Abstract
Squamous cell carcinoma of the head and neck (SCCHN) is an aggressive disease with poor survival and is the sixth most common cancer worldwide. Gastroesophageal reflux is a common event in SCCHN patients. GPR4 is a proton-sensing G-protein coupled receptor, which can be activated by acidosis. The objective of this study was to explore the role of GPR4 in acid exposure and tumor angiogenesis in SCCHN. In this study, we confirmed that overexpressing GPR4 in SCCHN cells could increase the expression and secretion of IL6, IL8 and VEGFA at pH 5.9. This effect could be inhibited by SB203580 (a p38 inhibitor). Western blot analysis indicated that phosphorylation of p38 increased in GPR4 infected cells at pH 5.9, which could be inhibited by SB203580. In tube formation assay, HMEC-1 cells were incubated with conditioned medium (CM, pH 5.9, 6.5, 7.4) derived from control and GPR4 infected SCCHN cells. Tube length was significantly increased in HMEC-1 cells incubated with CM from GPR4 infected cells compared with control cells at pH5.9, which indicated the pro-angiogenic effect of GPR4 in acidic pH. The neutralizing antibodies of IL6, IL8 and VEGFA could inhibit tube formation of HMEC-1 cells. In vivo, the effect of GPR4 on angiogenesis was investigated with the chick chorioallantoic membrane (CAM) model. Control and GPR4 infected SCCHN cells were seeded onto the upper CAM surface (n = 5 in each group) and 5 μL DMEM/F12 (pH 5.9, 6.5, 7.4) was added to the surface of the cell every 24 h. Four days later, the upper CAM were harvested and the ratio of the vascular area to the CAM area was quantified using Image-Pro Plus 6.0 software. GPR4 infected cells could recruit more vascular than control cells at pH5.9. In conclusion, we suggested that GPR4 induces angiogenesis via GPR4-induced p38-mediated IL6, IL8 and VEGFA secretion at acidic extracellular pH in SCCHN.
Introduction
Squamous cell carcinoma of the head and neck (SCCHN) is an aggressive disease with poor survival and is the sixth most common cancer worldwide [1]. The global incidence is approximately 600,000 cases each year [2]. Smoking and alcohol abuse are major risk factors for SCCHN. Gastroesophageal reflux is a common event in SCCHN patients [3], which indicates that it is associated with an elevated risk of laryngeal cancer and pharyngeal cancer [4]. Twenty-four-hour double-probe pH monitoring indicates the pH of the upper esophageal sphincter as being below 4 [5]. Acid exposure can lead to various otolaryngological disorders such as chronic laryngitis, vocal nodules, Reinke’s edema, contact ulcer and granuloma, laryngeal stenosis, and paroxysmal laryngospasm [6]. In esophageal squamous cell carcinoma, continuous acid exposure promotes vascular development [7], which plays a critical role during tumor initiation and malignant progression [8].
The proton-sensing G protein-coupled receptors (GPCRs) including GPR4, GPR65 (TDAG8), GPR68 (OGR1), and GPR132 (G2A) have recently been identified as novel pH sensors that are proposed to be activated by acidic extracellular pH [9]. It has been demonstrated that GPR4 is overexpressed in various types of malignancies [10, 11]. In epithelial ovarian carcinoma, GPR4 expression density is accompanied by a higher microvascular density (MVD) [10], and this correlated with the status of lymph node metastasis and clinical stage. This indicates that GPR4 may be involved in cancer-related angiogenesis. In SCCHN, the correlation between acid exposure, tumor angiogenesis and GPR4 has not been well studied. In a previous study, we confirmed that GPR4 could activate AP1 and ERK signaling pathways at acidic extracellular pH [12]. AP1 is one of the regulatory sites of IL6, IL8 and VEGFA [13–15]. GPR4 as a pH sensor is positively correlated with higher microvascular density, so we hypothesized that it could increase the expression of IL6, IL8 and VEGFA and induce angiogenesis at acidic extracellular pH. In this study, we suggested that GPR4 induced angiogenesis via GPR4-induced IL6, IL8 and VEGFA secretion at acidic extracellular pH in SCCHN.
Materials and Methods
Ethics Statement
Ethical permission for this study was granted by the Research Ethics Committee of Beijing Tongren Hospital (Beijing, China).
Cell Culture
The SCCHN cell lines FaDu cells and Tca8113 cells, human microvascular endothelial cells (HMEC-1) were used in the study. FaDu cells were obtained from State Key Laboratory of Oral Diseases, Sichuan University [16]. Tca8113 cells were obtained from China Infrastructure of Cell Line Resources. HMEC-1 cells, obtained from Thermo Fisher, was generated by Ades et al [17]. All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Hyclone, USA) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin/streptomycin. All cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Generation of Recombinant Adenovirus Carrying GPR4 Gene
The recombinant adenovirus expressing GPR4 (Ad-GPR4) was generated according to the methods described previously [18]. Briefly, GPR4 was sub-cloned into the adenoviral vector pAdxsi (Sinogenomax, China). The recombinant adenoviral construct was transfected into the packaging HEK293 cells and then these cells were freeze–thawed several times to release intracellular viral particles. The viral titers were tested in HEK293 cells and the number of plaque-forming units (pfu) was obtained using a plaque formation assay.
Cell Infection and Acid Stimulation
FaDu cells and Tca8113 cells were seeded in six-well plates, reaching 80% confluence by the following day. Then the cells were infected with Ad-GPR4 or Ad-null (a blank recombinant adenovirus as control). Eighteen hours later, acid stimulation was performed as described previously [12]. Briefly, 18h after infection, the culture media were replaced by serum-free DMEM/F12 (pH 5.9, 6.5 and 7.4). The cells were harvested after stimulation for 6h. SB203580 (a p38 inhibitor, 1 μM) was used to determine whether the p38 mediated the secretion of the pro-angiogenic cytokines. To perform p38 inhibition, GPR4 infected cells were serum-starved for 4h and treated with 1 μM SB203580 for 1h before acid stimulation and then the culture media were replaced by serum-free DMEM/F12 (pH 5.9). The cells were harvested 6h later.
