Summary
Background
An affordable, highly immunogenic Neisseria meningitidis serogroup A meningococcal conjugate vaccine (PsA–TT) was licensed for use in sub-Saharan Africa in 2009. In 2010, Burkina Faso became the first country to implement a national prevention campaign, vaccinating 11.4 million people aged 1–29 years. We analysed national surveillance data around PsA–TT introduction to investigate the early effect of the vaccine on meningitis incidence and epidemics.
Methods
We examined national population-based meningitis surveillance data from Burkina Faso using two sources, one with cases and deaths aggregated at the district level from 1997 to 2011, and the other enhanced with results of cerebrospinal fluid examination and laboratory testing from 2007 to 2011. We compared mortality rates and incidence of suspected meningitis, probable meningococcal meningitis by age, and serogroup-specific meningococcal disease before and during the first year after PsA–TT implementation. We assessed the risk of meningitis disease and death between years.
Findings
During the 14 year period before PsA–TT introduction, Burkina Faso had 148 603 cases of suspected meningitis with 17 965 deaths, and 174 district-level epidemics. After vaccine introduction, there was a 71% decline in risk of meningitis (hazard ratio 0.29, 95% CI 0.28–0.30, p<0.0001) and a 64% decline in risk of fatal meningitis (0.36, 0.33–0.40, p<0.0001). We identified a statistically significant decline in risk of probable meningococcal meningitis across the age group targeted for vaccination (62%, cumulative incidence ratio [CIR] 0.38, 95% CI 0.31–0.45, p<0.0001), and among children aged less than 1 year (54%, 0.46, 0.24–0.86, p=0.02) and people aged 30 years and older (55%, 0.45, 0.22–0.91, p=0.003) who were ineligible for vaccination. No cases of serogroup A meningococcal meningitis occurred among vaccinated individuals, and epidemics were eliminated. The incidence of laboratory-confirmed serogroup A N meningitidis dropped significantly to 0.01 per 100 000 individuals per year, representing a 99.8% reduction in the risk of meningococcal A meningitis (CIR 0.002, 95% CI 0.0004–0.02, p<0.0001).
Interpretation
Early evidence suggests the conjugate vaccine has substantially reduced the rate of meningitis in people in the target age group, and in the general population because of high coverage and herd immunity. These data suggest that fully implementing the PsA–TT vaccine could end epidemic meningitis of serogroup A in sub-Saharan Africa.
Funding
None.
Introduction
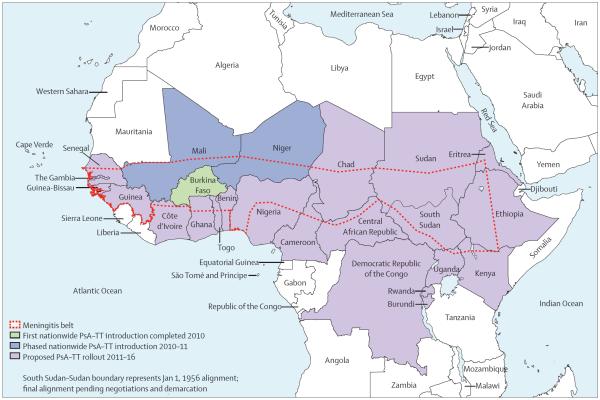
For at least 100 years, the meningitis belt of sub-Saharan Africa—stretching from Senegal to Ethiopia (figure 1) and home to 430 million people—has had high endemic rates of meningitis, annual seasonal outbreaks, and explosive epidemics occurring every 5–12 years.1,2 About 90% of cases during epidemics are attributable to Neisseria meningitidis serogroup A.3 Burkina Faso, a landlocked west African country with a population of roughly 16 million, is one of the few countries entirely located within the meningitis belt and has hyperendemic rates of meningitis.3,4 Annually, the government of Burkina Faso spends about 2% of its health budget on responding to epidemic meningitis.5 During the 2007 epidemic, households with an affected family member incurred an average cost equivalent to a third of their household income.6
Figure 1.
The meningitis belt of Africa and meningococcal serogroup A conjugate vaccine (PsA–TT) rollout plan 2010–16
In late 2009, a novel meningococcal serogroup A polysaccharide–tetanus toxoid conjugate vaccine (PsA–TT, MenAfriVac) was licensed and subsequently pre qualified by WHO—a requirement for purchase by UN agencies—based on results of clinical trials assessing safety and immunogenicity, but without efficacy trials.7–9 After pilot implementation in the health district of Kaya in September, 2010, PsA–TT was introduced in the remaining 62 health districts through a national mass vaccination campaign in Burkina Faso. More than 11 million people were vaccinated in about 10 days in December, 2010, resulting in 11 466 950 vaccinees in the target population of people aged 1–29 years.10 The vaccine and the aggressive strategy of rolling national vaccination campaigns in up to 26 at-risk countries within or bordering the meningitis belt (figure 1) over the next 5 years form an example of a new approach to control epidemic-prone, orphan diseases.11 Successful demonstration of the early effect of vaccination will validate this strategy and inform implementation plans for subsequent country campaigns. Toward this goal, we analysed national surveillance data around PsA–TT introduction and report the early effect of the vaccine on meningitis incidence and epidemics in Burkina Faso.
