Abstract
Improvement in litter size has become of great interest in the pig industry because fecundity is directly related to sow reproductive life. Improved reproduction has thus been achieved by elucidating the molecular functions of genes associated with fecundity. In the present study, we identified differentially expressed genes (DEGs) via transcriptomic analysis using RNA-sequencing (RNA-Seq) in Berkshire pig placentas from larger (LLG, mean litter size >12) and smaller (SLG, mean litter size < 6.5) litter size groups. In total 588 DEGs were identified (p < 0.05, > 1.5-fold change), of which 98 were upregulated, while 490 were downregulated in the LLG compared with the SLG. Gene Ontology (GO) enrichment was also performed. We concluded that 129 of the 588 DEGs were closely related to litter size according to reproduction related genes selected based on previous reports, as 110 genes were downregulated and 19 upregulated in the LLG compared with the SLG. RT-qPCR utilizing specific primers targeting the early growth response 2 (EGR2), pheromaxein c subunit (PHEROC) and endothelial lipase (LIPG) genes showed high accordance with RNA-Seq results. Furthermore, we investigated the upstream regulators of these three genes in the placenta. We found that WNT9B, a Wnt signaling pathway molecule, and IL-6, known inducers of EGR2 and LIPG, respectively, were significantly increased in LLG compared with SLG. We believe that the induction of IL-6 and LIPG may play an important role in increasing nutrition supply through the placenta from the sow to the piglet during gestation. These results provide novel molecular insights into pig reproduction.
Introduction
Improved litter size is a principal interest in the pig industry and among breeders. Litter size is a complex trait composed of many subordinated traits, such as ovulation rate, embryonic/fetal survival, uterine capacity, and others. Moreover, since selection by litter size has its limitations, including low heritability and sex-restriction, various efforts have been made to identify factors influencing litter size. These efforts have included optimizing nutrition and husbandry, management of sows, and genetic factors [1]. Genetic selection for increased litter size has in turn increased the prolificacy of sows and has drastically improved over the past 10 years, resulting in an average increase of 1.8 piglets per litter [2].
The first successful evidence of a candidate gene associated with litter size was estrogen receptor 1 (ESR1), which was identified using restriction fragment length polymorphism (RFLP) analysis [3]. Retinol-binding protein 4 (RBP4), erythropoietin receptor (EPOR), amphiregulin (AREG), epidermal growth factor (EGF), and others have also been determined to be associated with litter size [1, 4–6]. In addition, several expression studies performed in pigs have developed a profile of differentially expressed genes (DEGs) and even microRNAs (miRNAs) in endometria, ovaries and placentas [7–9]. The mRNA of the oligoadenylate synthetase 1 (OAS1) gene was highly expressed, whereas that of the RBP4, thiosulfate sulfurtransferase (TST) and vitronectin (VTN) genes were lowly expressed in the ovaries of a high prolificacy group compared with a low prolificacy group [7]. Moreover, the investigation uncovered the location of candidate genes according to significant quantitative trait loci (QTL) previously reported by Noguera et al. [7, 10]. The porcine endometrial transcriptome was also analyzed during the implantation period [8]. The androgen receptor (AR) was revealed to have a close interaction with genes associated with important processes during early pregnancy [8]. Recent reports on differential expression of miRNAs determined that selected miRNAs affect cell growth, angiogenesis and trophoblast differentiation, and these impacts may be important for placental growth and function [9]. Moreover, fetal loss in the pig is mostly a result of decreased uterine capacity and placental efficiency [11, 12]. Therefore, to understand the molecular mechanisms by which the structure and function of the placenta may be regulated is essential to increasing litter size [11]. Historically, the Chinese Meishan pig breed is known for its prolificacy and large litter size [13]. Some evidence also suggests that the Chinese Meishan may have improved uterine capacity and placental efficiency [14]. However, the reproductive rate of Berkshire pigs is lower than that of other pig breeds, although the production of Berkshire pigs has been increasing recently [15]. Improvement in the litter size of Berkshire pigs is essential for Berkshire pig breeders. In this context, we hypothesized that DEGs in the placenta play a critical role in the determination of litter size. However, the placental transcriptome of Berkshire pigs has not yet been elucidated. Therefore, this study analyzed the RNA-Seq of Berkshire placentas, which revealed novel genes that may be involved in placental development and function, thus playing a role in determining litter size. These results provide molecular insights into the genes underlying pig fecundity.
Materials and Methods
Ethics statement
There is an exception to the application of the Enforcement Rule of the Korean Animal Protection Act from Article 23 in the case of experiments conducted for scientific purposes concerning the relevant animal, ecology, and habitude of species. We did not need to obtain approval from the Animal Care and Use Committee of Gyeongnam National University of Science and Technology (ACUC of GNTECH) because all of the placentas used in this study were a natural by-product. Moreover, no ethics committee approval for the study was required for livestock in the Republic of Korea. We have enclosed the ACUC of GNTECH waiver (Supplementary information). The pigs used in this study were raised according to the guidelines on animal care and use established by ACUC of GNTECH and with the Korean Animal Protection Act and related laws.
