Abstract
OBJECTIVE
To assess the efficacy and safety of dapagliflozin, a selective sodium-glucose cotransporter 2 inhibitor, compared with placebo in patients with type 2 diabetes (T2D), documented pre-existing cardiovascular disease (CVD), and a history of hypertension.
RESEARCH DESIGN AND METHODS
Patients (N = 922) were randomized to receive 10 mg dapagliflozin or placebo in a double-blind trial for 24 weeks, followed by a 28-week extension period. In patients receiving insulin, the insulin dose was reduced by 25% at randomization. Patients were stratified by age, insulin use, and time from the most recent qualifying cardiovascular (CV) event. Co-primary end points were a change from baseline in hemoglobin A1c (HbA1c) and the proportion of patients achieving a combined reduction in HbA1c of ≥0.5% (5.5 mmol/mol), body weight (BW) of ≥3%, and systolic blood pressure (SBP) of ≥3 mmHg.
RESULTS
At 24 weeks, dapagliflozin significantly reduced HbA1c (−0.38% [−4.2 mmol/mol]) from baseline (8.18%) compared with a slight increase with placebo from baseline (8.08%) (0.08% [0.9 mmol/mol]). Significantly more patients met the three-item end point with treatment with dapagliflozin than with placebo (11.7% vs. 0.9%, respectively). Changes were maintained over 52 weeks. Although ∼42% of patients were ≥65 years old, similar results were observed in both age-stratified groups. Serious adverse events, hypoglycemia, urinary tract infections, and cardiac disorders were similar between groups. Adverse events of hypotension, dehydration, hypovolemia, genital infection, and renal failure or impairment occurred more often with dapagliflozin treatment.
CONCLUSIONS
In this study that evaluated T2D patients who were at high risk for future CVD events, dapagliflozin administration had significantly greater effects in reducing HbA1c, BW, and SBP, without adversely impacting CV safety when compared with placebo treatment.
Introduction
With advancing age, patients with type 2 diabetes often advance to a regimen that consists of a combination of antidiabetic agents and other concomitant medications to manage comorbid conditions (e.g., cardiovascular disease [CVD], hypertension, and obesity [1–4]). Chronic hyperglycemia contributes to the development of macrovascular and microvascular complications, yet, in the U.S., only 52% of patients with type 2 diabetes reach the recommended goal for hemoglobin A1c (HbA1c) concentration of <7.0% (53 mmol/mol) (5,6). Optimal glycemic control may be hampered by side effects associated with specific antidiabetic agents, challenges associated with an aging population, and concerns related to polypharmacy. Given the clinical consequences and the increased health risks conferred by comorbid conditions, effective management of type 2 diabetes must achieve glycemic control and include management strategies that address CVD risk, hypertension, and obesity (1–4).
Patients with type 2 diabetes who are of advanced age and/or are at a higher risk of a future cardiovascular (CV) event have been understudied and under-represented in clinical trials evaluating antidiabetic agents. In addition, older patients tend to have more advanced disease and require different therapeutic approaches. Therefore, it is imperative that clinicians are provided with additional evidence on effective therapies to use when designing therapeutic regimens for high-risk patients. The already increased risk of CV events in patients with type 2 diabetes is a well-recognized problem that has contributed to the U.S. Food and Drug Administration requirement for new diabetes therapies to demonstrate CV safety. For both populations, safe and effective therapeutic strategies are required that provide hyperglycemic control and added clinical benefit.
Dapagliflozin is an approved, first-in-class selective sodium-glucose cotransporter 2 inhibitor (SGLT2i) that reduces hyperglycemia through the reduction of glucose reabsorption into the kidney and circulation and through the promotion of urinary glucose excretion (glucuresis). Observations to date (7–12) for dapagliflozin have demonstrated reductions in fasting plasma glucose (FPG) within 1 week, reductions in HbA1c in patients in all stages of type 2 diabetes, a moderate lowering of blood pressure, and favorable effects on body weight (BW). Thus, the SGLT2i class of drugs represents a new insulin-independent means to achieve glycemia. In clinical trials, dapagliflozin is well tolerated as monotherapy or as combination therapy with metformin, sulfonylureas, and insulin (7–12).
Despite the clinical data to date, there is a paucity of data on the efficacy of SGLT2i agents in subjects with type 2 diabetes who are at high risk of CVD. Thus, we sought to determine the effect of dapagliflozin on HbA1c lowering, BW reduction, and systolic blood pressure (SBP) reduction in patients with a high risk for future CVD events. We defined “high risk” as consisting of an older aged cohort with increased risk of CV complications due to pre-existing CVD and hypertension, and patients with a regimen of concomitant medications such as oral antidiabetic drugs (OADs), insulin, antihypertensive drugs, and diuretic agents, including loop diuretics. Although not designed as a CV safety study, we conducted a 24-week, double-blinded, randomized control trial in this high-risk cohort and additionally assessed the long-term safety and efficacy of dapagliflozin up to 52 weeks.
Research Design and Methods
Study Design
The study was a multicenter, randomized, double-blind, placebo-controlled, international, phase 3 study of 24 weeks duration with a 28-week extension period (clinical trial reg. no. NCT01031680, clinicaltrials.gov), which was conducted in Europe, Asia, the U.S., Canada, and Argentina. An additional 52-week long-term extension study is ongoing (104 weeks in total). The study was designed and monitored in accordance with the ethical principles of Good Clinical Practice, as defined by the International Conference on Harmonisation and the Declaration of Helsinki. The protocol was approved by an institutional review board, and all patients gave written, informed consent.