Methods
Data collection
In Burkina Faso, two complementary systems of population-based meningitis surveillance exist. Surveillance for reportable diseases is done by the Télégramme Lettre Official Hebdomadaire (TLOH), to which district-level aggregate reports of clinically defined meningitis cases and meningitis-related deaths are transmitted weekly. Functional since 1997, this system contains no identifying information or laboratory data, and only scarce demographic information. A second system—enhanced surveillance for Maladies Potentiel Épidémie (MPE)—was implemented in 2003, in response to the first large meningitis outbreak caused by serogroup W135.12,13 This system collects enhanced case-level demographic information and results of cerebrospinal fluid examination and laboratory testing for a proportion of TLOH cases, using integrated disease surveillance and response instruments. The standard operating procedures for MPE surveillance have been modified over time, but were consistent between 2007 and 2010. In 2009, substantial efforts were made to improve specimen collection and transport to a national reference laboratory, pathogen confirmation, links between laboratory and demographic information, and case-based data management and quality (eg, completeness and timeliness)—these efforts to strengthen MPE surveillance were concentrated in ten districts. After the 2010 meningitis season and before the national PsA–TT vaccination campaign, revised case-based MPE surveillance standard operating procedures were implemented nationwide.
Cases were classified according to WHO case definitions.14 Suspected cases of meningitis are defined as sudden onset of fever with a stiffneck or, in infants, a bulging fontanelle. Probable bacterial meningitis is a suspected case with turbid cerebrospinal fluid. A probable case of meningococcal meningitis is a suspected case with either a petechial or purpuric rash, Gram-negative diplococci on cerebrospinal fluid Gram stain, or in the setting of a continuing meningococcal meningitis epidemic. A confirmed case of meningitis is a suspected or probable case with Neisseria meningitidis, Haemophilus influenzae type b, or Streptococcus pneumoniae antigen detected in cerebrospinal fluid or isolated in culture from blood or cerebrospinal fluid. Beginning in 2010, real-time PCR capacity was implemented at the national reference laboratory level, and detection of N meningitidis, H influenzae type b, or S pneumoniae genetic material by PCR was deemed confirmatory.15,16 Serogroup determination was made either by antigen detection or PCR, with PCR deemed definitive.
This assessment was deemed to be a public health programme evaluation and was therefore exempted from ethical review by the US Centers for Disease Control and Prevention and Burkina Faso Ministry of Health.
Statistical analyses
To assess the effect of PsA–TT on epidemic meningitis, we compared national and district level incidence of meningitis, overall meningitis mortality rate, and occurrence of epidemics before and during the first year after PsA–TT implementation. Suspected cases and deaths reported through TLOH from 1997 to 2010 comprised the before PsA–TT period, and 2011 the after PsA–TT period. The analysis of these TLOH data was restricted to epidemiological weeks 1–24 (meningitis season) to represent the period of highest predictive value for serogoup A meningococcal infection in the absence of causal information in the dataset. Annual and weekly cumulative incidence rates were calculated with national and district population estimates from the Institut National de la Statistique et de la Démographie (INSD) census.17 To account for redefinition of districts done during the assessment period, some health districts were combined, resulting in a total of 55 districts. District-level epidemics were defined by an annual incidence rate exceeding 100 per 100 000 population.18–20 We defined an epidemic year as a year in which the national incidence rate exceeded 100 per 100 000 population; other years were defined as endemic years. We used a piecewise exponential model for grouped survival data to assess for significant differences in the risk of disease and death during the meningitis season between years. Hazard ratios (HR) were calculated comparing all years combined, epidemic years only, endemic years only, or each individual year compared with 2011.
To assess the age-specific and pathogen-specific effect of vaccination, we analysed MPE data for each year from 2007 to 2010 compared with 2011, excluding cases among known non-residents. PsA–TT was implemented in one district during epidemiological week 38, thus the analysis of MPE data was restricted to weeks 1–37. We calculated the population-weighted cumulative incidence of probable meningococcal meningitis by age group, using the age distribution from the 2006 INSD census applied to each year's estimated total population. These yearly age-specific incidence rates were compared by a log-Poisson regression model. We compared age-specific cumulative incidence rate ratios for each year compared with 2011. We used log-binomial regression to assess for differences in the proportion of bacterial meningitis cases attributed to N meningitidis serogroup A between years. To account for possible bias resulting from changes identified in S pneumoniae incidence over time, analyses were repeated excluding confirmed S pneumoniae cases from the yearly proportion denominators. We compared pathogen-specific cumulative incidence of confirmed meningitis disease by year using the same log-Poisson regression method described above, assuming that the distribution of laboratory results for meningitis cases with specimens collected but not sent for confirmatory tests was the same as the distribution for cases with laboratory test results available. We used SAS version 9.2 for analyses.
