.Wen J, Jiang X, Dai Y, Zhang Y, Tang Y, Sun H, Mi T, Phatarpekar PV, Kellems RE, Blackburn MR, Xia Y. Increased adenosine contributes to penile fibrosis, a dangerous feature of priapism, via A2B adenosine receptor signaling. FASEB J. 2010;24:740–749. doi: 10.1096/fj.09-144147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo YA, Kang MH, Kim JS, Oh SC. Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-Smad 3 pathway. Carcinogenesis. 2008;29:480–490. doi: 10.1093/carcin/bgm281. [DOI] [PubMed] [Google Scholar]
- 13.Alexaki VI, Javelaud D, Van Kempen LC, Mohammad KS, Dennler S, Luciana F, Hoek KS, Juarez P, Goydos JS, Fournier PJ, Sibon C, Bertolotto C, Verrecchia F, Saule S, Delmas V, Ballotti R, Larue L, Saiag P, Guise TA, Mauviel A. GLI2-mediated melanoma invasion and metastasis. J Natl Cancer Inst. 2010;102:1148–1159. doi: 10.1093/jnci/djq257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cigna N, Farrokhi ME, Brayer S, Marchal-Somme J, Wemeau-Stervinou L, Fabre A, Mal H, Leseche G, Dehoux M, Soler P, Crestani B, Mailleux AA. The hedgehog system machinery controls transforming growth factor-beta-dependent myofibroblastic differentiation in humans: involvement in idiopathic pulmonary fibrosis. Am J Pathol. 2012;181:2126–2137. doi: 10.1016/j.ajpath.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Hu B, Liu J, Wu Z, Liu T, Ullenbruch MR, Ding L, Henke CA, Bitterman PB, Phan SH. Peemergence of hedgehog mediates epithelial-mesenchymal crosstalk in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2015;52:418–428. doi: 10.1165/rcmb.2014-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Podlasek CA, Zelner DJ, Jiang HB, Tang Y, Houston J, McKenna KE, McVary KT. Sonic hedgehog cascade is required for penile postnatal morphogenesis, differentiation and adult homeostasis. Biol Reprod. 2003;68:423–438. doi: 10.1095/biolreprod.102.006643. [DOI] [PubMed] [Google Scholar]
- 17.Podlasek CA, Zelner DJ, Harris JD, Meroz CL, Tang Y, McKenna KE, McVary KT. Altered sonic hedgehog signaling is associated with morphological abnormalities in the penis of the BB/WOR diabetic rat. Biol Reprod. 2003;69:816–827. doi: 10.1095/biolreprod.102.013508. [DOI] [PubMed] [Google Scholar]
- 18.Podlasek CA, Meroz CL, Tang Y, McKenna KE, McVary KT. Regulation of cavernous nerve injury-induced apoptosis by sonic hedgehog. Biol Reprod. 2007;76:19–28. doi: 10.1095/biolreprod.106.053926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bond C, Tang Y, Podlasek CA. Neural influences on sonic hedgehog and apoptosis in the penis. Biol Reprod. 2008;78:947–956. doi: 10.1095/biolreprod.107.064766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bond CW, Angeloni NL, Harrington DA, Zhang S, Stupp SI, McKenna K, Podlasek CA. Peptide amphiphile nanofiber delivery of sonic hedgehog protein to reduce smooth muscle apoptosis in the penis after cavernous nerve resection. J Sex Med. 2011;8:78–89. doi: 10.1111/j.1743-6109.2010.02001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angeloni NL, Bond CW, Tang Y, Harrington DA, Zhang S, Stupp SI, McKenna K, Podlasek CA. Regeneration of the cavernous nerve by sonic hedgehog using aligned peptide amphiphile nanofibers. Biomaterials. 2011;32:1091–1101. doi: 10.1016/j.biomaterials.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angeloni N, Bond CW, Harrington D, Stupp S, Podlasek CA. Sonic hedgehog is neuroprotective in the cavernous nerve with crush injury. J Sex Med. 2013;10:1240–1250. doi: 10.1111/j.1743-6109.2012.02930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angeloni NL, Bond CW, McVary KT, Podlasek CA. Sonic hedgehog protein is decreased and penile morphology is altered in prostatectomy and diabetic patients. PLOS ONE. 2013;8:e70985. doi: 10.1371/journal.pone.0070985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding H, Zhou D, Hao S, Zhou L, He W, Nie J, Hou FF, Liu Y. Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol. 2012;23:801–813. doi: 10.1681/ASN.2011060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horn A, Palumbo K, Cardazzo C, Dees C, Akhmetshina A, Tomcik M, Zerr P, Avouac J, Gusinde J, Zwerina J, Roudaut H, Traiffort E, Ruat M, Distler O, Schett G, Distler JH. Hedgehog signaling controls fibroblast activation and tissue fibrosis in systemic sclerosis. Arthritis Rheum. 2012;64:2724–2733. doi: 10.1002/art.34444. [DOI] [PubMed] [Google Scholar]