Treatments and Interventions
Patients (N = 922) were randomized 1:1 to receive once-daily dapagliflozin 10 mg or a matched placebo dose plus pre-existing stable background treatment, excluding rosiglitazone. Patients were stratified by age at enrollment (<65 or ≥65 years), insulin use at randomization (no or yes), and time from the most recent qualifying CV event (>1 or ≤ 1 year). In patients treated with insulin at randomization, the mean daily insulin dose used in the 2 weeks before randomization was reduced by 25% according to a prespecified algorithm; however, insulin up-titration was allowed per the rescue protocol. During the 28-week extension period, patients continued treatment as administered during the 24-week treatment period. Patients were permitted antidiabetic and/or antihypertensive rescue medication per protocol. Details regarding background treatment stabilization, key inclusion and exclusion criteria, randomization, rescue criteria, and treatment are provided in Supplementary Table 1.
Efficacy and Safety End Points
The primary end points evaluated in the overall population and in the predefined age strata included the mean change in HbA1c from baseline to week 24 and the proportion of responders achieving a three-item end point of combined clinical benefit at week 24. The three-item composite end point consisted of an absolute drop from baseline in HbA1c of ≥0.5% (5.5 mmol/mol), a relative drop of ≥3% for total BW, and an absolute drop of ≥3 mmHg from baseline in seated SBP. These end points were also evaluated in a post hoc subgroup analysis of insulin use.
Key secondary variables included the mean change in seated SBP from baseline (at weeks 8 and 24), the mean percent change in BW, and the proportion of patients with baseline BMI of ≥27 kg/m2 with a ≥5% reduction in BW. Other secondary end points included the mean change in seated diastolic blood pressure (DBP); the proportion of patients with seated SBP of <130 mmHg in the group of patients with a baseline seated SBP of ≥130 mmHg; the mean change in BW from baseline; the mean change in HbA1c in patients with a baseline HbA1c ≥8.0% (64 mmol/mol) and an HbA1c ≥9.0% (75 mmol/mol); the proportion of patients achieving an HbA1c <7.0% (53 mmol/mol); the mean change in FPG at weeks 1 and 24; the proportion of patients rescued for failing to maintain FPG/HbA1c below the prespecified rescue criteria at weeks 4, 8, 16, 24, and 52 (see Supplementary Data); the proportion of patients achieving a reduction in HbA1c of ≥0.5% (5.5 mmol/mol); the proportion of patients achieving a reduction in seated SBP from baseline of ≥3 or ≥5 mmHg; and the mean change in calculated average daily insulin dose in patients treated with insulin at baseline.
The safety analysis set included all patients who received one or more doses of randomized study medication and who provided safety records. The safety and tolerability of dapagliflozin versus placebo were assessed by the evaluation of adverse events (AEs), including CV events, laboratory values, electrocardiogram results, vital signs, hypoglycemic events, calculated creatinine clearance, estimated glomerular filtration rate (eGFR), and physical examination findings over 52 weeks (see Supplementary Data).
Statistical Analysis
The last observation carried forward approach was used for all variables at 24 weeks. The primary end points were tested by an ANCOVA model and the Cochran-Mantel-Haenszel method, respectively (13). The ANCOVA model examined the change from baseline in HbA1c with terms for treatment group, strata, and baseline HbA1c (covariate). The Cochran-Mantel-Haenszel test controlled for stratification in the study. Primary analyses of HbA1c and the three-item composite excluded data after patients received glycemic rescue treatment. The three-item composite end point also excluded data after patients received hypertensive rescue treatment. Results from the extension study were analyzed by longitudinal repeated-measures analysis. Primary end points were controlled for type I error by using a two-sided α = 0.025, a Bonferroni adjustment. If statistically significant for the overall study population, testing was performed within the age strata using a further Bonferroni adjustment with α = 0.0125. Key secondary end points were submitted to a hierarchical testing procedure for the overall study population and the two age strata in parallel streams, with the α level dependent upon whether statistical significance was reached for one or both of the primary end points. Statistical testing was not performed for exploratory (52-week) end points. Results for continuous data up to 52 weeks were analyzed by longitudinal, repeated-measures models. Planned sensitivity analyses were also conducted for efficacy end points that included data after rescue. Safety end points were evaluated for all patients. A full description of the statistical analysis used is included in the Supplementary Data.
Results
Efficacy
Demographic and Baseline Characteristics
Demographic and baseline characteristics were balanced between treatment groups (Table 1). In total, 807 of 922 patients completed 52 weeks of the study (Supplementary Fig. 1). The most common reasons for study discontinuation were meeting a study discontinuation criterion, withdrawal of consent, and AEs.
Table 1.