Placenta collection
Berkshire placentas were collected by a trained veterinarian from sows reared in the same environment (Dasan Pig Breeding Company, South Korea). We divided the placentas into two groups according to litter size: the larger (LLG) and smaller (SLG) litter size groups. The LLG was defined by an estimated breeding value (EBV) ≥ 0.8 and litter size ≥ 12, whereas the SLG was selected by an EBV ≤ –0.7 and litter size ≤ 6.5. Detailed information for sows is described in Table 1. Farrowing was allowed to start naturally. After delivery, the placental tissues were collected as part of the routine care of pigs. Samples were excised from the maternal side of the placenta 2 cm from the umbilical cord insertion site and free of maternal decidua corresponding to the chorioallantoic placenta. After collection, placental tissues were rinsed with PBS and frozen rapidly in liquid nitrogen.
Table 1. Characteristics of the Berkshire pigs used in this study.
| Group | sample ID | months of age | body weight | total parity numbers | mean of litter size | EBV |
|---|---|---|---|---|---|---|
| SLG | SLG1 | 23 | 175 | 4 | 5.5 | -0.78 |
| SLG2 | 23 | 170 | 4 | 6 | -0.8 | |
| SLG3 | 25 | 180 | 4 | 6.5 | -0.81 | |
| LLG | LLG1 | 27 | 180 | 4 | 12.25 | 0.9 |
| LLG2 | 28 | 200 | 4 | 12 | 0.8 | |
| LLG3 | 28 | 200 | 3 | 12 | 0.85 |
RNA isolation and RNA-Seq
Total RNA was isolated from three Berkshire pig placentas in each group using TRIzol reagent according to the manufacturer’s instructions (Molecular Research Center, Cincinnati, OH, USA). We pooled the isolated RNA from the three different pigs in a ratio of 1:1:1 before library preparation. The quality of the resulting total RNA was measured using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). All extractions exhibited an RNA integrity number > 7.0 and a 28S:18S ratio > 1.0.
RNA-Seq libraries were prepared using a TruSeq RNA Sample Prep kit (Illumina, San Diego, CA, USA). The poly A-containing mRNA molecules were purified from 2 μg total RNA from each sample using poly-T oligo-attached magnetic beads. The mRNA was fragmented into approximately 200 base pair pieces. Using reverse transcriptase and random hexamer primers, the first-strand cDNA was synthesized from the mRNA fragments. Second-strand cDNA was then synthesized using DNA polymerase I and RNaseH. The end of the cDNA fragment was then subjected to an end repair process that included the addition of a single ‘A’ base, followed by ligation of the adapters. Products were purified and enriched by polymerase chain reaction (PCR) to amplify the library DNA. Libraries were quantified using a KAPA Library Quantification kit (KAPA Biosystems, South Africa) and an Agilent 2100 Bioanalyzer. After quantitative reverse transcription-polymerase chain reaction (RT-qPCR) validation, libraries were subjected to paired-end sequencing with 100-base pair read lengths using the Illumina HiSeq 2500 platform (Illumina).
RNA-Seq analysis
High-quality reads were obtained by removing adaptor sequences, reads in which the percentage of unknown bases was greater than 10% or low quality reads (more than 20% of <Q20 bases). Selected reads were aligned to the susScr3 reference sequence using STAR (version 2.3.0e) [16], and the gene description in the Ensembl gene database (version 72) [17] was used to increase the accuracy of quantification. Expression levels were quantified using htseq-count (ver. 0.5.4p3) [18], while considering intersection-nonempty rules and paired-end alignments. DEGs were analyzed using TCC [19] with the negative binomial (NB) statistical test available in two R packages iDEGES/edgeR and cutoff values of p < 0.05 and 1.5-fold change.
Pearson correlation and functional enrichment analysis
A Pearson correlation coefficient was computed for fragments per kilobase of exon per million fragments mapped (FPKM) and log2 (FPKM) values for genes from each group. Gene ontology (GO) [17] annotation and KEGG pathway analysis were performed on all DEGs using the Database for Annotation, Visualization and Integrated Discovery tool (DAVID; National Institute of Health; http://david.abcc.ncifcrf.gov/) Bioinformatics Resources 6.7. Statistics were applied for the selection of GO categories using a modified Fisher’s exact p-value < 0.01 (EASE score in DAVID).