Conditioned Medium
To prepare conditioned medium (CM), the FaDu cells and Tca8113 cells were infected with Ad-GPR4 or Ad-null for 18h, the culture media were replaced by serum-free DMEM/F12 (pH 5.9, 6.5 and 7.4), and cells without infection acted as a control. Six hours later, the medium was centrifuged (4°C, 1500 rpm) and the supernatant was collected and stored at –80°C until use.
Real Time PCR (qPCR) and Semiquantitative PCR
Total RNAs of FaDu and Tca8113 cells and SCCHN tissues were isolated using TRIzol Reagent (Life Technologies, USA). Reverse transcription was performed using a FastQuant RT Kit (TIANGEN, China). qPCR was performed as recommended by the manufacturer. The following primers were used for qPCR: IL6 sense 5′-TGAGAGTAGTGAGGAACAAG-3′, antisense 5′-CGCAGAATGAGATGAGTTG-3′; IL8 sense 5′-CCTGATTTCTGCAGCTCTGTGT-3′, antisense 5′-GGTGGAAAGGTTTGGAGTATGTCT-3′; VEGFA sense 5′-GCCCACTGAGGAGTCCAACATC-3′, antisense 5′-CAAGGCCCACAGGGATTTTCT-3′. The primers for PCR of GPR4 were as follows: sense 5′-AACCTCTATCGGGTGTTCGTG-3′, antisense 5′-TTGGCTGTGCTGTTCCTCTTGG-3′. GAPDH was amplified as an internal standard. The relative RNA level in each sample was determined by the 2(–Delta Delta C(T)) method.
Enzyme-Linked Immunosorbent Assay (ELISA)
The control cells and GPR4 infected cells were stimulated for 6h using serum-free DMEM/F12 (pH 5.9). Then the medium was centrifuged (4°C, 1500 rpm) and the supernatant was collected. The concentration of IL6, IL8, and VEGFA in the culture medium was quantified with an ELISA kit according to the manufacturer’s instructions (Abcam).
Western Blot Analysis
Cells were lysed in RIPA buffer (Beyotime, China) supplemented with a 1 mM PMSF. A total of 100 μg proteins were electrophoresed on 10% SDS–PAGE gels, and electrotransferred onto nitrocellulose membranes (Invitrogen, USA). Western blot was performed as previously described [12]. GPR4 antibody was purchased from LifeSpan BioSciences (LifeSpan, USA). Phospho-p38 (p-p38), total p38 (p38), phospho-VEGF receptor 2 (Tyr1175) and VEGF receptor 2 antibody were purchased from Cell Signaling Technology (Cell Signaling, USA). GAPDH (Santa Cruz, CA) was used as a lysate loading control.
Tube Formation Assay
To confirm the effect of GPR4 on tube formation in HMEC-1, we performed an angiogenesis assay on growth factor-reduced Matrigel (BD Biosciences). One day before tube formation assay, HMEC-1 cells were seeded on a 10-cm disk. After coating the 96-well plate with Matrigel following the manufacturer’s instructions, HMEC-1 cells were harvested and resuspended at 1×105/mL using CM supplemented with 2% FBS. Then 0.1 mL of cell suspension was added to the 96-well plate. VEGFA (1 ng/mL) was used as a positive control and DMEM served as a negative control. The plate was incubated at 37°C, 5% CO2 for 12h to allow formation of capillary-like structures. Pictures were taken using a light microscope and the tube length (pixels) was counted using Image-Pro Plus 6.0 software.
Chick Chorioallantoic Membrane (CAM) Model
Fertilized Specific Pathogen Free (SPF) eggs were obtained from Beijing Laboratory Animal Research Center. Eggs were hatched in a humidified incubator at 37.0°C with 60% humidity. On day 10, large blood vessels and sac were labeled on the egg using Cold Light Fountain (STORZ, Germany). After disinfection using 75% alcohol, a round window was opened on the top of the eggshell, maintaining the outer eggshell membrane. A pinpoint hole was drilled at the location of the air sac. Then 20 μL of normal saline was added to the eggshell membrane. The eggshell membrane was punctured using a fine needle and the small pinpoint hole in the air sac was vacuumed using a rubber pipette bulb, so that the normal saline separated the outer eggshell membrane from the CAM. The eggshell membrane was peeled off without disturbing the CAM. The window was covered with parafilm and the egg was placed in the incubator for another day. Then 1×106control and GPR4 infected cells were seeded onto the upper CAM surface (n = 5 in each group). The square window on the egg was sealed. Then 5 μL DMEM/F12 (pH 5.9, 6.5, 7.4) was added to the surface of the cell every 24h. Four days later, the upper CAM were harvested and the ratio of the vascular area to the CAM area was quantified using Image-Pro Plus 6.0 software.
Bioinformatics
Bioinformatics method was used as Li [19] described, briefly, array data related to cancers from the Affymetrixhuman genome U133 plus 2.0 platform were down loaded from the GEO database (http://www.ncbi.nlm.nih.gov/geo/), and differentially expressed genes in tumors were analyzed using TumourProfile database (http://tumour.bjmu.edu.cn/, unpublished) as Wang et al previously described data processing and microarray analysis methods [20, 21]. The expression profile of GPR4 in a variety of cancers and the corresponding control (normal or non-tumor) tissues was searched in this database, and the expression levels were represented as average rank scores (ARS).
Immunohistochemistry (IHC)
Paraffin-embedded specimens of laryngeal or hypopharyngeal squamous cell carcinoma samples coupled with peri-carcinoma normal tissue samples were obtained from the head and neck tumor specimen bank of Beijing Tongren Hospital [12]. Paraffin tissue slides were dewaxed, rehydrated and placed in 10 mmol/L citrate buffer (pH 6.0), and heated twice in a microwave oven for 5 min each. Slides were incubated with 3% H2O2 for 10 min, washed with PBS, blocked with 10% normal goat serum for 30 min, and then incubated with anti-GPR4 (diluted 1:100; final concentration, 2 μg/mL, LifeSpan, USA) or normal rabbit IgG as a control at 4°C overnight. After washing, the slides were stained with the catalyzed signed amplification system kit (DAKO code k5007) and images were photographed with an Olympus IX70 microscope. Semiquantitative expression of GPR4 was calculated using Image-Pro Plus 6.0 software.
