Abstract
With effective antiretroviral therapy (ART), many HIV-infected people die of diseases other than acquired immune deficiency syndrome (AIDS). In particular, coronary artery disease has emerged as one of most critical complications of HIV infection and a major cause of morbidity and mortality. Although reportedly antiretroviral combination therapy itself may accelerate atherosclerosis by enhancing dyslipidemia, most recent epidemiological studies support the notion that HIV infection itself contributes to cardiovascular disease. However, it is still a mystery how the virus can contribute to cardiovascular disease development even while suppressed by ARTs. This review discusses the current understanding of interactions between HIV infection and cardiovascular diseases in both clinical and experimental studies with special focus on those viral proteins which are still produced by HIV. This will help infectious disease/vascular biology experts to gain insights into the pathophysiological mechanisms of HIV associated cardiovascular disease and new trends to treat and prevent cardiovascular disease in the HIV infected population.
Cardiovascular disease (CVD) as a severe complication in HIV patients
Due to the successful control of HIV viremia and HIV-induced AIDS through antiretroviral therapy (ART), CVD has emerged as a leading cause of death in those infected with human immunodeficiency virus (HIV) [1–3]. Interestingly, HIV-infected individuals have an increased risk of CVD both in the absence and presence of virologically suppressive ART [4–8]. Triant et al have shown that acute myocardial infarction (AMI) rates and cardiovascular risk factors were increased in HIV patients compared with non-HIV patients as indicated by a relative risk of 1.75 (95% CI 1.51–2.02, p<0.0001) for AMI [6]. This increased risk is magnified exponentially in the older HIV-infected population. Freiberg et al analyzed over 81,000 HIV-positive and HIV-negative individuals from the Virtual Cohort of the Veterans Aging Cohort Study from 2003 to 2008, and showed that even after accounting for other traditional risk factors, HIV infection resulted in a two-fold higher risk of heart attack [9].
Atherosclerosis in the HIV infected population
Several studies have addressed the nature of atherosclerotic lesions in the HIV infected population [10–13]. Fitch et al. reported [10, 14] that the presence of plaque and numbers of noncalcified plaque segments were increased in the HIV-infected group compared with HIV negative controls. They concluded that HIV patients without significant metabolic abnormalities may still develop non-calcified plaque and therefore are at increased risk for CAD [10]. Another study assessed the increased risk of atherosclerosis in HIV-infected patients by measuring carotid intima-medial thickness in 145 HIV patients on ART for at least 6 months. They revealed that 34 (23.4%) of these patients had carotid plaques that were associated with three independent risk factors: old age [odds ratio (OR) 6.16, 95% confidence interval (CI) (1.09–34.88; p=0.040), hypertension (OR 12.62, 95% CI 1.72–92.49; p=0.013) and higher low-density lipoprotein cholesterol (LDL-C) (OR 1.08, 95% CI 1.01–1.16; p=0.039) [11].
Is ART a culprit for HIV-related CVD?
Although the increased risk of CVD in HIV-infected patients is still not fully understood yet, one explanation could be HIV virus- or ART-induced hyperlipidemia and hypercholesterolemia, conditions known to promote progression of coronary atherosclerosis [15]. Indeed, HIV itself [16, 17] and a few antiretroviral drugs [18], in particular HIV protease inhibitors, reportedly can cause dyslipidemia in the HIV-positive population, thus contributing to the increased risk for CVD. However, the heightened risk of CVD persists even in the current treatment era, in which new generation ART have significantly reduced dysmetabolic side effects (e.g. insulin resistance, dyslipidemia, and hypertension) [19, 20].