RT-qPCR
Total RNA was isolated from three different placentas and was used for RT-qPCR. RNA (1 μg) was then used for reverse transcription in a 20-μL reaction volume using Superscript II (Invitrogen). RT-qPCR was performed using a Rotor Gene Q Thermocycler (QIAGEN) with SYBR green. Gene-specific exon spanning primers were designed using the Primer3 program as described previously (S1 Table) [20]. RT-qPCR was performed in a 10-μL reaction volume containing 1 μL cDNA (50 ng) as the template, 5 μL Rotor Gene SYBR Green PCR Master Mix, 1 μL each of the forward and reverse primers (10 pmol), and 3 μL H2O using the following cycling parameters: 40 cycles at 94°C for 5 s and 60°C for 10 s. A melting curve was performed at the end of the PCR for 5 s from 60°C to 95°C to identify unique PCR products amplified during the reaction. A no template control was used as a negative control. The qPCR reaction was repeated three times independently. PPIA was used as a reference gene [21]. Amplification efficiencies and correlation coefficients (R2 values) were generated using the slopes of standard curves obtained from serial dilutions. Standard curves with a 10-fold dilution series were used to calculate the amplification efficiency. The amplification efficiency was calculated according to the formula: efficiency (%) = (10(-1/slope)-1) × 100. The efficiency of all of the RT-qPCR amplifications was almost 90%. Data were analyzed by the relative quantification method using 2-ΔΔCt. The control sample used to determine fold induction was the lowest expressed sample for the target gene. The significance of differences was analyzed using Student’s t-test or the Mann–Whitney test with p < 0.05 considered significant.
Results
RNA-Seq analysis
In this study, DEGs were identified from Berkshire pig placentae, and the role of candidate genes influencing litter size was investigated. To identify DEGs, we divided the placentae into two groups (LLG and SLG) according to litter size. RNA-Seq was performed on total RNA pooled from three placentae of Berkshire pigs. The total numbers of clean reads were 47,289,182 (90.8%) and 45,580,292 (95.3%) and mapped reads were 40,421,582 (85.5%) and 39,219,270 (86.0%) in LLG and SLG, respectively (data not shown). The number of genes expressed was quantified using htseq-count and was 14,824 in the LLG and 14,511 in the SLG. The number of genes commonly expressed between the two groups was 13,970 (Fig 1).
Fig 1. Identification of DEGs by RNA-Seq analysis.
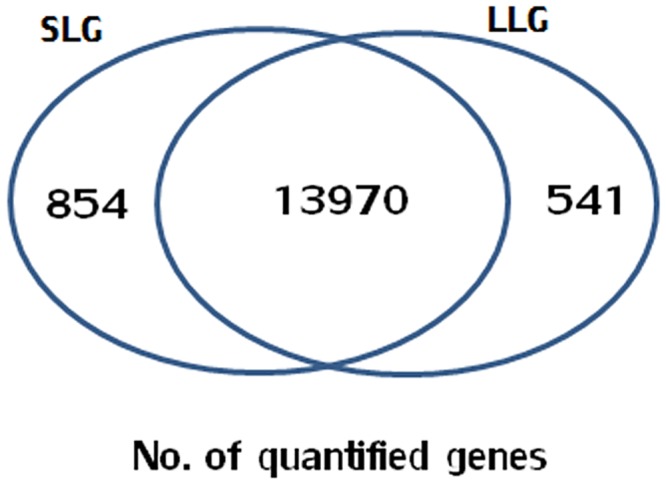
The number of annotated genes using RNA-seq is shown. A total of 14,511 genes in the LLG and 14,824 genes in the SLG were detected. The number of commonly expressed genes among both groups was 13,970.
Analysis of annotated genes
Pearson correlation coefficients were calculated for each group. The Pearson correlation coefficient between the FPKM of genes from each group was 0.962. Similarly, the Pearson correlation coefficient between the log2(FPKM) of genes was 0.978. These results indicate a strong correlation between the two groups.