Data Analysis
The bioinformatics analysis of the differences in GPR4 expression between the cancers and control tissues were evaluated using the Wilcoxon rank-sumtest in the R (http://www.r-project.org/) software environment. Bonferroni’s correction of the R function “p.adjust” was used to adjust the P-values. The experimental data were analyzed using Student’s t test with SPSS19.0 software. A p-value <0.05 was considered to be statistically significant.
Results
GPR4 Stimulates Pro-Angiogenic Cytokine Secretion at Acidic Extracellular pH
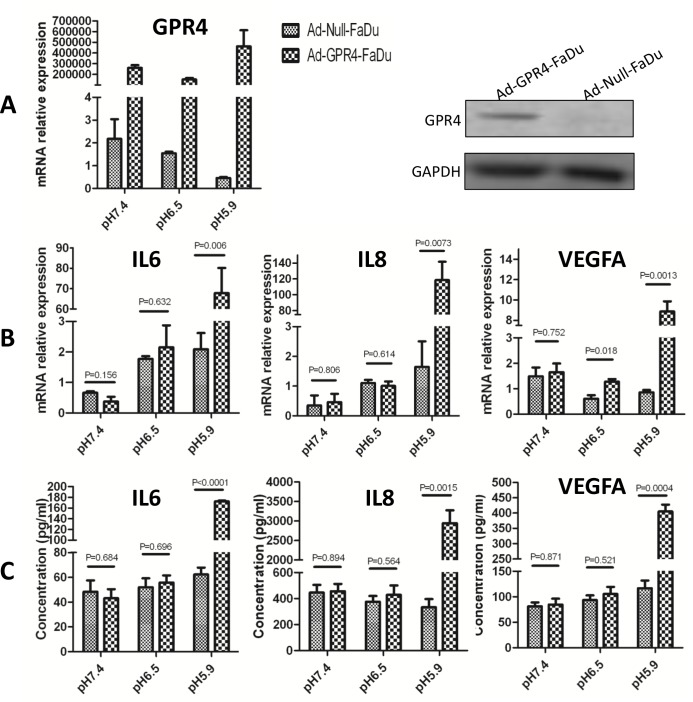
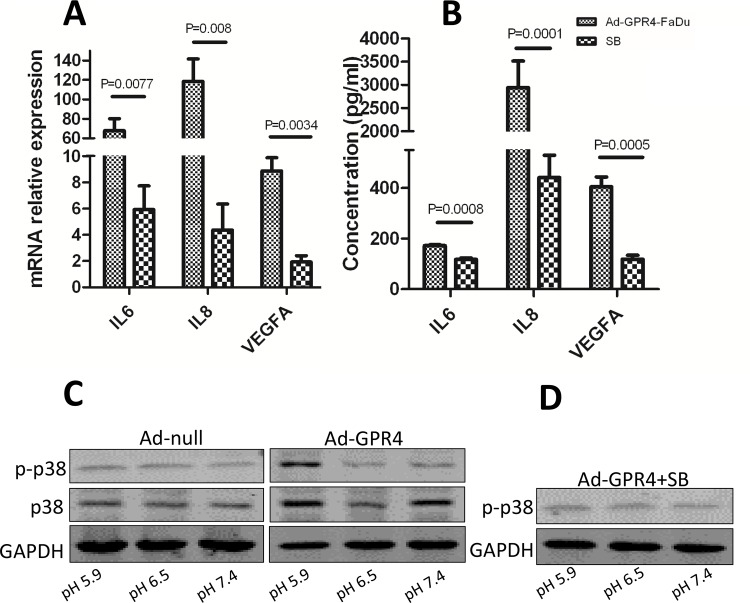
To investigate whether GPR4 could induce the expression of pro-angiogenic cytokines at acidic extracellular pH, we detected the expression of IL6, IL8, and VEGFA at different pH values (pH 7.4, 6.5 and 5.9). The overexpression of GPR4 in GPR4 infected cells was validated by qPCR and western blot (Fig 1A). The mRNA levels of IL6, IL8, and VEGFA in the Ad-GPR4 cells increased statistically significantly compared with the Ad-null cells only at pH 5.9 (Fig 1B). Compared with the controls, there was 67.8-, 118- and 12.5-fold induction for IL6, IL8 and VEGFA, respectively, in the Ad-GPR4 cells. This indicated that acidic extracellular pH enhanced pro-angiogenic cytokine expression via GPR4, which was further confirmed at the protein level. The concentrations of IL6, IL8, and VEGFA were increased significantly in the supernatant of GPR4 overexpressed cells compared with the control cells at pH 5.9 (Fig 1C). The inhibitor, SB203580, could reduce the expression and secretion of IL6, IL8, and VEGFA (Fig 2A and 2B), suggesting that p38 may be involved in cytokine induction by GPR4. Western blot was performed to investigate if GPR4 could increase p38 phosphorylation at different pH. Phosphorylation of p38 increased in GPR4 overexpressed cells at pH 5.9 (Fig 2C), which could be inhibited by SB203580 (Fig 2D). Our results was correspondent with that, in SCCHN, p38 is constitutively activated and leads to increasing secretion of cytokines IL6 and IL8 [22].
Fig 1. GPR4 stimulates cytokine secretion at acidic pH.
Total RNA and protein of Ad-GPR4-FaDu cells, Ad-null-FaDu cells were isolated after acid stimulation for 6 h. qPCR and western blot were performed. (A) Overexpression of GPR4 in Ad-GPR4-FaDu cells was comfirmed by qPCR and western blot. (B) The expression of IL6, IL8, and VEGFA increased significantly in GPR4 infected cells at pH 5.9. (C) The supernatants of the cells were collected and the concentrations of IL6, IL8 and VEGFA were detected by ELISA. Similar results were observed in Tca8113 cells (S1 Fig).
Fig 2. SB203580 reduces cytokine secretion by inhibiting p38 phosphorylation.
(A) In Ad-GPR4-FaDu cells, SB203580 reduced the expression of IL6, IL8 and VEGFA at pH 5.9. (B) SB203580 reduced secretion of IL6, IL8 and VEGFA. (C) GPR4 increased p38 phosphorylation at pH5.9. (D) SB203580 inhibit phosphorylation of p38 in GPR4 overexpressed cells. Similar results were observed in Tca8113 cells (S2 Fig).