Currently, there is a controversial discussion whether living with HIV or being on ART produces greater cardiovascular risk than being treatment-naïve. Untreated HIV infection has been found to associate with increased levels of IL-6, a pro-inflammatory cytokine and a stimulus for hepatic C-reactive protein production, a surrogate marker for systemic inflammation. Higher levels of IL-6 strongly predict cardiovascular events and overall mortality in antiretroviral-untreated and treated HIV infection [21]. This and several other studies suggest that ongoing HIV replication and immune depletion significantly contribute to increased prevalence of elevated inflammation biomarkers, altered coagulation, and monocyte activation, and this contribution is independent of the substantial contribution from comorbid conditions [22–24]. Hsue and colleagues [25] concluded that HIV infection correlates with premature atherosclerosis even in the absence of detectable viremia, overt immunodeficiency and exposure to ART, and appears to be independent of traditional cardiac risk factors. They found that Intima-media thickness (IMT) and C-reactive protein was strongly associated with the presence of HIV disease rather than viral load or CD4+ T cell count. However, antiretroviral drug exposure was also associated with higher IMT [25]. Nevertheless, the Women’s Interagency HIV Study measured Carotid Artery Stiffness via ultrasound 6.5 years after measurement of T Cell activation, and showed that even after the initiation of ART, persistently activated T Cells strongly correlated with increased carotid artery stiffness only in HIV patients, but not in HIV-negative patients [26]. This conclusion that HIV itself increases the risk of CVD is further supported by the National Institutes of Health’s Strategies for Management of Antiretroviral Therapy (SMART) study, which utilizes CD4+ cell counts to determine the starting and stopping point of intermittent therapy. It reported that the interruption of ART was surprisingly associated with increased risk of CVD [27]. This study suggests that ART is not the prevailing reason for increased CVD risk in those infected with HIV, and that HIV viral activities might also contribute. Hansen et. al showed impaired aortic endothelial function, increased c-IMT, and increased arterial stiffness in a Transgenic (Tg) mouse model expressing HIV viral proteins env, tat, nef, vpu, vpr, gp120 and rev. They also found markers for vascular remodeling such as decreased elastin content, increased cathepsin K and cathepsin S activity, and increased mechanical residual stress in the arteries of these Tg mice [28]. This is in line with previous conclusions that HIV infection itself has emerged as an independent contributor to cardiovascular disease in this population [5–7, 29].
Effects of smoking, alcohol and drug abuse for CVD in HIV patients
Smoking has been found to be an independent factor that is associated with CVD [30, 31]. Reportedly, 40–70% of HIV-infected population is current smokers. A cohort study of 33,308 HIV patients showed that the risk of myocardial infarction and CVD decreased with each passing year after smoking cessation, and that after 3 years the risk was almost half that of the first year. Smoking tobacco inhibits effective T cells function, which may result in increased risk of infection such as pulmonary infections. In addition, HIV infection is associated with a chronic state of persistent inflammation which increases the risk for CVD, chronic obstructive pulmonary disease and non-AIDS defining cancers.
The Veterans Aging Cohort Study showed that among HIV-infected men, alcohol abuse was associated with a higher prevalence of CVD compared with infrequent and moderate drinking, even after adjusting for traditional CVD risk factors, antiretroviral therapy, and CD4 count [32]. In addition, recreational drug use including cocaine and methamphetamine contributes to cardiac toxicity [33].
Endothelial dysfunction in HIV patients
Endothelial dysfunction is associated with an impaired ability of the vascular lining to maintain normal homeostasis. In vivo, it predominantly but not exclusively describes the reduced ability of arteries to dilate in response to flow-induced nitric oxide (NO) production. In vitro, correlates for vascular dysfunction include the NO-quenching reactive oxygen species (ROS), endothelial chemokine/adhesion protein expression and endothelial cell death (apoptosis or necrosis) because of the contribution of local inflammation and microthromboses to endothelial dysfunction in vivo. Endothelial dysfunction can progress to atherosclerosis and has been shown to predict future cardiovascular events in most population studies [34, 35]. The untreated HIV state has been associated with impaired endothelial function [36, 37]. This condition can be best experimentally assessed using whole virus or viral envelope protein, HIV gp120, which is essential for virus entry by binding CXCR4 or CCR5 receptors, and by activating these receptors to affect T lymphocytes, macrophages, cardiomyocytes, endothelial cells and central nervous system cells [38–41]. HIV gp120 was found in inflammatory cells, endothelial cells and cardiomyocytes in heart tissues from HIV patients with or without HIV cardiomyopathy, while HIV DNA or RNA was only in inflammatory cells [42]. Studies with HIV envelope as recombinant gp120 protein from CXCR4 binding strains have shown it induces endothelial apoptosis by CXCR4-dependent caspase activation [42–43]. Endothelium-derived nitric oxide, synthesized by the endothelial NO synthase (eNOS), is a major mediator of endothelium-dependent vasorelaxation and was reduced by HIV gp120 in TNF-α-activated endothelial cells [44]. Interestingly, exposure to cigarette smoke and HIV gp120 causes a synergistic increase in endothelial cell death[45].