Role of the funding source
We had no external funding sources—the investigators were responsible for study design, data collection, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
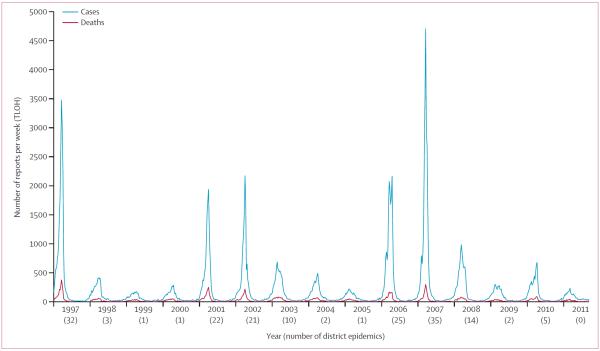
From Jan 1, 1997, to Dec 31, 2010, 148 603 cases of suspected meningitis were reported through TLOH in Burkina Faso, with 17 965 deaths, corresponding to an annual median of 7757 cases (IQR 5082–13 886) and incidence of 61·0 per 100 000 population (IQR 37·8–117·3, figure 2). Overall, 92% (136 831 of 148 603) of cases occurred during the meningitis season (weeks 1–24). During this 14 year period, 174 district-level epidemics occurred; at least one district had an epidemic during each year (range 1–35). 5 epidemic years—1997, 2001, 2002, 2006, and 2007—accounted for 78% (135 of 174) of district-level epidemics, and 54 of 55 districts had at least one epidemic year during those 5 years. The median epidemic-year incidence was 131·5 per 100 000 individuals (IQR 117·3–179·1), and a median of 25 district epidemics (22–32) were recorded during these 5 years. By contrast, in the 9 endemic years the median incidence was 43·5 per 100 000 individuals (IQR 31·6–52·6), and a median of two districts had epidemics (IQR one to five).
Figure 2.
Suspected cases of meningitis and deaths reported by the Télégramme Lettre Official Hebdomadaire surveillance system, by week, and number of health districts experiencing epidemics by year in Burkina Faso, 1997–2011
In 2011, after the national PsA–TT campaign, 3875 suspected meningitis cases and 588 deaths were reported—the corresponding cumulative incidence of 24·1 per 100 000 individuals represented a decline of 60% from the median incidence 1997–2010. 71% (2748 of 3875) of cases occurred during the meningitis season, and no districts had epidemics in 2011—this finding was a break from the periodicity recorded in the previous 14 years. Comparing 2011 district-level meningitis season incidence to each of the 14 before PsA–TT years, incidence decreased in all health districts by a median of 79% (IQR 71–86% decrease).
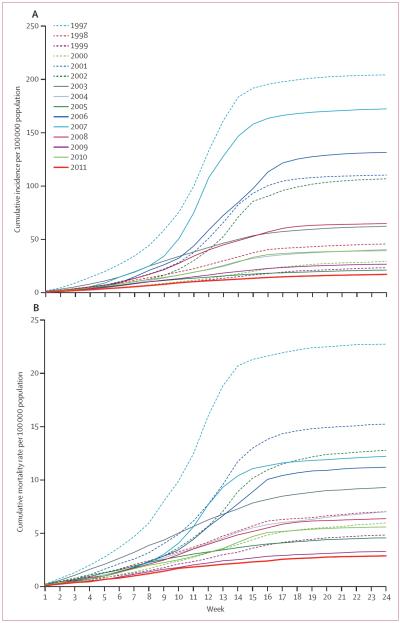
With all 14 before PsA–TT years as a baseline (figure 3), risk of meningitis decreased by 71% (HR 0·29, 95% CI 0·28–0·30, p<0·0001) and risk of death decreased by 64% (0·36, 0·33–0·40, p<0·0001) after PsA–TT implementation. Significant declines in risk (p<0·0001 for all comparisons except p=0·04 for deaths in 2009) were identified when the analysis was repeated comparing 2011 to grouped epidemic years (88% disease, 80% deaths), grouped endemic years (54% disease, 51% deaths), and each year individually for both meningitis disease (range 21% [2005] to 92% [1997]) and deaths (range 13% [2009] to 87% [1997]).
Figure 3.
Cumulative curves of rates per 100 000 population of suspect meningitis cases (A) and deaths (B) reported through Télégramme Lettre Official Hebdomadaire by week during the meningitis season in Burkina Faso, 1997–2011
From 2007 to 2011, 25 220 cases of suspected meningitis were reported through the MPE enhanced meningitis surveillance, compared with 51 700 meningitis cases through TLOH. During this time, the sensitivity of MPE surveillance to detect suspected meningitis cases—with TLOH for comparison—improved from 41% (10 614 of 25 695) to 88% (3412 of 3875), the proportion of MPE-reported suspect cases with a cerebrospinal fluid specimen that was transported to a reference laboratory for confirmatory testing increased from 29% (2898 of 9824) to 99% (3399 of 3412), and the proportion of case-patient specimens with a bacterial meningitis pathogen identified increased from 7% (685 of 9824) to 35% (1157 of 3318).