Demographic and baseline characteristics for the overall population (full analysis set)
| Placebo group (n = 459) | Dapagliflozin 10 mg group (n = 455) | |
|---|---|---|
| Age, mean (SD), years | 63.0 (7.7) | 62.8 (7.0) |
| Subjects <65 years of age, n (%) | 263 (57.3) | 263 (57.8) |
| Subjects ≥65 years of age, n (%) | 196 (42.7) | 192 (42.2) |
| Female sex, n (%) | 144 (31.4) | 146 (32.1) |
| Race, n (%) | ||
| White | 391 (85.2) | 376 (82.6) |
| Black/African American | 27 (5.9) | 26 (5.7) |
| Asian | 38 (8.3) | 49 (10.8) |
| Other | 3 (0.7) | 4 (0.9) |
| BW, mean (SD), kg | 93.6 (19.5) | 92.6 (20.5) |
| BMI, mean (SD), kg/m2 | 32.9 (6.1) | 32.6 (5.9) |
| Qualifying CV event, n (%) | ||
| Coronary heart disease | 349 (76.0) | 338 (74.3) |
| Stroke or TIA | 89 (19.4) | 100 (22.0) |
| Peripheral artery disease | 18 (3.9) | 15 (3.3) |
| Not reported | 3 (0.7) | 2 (0.4) |
| Time from most recent qualifying CV event, years | 6.2 (5.9) | 5.7 (5.6) |
| Duration of hypertension, n (%) | ||
| <3 years | 55 (12) | 56 (12.3) |
| ≥3 and <10 years | 173 (37.7) | 173 (38.0) |
| ≥10 years | 230 (50.1) | 225 (49.5) |
| Not reported | 1 (0.2) | 1 (0.2) |
| Seated SBP, mean (SD), mmHg | 133.0 (13.8) | 133.5 (13.5) |
| Seated DBP, mean (SD), mmHg | 76.9 (9.0) | 77.0 (9.1) |
| Type 2 diabetes duration, years | 12.3 (8.2) | 12.6 (8.7) |
| HbA1c, mean (SD), % [mmol/mol] | 8.08 (0.80) [65 (8.7)] | 8.18 (0.84) [66 (9.2)] |
| FPG, mean (SD), mmol/L | 8.8 (2.3) | 8.9 (2.6) |
| Type of treatment, n (%) | ||
| OAD | 217 (47.3) | 221 (48.6) |
| OAD plus insulin | 165 (35.9) | 158 (34.7) |
| Insulin only | 77 (16.8) | 76 (16.7) |
| Daily insulin dose, mean (SD), IU | 49.2 (28.7) | 56.8 (37.4) |
| OADs, n (%) | ||
| 0 | 77 (16.8) | 76 (16.7) |
| 1 | 185 (40.3) | 192 (42.2) |
| 2 | 195 (42.5) | 183 (40.2) |
| >2 | 2 (0.4) | 4 (0.9) |
| Concomitant medications,* n (%) | ||
| Antihypertensive | 454 (98.3) | 455 (98.9) |
| ACEIs/ARBs | 409 (88.5) | 408 (88.7) |
| Diuretics | 241 (52.24) | 212 (46.1) |
| Loop diuretics | 100 (21.6) | 81 (17.6) |
| Lipid-lowering medications | 409 (88.5) | 387 (84.1) |
| Acetylsalicylic acid | 341 (73.8) | 329 (71.5) |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; n, number of randomized subjects with at least one dose of study medication, and with baseline and at least one postbaseline efficacy observation; TIA, transient ischemic attack.
*A prespecified list of drugs was used to assess the percentage of patients receiving antihypertensive, ARB/ACEi, lipid-lowering, and loop diuretic medications; assessments were made through 52 weeks for these parameters. For diuretic medications and acetylsalicylic acid, the numbers were determined based on the overall therapeutic classes.
Glycemic Efficacy
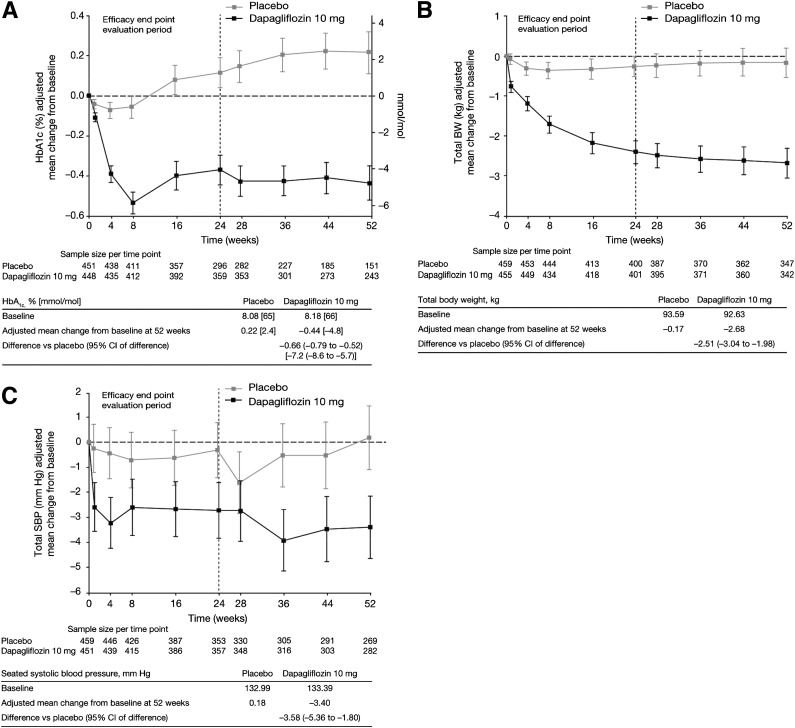
At week 24, a reduction in mean HbA1c from baseline (8.18%) was observed with dapagliflozin (−0.38% [−4.2 mmol/mol]) versus a slight increase with placebo (baseline 8.08%; 0.08% [0.9 mmol/mol]) (Table 2). The placebo-corrected reduction in HbA1c with dapagliflozin treatment was significant at week 24 (−0.46% [−5.0 mmol/mol], P < 0.0001) and was maintained at week 52 (−0.66% [−7.2 mmol/mol]) (Fig. 1A and Supplementary Table 2). Significantly greater HbA1c reductions were observed with dapagliflozin versus placebo treatment in patients with baseline HbA1c ≥8.0% (−0.56% [−6.1 mmol/mol] vs. −0.08% [0.9 mmol/mol]) and ≥9.0% (−0.99% [−10.8 mmol/mol] vs. −0.35% [−3.8 mmol/mol]) at week 24, and these larger reductions persisted at 52 weeks. More patients in the dapagliflozin group with a baseline HbA1c >7.0% achieved the predefined measure of HbA1c <7.0% than in the placebo group (16.4% vs. 8.4%, nominal P < 0.05), and the difference was maintained to week 52 (14.6% vs. 5.1%).
Table 2.