However, as discussed in the paragraphs above, ART and its associated reduction in viral replication does not fully normalize endothelial activation and dysfunction. Thus, a better understanding of mechanisms for continued endothelial dysfunction in ART-treated HIV patients is needed to devise new therapeutic strategies to decrease cardiovascular risk in HIV-infected patients.
Early HIV-encoded proteins and endothelial dysfunction
The ongoing viral contribution to vascular dysfunction and CVD may be partially explained by the presence of HIV-infected reservoir cells, as reservoir cells and associated cytokine signaling are suggested to be important in the development and progression of cardiomyopathy and encephalopathy [46]. According to a prevailing view, infected cells hide in tissues such as the lymphatic system [47]. But how can these reservoir T cells possibly influence atherosclerosis development when systemic levels of released HIV proteins and proinflammatory cytokines are largely reduced when virus production is halted? Certainly, low-level transcription of HIV genes continues even after years of ART [48–50], but their relevance for disease is unclear. Interestingly, further analysis has shown that the majority of these transcripts represent the “early” HIV genes Tat, Rev, and Nef. As Rev is a nuclear protein [51] with no significant effects on vascular biology, we will focus on HIV-Tat and HIV-Nef proteins in the following paragraphs.
1. Effects of HIV-Tat on the vasculature
HIV Trans-Activator of Transcription (Tat) protein, a regulatory protein, is essential for efficiency of viral transcription [52] and has been implicated in several disease conditions ranging from pulmonary hypertension to sleep disorder [42] [53] [54]. Several in vivo studies suggest that Tat causes aberrant cell signaling and leads to altered endothelial cell morphology, gene expression, and survival. HIV-Tat has been suggested to play a role in HIV-related Kaposi sarcoma by promoting endothelial cell proliferation and tumor angiogenesis [55]; and Tat protein was shown to promote inflammation by activating human endothelial cells [56]. In contrast to its roles in angiogenesis, Tat can also cause apoptosis of primary microvascular endothelial cells of lung origin via either tumor necrosis factor secretion or the Fas pathway [57].
2. Role of HIV-Nef in endothelial dysfunction
Negative factor (Nef) is decreased to a much lesser extent than other HIV gene products after initiation of antiretroviral treatment [58, 59] suggesting that Nef could play a role in mechanisms of cardiovascular dysfunction in HIV patients on ART. In general, Nef is known as an important HIV pathogenic factor [60]. Nef is also responsible for T cell activation in infected cells [61, 62] and enhances virus production in vivo [63]. In fact, transgenic mice expressing CD4-promoter-driven Nef develop a spectrum of pathologies including AIDS-like disease [64] and vasospasm in the heart [65], and certain Nef gene variants were linked to pulmonary hypertension [66, 67]. We have recently reported that HIV-infected T cells are more potent than free virus in activating coronary arterial endothelial cells [68]. There is evidence that this effect is Nef-dependent. Nef-deleted virus shows only residual activity, suggesting that Nef, when compared to all other proteins including envelope gp120 and Tat, is the main contributor of HIV-induced endothelial activation.