When comparing 2011 to the lowest incidence year before PsA–TT (either 2009 or 2010), a 62% decline in risk of probable meningococcal meningitis was identified across the age group targeted for vaccination (cumulative incidence ratio [CIR] 0·38, 95% CI 0·31–0·45, p<0·0001; table). A statistically significant decline (p<0·0001) was also recorded for the comparison of 2011 to each age group individually, with the largest decline among people aged 5–14 years. Among age groups not eligible for vaccine, a 55% decline in risk of bacterial meningitis was indentified among people aged 30 years and older (p=0·003, table), and a 54% decline in risk among children aged younger than 1 year (p=0·02, table).
Table.
Cumulative incidence per 100 000 population of probable meningococcal meningitis by age category reported through Maladies Potentiel Épidémie surveillance during weeks 1–37 in Burkina Faso, 2007–11
| Cumulative incidence (n) |
Baselineyear* | 2011 compared to baselineyear |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 2007 | 2008 | 2009 | 2010 | 2011 | CIR (95%CI) | p value | Relative risk reduction† | ||
| Age (years) | |||||||||
| <1 | 26.9 (167) | 14.4 (92) | 6.3 (42) | 4.4 (30) | 2.0 (14) | 2010 | 0.46 (0.24–0.86) | 0.02 | 54% |
| 1–4 | 30.6 (652) | 14.2 (312) | 3.6 (83) | 6.0 (140) | 1.5 (37) | 2009 | 0.43 (0.29–0.63) | <0.0001 | 57% |
| 5–4 | 32.6 (1339) | 15.3 (649) | 5.5 (243) | 10.9 (492) | 2.0 (92) | 2009 | 0.36 (0.29–0.46) | <0.0001 | 64% |
| 15–29 | 11.6 (417) | 5.3 (195) | 1.9 (72) | 1.8 (71) | 0.7 (28) | 2010 | 0.39 (0.25–0.6) | <0.0001 | 61% |
| ≥30 | 4.8 (185) | 3.3 (132) | 0.7 (30) | 0.6 (24) | 0.3 (11) | 2010 | 0.45 (0.22–0.91) | 0.003 | 55% |
| Total (1–29) | 24.4 (2408) | 11.4 (1156) | 3.8 (398) | 6.5 (703) | 1.4 (157) | 2009 | 0.38 (0.31–0.45) | <0.0001 | 62% |
| Total (<1, ≥30) | 7.8 (352) | 4.8 (224) | 1.5 (72) | 1.1 (54) | 0.5 (25) | 2010 | 0.45 (0.28–0.73) | 0.001 | 55% |
CIR=cumulative incidence ratio.
The year with the lowest incidence before implementation was used as the baseline year for comparison to 2011.
Relative risk reduction indicates a decrease in risk of meningitis in the aff ected population relative to the baseline after vaccine introduction calculated as (1–CIR)×100.
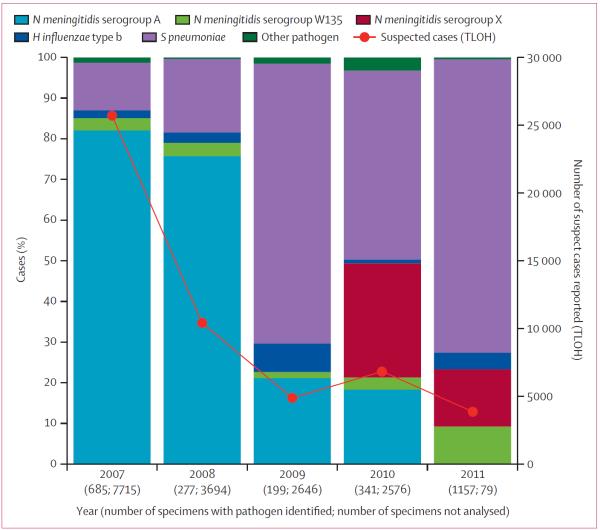
The overall proportion of confirmed cases of meningococcal meningitis during the before PsA–TT period was 68% (1020 of 1505), of which 86% (875 of 1020) were caused by serogroup A N meningitidis (figure 4). Among all identified pathogens, the proportion of serogroup A was higher during 2007–08 (80%, 771 of 965) than the subsequent endemic years (19%, 104 of 540, p<0·0001). In 2011, with cerebrospinal fluid obtained for laboratory confirmation from 99% of all patients with suspected meningitis, only one case of serogroup A meningococcal meningitis was confirmed among resi dents of Burkina Faso—who had not received PsA–TT—representing a decrease in serogroup A to less than 0·1% (one of 1157) of confirmed meningitis cases. This proportion was significantly (p<0·0001) lower than for each individual year from 2007 to 2010, whether S pneumoniae cases were included (relative risk range 0·005, 95% CI 0·0007–0·03 [2010] to 0·002, 0·0003–0·01 [2007]) or omitted (data not shown) from the proportion denominators.
Figure 4.
Proportions of confirmed meningitis cases by year attributable to Neisseria meningitidis, Haemophilus influenzae type b, and Streptococcus pneumoniae from Maladies Potentiel Épidémie surveillance compared with suspected cases reported by TLOH in Burkina Faso, 2007–11 TLOH=Télégramme Lettre Official Hebdomadaire.