Efficacy measurements in the overall study population and in the corresponding strata of age <65 years and age ≥65 years (full analysis set)
| Full analysis set |
Age <65 years |
Age ≥65 years§* |
||||
|---|---|---|---|---|---|---|
| Placebo group (n = 459) | Dapagliflozin 10 mg group (n = 455) | Placebo group (n = 263) | Dapagliflozin 10 mg group (n = 263) | Placebo group (n = 196) | Dapagliflozin 10 mg group (n = 192) | |
| HbA1c at 24 weeks* | ||||||
| n | 451 | 448 | 259 | 260 | 192 | 188 |
| Baseline, mean (SD) | ||||||
| % | 8.08 (0.80) | 8.18 (0.84) | 8.06 (0.82) | 8.22 (0.86) | 8.10 (0.79) | 8.13 (0.81) |
| mmol/mol | 65 (8.7) | 66 (9.2) | 65 (9.0) | 66 (9.4) | 65 (8.6) | 65 (8.9) |
| Change from baseline, adjusted mean (95% CI) | ||||||
| % | 0.08 (0.01–0.16) | −0.38# (−0.46 to −0.30) | 0.02 (−0.08 to 0.12) | −0.40# (−0.50 to −0.30) | 0.16 (0.04–0.28) | −0.37# (−0.49 to −0.25) |
| mmol/mol | 0.9 (0.1–1.7) | –4.2# (−5.0 to 3.3) | 0.2 (−0.9 to 1.3) | −4.4# (−5.5 to −3.3) | 1.7 (0.4–3.1) | −4.0# (−5.4 to −2.7) |
| Responders of three-item end point at 24 weeks* | ||||||
| x/n | 4/451 | 52/444 | 1/259 | 29/258 | 3/192 | 23/186 |
| % (95% CI) | 0.9 (0.0–1.8) | 11.7# (8.7–14.7) | 0.4 (−0.4 to 1.1) | 11.2# (7.4–15.1) | 1.6 (−0.2 to 3.3) | 12.4# (7.6–17.1) |
| Seated SBP at 8 weeks, mmHg†** | ||||||
| n | 459 | 451 | 263 | 261 | 196 | 190 |
| Baseline, mean (SD) | 132.99 (13.81) | 133.39 (13.48) | 131.51 (14.09) | 132.72 (13.44) | 134.96 (13.20) | 134.31 (13.51) |
| Change from baseline, adjusted mean (95% CI) | −0.99 (−2.29 to 0.32) | −2.96§ (−4.29 to −1.64) | 0.01 (−1.67 to 1.70) | −3.10‖ (−4.79 to −1.42) | −2.30 (−4.29 to −0.31) | −2.77 (−4.82 to −0.73) |
| BW at 24 weeks, kg† | ||||||
| n | 459 | 455 | 263 | 263 | 196 | 192 |
| Baseline, mean (SD) | 93.59 (19.47) | 92.63 (20.50) | 93.74 (20.18) | 94.27 (20.70) | 93.38 (18.51) | 90.39 (20.07) |
| Change from baseline, adjusted mean (95% CI), % | −0.30 (−0.62 to 0.03) | −2.56# (−2.88 to −2.24) | −0.11 (−0.52 to 0.31) | −2.40# (−2.80 to −2.00) | −0.49 (−0.98 to 0.01) | −2.73†† (−3.23 to −2.23) |
| Seated SBP at 24 weeks, mmHg† | ||||||
| n | 459 | 451 | 263 | 261 | 196 | 190 |
| Baseline, mean (SD) | 132.99 (13.81) | 133.39 (13.48) | 131.51 (14.09) | 132.72 (13.44) | 134.96 (13.20) | 134.31 (13.51) |
| Change from baseline, adjusted mean (95% CI) | −1.03 (−2.39 to 0.32) | −2.99§ (−4.36 to −1.61) | 0.05 (−1.57 to 1.66) | −2.94‖ (−4.56 to −1.32) | −2.38 (−4.66 to −0.10) | −3.03†† (−5.37 to −0.69) |
| Patients with BW decrease of ≥5% in patients with baseline BMI of ≥27 kg/m2† | ||||||
| x/n | 16/397 | 64/388 | 8/221 | 36/224 | 8/176 | 28/164 |
| % (95% CI) | 4.0 (2.1–5.9) | 16.5# (12.8–20.2) | 3.6 (1.2–6.1) | 16.1# (11.3–20.9) | 4.5 (1.4–7.6) | 17.1†† (11.2–22.8) |
| FPG, mmol/L‡ | ||||||
| n | 441 | 437 | 255 | 256 | 186 | 181 |
| Baseline, mean (SD) | 8.77 (2.34) | 8.89 (2.59) | 8.95 (2.39) | 8.92 (2.49) | 8.52 (2.24) | 8.84 (2.74) |
| Change from baseline, adjusted mean (95% CI) | 0.35 (0.13–0.57) | −0.57§ (−0.78 to −0.34) | 0.23 (−0.04 to 0.49) | −0.56# (−0.83 to −0.30) | 0.52 (0.17–0.87) | −0.57# (−0.93 to −0.22) |
This was a last observation carried forward analysis. x, number of responders; n, number of patients in the full analysis set with nonmissing baseline and week t (last observation carried forward) values.
*Co-primary end point. †Key secondary end point. ‡Other end point. §P < 0.05. ‖P < 0.01. #P < 0.0001. **The protocol mandated that antihypertensive medication was unchanged during the first 8 weeks of the study. Analyses excluded data after glycemic rescue and included data after antihypertensive rescue for HbA1c, excluded data after glycemic and hypertension rescue for the three-item end point, included data after glycemic and antihypertensive rescue for BW end points, and included data after glycemic rescue and excluded data after antihypertensive rescue for seated SBP end points. ††Statistical testing was not performed in the stratum of age ≥65 years for the key secondary end points of change in BW, change in seated SBP at 24 weeks, and patients with a BW decrease of ≥5% among patients with a baseline BMI of ≥27 kg/m2, because the first key secondary end point did not meet statistical significance.
Figure 1.
