Abstract
Objective
Concerns about over-treatment have led to practice guidelines discouraging active treatment of prostate cancer (PCa) in men with limited life expectancies and/or low-risk tumors. We evaluated treatment patterns for older veterans with localized PCa, particularly those with low-risk features.
Methods
We used VA Cancer Registry data to identify men aged 65+ diagnosed with clinically localized PCa between January 1st 2003 and December 31st 2008. We obtained baseline data on demographics, tumor characteristics, comorbidities, and initial treatment within 6 months of diagnosis: radical prostatectomy, radiotherapy, primary androgen-deprivation therapy (PADT), or no active treatment. National VA surveys provided facility data, including academic affiliation, availability of oncologic specialists, and distance to radiotherapy facilities. Multinomial regression analyses determined associations between patient and facility characteristics and cancer treatment for men with localized and low-risk PCa (stage ≤ IIa, PSA <10 ng/mL, Gleason ≤6).
Results
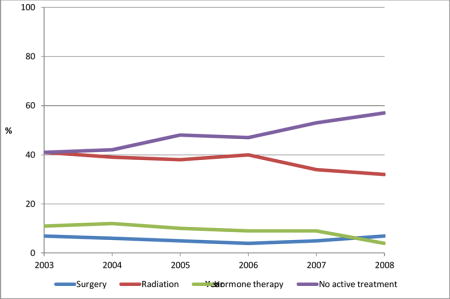
17,206 veterans had localized PCa, 32% age 75+ had 12% comorbidity scores ≥ 3, and 33% had low-risk tumors. Overall, 39% received radiotherapy, 6% surgery, 20% PADT, and 35% no active treatment. For those with low-risk cancers, older men (RR = 0.36, 95% CI 0.30–0.43) and sicker men (RR = 0.75, 95% CI 0.62–0.90) were less likely to receive surgery or radiotherapy versus no active treatment. Over time, more of these men received no active treatment (41% to 57%, P < 0.001) while fewer received PADT (11% to 4%, P < 0.001).
Conclusion
VA treatment patterns followed evidence-based guidelines against treating older and sicker men with surgery or radiotherapy, for decreasing use of PADT, and for increasingly withholding active treatment, particularly for men with low-risk PCa.
Keywords: prostatic neoplasms, prostatectomy, radiotherapy, watchful waiting, aged, physician’s practice patterns
Graphical abstract
Introduction
Prostate-specific antigen (PSA) testing has dramatically increased the observed incidence of localized prostate cancer.[1] Most men with these cancers undergo surgery or radiotherapy,[2] even though a high proportion of screen-detected cancers are not likely to benefit from treatment.[3] Furthermore, the optimal treatment for localized prostate cancer is uncertain because no randomized trials have published results comparing surgery versus radiotherapy. Treatment options are even more uncertain for older men because they have been excluded from randomized treatment trials.[4–6] Accordingly, treatment guidelines recommend observation – either watchful waiting or active surveillance – as an appropriate option for older men with a localized cancer, particularly those with low-risk tumor characteristics (based on clinical stage, PSA levels, Gleason scores, and biopsy tumor burden).[7, 8] Nonetheless, data from Surveillance, Epidemiology, and End Results (SEER)–Medicare[9–11] and the Cancer of the Prostate Strategic Urological Research Endeavor (CaPSURE)[2] document that over 70% of older men with a low-risk cancer undergo surgery or radiotherapy.
Nambudiri et al. previously used Veterans Affairs (VA) Central Tumor Registry and national VA facility survey data to characterize variation in prostate cancer treatment for veterans of all ages diagnosed with local/regional cancers during 2001–2004.[12] We updated this analysis by using more recent VA data, and focused on the patient, tumor, and facility factors associated with treatment selection among older men with localized cancer, particularly those with low-risk tumor characteristics.
Methods
Data and patients
The VA Central Cancer Registry collects uniformly reported information on all US veterans who receive a diagnosis of prostate cancer or receive their first course of cancer treatment at a VA Medical Center facility. We used registry data to identify a cohort of veterans aged 65 or older with an incident of clinically localized prostate cancer (clinical tumor stages T1/T2)[13] diagnosed between January 1st, 2003 and December 31st, 2008. “Clinically localized” was defined as a cancer confined to the prostate on the basis of digital rectal examination with or without imaging studies. We used linked VA and Medicare inpatient and outpatient claims to exclude men with a history of prostate cancer, radical prostatectomy, or androgen-deprivation therapy (ADT) between January 1st 1999 and their VA cancer diagnosis date.