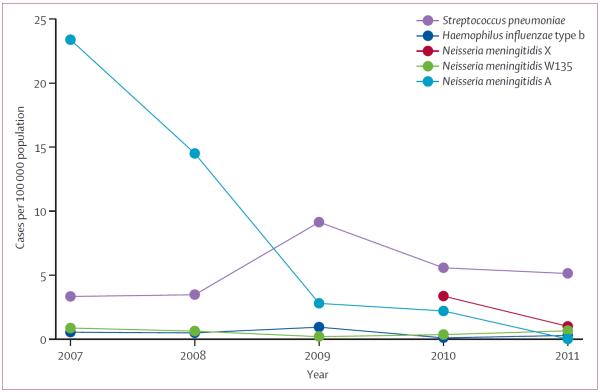
In 2011, one confirmed case of serogroup A meningococcal disease was identified in Burkina Faso, resulting in an incidence of 0·01 cases per 100 000 individuals (figure 5). This finding represents a 99·8% decline in risk compared with 2010 (CIR 0·002, 95% CI 0·0004–0·02, p<0·0001). Among other meningococcal serogroups, the incidence of serogroup W135 was fairly constant over time from 2007. Although serogroup X incidence declined in 2011, the number of districts with confirmed serogroup X cases increased from 6% (four of 63 districts, two epidemics) in 2010, to 67% (42 of 63 districts, no epidemics). By contrast, the mean incidence of pneumococcal meningitis increased from 3·4 (2007–08) to 6·6 per 100 000 (2009–11), and a slight seasonality was identified with a peak in February and March (data not shown).
Figure 5.
Estimated annual incidence of confirmed meningitis by pathogen and year from Maladies Potentiel Épidémie surveillance in Burkina Faso, 2007–11
Discussion
Observational data after the first national meningococcal A conjugate vaccination campaign provide evidence that serogroup A meningococcal conjugate vaccine has substantially reduced the burden of meningitis in Burkina Faso. Significant reductions were achieved both nationally and at the district level in the occurrence of meningitis epidemics and cases of suspected and probable meningococcal meningitis. The unprecedented low incidence of serogroup A disease in view of exceptional laboratory confirmation provides strong evidence for a great short-term vaccine effect.
Trials done before licensure showed that PsA–TT vaccination results in high titres of antibodies in both adults and children, which would be expected to lead to a high degree of direct individual protection.8,9,21 Results from a coverage survey completed in December, 2011, showed national coverage of 95% among the target population of the 2010 PsA–TT campaign. Therefore, the 5 million individuals either too young or too old to be vaccinated were likely to indirectly benefit from high population immunity. The complete absence of confirmed serogroup A meningococcal disease among vaccine recipients is attributable in large measure to the immunogenicity of the vaccine. However, one serogroup A case was confirmed in an unvaccinated resident of Burkina Faso and three additional cases were confirmed in Burkina Faso among unvaccinated non-residents, showing that serogroup A has not completely been eradicated.
Epidemic waves are postulated to occur when epidemic cofactors increase and novel strains are introduced into an immunologically naive population.22,23 The experience with H influenzae type b and meningococcal serogroup C clearly shows the substantial effect conjugate vaccines can have on nasopharyngeal carriage of bacteria, a necessary precursor to invasive disease.24–28 Our finding of a significant reduction in risk of probable meningococcal meningitis not only in the vaccinated age group, but also in the population broadly suggests a herd effect resulting from reduction in carriage and interruption of transmission. Studies are underway in Burkina Faso to assess herd immunity, but this broad risk reduction is consistent with evidence of a substantial reduction in serogroup A carriage prevalence after the vaccination campaign (DA Caugant, Norwegian Institute of Public Health, a WHO Collaborating Centre for Reference and Research on Meningococci, personal communication).29
The continued near elimination of the hyperendemic ST-11 serogroup C clone in the UK after its serogroup C vaccination programme might be largely attributable to a sustained herd effect despite waning titres of protective antibody among individual vaccinees.30,31 Molecular subtyping of carriage and invasive isolates from Burkina Faso identified a high degree of homogeneity among serogroup A isolates in the past 5 years.10 The potential for sustained reductions in carriage (or even elimination) of hyperendemic serogroup A meningococci is unclear.
2011 showed a break from the cyclic meningitis epidemic patterns recorded between 1997 and 2010. Improved surveillance nationally after the 2007 serogroup A epidemic has led to recognition that S pneumoniae was the most common cause of bacterial meningitis in recent endemic years—the proportion of S pneumoniae increased from less than 20% in 2007–08 to greater than 50% during 2009–11. This finding might be an actual increase in disease burden, potentially from the introduction of a more virulent serotype.32,33 We compared these data to regional data from the endemic period before 2007, and identified similarities in both the relative rates of pneumococcal to meningococcal meningitis and the slight seasonality of pneumococcal meningitis.34,35 Thus, the difference in incidence could equally be confounded by improved surveillance, with the most recent data showing the true endemic rate. Although we did not report pneumococcal serotype, data from one Burkina Faso region suggested that the seven-valent pneumococcal conjugate vaccine would only cover less than a third of serotypes identified in children aged younger than 5 years, but ten-valent and 13-valent vaccines that are now becoming available could improve serotype coverage to 67%.35 In-country capacity for molecular serotype determination is being established in Burkina Faso to assist the country in planning and assessment of the pneumococcal vaccine programme.