Our and other groups [66, 68] have demonstrated Nef protein in endothelium of coronary and pulmonary arteries in SIV-HIV-Nef-infected macaques, which showed a relatively high percentage of blood cells constantly probing vascular endothelial cells [69], Thus, endothelial cells, especially those in developing atherosclerotic plaques, are constantly in direct contact with circulating monocytes and T cells and in prime physical position to receive Nef transfer. In the proinflammatory conditions associated with viremic and even aviremic HIV infection, endothelial cell activation leads to increased vascular adhesion protein and chemokine expression. This activation promotes T cell and monocyte adherence to endothelial cells, often followed by diapedesis. Importantly, Nef expressing T cells have been shown to exhibit increased adherence to endothelial cells based on their impaired diapedesis and migration into the subendothelium space [70].
In summary, the present review highlights that HIV early gene-encoded proteins, in particular Tat and Nef, could play a role in the development of CVD in the HIV-infected population independent of traditional risk factors (e.g. ART treatment and smoking/alcohol/drug use) (Table 1). By understanding the mechanisms of these risk factors, we may ultimately discover new ways to treat and prevent CVD in the HIV infected population.
Table 1.
Concluded the risk factors and their main contributions to CVD in HIV infected population.
| Category of risk factor | Risk factor | Main contribution to CAD |
|---|---|---|
| ART | Protease inhibitors | Induce ROS activation and may induce endothelial cell apoptosis Induces dyslipidemia |
| NRTI | Increase platelet reactivity | |
| NNRTI | Induce monocytes to adhere to vascular endothelium Induces dyslipidemia | |
| HIV | Virus | Induces MCP-1 production |
| Nef protein | Induces MCP-1 production, ROS activity and endothelial apoptosis eNOS downregulation | |
| Tat Protein | Induces expression of MCP-1 and adhesion molecules, including VCAM-1.ICAM-1 and E-selectin | |
| Behaviors | Smoking, alcohol and drug abuse | Inflammation, Immune dysfunction, Synergy with gp120 to induce vascular endothelial cell death |
| Co-infection | Virus such as Hepatitis C,human herpesvirus 4 and Cytomegalovirus Bacteria | inflammation |
Schematic 1.
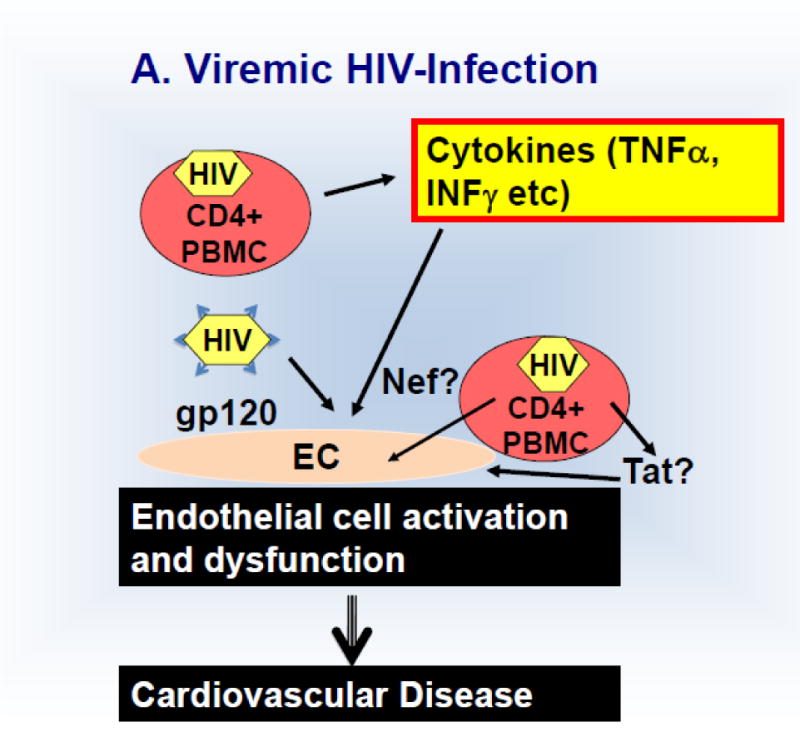
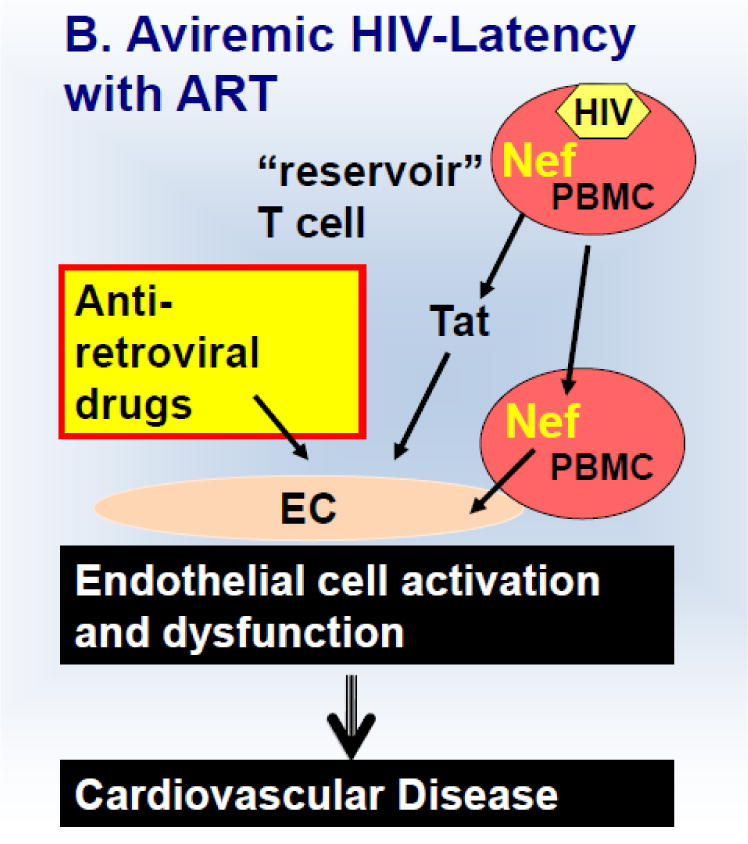
Discussed mechanism of HIV-induced endothelial activation and dysfunction in untreated viremic (A) HIV patients and those on anti-retroviral treatment (B).
Acknowledgments
Funding Sources
This review was supported by grants from the National Institutes of Health (HL095149, HL120390 to MC) and a pre-doctoral fellowship grant from the American Heart Association (13PRE14780025 to TW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All authors report no conflicts.
References
- 1.Grinspoon SK, et al. State of the science conference: Initiative to decrease cardiovascular risk and increase quality of care for patients living with HIV/AIDS: executive summary. Circulation. 2008;118(2):198–210. doi: 10.1161/CIRCULATIONAHA.107.189622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamin DS, Grinspoon SK. Cardiovascular disease in HIV-positive patients. AIDS. 2005;19(7):641–52. doi: 10.1097/01.aids.0000166087.08822.bc. [DOI] [PubMed] [Google Scholar]