Analysis of DEG and GO enrichment analysis
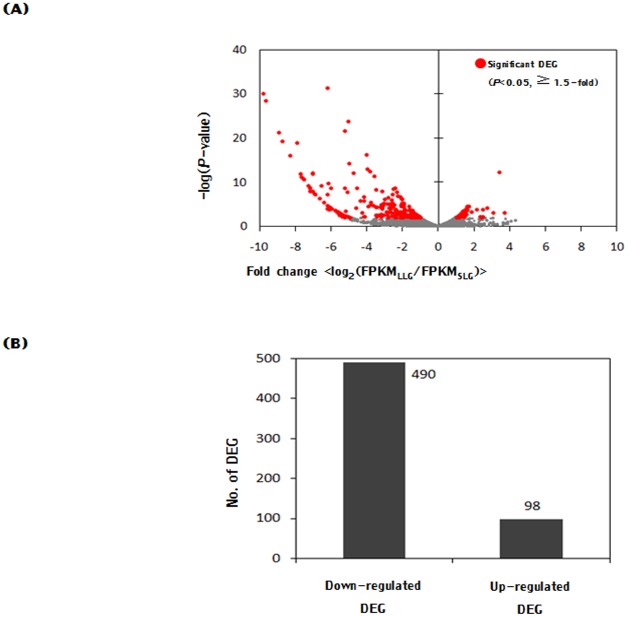
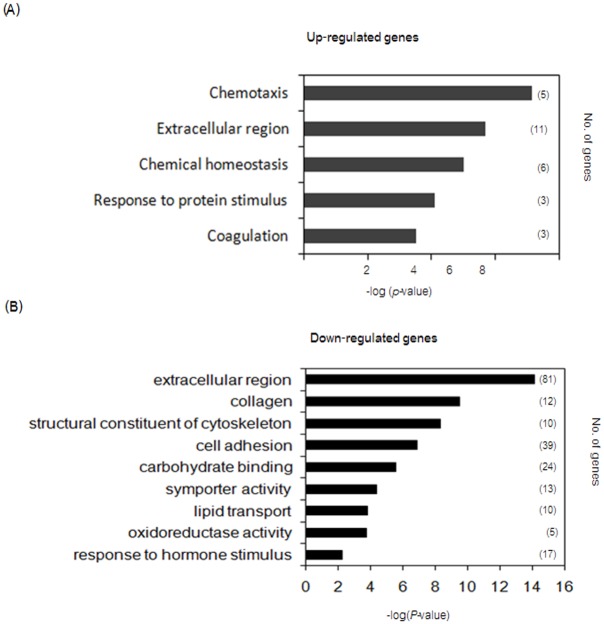
Each gene annotated as a DEG is shown as a dot graph in Fig 2A. The number of downregulated DEGs was higher than the number of upregulated DEGs in the LLG compared with the SLG. The significantly altered DEGs are indicated as red dots. A total of 490 and 98 genes were annotated among the downregulated DEGs and upregulated DEGs in the LLG, respectively (Fig 2B). Next, GO enrichment analysis was performed on the upregulated genes in the LLG. The annotated genes were associated with chemotaxis, extracellular region, chemical homeostasis, response to protein stimulus and coagulation. Eleven genes were associated with the extracellular region, including PLAT, INHBA, CCL2 and IL8. Six genes were associated with chemical homeostasis, including ALAS2, CCL2 and EGR2. Five genes were associated with chemotaxis, including PLAU and PLAUR (Fig 3A). We next compared GO enrichment analysis results with the downregulated genes in the LLG. The genes were categorized into extracellular region, collagen, structural constituent of cytoskeleton, cell adhesion, carbohydrate binding, symporter activity, lipid transport, oxidoreductase activity and response to hormone stimulus. Eighty-one genes were associated with the extracellular region, including LTBP2 and FAM3D. In addition, 39 genes were associated with cell adhesion, while 24 genes were related to carbohydrate binding (Fig 3B).
Fig 2. Distribution of DEGs.
(A) The dot graph shows each DEG between the LLG and SLG. log2-fold change between fragments per kilobase of exon per million reads mapped (FPKM) in the SLG and LLG according to the Cufflinks package are presented along the x axis. Red dots represent significantly altered DEGs between the LLG and SLG (p < 0.05, fold change > 1.5, the decimal system). The y axis indicates significance as -log (p value). (B) The number of downregulated and upregulated DEGs in the LLG compared with the SLG.
Fig 3. GO enrichment analysis.
GO enrichment analysis was performed for the upregulated (A) and downregulated (B) DEGs. The x axis indicates the significance of DEGs as −log (p value). The number of genes in each category is shown on the y axis, as indicated in parentheses.
Analysis of DEGs associated with litter size
Next, we investigated the genes associated with litter size among all DEGs by searching for terms related to litter size such as reproduction, fecundity, prolificacy and litter size in the NCBI PubMed. A total of 129 (22%) of the 588 DEGs were selected. The upregulated DEGs included 19 genes; whereas, the downregulated DEGs included 110 genes (S2 and S3 Tables). The representative genes are listed in Table 2. Among the upregulated DEGs, PHEROC and XIRPL levels showed approximately 5.3- to 5.6-fold change in the LLG compared with the SLG, with the decimal system (Table 2). Interestingly, KRT1 was decreased more than 1,200-fold in the LLG compared with the SLG (S3 Table). Of note, some genes described as downregulated DEGs, such as USH1C, LCN15, DAO, MUC13A, REG4, EPS8L3, KRT77, SLC6A19, APO8, HNF4A, LGALS2, IGSF23, RBP2 and TM4SF20, were determined to lack transcripts in the LLG. Presumably, these genes might play a negative role in reproduction regulation.