These data provide compelling first evidence of a substantial effect of serogroup A meningococcal conjugate vaccine when applied in a public health programme (panel). Although this is an observational study assessing only the first year of follow-up after implementation, a decrease in suspect cases and probable meningococcal meningitis in 2011 was identified even when compared with the lowest endemic years, suggesting an effect of vaccination as opposed to only secular variation in incidence. Strengthened surveillance provided early evidence of effective prevention of serogroup A disease and elimination of epidemics in the short term—continued investment to sustain high-quality surveillance in Burkina Faso is necessary to monitor vaccine effect and modification of secular epidemic trends over time. Although serogroup X has not been documented to cause epidemics similar in scale to serogroups A or W135, improved surveillance has detected the emergence and geographic expansion of serogroup X from four districts in 2010, to 42 in 2011.36,37 Serogroup replacement has not been identified in countries that have used monovalent serogroup C conjugate vaccine. However, the recent localised epidemics of serogroups X and W135, and historically C are a cautionary reminder that maintenance of high-quality laboratory confirmation is crucial beyond the assessment of the immediate effect of the PsA–TT mass vaccination programme.27 Other important assessments that should come from the first national implementation of PsA–TT include measures of vaccine effectiveness and causes of vaccine failures.38,39
Burkina Faso achieved high vaccine coverage in a period of only 10 days in December, 2010, as a result of experience in doing mass vaccination campaigns for meningitis epidemic response and other vaccine preventable diseases, and years of concerted effort to advocate with policy makers, sensitise the population, and organise the logistics necessary to fully implement the first national PsA–TT campaign. Surveillance systems in Burkina Faso can be viewed as an example of the high quality that can be achieved in developing countries through concerted collaboration. Although this effort is not warranted in all countries that implement the vaccine, other at-risk countries of the meningitis belt present logistical challenges to achieving full implementation of vaccination campaigns, and some degree of infrastructure strengthening of surveillance health systems is essential to show the effectiveness of the vaccine and effect of vaccination under variable conditions. Longitudinal surveillance should be sustained in some countries to measure long-term effect and assess the need for booster vaccination or other maintenance strategies. Without adequate surveillance infrastructure, system weaknesses could be interpreted as vaccine failure and threaten the implementation strategy.
Epidemic meningitis has been a devastating problem in Africa for the past century. The true goal must be to sustain its elimination. Future challenges such as accumulation of susceptible cohorts due to waning immunity, introduction across porous national borders of novel serogroup A strains, and the potential for other serogroups to emerge all argue for sustained investment in surveillance and response capacity. PsA–TT is not licensed for use in infants, and appropriate strategies for maintenance of epidemic elimination should be planned.11,40 Affordable multivalent conjugate vaccines are needed, because other serogroups are proven causes of meningitis outbreaks. Despite these future challenges, this early success in Burkina Faso should strengthen momentum toward achieving the goal of ending epidemic meningitis as a public health concern in sub-Saharan Africa.
Panel: Research in context.
Systematic review
We searched PubMed with the terms “MenAfriVac” OR “PsA–TT” OR “meningococcal A conjugate vaccine” OR “group A meningococcal conjugate vaccine” AND “epidemic meningitis” OR “epidemic meningococcal meningitis” AND “Africa” for reports from December, 2009, onward, since the first monovalent conjugate meningococcal serogroup A meningitis vaccine was licensed in 2009. The date of the search was May 15, 2012. We identified 32 articles but no population-based reports of the effect of vaccination on meningitis epidemics.
Interpretation
Our study is the first to report the effects of a national meningococcal serogroup A conjugate vaccination programme on epidemic meningitis at a population level. We have shown a substantial decrease in all-cause meningitis, meningitis epidemics, and serogroup A meningococcal disease in Burkina Faso after the implementation of the mass vaccination programme. Continued progress toward elimination of serogroup A meningococcal meningitis epidemics in sub-Saharan Africa will require high vaccination coverage in at-risk countries, adequate surveillance to monitor vaccine effect and the potential re-emergence of disease, and implementation of a vaccination programme to maintain epidemic elimination.
Acknowledgments
We thank the residents in all the districts of Burkina Faso for their participation in the surveillance of meningitis, and the health-care workers from districts and regions involved. We thank the public health workers at the Direction de la Prevention par la Vaccination, the Direction de la Lutte contre la Maladie, WHO country office in Ouagadougou, WHO Intercountry Support Team for West Africa in Ouagadougou, WHO Regional Office for Africa in Brazzaville, and WHO Headquarter in Geneva for their contribution. We especially thank Dr Bokar Touré and Dr Djamila Cabral for their support to meningitis surveillance. The views expressed in this Article are those of the authors and do not necessarily represent the decisions, policies, or views of the Burkina Faso Ministry of Health, CDC, PATH, or WHO.