- 3.Sackoff JE, et al. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145(6):397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 4.Currier JS, et al. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118(2):e29–35. doi: 10.1161/CIRCULATIONAHA.107.189624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obel N, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis. 2007;44(12):1625–31. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 6.Triant VA, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grunfeld C, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23(14):1841–9. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freiberg MS, et al. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA Intern Med. 2013:1–9. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freiberg MS, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ullrich CK, Groopman JE, Ganju RK. HIV-1 gp120- and gp160-induced apoptosis in cultured endothelial cells is mediated by caspases. Blood. 2000;96(4):1438–42. [PubMed] [Google Scholar]
- 11.Jeong SJ, et al. Clinical factors associated with carotid plaque and intima-medial thickness in HIV-infected patients. Yonsei Med J. 2013;54(4):990–8. doi: 10.3349/ymj.2013.54.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Post WS, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160(7):458–67. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowe SM, et al. The macrophage: the intersection between HIV infection and atherosclerosis. J Leukoc Biol. 2010;87(4):589–98. doi: 10.1189/jlb.0809580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitch KV, et al. Increased coronary artery calcium score and noncalcified plaque among HIV-infected men: relationship to metabolic syndrome and cardiac risk parameters. J Acquir Immune Defic Syndr. 2010;55(4):495–9. doi: 10.1097/QAI.0b013e3181edab0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown TT, Glesby MJ. Management of the metabolic effects of HIV and HIV drugs. Nat Rev Endocrinol. 2012;8(1):11–21. doi: 10.1038/nrendo.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein JH, Currier JS, Hsue PY. Arterial disease in patients with human immunodeficiency virus infection: what has imaging taught us? JACC Cardiovasc Imaging. 2014;7(5):515–25. doi: 10.1016/j.jcmg.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chanthong P, et al. Echocardiography and carotid intima-media thickness among asymptomatic HIV-infected adolescents in Thailand. AIDS. 2014;28(14):2071–9. doi: 10.1097/QAD.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallon PW. Impact of nucleoside reverse transcriptase inhibitors on coronary heart disease. Rev Cardiovasc Med. 2014;15(Suppl 1):S21–9. [PubMed] [Google Scholar]
- 19.Cao R, et al. Prevention of HIV protease inhibitor-induced dysregulation of hepatic lipid metabolism by raltegravir via endoplasmic reticulum stress signaling pathways. J Pharmacol Exp Ther. 2010;334(2):530–9. doi: 10.1124/jpet.110.168484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo PT. Dyslipidemia and coronary artery disease. Clin Cardiol. 1994;17(10):519–27. doi: 10.1002/clc.4960171003. [DOI] [PubMed] [Google Scholar]