- 26.Fitch PM, Howie SE, Wallace WA. Oxidative damage and TGF-beta differentially induce lung epithelial cell sonic hedgehog and tenascin-C expression:implications for the regulation of lung remodeling in idiopathic interstitial lung disease. Int J Exp Pathol. 2011;92:8–17. doi: 10.1111/j.1365-2613.2010.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amankulor NM, Hambardzumyan D, Pyonteck SM, Becher OJ, Joyce JA, Holland EC. Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation. J Neurosci. 2009;29:10299–10308. doi: 10.1523/JNEUROSCI.2500-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolanos AL, Milla CM, Lira JC, Ramirez R, Checa M, Barrera L, Garcia-Alvarez J, Carbajal V, Becerril C, Gaxiola M, Pardo A, Selman M. Role of sonic hedgehog in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303:L978–L990. doi: 10.1152/ajplung.00184.2012. [DOI] [PubMed] [Google Scholar]
- 29.Lin Y, Zhang S, Rehn M, Itaranta P, Tuukkanen J, Heljasvaara R, Peltoketo H, Pihlajaniemi T, Vainio S. Induced repatterning of type XVIII collagen expression in ureter bud from kidney to lung type: association with sonic hedgehog and ectopic surfactant protein C. Development. 2001;128:1573–1585. doi: 10.1242/dev.128.9.1573. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Kugler MC, Loomis CA, Samdani R, Zhao Z, Chen GJ, Brandt JP, Brownell I, Joyner AL, Rom WN, Menger JS. Hedgehog signaling in neonatal and adult lung. Am J Respir Cell Mol Biol. 2013;48:703–710. doi: 10.1165/rcmb.2012-0347OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsang SW, Zhang H, Lin C, Xiao H, Wong M, Shang H, Yang Z-J, Lu A, Yung KK-L, Bian Z. Rhein, a natural anthraquinone derivative, attenuates the activation of pancreatic stellate cells and ameliorates pancreatic fibrosis in mice with experimental chronic pancreatitis. PLOS ONE. 2013;8:e82201. doi: 10.1371/journal.pone.0082201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S, Greenfield MA, Mata A, Palmer LC, Bitton R, Mantei JR, Aparicio C, de la Cruz MO, Stupp SI. A self-assembly pathway to aligned monodomain gels. Nat Materials. 2010;9:594–601. doi: 10.1038/nmat2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berns EJ, Sur S, Pan L, Goldberger JE, Suresh S, Zhang S, Kessler JA, Stupp SI. Aligned neurite outgrowth and directed cell migration in self-assembled monodomain gels. Biomaterials. 2014;35:185–195. doi: 10.1016/j.biomaterials.2013.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gefen A, Chen J, Elad D. A biomechanical model of Peyronie’s disease. Journal of Biomechanics. 2000;33:1739–1744. doi: 10.1016/s0021-9290(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 35.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clinical Biochemistry. 1996;29:225–229. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 36.Sheehan DC, Hrapchak BB. Theory and practice of histotechnology. 2. St. Louis: C.V. Mosby Company; 1980. pp. 141–154. [Google Scholar]
- 37.Mullerad M, Donohue JF, Li PS, Scardino PT, Mulhall JP. Functional Sequelae of Cavernous Nerve Injury in the Rat: is There Model Dependency. J Sex Med. 2006;3:77–83. doi: 10.1111/j.1743-6109.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 38.Nangle MR, Keast JR. Reduced Efficacy of Nitrergic Neurotransmission Exacerbates Erectile Dysfunction After Penile Nerve Injury Despite Axonal Regeneration. Exp Neurol. 2007;207:30–41. doi: 10.1016/j.expneurol.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Podlasek CA, Meroz CL, Korolis H, Tang Y, McKenna KE, McVary KT. Sonic hedgehog, the penis and erectile dysfunction: A review of sonic hedgehog signaling in the penis. Current Pharmaceutical Design. 2005;11:4011–4027. doi: 10.2174/138161205774913408. [DOI] [PubMed] [Google Scholar]
- 40.Boot-Handford RP, Tuckwell DS. Fibrillar collagen: the key to vertebrate evolution? A tale of molecular incest. Bio Essays. 2003;25:142–151. doi: 10.1002/bies.10230. [DOI] [PubMed] [Google Scholar]
- 41.Pinheiro ACAD, Costa WS, Cardoso LEM, Sampaio FJB. Organization and relative content of smooth muscle cells, collagen and elastic fibers in the corpus cavernosum of rat penis. J Urol. 2000;164:1802–1806. [PubMed] [Google Scholar]
- 42.Lu BD, Nian JH, Huang XJ, Zhang SG, Geng Q, Chen G, Chen ST. Hypoxia induces fibrosis of corpus cavernosum smooth muscle in SD rats. Zhonghua Nan Ke Xue. 2011;17:121–125. [PubMed] [Google Scholar]
- 43.Majeed SK. Survey on spontaneous peripheral neuropathy in aging rats. Arzneimittelforschung. 1992;42:986–990. [PubMed] [Google Scholar]
- 44.Murakami R. Development of the os penis in genital tubercle cultured beneath the renal capsule of adult rats. J Anat. 1986;149:11–20. [PMC free article] [PubMed] [Google Scholar]
- 45.Leeson TS, Leeson CR. Penile cavernous tissue: an electron microscopic study of its development in the rat. Acta Anat. 1966;63:404–417. doi: 10.1159/000142803. [DOI] [PubMed] [Google Scholar]