Footnotes
Contributors RTN developed methods, led the analysis and interpretation of data, and drafted the paper. SWM contributed to the statistical analysis, and assisted with the review of available studies. JLK, FVKD, TFT, and SRT contributed to developing methods, study implementation, data collection, data interpretation, and critical revision of the paper for important intellectual content. RO-T, LS, CL, CH, LWM, FA, and MHD were involved in study implementation and data collection, primary data collection, and technical support. FML contributed to data interpretation and report revisions. NEM and TAC provided conceptual and technical guidance and contributed to critical revision of the paper for important intellectual content.
Conflicts of interest We declare that we have no conflicts of interest.
References
- 1.Lapeyssonnie L. La méningite cérébrospinale en Afrique. Bull World Health Organ. 1963;28(suppl):1–114. [PMC free article] [PubMed] [Google Scholar]
- 2.Greenwood B. Manson lecture. Meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg. 1999;93:341–53. doi: 10.1016/s0035-9203(99)90106-2. [DOI] [PubMed] [Google Scholar]
- 3.Djingarey M, Noazin S, Préziosi MP. A 20-year retrospective analysis of epidemic meningitis surveillance data in Burkina Faso, Mali, and Niger. 16th International Pathogenic Neisseria Conference; Rotterdam, Netherlands. Sept 7–12, 2008.p. P166. [Google Scholar]
- 4.Molesworth AM, Cuevas LE, Connor SJ, Morse AP, Thomson MC. Environmental risk and meningitis epidemics in Africa. Emerg Infect Dis. 2003;9:1287–93. doi: 10.3201/eid0910.030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombini A, Badolo O, Gessner BD, Jaillard P, Seini E, Da Silva A. Costs and impact of meningitis epidemics for the public health system in Burkina Faso. Vaccine. 2011;29:5474–80. doi: 10.1016/j.vaccine.2011.05.058. [DOI] [PubMed] [Google Scholar]
- 6.Colombini A, Bationo F, Zongo S, et al. Costs for households and community perception of meningitis epidemics in Burkina Faso. Clin Infect Dis. 2009;49:1520–25. doi: 10.1086/644623. [DOI] [PubMed] [Google Scholar]
- 7.WHO . Procedure for assessing the acceptability, in principle, of vaccines for purchase by United Nations agencies. WHO Press; Geneva: 2010. [Google Scholar]
- 8.Kshirsagar N, Mur N, Thatte U, et al. Safety, immunogenicity, and antibody persistence of a new meningococcal group A conjugate vaccine in healthy Indian adults. Vaccine. 2007;25(suppl 1):A101–07. doi: 10.1016/j.vaccine.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 9.Sow SO, Okoko BJ, Diallo A, et al. Immunogenicity and safety of a meningococcal A conjugate vaccine in Africans. N Engl J Med. 2011;364:2293–304. doi: 10.1056/NEJMoa1003812. [DOI] [PubMed] [Google Scholar]
- 10.Djingarey MH, Barry R, Bonkoungou M, et al. Effectively introducing a new meningococcal A conjugate vaccine in Africa: the Burkina Faso experience. Vaccine. 2012;30:B40–45. doi: 10.1016/j.vaccine.2011.12.073. [DOI] [PubMed] [Google Scholar]
- 11.Laforce FM, Okwo-Bele JM. Eliminating epidemic group A meningococcal meningitis in Africa through a new vaccine. Health Aff (Millwood) 2011;30:1049–57. doi: 10.1377/hlthaff.2011.0328. [DOI] [PubMed] [Google Scholar]
- 12.WHO Enhanced surveillance of epidemic meningococcal meningitis in Africa: a three-year experience. Wkly Epidemiol Rec. 2005;80:313–20. [PubMed] [Google Scholar]
- 13.Koumare B, Ouedraogo-Traore R, Sanou I, et al. The first large epidemic of meningococcal disease caused by serogroup W135, Burkina Faso, 2002. Vaccine. 2007;25(suppl 1):A37–41. doi: 10.1016/j.vaccine.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 14.WHO . Control of epidemic meningococcal disease. WHO practical guidelines. 2nd edn World Health Organization; Geneva: 1998. [Google Scholar]
- 15.Mothershed EA, Sacchi CT, Whitney AM, et al. Use of real-time PCR to resolve slide agglutination discrepancies in serogroup identification of Neisseria meningitidis. J Clin Microbiol. 2004;42:320–28. doi: 10.1128/JCM.42.1.320-328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO . Laboratory methods for the diagnosis of meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. 2nd edn World Health Organization; Geneva: 2011. [Google Scholar]
- 17. [accessed June 1, 2012];Burkina Faso Institut National de la Statistique et de la Démographie. 2012 http://www.insd.bf.