GPR4 Promotes Angiogenesis In Vitro
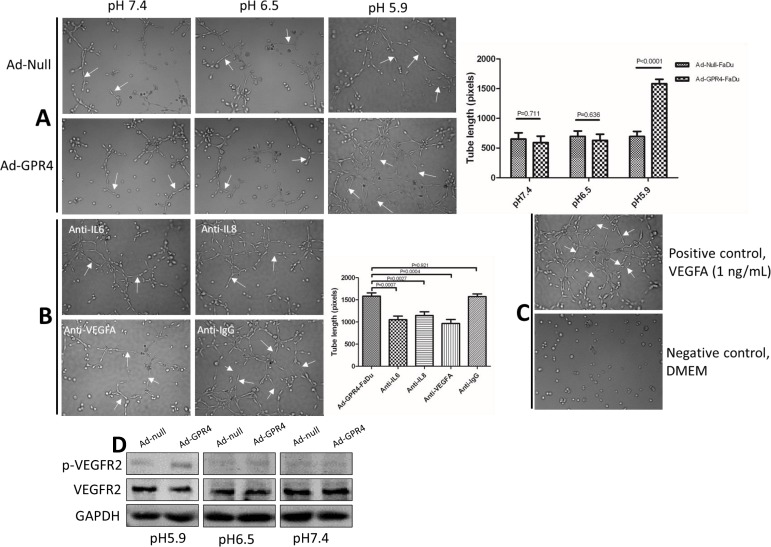
To test whether GPR4 could promote tube formation, HMEC-1 cells were incubated with CM derived from control cells and GPR4 overexpressed cells. The lengths of tubes (arrows) were significantly increased in HMEC-1 cells incubated with CM from GPR4 infected cells compared with control cells at pH 5.9 (Fig 3A). This indicated that overexpression of GPR4 in SCCHN could induce angiogenesis in vitro at acidic pH. In SCCHN, angiogenesis is triggered by IL-8, IL-6 and VEGF [23, 24]. To test whether the effect of GPR4-induced angiogenesis was directly mediated by IL6, IL8 and VEGFA secretion, monoclonal neutralizing antibodies (R&D Systems) of IL6, IL8 and VEGFA were added to the CM of Ad-GPR4-FaDu cells, respectively, isotype IgG antibody was used as a negative control (Fig 3B). These neutralizing antibodies reduced the tube length of HMEC-1, and moreover, the neutralizing antibody effects could be reversed by the addition of the excess amount of these factors (S3 Fig). This suggested that GPR4 induced angiogenesis via IL6, IL8 and VEGFA secretion. Vascular endothelial growth factor receptor 2 (VEGFR2) is the primary receptor of VEGF and the major mediator of VEGF-induced pro-angiogenesis signaling in endothelial cells. To test whether GPR4 increased VEGFR2 phosphorylation in HMEC-1 cells, western blot was performed. The results showed that VEGFR2 phosphorylation increasing in HMEC-1 cells incubated with CM (pH 5.9) derived from GPR4 overexpressed cells (Fig 3D).
Fig 3. Tube formation assay.
(A) The tube length (arrows) of HMEC-1 cells was increased in CM derived from Ad-GPR4-FaDu cells compared with Ad-null-FaDu cells at pH 5.9. (B) The neutralizing antibodies of IL6, IL8 and VEGFA inhibited tube formation in HMEC-1 cells. Isotype IgG antibody was used as a control in neutralizing antibody test. (C) VEGFA (1 ng/mL) was used as a positive control and DMEM served as a negative control in tube formation assay. Tube lengths (pixels) were quantified from 6 representative fields using Image-Pro Plus 6.0 software. (D) GPR4 increased VEGFR2 phosphorylation in HMEC-1 cells at pH 5.9. Similar results were observed in Tca8113 cells (S4 Fig).
GPR4 Promotes Angiogenesis In Vivo
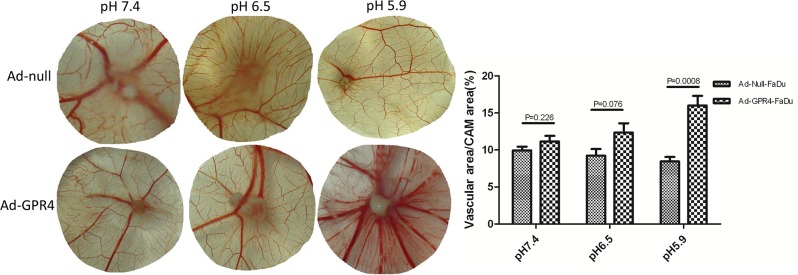
In vivo, the angiogenic potential of GPR4 in SCCHN was verified in the CAM model. GPR4 infected cells seeded on the CAM recruited more vascular than control cells at pH 5.9. The vascular density was quantified by calculating the ratio of the vascular area to the CAM area. In the Ad-null and Ad-GPR4-FaDu cells, the vascular densities surrounding the tumor were 8.46%, and 16%, respectively (Fig 4). This indicated that overexpression of GPR4 in SCCHN could promote angiogenesis in vivo at acidic pH. The in-vivo and in-vitro studies indicated that GPR4 induces angiogenesis at acidic pH, a hallmark of tumor progression.
Fig 4. GPR4 induced angiogenesis in CAM model.
The Ad-GPR4-FaDu cells recruited more vascular than Ad-null-FaDu cells only at pH 5.9. No differences were observed between Ad-GPR4-FaDu cells and Ad-GPR4-FaDu cells at pH 7.4 and 6.5. The ratios of vascular area to CAM area were quantified using Image-Pro Plus 6.0 software. Similar results were observed in Tca8113 cells (S5 Fig).
GPR4 Is Overexpressed in SCCHN
GPR4 is overexpressed in various types of malignancies including breast cancers, ovarian cancers, colon cancers, liver cancers and kidney cancers [11]. To assess the differential expression of GPR4 across multiple cancers and their corresponding control (normal or non-tumor) tissues at the mRNA level, an integrated bioinformatics analysis were performed based on the omic tumor data set from the GEO database. The average rank scores (ARS), Bonferroni correction adjusted P-values (Table 1) were synthesized, and GPR4 was upregulated in kidney clear cell renal cell carcinoma, SCCHN and colorectal adenocarcinoma. The overexpression of GPR4 in SCCHN is further supported by the BioXpress (http://hive.biochemistry.gwu.edu/tools/bioxpress) [25]. The BioXpress database indicates that GPR4 is upregulated in 85.37% of SCCHN samples compared with their paired normal samples based on RNA sequencing (RNA-seq); the data set deposited in the Cancer Genome Atlas (TCGA) from a total of 72 patients has been collected and used for the analysis [25].