- 21.Kuller LH, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armah KA, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis. 2012;55(1):126–36. doi: 10.1093/cid/cis406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandler NG, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203(6):780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duprez DA, et al. N-terminal-proB-type natriuretic peptide predicts cardiovascular disease events in HIV-infected patients. AIDS. 2011;25(5):651–7. doi: 10.1097/QAD.0b013e32834404a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsue PY, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23(9):1059–67. doi: 10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karim R, et al. T-Cell Activation, Both Pre- and Post-HAART Levels, Correlates With Carotid Artery Stiffness Over 6.5 Years Among HIV-Infected Women in the WIHS. J Acquir Immune Defic Syndr. 2014;67(3):349–56. doi: 10.1097/QAI.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strategies for Management of Antiretroviral Therapy Study, G. et al. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ Cell counts and HIV RNA levels during follow-up. J Infect Dis. 2008;197(8):1145–55. doi: 10.1086/529523. [DOI] [PubMed] [Google Scholar]
- 28.Hansen L, et al. Endothelial dysfunction, arterial stiffening, and intima-media thickening in large arteries from HIV-1 transgenic mice. Ann Biomed Eng. 2013;41(4):682–93. doi: 10.1007/s10439-012-0702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freiberg MS, et al. The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circ Cardiovasc Qual Outcomes. 2011;4(4):425–32. doi: 10.1161/CIRCOUTCOMES.110.957415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friis-Moller N, et al. Cardiovascular disease risk factors in HIV patients–association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17(8):1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 31.Petoumenos K, et al. Rates of cardiovascular disease following smoking cessation in patients with HIV infection: results from the D:A:D study(*) HIV Med. 2011;12(7):412–21. doi: 10.1111/j.1468-1293.2010.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freiberg MS, et al. The association between alcohol consumption and prevalent cardiovascular diseases among HIV-infected and HIV-uninfected men. J Acquir Immune Defic Syndr. 2010;53(2):247–53. doi: 10.1097/QAI.0b013e3181c6c4b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gopal M, et al. Heart Disease in Patients with HIV/AIDS-An Emerging Clinical Problem. Curr Cardiol Rev. 2009;5(2):149–54. doi: 10.2174/157340309788166705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gokce N, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41(10):1769–75. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 35.Suwaidi JA, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 36.Torriani FJ, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52(7):569–76. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf K, et al. Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients infected with human immunodeficiency virus type 1. J Infect Dis. 2002;185(4):456–62. doi: 10.1086/338572. [DOI] [PubMed] [Google Scholar]
- 38.Hogan CM, Hammer SM. Host determinants in HIV infection and disease. Part 2: genetic factors and implications for antiretroviral therapeutics. Ann Intern Med. 2001;134(10):978–96. doi: 10.7326/0003-4819-134-10-200105150-00012. [DOI] [PubMed] [Google Scholar]