Outcome measure
The major outcome was primary prostate cancer treatment within 6 months of the registry date of diagnosis, including a radical prostatectomy, external-beam radiation therapy or brachytherapy (radiotherapy) with or without concomitant ADT, primary ADT (PADT), and no active treatment (we could not determine whether men opted for watchful waiting or active surveillance). Men who underwent multiple treatments in the 6 months after diagnosis were assigned to the most aggressive treatment (radical prostatectomy > radiotherapy > PADT).
Patient-level characteristics
We obtained registry data on demographic characteristics, including age (stratified as 65–69, 70–74, ≥75), race/ethnicity (white, black, other), marital status, US Census geographic regions,[14] distance to the diagnosing VA facility (stratified as <10, 10–50, >50 miles and based on distance in linear miles from the centroid of the patient’s zip code to the VA facility), whether or not patients were followed at a community-based outpatient clinic, and residence in a zip code area with >25% college graduates based on 2000 Census data.[15]
Additional patient-level variables included year of diagnosis, comorbidity (based on Charlson scores from VA and Medicare claims in the year before diagnosis), nearest PSA result before date of cancer diagnosis, and tumor characteristics (clinical stage, tumor grade).
Facility-level characteristics
We used a survey of 138 Veterans Health Affairs Medical Centers conducted in December 2005 (response rate 100%) to obtain data on academic affiliations, based on training residents and staff physicians having faculty appointments, and the availability of radiation and surgical oncologists.[12] A subsequent survey of these Medical Centers collected data on the availability of a urology faculty and urology residents and distance to the nearest facility for external-beam radiation and brachytherapy.[12] We assigned facility characteristics to the diagnosing facility even if patients were treated elsewhere.
Statistical analyses
We used descriptive statistics to characterize baseline characteristics, including patient-level, facility-level, and time period. We compared differences across treatment groups (surgery, radiotherapy, PADT, and no active treatment) with chi-square tests. We used multinomial logistic regression analyses to assess the associations between patient- and facility-level characteristics with primary cancer treatment, accounting for clustering at the level of the facility using a robust variance estimator. We evaluated these associations for the full cohort of men diagnosed with clinically localized cancers and for the subgroup of men with low-risk cancers, defined as clinical stage T1c (tumor identified by needle biopsy e.g. because of elevated PSA) or T2a (tumor involves one-half of one lobe or less), PSA level < 10 ng/mL, and Gleason score ≤ 6. We performed all analyses using statistical software SAS 9.4 (SAS Institute, Cary, North Carolina, USA) and STATA 12.1 (College Station, Texas, USA). All tests of statistical significance were two-sided. This study was approved by the Committee on Human Research at the University of California, San Francisco, and the Committee for Research and Development at the San Francisco Veterans Affairs Medical Center.
Results
Study cohorts
We identified 17,206 Veterans aged 65 and older who were diagnosed with or received their first course of treatment for localized prostate cancer at a Veterans Affairs Medical Center between January 1st, 2003 and December 31st, 2008. Among these men, about one third were older than 75, 76% were white, 59% were married, and nearly half were from the South (Table 1). About 12% had a Charlson comorbidity score ≥ 3; 64% were clinical stage T1c, 31% had a PSA level ≥ 10 ng/mL, and 48% had a Gleason score > 6. Overall, 5616 (33%) of men with a localized cancer had low-risk features. Baseline characteristics of the men with low-risk cancers were generally similar to the entire cohort of men with localized cancer, though men with low-risk cancers were more likely to be younger than 70 (Table 2).
Table 1.
Baseline patient and facility characteristics and unadjusted rates of primary treatments for men with localized prostate cancers.