- 18.Moore PS, Plikaytis BD, Bolan GA, et al. Detection of meningitis epidemics in Africa: a population-based analysis. Int J Epidemiol. 1992;21:155–62. doi: 10.1093/ije/21.1.155. [DOI] [PubMed] [Google Scholar]
- 19.Lewis R, Nathan N, Diarra L, Belanger F, Paquet C. Timely detection of meningococcal meningitis epidemics in Africa. Lancet. 2001;358:287–93. doi: 10.1016/S0140-6736(01)05484-8. [DOI] [PubMed] [Google Scholar]
- 20.Leake JA, Kone ML, Yada AA, et al. Early detection and response to meningococcal disease epidemics in sub-Saharan Africa: appraisal of the WHO strategy. Bull World Health Organ. 2002;80:342–49. [PMC free article] [PubMed] [Google Scholar]
- 21.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–26. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leimkugel J, Hodgson A, Forgor AA, et al. Clonal waves of Neisseria colonisation and disease in the African meningitis belt: eight-year longitudinal study in northern Ghana. PLoS Med. 2007;4:e101. doi: 10.1371/journal.pmed.0040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenwood B. Editorial: 100 years of epidemic meningitis in West Africa—has anything changed? Trop Med Int Health. 2006;11:773–80. doi: 10.1111/j.1365-3156.2006.01639.x. [DOI] [PubMed] [Google Scholar]
- 24.Singleton R, Bulkow LR, Levine OS, Butler JC, Hennessy TW, Parkinson A. Experience with the prevention of invasive Haemophilus influenzae type b disease by vaccination in Alaska: the impact of persistent oropharyngeal carriage. J Pediatr. 2000;137:313–20. doi: 10.1067/mpd.2000.107843. [DOI] [PubMed] [Google Scholar]
- 25.Wenger JD. Epidemiology of Haemophilus influenzae type b disease and impact of Haemophilus influenzae type b conjugate vaccines in the United States and Canada. Pediatr Infect Dis J. 1998;17:S132–36. doi: 10.1097/00006454-199809001-00008. [DOI] [PubMed] [Google Scholar]
- 26.Ramsay ME, Andrews NJ, Trotter CL, Kaczmarski EB, Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ. 2003;326:365–66. doi: 10.1136/bmj.326.7385.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maiden MCJ, Ibarz-Pavón AB, Urwin R, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197:737–43. doi: 10.1086/527401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trotter CL, Maiden MC. Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert Rev Vaccines. 2009;8:851–61. doi: 10.1586/erv.09.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kristiansen PA, Diomande F, Wei SC, et al. Baseline meningococcal carriage in Burkina Faso before the introduction of a meningococcal serogroup A conjugate vaccine. Clin Vaccine Immunol. 2011;18:435–43. doi: 10.1128/CVI.00479-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell H, Andrews N, Borrow R, Trotter C, Miller E. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol. 2010;17:840–47. doi: 10.1128/CVI.00529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibarz-Pavón AB, MacLennan J, Andrews NJ, et al. Changes in serogroup and genotype prevalence among carried meningococci in the United Kingdom during vaccine implementation. J Infect Dis. 2011;204:1046–53. doi: 10.1093/infdis/jir466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leimkugel J, Adams Forgor A, Gagneux S, et al. An outbreak of serotype 1 Streptococcus pneumoniae meningitis in northern Ghana with features that are characteristic of Neisseria meningitidis meningitis epidemics. J Infect Dis. 2005;192:192–99. doi: 10.1086/431151. [DOI] [PubMed] [Google Scholar]
- 33.Yaro S, Lourd M, Traoré Y, et al. Epidemiological and molecular characteristics of a highly lethal pneumococcal meningitis epidemic in Burkina Faso. Clin Infect Dis. 2006;43:693–700. doi: 10.1086/506940. [DOI] [PubMed] [Google Scholar]
- 34.Campagne G, Schuchat A, Djibo S, Ousseini A, Cisse L, Chippaux JP. Epidemiology of bacterial meningitis in Niamey, Niger, 1981–96. Bull World Health Organ. 1999;77:499–508. [PMC free article] [PubMed] [Google Scholar]
- 35.Traore Y, Tameklo TA, Njanpop-Lafourcade B-M, et al. Incidence, seasonality, age distribution, and mortality of pneumococcal meningitis in Burkina Faso and Togo. Clin Infect Dis. 2009;48:S181–89. doi: 10.1086/596498. [DOI] [PubMed] [Google Scholar]
- 36.Delrieu I, Yaro S, Tamekloe TA, et al. Emergence of epidemic Neisseria meningitidis serogroup X meningitis in Togo and Burkina Faso. PLoS One. 2011;6:e19513. doi: 10.1371/journal.pone.0019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Traore Y, Njanpop-Lafourcade BM, Adjogble KL, et al. The rise and fall of epidemic Neisseria meningitidis serogroup W135 meningitis in Burkina Faso, 2002–2005. Clin Infect Dis. 2006;43:817–22. doi: 10.1086/507339. [DOI] [PubMed] [Google Scholar]
- 38.Soriano-Gabarro M, Rosenstein N, LaForce FM. Evaluation of serogroup A meningococcal vaccines in Africa: a demonstration project. J Health Popul Nutr. 2004;22:275–85. [PubMed] [Google Scholar]
- 39.Terranella A, Cohn A, Clark T. Meningococcal conjugate vaccines: optimizing global impact. Infect Drug Resist. 2011;4:161–69. doi: 10.2147/IDR.S21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levine OS, Bloom DE, Cherian T, et al. The future of immunisation policy, implementation, and financing. Lancet. 2011;378:439–48. doi: 10.1016/S0140-6736(11)60406-6. [DOI] [PubMed] [Google Scholar]