- 39.Ahr B, et al. Apoptosis of uninfected cells induced by HIV envelope glycoproteins. Retrovirology. 2004;1:12. doi: 10.1186/1742-4690-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Twu C, et al. Cardiomyocytes undergo apoptosis in human immunodeficiency virus cardiomyopathy through mitochondrion- and death receptor-controlled pathways. Proc Natl Acad Sci U S A. 2002;99(22):14386–91. doi: 10.1073/pnas.212327899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanmogne GD, Kennedy RC, Grammas P. HIV-1 gp120 proteins and gp160 peptides are toxic to brain endothelial cells and neurons: possible pathway for HIV entry into the brain and HIV-associated dementia. J Neuropathol Exp Neurol. 2002;61(11):992–1000. doi: 10.1093/jnen/61.11.992. [DOI] [PubMed] [Google Scholar]
- 42.Fiala M, et al. HIV-1 induces cardiomyopathyby cardiomyocyte invasion and gp120, Tat, and cytokine apoptotic signaling. Cardiovasc Toxicol. 2004;4(2):97–107. doi: 10.1385/ct:4:2:097. [DOI] [PubMed] [Google Scholar]
- 43.Fiala M, et al. HAART drugs induce mitochondrial damage and intercellular gaps and gp120 causes apoptosis. Cardiovasc Toxicol. 2004;4(4):327–37. doi: 10.1385/ct:4:4:327. [DOI] [PubMed] [Google Scholar]
- 44.Jiang J, et al. HIV gp120 induces endothelial dysfunction in tumour necrosis factor-alpha-activated porcine and human endothelial cells. Cardiovasc Res. 2010;87(2):366–74. doi: 10.1093/cvr/cvq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green LA, et al. HIV envelope protein gp120-induced apoptosis in lung microvascular endothelial cells by concerted upregulation of EMAP II and its receptor, CXCR3. Am J Physiol Lung Cell Mol Physiol. 2014;306(4):L372–82. doi: 10.1152/ajplung.00193.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orenstein JM. Replication of HIV-1 in vivo and in vitro. Ultrastruct Pathol. 2007;31(2):151–67. doi: 10.1080/01913120701344343. [DOI] [PubMed] [Google Scholar]
- 47.Haase AT. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu Rev Immunol. 1999;17:625–56. doi: 10.1146/annurev.immunol.17.1.625. [DOI] [PubMed] [Google Scholar]
- 48.Furtado MR, et al. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N Engl J Med. 1999;340(21):1614–22. doi: 10.1056/NEJM199905273402102. [DOI] [PubMed] [Google Scholar]
- 49.Fischer M, et al. Residual HIV-RNA levels persist for up to 2.5 years in peripheral blood mononuclear cells of patients on potent antiretroviral therapy. AIDS Res Hum Retroviruses. 2000;16(12):1135–40. doi: 10.1089/088922200414974. [DOI] [PubMed] [Google Scholar]
- 50.Gunthard HF, et al. Residual human immunodeficiency virus (HIV) Type 1 RNA and DNA in lymph nodes and HIV RNA in genital secretions and in cerebrospinal fluid after suppression of viremia for 2 years. J Infect Dis. 2001;183(9):1318–27. doi: 10.1086/319864. [DOI] [PubMed] [Google Scholar]
- 51.Cochrane A, et al. The human immunodeficiency virus rev protein is a nuclear phosphoprotein. Virology. 1989;171(1):264–6. doi: 10.1016/0042-6822(89)90535-7. [DOI] [PubMed] [Google Scholar]
- 52.Debaisieux S, et al. The ins and outs of HIV-1 Tat. Traffic. 2012;13(3):355–63. doi: 10.1111/j.1600-0854.2011.01286.x. [DOI] [PubMed] [Google Scholar]
- 53.Aoki Y, Tosato G. HIV-1 Tat enhances Kaposi sarcoma-associated herpesvirus (KSHV) infectivity. Blood. 2004;104(3):810–4. doi: 10.1182/blood-2003-07-2533. [DOI] [PubMed] [Google Scholar]
- 54.Wang T, et al. Intracellular Nef protein detected in PBMCs from HIV patients. AIDS Res Hum Retroviruses. 2014 doi: 10.1089/aid.2013.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Albini A, et al. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat Med. 1996;2(12):1371–5. doi: 10.1038/nm1296-1371. [DOI] [PubMed] [Google Scholar]