Table 1. The average expression intensities of GPR4 in multiple cancers.
| Tissue | Sample size | ARSa | P valueb | Bonferronic |
|---|---|---|---|---|
| bladder cancer | 186 | 56.15 | 0.780492675 | 1 |
| bladder control | 64 | 55.34 | ||
| breast cancer | 3080 | 55.68 | 0.019528491 | 1 |
| breast control | 374 | 55.94 | ||
| colorectal adenocarcinomad | 1841 | 55.36 | 9.56E-25 | 5.21E-20 |
| colon mucosa normal | 273 | 47.41 | ||
| gastric cancer | 681 | 58.35 | 0.132038546 | 1 |
| gastric normal | 61 | 56.64 | ||
| SCCHNd | 220 | 63.44 | 7.27E-06 | 0.397103264 |
| SCCHN control | 59 | 51.29 | ||
| kidney clear cell renal cell carcinomad | 652 | 74.97 | 2.77E-77 | 1.51E-72 |
| kidney control | 244 | 56.72 | ||
| hepatocellular carcinoma | 263 | 53.96 | 0.11868146 | 1 |
| liver control | 62 | 56.89 | ||
| oral squamous cell carcinoma | 339 | 53.82 | 4.97E-06 | 0.271182795 |
| oral squamous cell control | 118 | 48.25 | ||
| ovarian cancer | 379 | 54.19 | 0.013964164 | 1 |
| ovary control | 120 | 50.28 | ||
| pancreatic cancer | 263 | 57.04 | 0.166179535 | 1 |
| pancreas control | 83 | 59.98 | ||
| prostate cancer | 345 | 58.62 | 0.46249681 | 1 |
| prostate control | 81 | 59.41 |
aARS denotes the average rank score.
bThe P-values were calculated using the Wilcoxon rank-sum test in the R (http://www.r-project.org/) software environment and are relative to the corresponding normal or non-tumor tissues.
cThe P-values were adjusted using Bonferroni correction in the function “p.adjust” in the R software.
dThe expression levels of GPR4 in the underlined tissues were considered to be upregulated by fully considering the differences in the ARS values, the Bonferroni correction adjusted P-values.
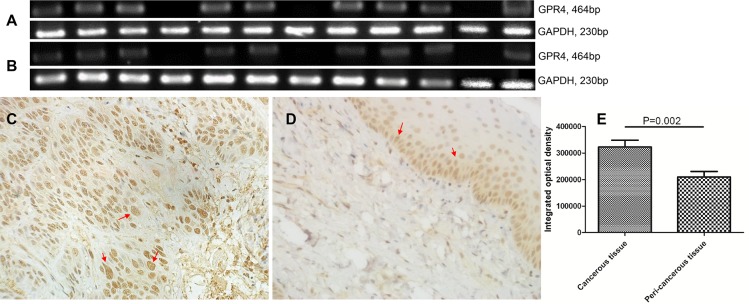
We collected 12 SCCHN samples (Table 2) and performed RT-PCR (Fig 5A and 5B) and immunohistochemical (IHC) staining (Fig 5C and 5D). All of the patients suffered from gastroesophageal reflux. As shown in Fig 5, GPR4 was amplified in 9/12 samples and upregulated in the tumor tissues (Fig 5A) compared with the peri-tumor normal tissues (Fig 5B). The GPR4 protein expressed at higher levels (red arrows) in the tumor cells compared with the epithelial cells of peri-tumor normal tissues. Similar results were observed in both laryngeal and hypopharyngeal cancers.
Table 2. Clinical features of the SCCHN samples and corresponding GPR4 expression in IHC.
| Case | Gender | Age | Clinical and pathological diagnosis | TNM staging | Differentiation | IOD of CT | IOD of PCT |
|---|---|---|---|---|---|---|---|
| 1 | M | 59 | SGC | T2N0M0 | High | 352567.565 | 255943.18 |
| 2 | M | 74 | GC | T2N0M0 | Moderate | 396264.045 | 173284.705 |
| 3 | M | 67 | GC | T3N2M0 | High | 249689.9 | 197848.23 |
| 4 | M | 47 | GC | T4N0M0 | Moderate | 340095.92 | 307834.09 |
| 5 | M | 78 | GC | T3N0M0 | Moderate | 379242.5 | 279154.405 |
| 6 | M | 66 | HPC | T2N2M0 | Moderate | 489210.845 | 219239.21 |
| 7 | M | 57 | HPC | T3N1M0 | Moderate | 275309.335 | 245821.32 |
| 8 | M | 50 | HPC | T3N2M0 | Low | 379145.465 | 134423.65 |
| 9 | M | 69 | HPC | T3N1M0 | Low | 306495.335 | 153739.275 |
| 10 | M | 71 | HPC | T2N1M0 | Moderate | 313586.58 | 350465.55 |
| 11 | M | 61 | HPC | T2N1M0 | Low | 273720.055 | 109265.1685 |
| 12 | M | 56 | HPC | T3N2M0 | Moderate | 357452.75 | 222803.135 |
CT: cancer tissue; PCT: peri-cancer tissue; IOD: integrated optical density; SGC: supraglottic cancer; GC: glottic cancer; HPC: hypopharyngeal cancer; All cases were squamous cell carcinoma. TNM staging is according to the AJCC cancer staging, 7th edition.
Fig 5. GPR4 is overexpressed in SCCHN.
(A) RT-PCR of GPR4 in cancerous tissues. (B) RT-PCR of GPR4 in peri-cancerous normal tissues. GAPDH was used as an internal standard. (C) Representative image of IHC of moderately differentiated hypopharyngeal cancer. (D) Peri-cancerous normal tissue from the same patient as C. (E) Integrated optical density (IOD) of positive expression cells (arrows) was calculated using Image-Pro Plus 6.0 software. In total, 12 cases of SCCHN were included. The level of immunoreactivity was graded by calculating the integrated optical density (IOD) of positive expression cells.