Table 2. List of DEGs between LLG and SLG.
| Accession | Symbol | Description | log2fc | p-value |
|---|---|---|---|---|
| ENSSSCG00000013067 | PHEROC | pheromaxein C subunit | 2.51 | 0.01 |
| ENSSSCG00000011265 | XIRP1 | xin actin-binding repeat containing 1 | 2.42 | 0.01 |
| ENSSSCG00000011951 | NFKBIZ | nuclear factor of kappa light polypeptide | 1.85 | < .001 |
| ENSSSCG00000010224 | EGR2 | early growth response 2 | 1.62 | < .001 |
| ENSSSCG00000003451 | PDPN | Podoplanin | 1.55 | < .001 |
| ENSSSCG00000004509 | LIPG | endothelial lipase | 1.51 | < .001 |
| ENSSSCG00000011663 | RBP2 | retinol binding protein 2, cellular | Only SLG | < .001 |
| ENSSSCG00000009558 | F10 | coagulation factor X protein | -5.16 | < .001 |
| ENSSSCG00000011453 | ITIH4 | inter-alpha-trypsin inhibitor heavy chain H4 | -4.99 | < .001 |
| ENSSSCG00000020694 | DSG1 | desmoglein 1 | -4.36 | < .001 |
| ENSSSCG00000016196 | VIL1 | Villin-1 | -4.18 | < .001 |
| ENSSSCG00000023949 | PIM3 | pim-3 oncogene | -3.50 | < .001 |
| ENSSSCG00000007507 | PCK1 | phosphoenolpyruvate carboxykinase 1 | -3.47 | < .001 |
Note: log2fc means the log2 value of the fold change in fragments per kilobase of exon per million fragments mapped (FPKM) by comparing of LLG with SLG.
Validation of DEGs associated with litter size by RT-qPCR
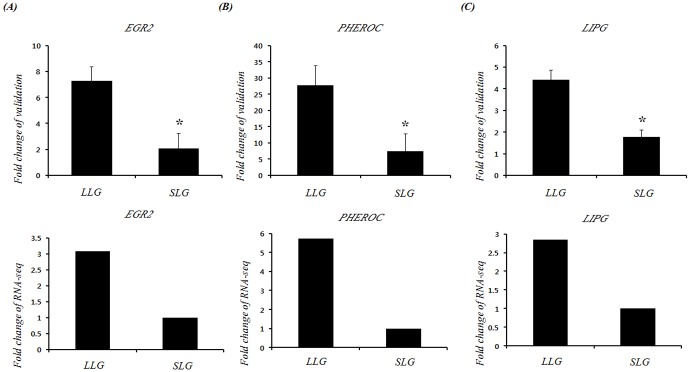
Next, RT-qPCR was performed to confirm the RNA-Seq results. The results from RT-qPCR were highly accordant with those from RNA-Seq. PCR products amplified using primers specific to early growth response 2 (EGR2), pheromaxein c subunit (PHEROC) and endothelial lipase (LIPG) are shown in Fig 4. In all cases, the relative fold changes in gene expression were in the same direction as those observed in the RNA-Seq results, and consistently showed a higher magnitude with RT-qPCR than RNA-Seq technology (Fig 4).
Fig 4. RT-qPCR analysis of EGR2, PHEROC and LIPG in placental tissue.
Three different placentas from LLG and SLG were prepared immediately following delivery and subjected to RT-qPCR analysis. The fold change of validation (upper panel) and RNA-Seq (lower panel) for the (A) EGR2, (B) PHEROC and (C) LIPG genes are presented. The RT-qPCR data were analyzed by relative quantification using 2-ΔΔCt. Data are expressed as means ± standard deviation (S.D.). Each experiment was performed in triplicate. *, p < 0.05 versus SLG.
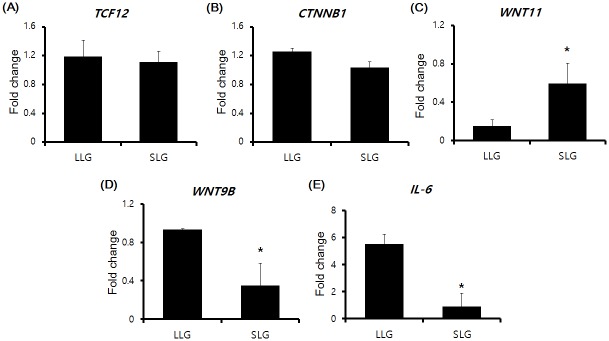
To examine the molecules upstream from the DEGs annotated in this study, we investigated the mRNA expression of genes related to the Wnt signaling pathway such as TCF12, CTNNB1, WNT11 and WNT9B, because Wnt signaling plays a crucial role in placenta and trophoblast development and differentiation [22]. In addition, several Wnt ligands are strongly expressed in first trimester trophoblasts, suggesting that these ligands could play a crucial role in early placental function and development [23]. Moreover, some evidence of EGR2 induction via the Wnt signaling pathway has been obtained in investigations in other fields [24–26]. When we examined gene expression in three different placental RNA samples from LLG and SLG using RT-qPCR, the mRNA levels of TCF12 and CTNNB1 were unchanged between the two groups (Fig 5A and 5B). In comparison, the expression of WNT11 mRNA was decreased and that of WNT9B was increased in LLG compared with SLG, respectively (Fig 5C and 5D).