A: Demonstration of the placebo-corrected reduction in HbA1c with dapagliflozin treatment at week 24, which was maintained through week 52. B: The placebo-corrected reduction in BW was significant at week 24 and persisted through week 52 when compared with placebo. C: Demonstration of the mean placebo-subtracted reduction in seated SBP. SBP was also statistically significant at week 8 and was maintained at weeks 24 and 52.
Dapagliflozin demonstrated efficacy in a high proportion of patients who had mild (eGFR ≥60 to <90 mL/min/1.73 m2, 58–61%) or moderate (eGFR ≥30 to <60 mL/min/1.73 m2, 15–20%) renal impairment.
Patients in the dapagliflozin group, excluding those who received rescue therapy, showed a rapid mean reduction in FPG from baseline at week 1 that was greater than that with placebo and was maintained through week 24 (−0.57 vs. 0.35 mmol/L, Table 2) and 52 weeks (−0.96 vs. –0.01 mmol/L, Supplementary Table 2). Approximately half of the dapagliflozin group (n = 116 [24.6%]) required rescue therapy prior to or at week 52 versus the placebo group (n = 233 [51.8%]) (see Kaplan-Meier plot depicting the time to rescue) (Supplementary Fig. 2). The adjusted mean change in daily insulin dose was 1.0 international units (IU)/day for dapagliflozin treatment versus 5.1 IU/day for placebo treatment at week 24 (nominal P < 0.05) and 4.7 IU/day versus 10.7 IU/day, respectively, at week 52, including data after rescue therapy.
Weight
A greater reduction in mean BW was observed in patients treated with dapagliflozin versus placebo at week 24 (−2.56% vs. −0.30%, Table 2) and was maintained through week 52 (−2.89% vs. −0.29%, Supplementary Table 2). The placebo-corrected reduction in BW was significant at week 24 (−2.10 kg, nominal P < 0.05) and persisted through week 52 (−2.51 kg, Fig. 1B). About four times more patients with a baseline BMI of ≥27 kg/m2 achieved a BW reduction of ≥5% at week 24 in the dapagliflozin group versus the placebo group (16.5% vs. 4.0%) and at week 52 (15.8% vs. 6.8%, Supplementary Table 2).
Blood Pressure
Greater reductions in mean seated SBP from baseline were observed at week 24 after treatment with dapagliflozin than with placebo. The mean placebo-subtracted seated reduction in SBP was statistically significant at week 8 (−1.97 mmHg), and was maintained at week 24 (−1.95 mmHg, Table 2) and week 52 (−3.58 mmHg, Fig. 1C and Supplementary Table 2) (P < 0.0001). The difference between dapagliflozin and placebo treatment was less pronounced for the proportion of patients with a baseline seated SBP of ≥130 mmHg who achieved an SBP of <130 mmHg at week 24 (29.4% vs. 24.2%, respectively) than for those at week 52 (24.0% vs. 13.2%). The dapagliflozin group showed a slight mean decrease in seated DBP from baseline at week 24 of −1.7 mmHg compared with a decrease in the placebo group of −0.4, and decreases at week 52 were −1.7 and −0.2 mmHg, respectively.
Three-Item Combined Clinical Benefit
Approximately 12% of patients receiving dapagliflozin, compared with ∼1% receiving placebo, responded simultaneously to the three-item end point of combined clinical benefit (Table 2), which was maintained through week 52 (6.6% vs. 0.7%, Supplementary Table 2). Approximately twice as many patients receiving dapagliflozin achieved an absolute reduction in HbA1c of ≥0.5% versus placebo at week 24 (45.3% vs. 20.6%, respectively, nominal P < 0.05), and these differences between the groups became greater at week 52 (30.6% vs. 7.0%). Almost three times as many patients receiving dapagliflozin than placebo achieved a relative reduction in BW of ≥3% at 24 weeks (40% vs. 13.9%), with similar results observed at 52 weeks (31.9% vs. 13.1%), whereas the difference in the absolute reduction of ≥3 mmHg SBP was less pronounced at 24 weeks (49.1% vs. 41.6%) than at 52 weeks (32.0% vs. 22.2%).
Safety and Tolerability
Overall AEs
Most AEs were mild to moderate in intensity (Supplementary Table 3). The majority of discontinuations were based on protocol-predefined laboratory discontinuation criteria. The study was not designed to evaluate CVD events between groups, but we have listed the causes of death in both groups (Supplementary Table 3). In the dapagliflozin group, causes of death (number of deaths) were sudden death (3), multiorgan failure (1), myocardial infarction (2), and cardiogenic/septic shock (1); and in the placebo group, were cerebrovascular accident (1) and pulmonary embolism (1). These deaths were not related to the study medication, as assessed by investigators (Supplementary Table 3, footnote). Safety data for subgroup analyses (patients with congestive heart failure and/or receiving therapy with loop diuretics) are presented (Supplementary Table 3). The numbers of hypoglycemic events were balanced between groups (Supplementary Table 3). Study discontinuations due to a hypoglycemic event were rare (dapagliflozin group n = 1, placebo group n = 2).
Events of Special Interest
AEs of fungal genital infection were more often reported in the dapagliflozin group, and none were assessed as serious AEs (SAEs); whereas AEs of urinary tract infection were reported by a similar number of patients in each group (Supplementary Table 3). A greater proportion of patients in the dapagliflozin group were reported to experience AEs of renal impairment or failure; these were predominantly abnormalities in laboratory values (Supplementary Table 3). For most patients, these AEs resolved or normalized (dapagliflozin group 49 of 55 AEs, placebo group 25 of 31 AEs). AEs of hypotension, dehydration, or hypovolemia occurred more often in the dapagliflozin group than in the placebo group (Supplementary Table 3), and none were considered SAEs. In both groups, serum electrolytes were mostly unchanged, and marked abnormalities and elevated liver test values were rare (<6% of patients per group).