| Baseline Characteristics | All (n, %) | RP (%) | Radiation (%) | PADT (%) | No active treatment (%) | P-value |
|---|---|---|---|---|---|---|
|
| ||||||
| N = 17,206 (100.0) |
N = 991 (5.8) |
N = 6,648 (38.8) |
N = 3,486 (20.3) |
N = 6,061 (35.2) |
||
|
| ||||||
| Age | <0.0001 | |||||
|
| ||||||
| 65–69 | 5,821 (33.8) | 10.3 | 44.5 | 10.5 | 34.7 | |
| 70–74 | 5,821 (33.8) | 5.1 | 44.9 | 15.6 | 34.4 | |
| 75+ | 5,564 (32.4) | 1.7 | 26.3 | 35.3 | 36.7 | |
|
| ||||||
| Race* | <0.0001 | |||||
|
| ||||||
| White | 12,987 (75.9) | 6.4 | 38.9 | 19.2 | 35.5 | |
| Black | 3,503 (20.5) | 3.5 | 39.7 | 21.3 | 35.5 | |
| Other | 611 (3.6) | 4.9 | 31.1 | 33.9 | 30.1 | |
|
| ||||||
| Marrieda | <0.0001 | |||||
|
| ||||||
| No | 6,935 (40.7) | 4.8 | 37.9 | 21.5 | 35.8 | |
| Yes | 10,112 (59.3) | 6.5 | 39.4 | 19.3 | 34.8 | |
|
| ||||||
| Lived in an area in which ≥25% of adults had a college educationa | <0.0001 | |||||
|
| ||||||
| No | 11,876 (71.6) | 5.9 | 39.1 | 17.1 | 37.9 | |
|
| ||||||
| Yes | 4,702 (28.4) | 5.7 | 38.6 | 21.6 | 34.1 | |
|
| ||||||
| Census regionb | <0.0001 | |||||
|
| ||||||
| Midwest | 3,676 (21.4) | 6.0 | 39.0 | 18.3 | 36.7 | |
| Northeast | 2,437 (14.2) | 5.5 | 40.3 | 16.7 | 37.5 | |
| South | 8,017 (46.5) | 4.9 | 38.9 | 23.9 | 32.3 | |
| West | 3,076 (17.9) | 7.9 | 36.7 | 16.1 | 39.3 | |
|
| ||||||
| Charlson comorbidity score | <0.0001 | |||||
|
| ||||||
| 0 | 9,952 (57.8) | 6.8 | 39.8 | 19.1 | 34.3 | |
| 1–2 | 5,148 (29.9) | 4.7 | 38.5 | 20.9 | 35.9 | |
| ≥ 3 | 2,106 (12.3) | 3.3 | 34.3 | 24.4 | 38.0 | |
|
| ||||||
| Clinical stagec | < 0.001 | |||||
|
| ||||||
| T1c | 10,988 (63.9) | 5.4 | 39.7 | 17.6 | 37.3 | |
| T2 | 872 (5.1) | 10.6 | 30.5 | 24.2 | 34.7 | |
| T2a/b/c | 5,346 (31.0) | 5.6 | 38.2 | 25.1 | 31.1 | |
|
| ||||||
| PSA (ng/mL, before cancer diagnosis) | <0.0001 | |||||
|
| ||||||
| < 4 | 2,062 (12.0) | 7.6 | 35.6 | 16.0 | 40.8 | |
| 4 to <10 | 9,849 (57.2) | 6.6 | 42.9 | 13.3 | 37.2 | |
| ≥ 10 | 5,295 (30.8) | 3.5 | 32.2 | 35.0 | 29.3 | |
|
| ||||||
| Gleason score (biopsy)a | <0.0001 | |||||
|
| ||||||
| 2–6 | 8,808 (51.7) | 8.4 | 47.7 | 15.5 | 28.4 | |
| 7–10 | 8,215 (48.3) | 8.2 | 53.9 | 26.7 | 11.2 | |
|
| ||||||
| Distance to diagnosing VA (miles)a | 0.02 | |||||
|
| ||||||
| 0 to <10 | 9,257 (53.8) | 5.3 | 38.8 | 19.8 | 36.1 | |
| 10 to <50 | 7,642 (44.5) | 6.1 | 38.8 | 20.9 | 34.2 | |
| 50+ | 290 (1.7) | 7.9 | 40.3 | 19.0 | 32.8 | |
|
| ||||||
| Seen at community-based outpatient clinic within 1 year before/after cancer diagnosis | <0.0001 | |||||
|
| ||||||
| No | 11,497 (66.8) | 6.5 | 38.9 | 20.2 | 34.4 | |
| Yes | 5,709 (33.2) | 4.2 | 38.6 | 20.3 | 36.9 | |
|
| ||||||
| Academic affiliation* | 0.39 | |||||
|
| ||||||
| No | 507 (3.0) | 6.9 | 51.7 | 22.5 | 18.9 | |
| Yes | 16,145 (97.0) | 8.2 | 50.3 | 20.9 | 20.6 | |
|
| ||||||
| Availability of urologists | <0.001 | |||||
|
| ||||||
| No | 880 (5.1) | 6.5 | 37.5 | 22.6 | 33.4 | |
| Urologists but no urology residents | 5,002 (29.1) | 5.6 | 41.6 | 22.9 | 29.9 | |
| Urologists and urology residents | 11,324 (65.8) | 5.8 | 37.6 | 18.9 | 37.7 | |
|
| ||||||
| Availability of radiation oncologists | <0.001 | |||||
|
| ||||||
| No | 7,749 (45.0) | 7.0 | 39.2 | 20.3 | 33.5 | |
| Yes | 9,457 (55.0) | 4.8 | 38.4 | 20.2 | 36.6 | |
|
| ||||||
| Year of cancer diagnosis | <0.001 | |||||
|
| ||||||
| 2003 | 2,286 (13.3) | 6.3 | 39.2 | 22.0 | 32.5 | |
| 2004 | 2,929 (17.0) | 6.3 | 38.9 | 22.4 | 32.4 | |
| 2005 | 2,735 (15.9) | 5.1 | 40.3 | 20.1 | 34.5 | |
| 2006 | 2,885 (16.8) | 4.3 | 41.2 | 20.3 | 34.2 | |
| 2007 | 3,309 (19.2) | 5.4 | 36.1 | 21.4 | 37.1 | |
| 2008 | 3,062 (17.8) | 7.1 | 37.5 | 15.8 | 39.6 | |
Missing values: race, 105; marital status, 159; college education, 628; VA distance, 17; Gleason score, 183; academic affiliation, 554.