Fig 5. RT-qPCR analysis to assess TCF12, CTNNB1, WNT11, WNT9B and IL-6 mRNA expression.
RNAs from three different individual placentas each were prepared from LLG and SLG. RT-qPCR was performed using gene-specific primers. PPIA was used as a universal control. The RT-qPCR data were analyzed by relative quantification using 2-ΔΔCt. Data are expressed as means ± standard deviation (S.D.). Each experiment was performed in triplicate. *, p < 0.05 versus SLG.
Nutrient supply is a crucial factor supporting piglet growth. The primary fetal nutrients that cross the placenta are glucose, free amino acids, ketone bodies, and free fatty acids [27]. The triglyceride lipase gene (TLG) family comprises secreted lipases that hydrolyze triglycerides and phospholipids [28]. LIPG is one of the most well studied lipases in the TLG family [28]. Of note, LIPG is expressed in trophoblasts in the first trimester in humans. The expression of LIPG mRNA was significantly lower in placentas from intrauterine growth restricted pregnancies compared with normal placentas [28]. In this regard, the upregulation of LIPG may accelerate nutrient supply via the placenta, strongly suggesting that LIPG contributes to increased litter size. A limited number of cytokines and hormones, such as TNF-α, IL-6, leptin, and adiponectin, have been determined to regulate LIPG mRNA expression [28–30]. The mRNA expression of TNF-α, leptin and adiponectin were unchanged between the LLG and SLG (data not shown). However, IL-6 was significantly increased in the LLG compared with the SLG (Fig 5E). Moreover, one of the pro-inflammatory cytokines, IL-1B was also upregulated in LLG compared with SLG (data not shown). IL-6, together with IL-1β might enhance the capacity of endothelial cells to bind and transport high-density lipoproteins, and could increase the permeability of the endothelial barrier to allow easy transport of nutrients across the placenta [31].
Discussion
Various research groups have investigated DEGs from reproductive tissues such as the endometrium in pigs [32, 33]. DEGs in the placenta have rarely been demonstrated using RNA-Seq. In this study, we revealed DEGs according to litter size in Berkshire pig placentas. We found 588 annotated DEGs between LLG and SLG. A total of 129 genes (19 upregulated genes and 110 downregulated genes in LLG compared with SLG) from the 588 DEGs were selected because they are associated with litter size [34–37]. Intriguingly, in an effort to delineate the molecular pathways responsible for the upregulation of EGR2 and LIPG, we discovered that the induction of WNT9B and IL-6 might stimulate the upregulation of EGR2 and LIPG, respectively, in LLG compared with SLG. Previously, Wnt signaling is known to increase EGR2 expression [23–25]. Moreover, the activation of Wnt signaling accelerates EGR2 expression and compromises the inhibitory effect of glucocorticoids on EGR2 expression [25]. Therefore, we evaluated the mRNA expression of the following Wnt ligands: WNT6, WNT7B WNT9B, WNT10A, and WNT11. WNT9B was upregulated in the LLG compared with the SLG, while no expression of WNT6, WNT7B or WNT10A mRNA was detected, and WNT11 was decreased in the LLG compared with the SLG. Although we could not exclude a role of WNT11 in SLG, WNT9B is a likely mediator of EGR2 induction in LLG. As we mentioned above, Wnt signaling plays a critical role in development and differentiation of the placenta and trophoblast. Wnt may also modulate other trophoblast processes such as phospholipid uptake and transport. However, further investigation is needed to fully understand the role of Wnt signaling in the placenta.
Since the pig industry is actively incorporating marker-associated selection to improve economical traits such as litter size, we searched for SNPs of the DEGs in this study. Four different missense SNPs were identified in the EGR2 gene. A future study should examine the correlation of polymorphisms in EGR2 and litter size, although we are not convinced whether the polymorphisms affect the mRNA expression or not.
Together with the RNA-Seq experiment, we originally performed whole genome bisulfite sequencing (WGBS) to analyze the correlation between DNA methylation and mRNA expression. DNA methylation is a process that adds methyl groups to DNA, which stably alters the expression of genes. Some methylation is heritable. Therefore, we believe that DNA markers in which the nucleotide sequence is not changed, but influences gene expression could be useful when breeders select breeding pigs. We also wanted to determine whether the differentially methylated region (DMR) within specific genes of interest could regulate their mRNA expression. For example, PCK1 was hypomethylated in gene body region (decrease methylated status) in LLG compared with SLG, and the expression of PCK1 mRNA was down-regulated in LLG compared with SLG, which indicates a correlation between DNA methylation and mRNA expression (in press).