Discussion
In this study, we have suggested that, when exposed to an acidic environment, GPR4, one of the proton-sensing GPCRs, could induce angiogenesis via pro-angiogenic cytokine secretion in SCCHN. The cytokines included IL6, IL8, and VEGFA, and this was confirmed by qPCR and ELISA. The pro-angiogenic effect of GPR4 was observed by the tube formation assay and CAM model.
Angiogenesis is one of the hallmarks of cancer [26]. Tumors cannot grow beyond a critical size or metastasize to another organ without blood vessels [27], so they must recruit new blood vessels by angiogenesis. The ‘angiogenic switch’ is balanced by pro-angiogenic and anti-angiogenic molecules [27]. One of the signals that trigger this switch is low pH. In human melanoma cells, acidic pH increases the expression of the proangiogenic factors VEGFA and IL8 [28]. GPR4 predominantly functions as a proton sensor and is expressed endogenous in HMEC-1 and HUVEC. Extracellular acidosis can activate GPR4 and lead to cAMP production [29]. Hypercapnic acidosis can also activate GPR4 to stimulate HUVEC adhesion molecule expression and adhesiveness [30]. In another study, it was demonstrated that GPR4 played pivotal roles in growth, migration, and tube formation by endothelial cell, but pH changes could not regulate cAMP production in HMEC-1 and HUVEC [31]. In HUVEC, acidosis activates GPR4 and increases the expression of IL1, IL2, IL6 and IL8 [32]. GPR4, as a pH sensor, is fully activated at acidic pH 6.8 and partially activated in the physiological range (pH 7.4–6.8) [33, 34]. Gastroesophageal reflux is a common event in head and neck cancer patients [3], which means that mucosa and carcinoma are often exposed to low pH. The relationship between low pH and tumor angiogenesis in SCCHN has not been well studied. In this study, we suggested that, in Ad-null-FaDu and Ad-null-Tca8113 cells, low pH could not increase the expression of IL6, IL8, and VEGFA. In Ad-GPR4 cells, the expression of IL6, IL8, and VEGFA increased significantly compared with control at pH 5.9 but not at pH 6.8 and pH 7.4, which may indicate that GPR4 is fully activated at pH 5.9 in SCCHN and this is consistent with our previous study [12].
The tube formation assay and CAM model verified the pro-angiogenic effect of GPR4. CM derived from Ad-GPR4-FaDu cells induced longer tubes in HMEC-1 cells and more vascular density in CAM than in the other groups at pH 5.9. The neutralizing antibodies of IL6, IL8 and VEGFA reduced the tube lengths of HMEC-1 cells, which indicates that GPR4 induced angiogenesis via IL6, IL8 and VEGF secretion. VEGFA and IL6 are recognized as the main pro-angiogenic molecules in SCCHN and have been linked with disease aggressiveness and poor patient outcome [35–40]. IL8, an end-product of the VEGF pathway, was also observed at high levels in SCCHN [40]. Because of the important role of angiogenesis in tumor progression, antiangiogenic agents have been developed. In FaDu SCCHN xenografts, long-term administration of bevacizumab, a vascular endothelial growth factor antibody, effectively modulated chemotherapeutic efficacy [41]. In phase II clinical trials in SCCHN patients, bevacizumab plus cetuximab or chemotherapy showed encouraging efficacy results [42, 43]. Overexpression of IL8 led to resistance to bevacizumab in SCCHN, so co-targeting of VEGF and IL8 may help overcome resistance and enhance therapeutic efficacy.
Antacid medications including histamine 2 receptor antagonist (H2RA) and proton pump inhibitors (PPI) are always used in the treatment of SCCHN patients. Some studies have revealed that the usage of antacid medications may have significant therapeutic benefits for SCCHN patients [44, 45]. One of the mechanisms may be because antacid medications can modulate adhesion of tumor cells to endothelium [44]. In the present study, we have shown that GPR4 promotes pro-angiogenic factors secretion at acidic pH. These results indicate that inhibition of GPR4 or acidic pH may lead to inhibition of tumor angiogenesis. So we speculate that another probable mechanism is that antacid medications can inhibit pro-angiogenic factors secretion by increasing the pH, and then reduce angiogenesis, which plays a critical role during tumor progression. Previously, we showed that acidic extracellular pH enhances matrix metalloproteinase (MMP) expression via the ERK signaling pathway in GPR4 overexpressed cells. In this study, we showed that GPR4 could increase phosphorylation of p38 at pH 5.9, which could be inhibited by SB203580. SB203580 could reduce the expression of pro-angiogenic cytokines, which may indicate p38 play an important role in mediating the expression of cytokines.
In conclusion, we suggested that GPR4 induces angiogenesis via GPR4-induced p38-mediated IL6, IL8 and VEGFA secretion at acidic extracellular pH in SCCHN.
Supporting Information
Total RNA and protein of Ad-GPR4-Tca8113 cells, Ad-null-Tca8113 cells were isolated after acid stimulation for 6 h. qPCR and western blot were performed. (A) Overexpression of GPR4 in Ad-GPR4-Tca8113 cells was comfirmed by qPCR and western blot. (B) The expression of IL6, IL8, and VEGFA increased significantly in GPR4 infected cells at pH 5.9. (C) The supernatants of the cells were collected and the concentrations of IL6, IL8 and VEGFA were detected by ELISA.
(TIF)
(A) In Ad-GPR4-Tca8113 cells, SB203580 reduced the expression of IL6, IL8 and VEGFA at pH 5.9. (B) SB203580 reduced secretion of IL6, IL8 and VEGFA. (C) GPR4 increased p38 phosphorylation at pH5.9. (D) SB203580 inhibit phosphorylation of p38 in GPR4 infected cells.
(TIF)
(TIF)
(A) The tube length (arrows) of HMEC-1 cells was increased in CM derived from Ad-GPR4-Tca8113 cells compared with Ad-null- Tca8113 cells at pH 5.9. (B) The neutralizing antibodies of IL6, IL8 and VEGFA inhibited tube formation in HMEC-1 cells. Isotype IgG antibody was used as a control in neutralizing antibody test.