- 56.Hofman FM, et al. Exogenous tat protein activates human endothelial cells. Blood. 1993;82(9):2774–80. [PubMed] [Google Scholar]
- 57.Park IW, et al. HIV-1 Tat induces microvascular endothelial apoptosis through caspase activation. J Immunol. 2001;167(5):2766–71. doi: 10.4049/jimmunol.167.5.2766. [DOI] [PubMed] [Google Scholar]
- 58.Fischer M, et al. Cellular viral rebound after cessation of potent antiretroviral therapy predicted by levels of multiply spliced HIV-1 RNA encoding nef. J Infect Dis. 2004;190(11):1979–88. doi: 10.1086/425983. [DOI] [PubMed] [Google Scholar]
- 59.Fischer M, et al. Biphasic decay kinetics suggest progressive slowing in turnover of latently HIV-1 infected cells during antiretroviral therapy. Retrovirology. 2008;5:107. doi: 10.1186/1742-4690-5-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foster JL, Garcia JV. HIV-1 Nef: at the crossroads. Retrovirology. 2008;5:84. doi: 10.1186/1742-4690-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lundquist CA, et al. Nef-mediated downregulation of CD4 enhances human immunodeficiency virus type 1 replication in primary T lymphocytes. J Virol. 2002;76(9):4625–33. doi: 10.1128/JVI.76.9.4625-4633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mangasarian A, et al. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J Virol. 1999;73(3):1964–73. doi: 10.1128/jvi.73.3.1964-1973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kestler HW, 3rd, et al. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65(4):651–62. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 64.Weng X, et al. CD4+ T cells from CD4C/HIVNef transgenic mice show enhanced activation in vivo with impaired proliferation in vitro but are dispensable for the development of a severe AIDS-like organ disease. J Virol. 2004;78(10):5244–57. doi: 10.1128/JVI.78.10.5244-5257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kay DG, et al. Cardiac disease in transgenic mice expressing human immunodeficiency virus-1 nef in cells of the immune system. Am J Pathol. 2002;161(1):321–35. doi: 10.1016/S0002-9440(10)64184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Almodovar S, et al. Pathogenesis of HIV-associated pulmonary hypertension: potential role of HIV-1 Nef. Proc Am Thorac Soc. 2011;8(3):308–12. doi: 10.1513/pats.201006-046WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Almodovar S, et al. Human Immunodeficiency Virus nef signature sequences are associated with pulmonary hypertension. AIDS Res Hum Retroviruses. 2012;28(6):607–18. doi: 10.1089/aid.2011.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang T, et al. Transfer of intracellular HIV Nef to endothelium causes endothelial dysfunction. PLoS One. 2014;9(3):e91063. doi: 10.1371/journal.pone.0091063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zarbock A, et al. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcR gamma to induce slow leukocyte rolling. J Exp Med. 2008;205(10):2339–47. doi: 10.1084/jem.20072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stolp B, et al. HIV-1 Nef interferes with T-lymphocyte circulation through confined environments in vivo. Proc Natl Acad Sci U S A. 2012;109(45):18541–6. doi: 10.1073/pnas.1204322109. [DOI] [PMC free article] [PubMed] [Google Scholar]