Patients Aged <65 and ≥65 Years
Analyses of age strata showed no meaningful differences in efficacy versus the overall study population. In both strata, the HbA1c reductions were significantly greater for the dapagliflozin group, and more patients in the dapagliflozin group met the three-item combined clinical end point at week 24 (Supplementary Table 2). In the ≥65 years stratum, the change in seated SBP at week 8 was not statistically significant; therefore, additional secondary efficacy measurements, such as BW, were not evaluated for statistical inference. However, the changes observed for these measurements were similar to those observed in the overall population. Decreases in HbA1c and weight were maintained over 52 weeks in both age strata.
The proportions of patients experiencing one or more treatment-related AEs were similar between treatment groups in the age stratum <65 years (dapagliflozin group 72.6%, placebo group 71.6%) and in the age stratum ≥65 years (dapagliflozin group 75.8%, placebo group 75.8%). The proportions of patients discontinuing with an AE or due to an AE were larger in the dapagliflozin group than in the placebo group, both in the age stratum <65 years (9.8% vs. 5.7%) and in the stratum ≥65 years (16.5% vs. 12.1%). The proportion of patients with one or more SAEs was similar between treatment groups in those <65 years of age, and the proportion was larger with dapagliflozin versus placebo treatment in those patients ≥65 years of age (15.5% vs. 11.1%, respectively). The total proportion of patients with one or more AEs of renal impairment or failure was larger in the age stratum ≥65 years than in the age stratum <65 years. In both age strata, the proportion of patients with one or more AEs of renal impairment or failure was larger with the dapagliflozin group than with the placebo group (<65 years 7.1% vs. 3.8%, ≥65 years 18.6% vs. 10.6%).
Patients Treated With Insulin
Efficacy results were generally similar in both insulin strata relative to the overall study population (Supplementary Table 4); dapagliflozin treatment was shown to have significantly greater effects for both primary efficacy outcomes at week 24 when compared with placebo treatment. Notably, the change in seated SBP at week 8 was not statistically significant for patients who used insulin at randomization versus those who did not. At 24 weeks, the proportion of patients experiencing hypoglycemia was similar in the dapagliflozin and placebo groups and was higher for insulin users (35.7% and 36.3%, respectively) versus patients who did not use insulin (14.0% and 14.7%).
Conclusions
This study provides new clinical information on the role of an SGLT2i in an important but understudied population of patients with type 2 diabetes who had documented CVD and (treated) hypertension. Specifically, we demonstrate in this high-risk cohort that dapagliflozin was superior to placebo when added to usual care in reducing HbA1c, as well as achieving a three-item combined clinical benefit end point of simultaneous HbA1c lowering, BW reduction, and SBP reduction. While the results appear to confirm observations from other clinical trials (7–12,14) with use of dapagliflozin as monotherapy or add-on therapy, the prior studies did not specifically evaluate subjects who were at such high risk for CVD; and therefore, the data provide new clinical information to guide physicians. Importantly, the data suggest that similar efficacy was observed for analyses by age strata (<65 years and ≥65 years) and insulin use at randomization. The efficacy of both primary end points was maintained over 52 weeks in the overall study population and in the age-groups.
As an important feature of this study, patients were required to be receiving stable regimens of antidiabetic, antihypertensive, antiplatelet, and lipid-lowering medications at randomization, and use of these medications was maintained throughout the study. However, the insulin dose was reduced by 25% at the onset of the study to minimize the risk of initial hypoglycemia when adding dapagliflozin to therapy.
A companion study (15) (clinical trial reg. no. NCT01042977, clinicaltrials.gov) enrolled patients with type 2 diabetes and pre-existing CVD; however, unlike the current study, a history of hypertension was not required for enrollment, but >90% of patients had hypertension. Another important aspect of the current study is that dapagliflozin treatment resulted in significant HbA1c lowering compared with placebo in a wide range of renal functions. These data appear to be in contrast to those from a report (16) suggesting reduced efficacy in patients with moderate renal impairment. Results from the companion study (15) appear to support the observations of this current report.
It is important for providers to consider therapies that address not only glycemia, but also other unmet clinical needs such as weight gain, hypoglycemia, and other CVD risk factors. In this regard, this study was much different from past studies as it is one of the first to assess the treatment of comorbid conditions in a high-risk cohort. Specifically, we evaluated a co-primary three-item end point of combined clinical benefit, which consisted of an absolute drop from baseline in HbA1c of ≥0.5% (5.5 mmol/mol), a relative drop of ≥3% in total BW, and an absolute drop of ≥3 mmHg from baseline in seated SBP. The choice of end points and the levels chosen as clinically significant were based on the expected efficacy of dapagliflozin-induced glucuresis, which are well-established benefits to patients, and accepted regulatory thresholds from prior studies (17–20) evaluating such clinical parameters. To our knowledge, this is the first time that this was tested in a prospective, randomized study. Dapagliflozin had significantly greater effects in achieving this three-item end point of combined clinical benefit compared with placebo. As outlined, 45% of dapagliflozin-treated patients had a reduction in HbA1c of ≥0.5%, 40% had a reduction in BW of ≥3%, 38% had a reduction in SBP of ≥3 mmHg, and ∼12% successfully met all three criteria for the overall response to the three-item end point at 24 weeks.
Additional measures of glycemic control (i.e., FPG reduction, the proportion of patients with an HbA1c <7.0%, and the proportion of patients requiring antidiabetic rescue [24.6% for the dapagliflozin group and 51.8% for the placebo group]) were significantly better in patients treated with dapagliflozin than placebo at 24 weeks and were maintained through the study extension. There are many factors that may have contributed to the rescue rate in both groups, including the higher HbA1c upper limit of 10.5%, as individuals in this upper range may have been considered more advanced in the disease process. In addition, it was the strategy to decrease insulin dosing by 25% at the time of randomization, which may have increased the failure rate of those patients receiving placebo.