(TIF)
(TIF)
(XLSX)
Acknowledgments
We thank Dr. Pingzhang Wang (Department of Immunology, School of Basic Medical Sciences, Peking University Health Science Center; Peking University Center for Human Disease Genomics) for the bioinformatics analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by grant from the Yangfan Program of Beijing Municipal Administration of Hospitals (no. XMLX201507). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(14):2137–50. [DOI] [PubMed] [Google Scholar]
- 2.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nature reviews Cancer. 2011;11(1):9–22. 10.1038/nrc2982 [DOI] [PubMed] [Google Scholar]
- 3.Copper MP, Smit CF, Stanojcic LD, Devriese PP, Schouwenburg PF, Mathus-Vliegen LM. High incidence of laryngopharyngeal reflux in patients with head and neck cancer. The Laryngoscope. 2000;110(6):1007–11. [DOI] [PubMed] [Google Scholar]
- 4.Sturgis EM, Wei Q, Spitz MR. Descriptive epidemiology and risk factors for head and neck cancer. Seminars in oncology. 2004;31(6):726–33. [DOI] [PubMed] [Google Scholar]
- 5.Smit CF, Tan J, Devriese PP, Mathus-Vliegen LM, Brandsen M, Schouwenburg PF. Ambulatory pH measurements at the upper esophageal sphincter. The Laryngoscope. 1998;108(2):299–302. [DOI] [PubMed] [Google Scholar]
- 6.Toohill RJ, Kuhn JC. Role of refluxed acid in pathogenesis of laryngeal disorders. The American journal of medicine. 1997;103(5a):100s–6s. [DOI] [PubMed] [Google Scholar]
- 7.Sato S, Yamamoto H, Mukaisho K, Saito S, Hattori T, Yamamoto G, et al. Continuous taurocholic Acid exposure promotes esophageal squamous cell carcinoma progression due to reduced cell loss resulting from enhanced vascular development. PloS one. 2014;9(2):e88831 10.1371/journal.pone.0088831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478(7369):399–403. 10.1038/nature10525 [DOI] [PubMed] [Google Scholar]
- 9.Justus CR, Dong L, Yang LV. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Frontiers in physiology. 2013;4:354 10.3389/fphys.2013.00354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren J, Jin W, Gao YE, Zhang Y, Zhang X, Zhao D, et al. Relations between GPR4 expression, microvascular density (MVD) and clinical pathological characteristics of patients with epithelial ovarian carcinoma (EOC). Current pharmaceutical design. 2014;20(11):1904–16. [DOI] [PubMed] [Google Scholar]
- 11.Sin WC, Zhang Y, Zhong W, Adhikarakunnathu S, Powers S, Hoey T, et al. G protein-coupled receptors GPR4 and TDAG8 are oncogenic and overexpressed in human cancers. Oncogene. 2004;23(37):6299–303. [DOI] [PubMed] [Google Scholar]
- 12.Xu H, Chen X, Huang J, Deng W, Zhong Q, Yue C, et al. Identification of GPR65, a novel regulator of matrix metalloproteinases using high through-put screening. Biochemical and biophysical research communications. 2013;436(1):96–103. 10.1016/j.bbrc.2013.05.065 [DOI] [PubMed] [Google Scholar]
- 13.Xiao W, Hodge DR, Wang L, Yang X, Zhang X, Farrar WL. NF-kappaB activates IL-6 expression through cooperation with c-Jun and IL6-AP1 site, but is independent of its IL6-NFkappaB regulatory site in autocrine human multiple myeloma cells. Cancer biology & therapy. 2004;3(10):1007–17. [DOI] [PubMed] [Google Scholar]
- 14.Schmeck B, Moog K, Zahlten J, van Laak V, N'Guessan PD, Opitz B, et al. Streptococcus pneumoniae induced c-Jun-N-terminal kinase- and AP-1 -dependent IL-8 release by lung epithelial BEAS-2B cells. Respiratory research. 2006;7:98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Q, Le X, Wang B, Abbruzzese JL, Xiong Q, He Y, et al. Regulation of vascular endothelial growth factor expression by acidosis in human cancer cells. Oncogene. 2001;20(28):3751–6. [DOI] [PubMed] [Google Scholar]
- 16.Ge L, Smail M, Meng W, Shyr Y, Ye F, Fan KH, et al. Yes-associated protein expression in head and neck squamous cell carcinoma nodal metastasis. PloS one. 2011;6(11):e27529 10.1371/journal.pone.0027529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, et al. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. The Journal of investigative dermatology. 1992;99(6):683–90. [DOI] [PubMed] [Google Scholar]
- 18.Wang P, Guo J, Wang F, Shi T, Ma D. Human SBK1 is dysregulated in multiple cancers and promotes survival of ovary cancer SK-OV-3 cells. Molecular biology reports. 2011;38(5):3551–9. 10.1007/s11033-010-0465-8 [DOI] [PubMed] [Google Scholar]
- 19.Li T, Cheng Y, Wang P, Wang W, Hu F, Mo X, et al. CMTM4 is frequently downregulated and functions as a tumour suppressor in clear cell renal cell carcinoma. Journal of experimental & clinical cancer research: CR. 2015;34:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Yang Y, Han W, Ma D. ImmuSort, a database on gene plasticity and electronic sorting for immune cells. Scientific reports. 2015;5:10370 10.1038/srep10370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang P, Qi H, Song S, Li S, Huang N, Han W, et al. ImmuCo: a database of gene co-expression in immune cells. Nucleic acids research. 2015;43(Database issue):D1133–9. 10.1093/nar/gku980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riebe C, Pries R, Kemkers A, Wollenberg B. Increased cytokine secretion in head and neck cancer upon p38 mitogen-activated protein kinase activation. International journal of molecular medicine. 2007;20(6):883–7. [PubMed] [Google Scholar]
- 23.Lingen MW. Angiogenesis in the Development of Head and Neck Cancer and Its Inhibition By Chemopreventive Agents. Critical Reviews in Oral Biology & Medicine. 1999;10(2):153–64. [DOI] [PubMed] [Google Scholar]
- 24.Pries R, Nitsch S, Wollenberg B. Role of cytokines in head and neck squamous cell carcinoma. Expert review of anticancer therapy. 2006;6(9):1195–203. [DOI] [PubMed] [Google Scholar]
- 25.Wan Q, Dingerdissen H, Fan Y, Gulzar N, Pan Y, Wu TJ, et al. BioXpress: an integrated RNA-seq-derived gene expression database for pan-cancer analysis. Database: the journal of biological databases and curation. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 27.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57. [DOI] [PubMed] [Google Scholar]