In conclusion, we identified DEGs associated with litter size from Berkshire pig placentas that may play crucial roles in pig prolificacy. The results of this study can provide important molecular insights into regulation of litter size. More detailed investigations will be necessary in the future, including SNP analysis of candidate genes, for selection of high prolificacy pigs in order to apply this knowledge to the pig industry.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We appreciate the support of professor and D.V.M. Dong-In Jung from Gyeongsang National University, College of Veterinary Medicine for placenta preparation. The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/G6ul87.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the Priority Research Centers Program (no.2011-0022965) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, from the Export Promotion Technology Development Program (no. 313012-05) of Ministry of Food, Agriculture, Forestry and Fisheries, Republic of Korea. The Dasan Pig Breeding Co. provided support in the form of a salary for author HCP, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific role of this author is articulated in the ‘author contributions’ section.
References
- 1.Spotter A, Distl O. Genetic approaches to the improvement of fertility traits in the pig. Vet J 2006;172:234–47. [DOI] [PubMed] [Google Scholar]
- 2.Quesnel H, Brossard L, Valancogne A, Quiniou N. Influence of some sow characteristics on within-litter variation of piglet birth weight. Animal: an international journal of animal bioscience 2008;2:1842–9. [DOI] [PubMed] [Google Scholar]
- 3.Rothschild M, Jacobson C, Vaske D, Tuggle C, Wang L, Short T, et al. The estrogen receptor locus is associated with a major gene influencing litter size in pigs. Proceedings of the National Academy of Sciences of the United States of America 1996;93:201–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haley CS, Lee GJ. Genetic basis of prolificacy in Meishan pigs. Journal of reproduction and fertility Supplement 1993;48:247–59. [PubMed] [Google Scholar]
- 5.Vallet JL, Freking BA, Leymaster KA, Christenson RK. Allelic variation in the erythropoietin receptor gene is associated with uterine capacity and litter size in swine. Animal genetics 2005;36:97–103. [DOI] [PubMed] [Google Scholar]
- 6.Spotter A, Kuiper H, Drogemuller C, Brenig B, Leeb T, Distl O. Assignment of the porcine epidermal growth factor (EGF) gene to SSC8q2.3-q2.4 by fluorescence in situ hybridization and radiation hybrid mapping. Animal genetics 2002;33:166–7. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Rodriguez A, Munoz M, Fernandez A, Pena RN, Tomas A, Noguera JL, et al. Differential gene expression in ovaries of pregnant pigs with high and low prolificacy levels and identification of candidate genes for litter size. Biology of reproduction 2011;84:299–307. 10.1095/biolreprod.110.085589 [DOI] [PubMed] [Google Scholar]
- 8.Franczak A, Wojciechowicz B, Kotwica G. Transcriptomic analysis of the porcine endometrium during early pregnancy and the estrous cycle. Reproductive biology 2013;13:229–37. 10.1016/j.repbio.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 9.Su L, Zhao S, Zhu M, Yu M. Differential expression of microRNAs in porcine placentas on days 30 and 90 of gestation. Reproduction, fertility, and development 2010;22:1175–82. 10.1071/RD10046 [DOI] [PubMed] [Google Scholar]
- 10.Noguera JL, Rodriguez C, Varona L, Tomas A, Munoz G, Ramirez O, et al. A bi-dimensional genome scan for prolificacy traits in pigs shows the existence of multiple epistatic QTL. BMC genomics 2009;10:636 10.1186/1471-2164-10-636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallet JL, Freking BA. Differences in placental structure during gestation associated with large and small pig fetuses. Journal of animal science 2007;85:3267–75. [DOI] [PubMed] [Google Scholar]
- 12.Spencer TE, Bazer FW. Uterine and placental factors regulating conceptus growth in domestic animals. Journal of animal science 2004;82 E-Suppl:E4–13. [DOI] [PubMed] [Google Scholar]
- 13.Christenson RK. Ovulation rate and embryonic survival in Chinese Meishan and white crossbred pigs. Journal of animal science 1993;71:3060–6. [DOI] [PubMed] [Google Scholar]
- 14.Vonnahme KA, Wilson ME, Ford SP. Conceptus competition for uterine space: different strategies exhibited by the Meishan and Yorkshire pig. Journal of animal science 2002;80:1311–6. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Song KD, Lee HK, Cho KH, Park HC, Park KD. Genetic Parameters of Reproductive and Meat Quality Traits in Korean Berkshire Pigs. Asian-Australasian journal of animal sciences 2015;28:1388–93. 10.5713/ajas.15.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29:15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flicek P, Amode MR, Barrell D, Beal K, Billis K, Brent S, et al. Ensembl 2014. Nucleic acids research 2014;42:D749–55. 10.1093/nar/gkt1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anders S, Pyl PT, Huber W. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J, Nishiyama T, Shimizu K, Kadota K. TCC: an R package for comparing tag count data with robust normalization strategies. BMC bioinformatics 2013;14:219 10.1186/1471-2105-14-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 2000;132:365–86. [DOI] [PubMed] [Google Scholar]