Following the baseline 25% reduction in insulin dose, the mean dose of insulin increased more in the placebo group than in the dapagliflozin group (5.1 vs. 1.0 IU/day), although doses remained lower than the prereduction level in either group. These findings, in addition to those from previous reports, suggest that dapagliflozin is efficacious in a broad spectrum of patients with type 2 diabetes.
Urinary frequency was not evaluated in this study, although early work on the compound (21) did demonstrate an increase in 24-h urine volume, supporting the osmotic diuresis. As a result of a mild osmotic diuresis, dapagliflozin has been observed to have blood pressure–lowering effects (SBP −2.3 to −7.2 mmHg, DBP −1.0 to −2.8 mmHg) (9). In the current study, the observed small reductions in SBP may also be attributed to the optimization of antihypertensive treatment in the lead-in period and the near-goal at baseline. The increase in renal impairment was thought to be mostly a result of patients meeting prespecified study discontinuation criteria for laboratory abnormalities assessed as renal impairment/failure, including decreased renal creatinine or increased blood creatinine. The reason that more patients overall, especially among those ≥65 years of age, met these criteria in the study may be the relatively higher proportion of patients with mild or moderate renal impairment.
The novelty of the current study relates to the evaluation of a new agent when used in a high-risk cohort. Patients are living longer with type 2 diabetes, and a greater percentage of older patients will have type 2 diabetes. Thus, a key focus of the current study was safety in patients who have multiple comorbid conditions and are treated with multiple concomitant medications. A limitation of our approach was that subjects with certain diseases and abnormalities were excluded from the study population for the purpose of limiting confounding factors that would complicate the interpretation of the results or to exclude subjects whose safety could be compromised by participation in the study. However, the changes observed in weight and blood pressure were in the range of clinical benefit, and no safety concerns were related to these measures. In particular, blood pressure changes are of potential concern in patients receiving therapy with loop diuretic drugs. Yet, the safety results in the patients in the dapagliflozin group who were treated with loop diuretic drugs were generally similar to those observed for patients treated with placebo, with the exception of an increased number of study discontinuations, which occurred mostly as a result of patients meeting prespecified discontinuation criteria for laboratory abnormalities assessed as renal impairment/failure, including decreased renal creatinine or increased blood creatinine.
In this population of patients with a history of CVD and hypertension, dapagliflozin was generally well tolerated over 52 weeks. Even with the additional concomitant medications, there was no increased risk of hypoglycemia with dapagliflozin treatment in these patients. The number of events of hypovolemia was elevated in the dapagliflozin group versus the placebo group, potentially reflecting the influence of the multiple concomitant medications being taken by this population. While the overall rates of volume-related events were low, caution should be exercised when administering dapagliflozin to patients who are at risk for volume depletion. An increased risk for events of fungal genital infections was observed with the use of dapagliflozin versus placebo, which is consistent with the findings of other reports on this class of drugs (22–24).
Safety results were largely similar for the current study and the companion study (15). In the current study, neoplasms were observed in 12 patients in the dapagliflozin group and in 2 patients in the placebo group, whereas in the parallel companion study fewer neoplasms were observed in the dapagliflozin group versus the placebo group (5 vs. 10, respectively). Similarly, an imbalance in the number of deaths considered unrelated to dapagliflozin was observed in the current study, which, again, was not observed in the companion study or in the overall dapagliflozin program. It is important to note that this sample size is too small to draw conclusions about rare events (25). In the overall dapagliflozin program, no imbalances were observed for the overall incidence rates of neoplasms/100 patient-years for patients receiving dapagliflozin (1.39) and control subjects (1.34) (26), and all-cause deaths were balanced in the dapagliflozin group (0.53%, 29 of 5,498 deaths) and the control group (0.60%, 19 of 3,184 deaths) (data on file). Moreover, in a meta-analysis (26) for major adverse cardiac events (i.e., CV death, myocardial infarction, and stroke) in all patients within the phase 2b/3 program (including this study and the companion study), the events hazard ratio was 0.819 (95% CI 0.583–1.152) in favor of dapagliflozin treatment.
The efficacy of dapagliflozin outlined in this study appears to be in line with or slightly greater than that of other agents in the class, including canagliflozin and those in development (i.e., empagliflozin) (27). The important and novel aspect of the study is that this study, along with its sister comparison trial, provided data on efficacy and weight loss in a high-risk group of patients, which separates this study from others (27). Clearly, the interest would be in having head-to-head comparisons with more recent agents (i.e., dipeptidyl peptidase-4 inhibitors), but this will need to be addressed in future studies (26). Although the 52-week data were felt to be exploratory, they do support the long-term durability of this agent.
In conclusion, this study clearly advances the field and provides significant new clinical information on SGLT2i use and efficacy. The strengths of the study are the large and well-characterized cohort, the observation period (i.e., 1 year), and the comprehensive clinical evaluation of the subjects. Given the paucity of available data, the current study assists clinicians when determining appropriate regimens for patients with type 2 diabetes who are at high risk for CVD. As such, dapagliflozin, when added to usual care in a population with a high CVD risk, was superior to placebo in reducing HbA1c, as well as demonstrating combined efficacy for HbA1c lowering, BW reduction, and SBP reduction, in a year-long study. These data indicate that the safety profile of dapagliflozin makes it appropriate for use in a population of patients with advanced type 2 diabetes, CVD, and hypertension, and, as such, provides significantly new clinical information.
Supplementary Material
Article Information
Acknowledgments. Initial medical writing assistance was provided by Alexandra Silveira, PhD, of PPSI (a PAREXEL company), and was funded by AstraZeneca.
Funding. W.T.C. was supported in part by a grant from the National Institute of General Medical Sciences of the National Institutes of Health (1-U54-GM-104940).