- 28.Rofstad EK, Mathiesen B, Kindem K, Galappathi K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer research. 2006;66(13):6699–707. [DOI] [PubMed] [Google Scholar]
- 29.Yang LV, Radu CG, Roy M, Lee S, McLaughlin J, Teitell MA, et al. Vascular abnormalities in mice deficient for the G protein-coupled receptor GPR4 that functions as a pH sensor. Molecular and cellular biology. 2007;27(4):1334–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen A, Dong L, Leffler NR, Asch AS, Witte ON, Yang LV. Activation of GPR4 by acidosis increases endothelial cell adhesion through the cAMP/Epac pathway. PloS one. 2011;6(11):e27586 10.1371/journal.pone.0027586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim KS, Ren J, Jiang Y, Ebrahem Q, Tipps R, Cristina K, et al. GPR4 plays a critical role in endothelial cell function and mediates the effects of sphingosylphosphorylcholine. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19(7):819–21. [DOI] [PubMed] [Google Scholar]
- 32.Dong L, Li Z, Leffler NR, Asch AS, Chi JT, Yang LV. Acidosis activation of the proton-sensing GPR4 receptor stimulates vascular endothelial cell inflammatory responses revealed by transcriptome analysis. PloS one. 2013;8(4):e61991 10.1371/journal.pone.0061991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Im DS. Two ligands for a GPCR, proton vs lysolipid. Acta pharmacologica Sinica. 2005;26(12):1435–41. [DOI] [PubMed] [Google Scholar]
- 34.Tobo M, Tomura H, Mogi C, Wang JQ, Liu JP, Komachi M, et al. Previously postulated "ligand-independent" signaling of GPR4 is mediated through proton-sensing mechanisms. Cellular signalling. 2007;19(8):1745–53. [DOI] [PubMed] [Google Scholar]
- 35.Eisma RJ, Spiro JD, Kreutzer DL. Vascular endothelial growth factor expression in head and neck squamous cell carcinoma. American journal of surgery. 1997;174(5):513–7. [DOI] [PubMed] [Google Scholar]
- 36.Kyzas PA, Cunha IW, Ioannidis JP. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11(4):1434–40. [DOI] [PubMed] [Google Scholar]
- 37.Kyzas PA, Stefanou D, Batistatou A, Agnantis NJ. Potential autocrine function of vascular endothelial growth factor in head and neck cancer via vascular endothelial growth factor receptor-2. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2005;18(4):485–94. [DOI] [PubMed] [Google Scholar]
- 38.Duffy SA, Taylor JM, Terrell JE, Islam M, Li Y, Fowler KE, et al. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113(4):750–7. 10.1002/cncr.23615 [DOI] [PubMed] [Google Scholar]
- 39.Van Tubergen E, Vander Broek R, Lee J, Wolf G, Carey T, Bradford C, et al. Tristetraprolin regulates interleukin-6, which is correlated with tumor progression in patients with head and neck squamous cell carcinoma. Cancer. 2011;117(12):2677–89. 10.1002/cncr.25859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu HW, Wall NR, Hsueh CT, Kim S, Ferris RL, Chen CS, et al. Combination antiangiogenic therapy and radiation in head and neck cancers. Oral oncology. 2014;50(1):19–26. 10.1016/j.oraloncology.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 41.Cao S, Durrani FA, Toth K, Rustum YM, Seshadri M. Bevacizumab enhances the therapeutic efficacy of Irinotecan against human head and neck squamous cell carcinoma xenografts. Oral oncology. 2011;47(6):459–66. 10.1016/j.oraloncology.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Argiris A, Kotsakis AP, Hoang T, Worden FP, Savvides P, Gibson MK, et al. Cetuximab and bevacizumab: preclinical data and phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2013;24(1):220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fury MG, Lee NY, Sherman E, Lisa D, Kelly K, Lipson B, et al. A phase 2 study of bevacizumab with cisplatin plus intensity-modulated radiation therapy for stage III/IVB head and neck squamous cell cancer. Cancer. 2012;118(20):5008–14. 10.1002/cncr.27498 [DOI] [PubMed] [Google Scholar]
- 44.Matossian M, Vangelderen C, Papagerakis P, Zheng L, Wolf GT, Papagerakis S. In silico modeling of the molecular interactions of antacid medication with the endothelium: novel therapeutic implications in head and neck carcinomas. International journal of immunopathology and pharmacology. 2014;27(4):573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papagerakis S, Bellile E, Peterson LA, Pliakas M, Balaskas K, Selman S, et al. Proton pump inhibitors and histamine 2 blockers are associated with improved overall survival in patients with head and neck squamous carcinoma. Cancer prevention research (Philadelphia, Pa). 2014;7(12):1258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Total RNA and protein of Ad-GPR4-Tca8113 cells, Ad-null-Tca8113 cells were isolated after acid stimulation for 6 h. qPCR and western blot were performed. (A) Overexpression of GPR4 in Ad-GPR4-Tca8113 cells was comfirmed by qPCR and western blot. (B) The expression of IL6, IL8, and VEGFA increased significantly in GPR4 infected cells at pH 5.9. (C) The supernatants of the cells were collected and the concentrations of IL6, IL8 and VEGFA were detected by ELISA.
(TIF)
(A) In Ad-GPR4-Tca8113 cells, SB203580 reduced the expression of IL6, IL8 and VEGFA at pH 5.9. (B) SB203580 reduced secretion of IL6, IL8 and VEGFA. (C) GPR4 increased p38 phosphorylation at pH5.9. (D) SB203580 inhibit phosphorylation of p38 in GPR4 infected cells.
(TIF)
(TIF)
(A) The tube length (arrows) of HMEC-1 cells was increased in CM derived from Ad-GPR4-Tca8113 cells compared with Ad-null- Tca8113 cells at pH 5.9. (B) The neutralizing antibodies of IL6, IL8 and VEGFA inhibited tube formation in HMEC-1 cells. Isotype IgG antibody was used as a control in neutralizing antibody test.
(TIF)
(TIF)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.