- 21.Park SJ, Kwon SG, Hwang JH, Park da H, Kim TW, Kim CW. Selection of appropriate reference genes for RT-qPCR analysis in Berkshire, Duroc, Landrace, and Yorkshire pigs. Gene 2015;558:152–8. 10.1016/j.gene.2014.12.052 [DOI] [PubMed] [Google Scholar]
- 22.Knofler M, Pollheimer J. Human placental trophoblast invasion and differentiation: a particular focus on Wnt signaling. Frontiers in genetics 2013;4:190 10.3389/fgene.2013.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonderegger S, Husslein H, Leisser C, Knofler M. Complex expression pattern of Wnt ligands and frizzled receptors in human placenta and its trophoblast subtypes. Placenta 2007;28 Suppl A:S97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaman G, Sunters A, Galea GL, Javaheri B, Saxon LK, Moustafa A, et al. Loading-related regulation of transcription factor EGR2/Krox-20 in bone cells is ERK1/2 protein-mediated and prostaglandin, Wnt signaling pathway-, and insulin-like growth factor-I axis-dependent. The Journal of biological chemistry 2012;287:3946–62. 10.1074/jbc.M111.252742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leclerc N, Noh T, Cogan J, Samarawickrama DB, Smith E, Frenkel B. Opposing effects of glucocorticoids and Wnt signaling on Krox20 and mineral deposition in osteoblast cultures. Journal of cellular biochemistry 2008;103:1938–51. [DOI] [PubMed] [Google Scholar]
- 26.Saint-Jeannet JP, He X, Varmus HE, Dawid IB. Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proceedings of the National Academy of Sciences of the United States of America 1997;94:13713–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson AF, Karp WB. Placental transport of nutrients. Southern medical journal 1976;69:1358–62. [DOI] [PubMed] [Google Scholar]
- 28.Gauster M, Hiden U, Blaschitz A, Frank S, Lang U, Alvino G, et al. Dysregulation of placental endothelial lipase and lipoprotein lipase in intrauterine growth-restricted pregnancies. The Journal of clinical endocrinology and metabolism 2007;92:2256–63. [DOI] [PubMed] [Google Scholar]
- 29.Robert J, Lehner M, Frank S, Perisa D, von Eckardstein A, Rohrer L. Interleukin 6 stimulates endothelial binding and transport of high-density lipoprotein through induction of endothelial lipase. Arteriosclerosis, thrombosis, and vascular biology 2013;33:2699–706. 10.1161/ATVBAHA.113.301363 [DOI] [PubMed] [Google Scholar]
- 30.Badellino KO, Wolfe ML, Reilly MP, Rader DJ. Endothelial lipase is increased in vivo by inflammation in humans. Circulation 2008;117:678–85. 10.1161/CIRCULATIONAHA.107.707349 [DOI] [PubMed] [Google Scholar]
- 31.Lager S, Jansson N, Olsson AL, Wennergren M, Jansson T, Powell TL. Effect of IL-6 and TNF-alpha on fatty acid uptake in cultured human primary trophoblast cells. Placenta 2011;32:121–7. 10.1016/j.placenta.2010.10.012 [DOI] [PubMed] [Google Scholar]
- 32.Fu Y, Zhou Y, Wang A, Li L, Liu H, Li B, et al. Tissue expression of EphB2 and RNA-seq analysis during embryo im-plantation in Meishan pigs. Yi chuan = Hereditas / Zhongguo yi chuan xue hui bian ji 2014;36:1243–8. 10.3724/SP.J.1005.2014.1243 [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Li A, Chen W, Wei J, Fu J, Wang A. Differential gene expression in uterine endometrium during implantation in pigs. Biology of reproduction 2015;92:52 10.1095/biolreprod.114.123075 [DOI] [PubMed] [Google Scholar]
- 34.Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, et al. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Molecular and cellular biology 2007;27:1914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humaidan P, Westergaard LG, Mikkelsen AL, Fukuda M, Yding Andersen C. Levels of the epidermal growth factor-like peptide amphiregulin in follicular fluid reflect the mode of triggering ovulation: a comparison between gonadotrophin-releasing hormone agonist and urinary human chorionic gonadotrophin. Fertility and sterility 2011;95:2034–8. 10.1016/j.fertnstert.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 36.Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, Demayo FJ, et al. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proceedings of the National Academy of Sciences of the United States of America 2007;104:18315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernandez SC, Hogg CO, Billon Y, Sanchez MP, Bidanel JP, Haley CS, et al. Secreted phosphoprotein 1 expression in endometrium and placental tissues of hyperprolific large white and meishan gilts. Biology of reproduction 2013;88:120 10.1095/biolreprod.112.104679 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.