Duality of Interest. This study was supported by Bristol-Myers Squibb and AstraZeneca. W.T.C. has served as the principal investigator of research studies awarded to his institution by AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Johnson & Johnson, Lexicon Pharmaceuticals, MannKind Corporation, and Sanofi. He has served as a consultant for Halozyme Therapeutics and Intarcia Therapeutics. L.A.L. has served in the roles of advisory panel member, consultant, member of a speaker’s bureau, or research support for AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline, Janssen Biotech, Novo Nordisk, Sanofi, Servier, and Takeda. T.W.A.d.B., I.G.-N., J.S., and S.J.P. are employees of AstraZeneca. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. W.T.C. was the principal investigator for this study, contributed to the study concept and design, analyzed and interpreted the data, and wrote and revised the article. L.A.L., J.S., and S.J.P. contributed to the study concept and design, analyzed and interpreted the data, and wrote and revised the article. T.W.A.d.B. contributed to the study concept and design; supervised the study; acquired, analyzed, and interpreted the data; and wrote and revised the article. I.G.-N. supervised the study; acquired, analyzed, and interpreted the data; and wrote and revised the article. W.T.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, PA, 8–12 June 2012.
Footnotes
Clinical trial reg. no. NCT01031680, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-0315/-/DC1.
References
- 1.American Diabetes Association . Standards of medical care in diabetes—2013. Diabetes Care 2013;36(Suppl. 1):S11–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodbard HW, Blonde L, Braithwaite SS, et al.; AACE Diabetes Mellitus Clinical Practice Guidelines Task Force . American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007;13(Suppl. 1):1–68 [DOI] [PubMed] [Google Scholar]
- 3.Inzucchi SE, Bergenstal RM, Buse JB, et al.; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD) . Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rydén L, Grant PJ, Anker SD, et al.; Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG) . ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on Diabetes, Pre-diabetes, and Cardiovascular Diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 2013;34:3035–3087 [DOI] [PubMed] [Google Scholar]
- 5.Cheung BM, Ong KL, Cherny SS, Sham PC, Tso AW, Lam KS. Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am J Med 2009;122:443–453 [DOI] [PubMed] [Google Scholar]
- 6.Dodd AH, Colby MS, Boye KS, Fahlman C, Kim S, Briefel RR. Treatment approach and HbA1c control among US adults with type 2 diabetes: NHANES 1999-2004. Curr Med Res Opin 2009;25:1605–1613 [DOI] [PubMed] [Google Scholar]
- 7.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 2010;375:2223–2233 [DOI] [PubMed] [Google Scholar]
- 8.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 2010;33:2217–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009;32:650–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care 2011;34:2015–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab 2011;13:928–938 [DOI] [PubMed] [Google Scholar]
- 12.Wilding JP, Norwood P, T’joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care 2009;32:1656–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsiatis AA, Davidian M, Zhang M, Lu X. Covariate adjustment for two-sample treatment comparisons in randomized clinical trials: a principled yet flexible approach. Stat Med 2008;27:4658–4677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Feng Y, List J, Kasichayanula S, Pfister M. Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: effects on glycaemic control and body weight. Diabetes Obes Metab 2010;12:510–516 [DOI] [PubMed] [Google Scholar]
- 15.Leiter LA, Cefalu WT, de Bruin TWA, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. J Am Geriatr Soc 2014;62:1252–1262 [DOI] [PubMed] [Google Scholar]
- 16.Kasichayanula S, Liu X, Pe Benito M, et al. The influence of kidney function on dapagliflozin exposure, metabolism and pharmacodynamics in healthy subjects and in patients with type 2 diabetes mellitus. Br J Clin Pharmacol 2013;76:432–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horton ES, Silberman C, Davis KL, Berria R. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care 2010;33:1759–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norris SL, Zhang X, Avenell A, et al. Efficacy of pharmacotherapy for weight loss in adults with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med 2004;164:1395–1404 [DOI] [PubMed] [Google Scholar]
- 19.Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration . Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003;362:1527–1535 [DOI] [PubMed] [Google Scholar]
- 20.Whelton PK, He J, Appel LJ, et al.; National High Blood Pressure Education Program Coordinating Committee . Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA 2002;288:1882–1888 [DOI] [PubMed] [Google Scholar]
- 21.Jabbour SA, Whaley JM, Tirmenstein M, et al. Targeting renal glucose reabsorption for the treatment of type 2 diabetes mellitus using the SGLT2 inhibitor dapagliflozin. Postgrad Med 2012;124:62–73 [DOI] [PubMed] [Google Scholar]
- 22.Geerlings S, Fonseca V, Castro-Diaz D, List J, Parikh S. Genital and urinary tract infections in diabetes: impact of pharmacologically-induced glucosuria. Diabetes Res Clin Pract 2014;103:373–381 [DOI] [PubMed] [Google Scholar]
- 23.Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin. J Diabetes Complications 2013;27:479–484 [DOI] [PubMed] [Google Scholar]
- 24.Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Urinary tract infections in patients with diabetes treated with dapagliflozin. J Diabetes Complications 2013;27:473–478 [DOI] [PubMed] [Google Scholar]
- 25.Berlin JA, Glasser SC, Ellenberg SS. Adverse event detection in drug development: recommendations and obligations beyond phase 3. Am J Public Health 2008;98:1366–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ptaszynska A, Johnsson KM, Parikh SJ, de Bruin TW, Apanovitch AM, List JF. Safety profile of dapagliflozin for type 2 diabetes: pooled analysis of clinical studies for overall safety and rare events. Drug Saf 2014;37:815–829 [DOI] [PubMed] [Google Scholar]
- 27.Hasan FM, Alsahli M, Gerich JE. SGLT2 inhibitors in the treatment of type 2 diabetes. Diabetes Res Clin Pract 2014;104:297–322 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.