States comprising census regions. Midwest: IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI; Northeast: CT, MA, ME, NJ, NY, PA, RI, VT; South: AR, AL, D.C., DE, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, WV; West: AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY.
Clinical tumor stage definitions: 1C, tumor identified by needle biopsy (e.g., because of elevated PSA); 2, tumor confined within prostate; 2A, tumor involves one half of one lobe or less; 2B, tumor involves more than one half of one lobe but not both lobes; 2C, tumor involves both lobes.
RP, radical prostatectomy; PADT, primary androgen deprivation; PSA, prostate-specific antigen; VA, Veterans Affairs.
Table 2.
Baseline patient and facility characteristics and unadjusted rates of primary treatments for men with low-risk prostate cancers
| Baseline characteristics | All (n, %) |
Radical prostatectomy (%) |
Radiation (%) |
PADT (%) |
No active treatment (%) |
P-value |
|---|---|---|---|---|---|---|
|
| ||||||
| N = 5,616 (100.0) | N = 312 (5.6) | N = 2,096 (37.3) | N = 509 (9.1) | N = 2,699 (48.1) | ||
|
| ||||||
| Agea | <0.0001 | |||||
|
| ||||||
| 65–69 | 2,371 (42.2) | 8.8 | 41.6 | 5.5 | 44.1 | |
| 70–74 | 2,005 (35.7) | 4.2 | 40.8 | 8.9 | 46.1 | |
| 75+ | 1,240 (22.1) | 1.5 | 23.4 | 16.2 | 58.9 | |
|
| ||||||
| Racea | 0.002 | |||||
|
| ||||||
| White | 4,403 (78.7) | 6.0 | 36.5 | 9.2 | 48.3 | |
| Black | 1,046 (18.7) | 3.5 | 40.7 | 7.7 | 48.1 | |
| Other | 146 (2.6) | 6.8 | 37.0 | 13.7 | 42.5 | |
|
| ||||||
| Marrieda | <0.0001 | |||||
|
| ||||||
| No | 2,118 (38.0) | 4.5 | 37.2 | 8.8 | 49.5 | |
| Yes | 3,451 (62.0) | 6.3 | 37.3 | 9.2 | 47.2 | |
|
| ||||||
| Lived in an area in which ≥25% of adults had a college educationa | <0.001 | |||||
|
| ||||||
| No | 3,800 (70.0) | 5.6 | 37.3 | 10.1 | 47.0 | |
| Yes | 1,631 (30.0) | 5.5 | 37.0 | 6.8 | 50.7 | |
|
| ||||||
| Census regionb | <0.0001 | |||||
|
| ||||||
| Midwest | 1,238 (22.1) | 5.0 | 37.5 | 7.7 | 49.8 | |
| Northeast | 893 (15.9) | 5.7 | 38.1 | 6.8 | 49.4 | |
| South | 2,490 (44.3) | 5.0 | 39.4 | 11.5 | 44.1 | |
| West | 995 (17.7) | 7.4 | 31.2 | 6.8 | 54.6 | |
|
| ||||||
| Charlson comorbidity score | <0.0001 | |||||
|
| ||||||
| 0 | 3,279 (58.4) | 6.3 | 38.7 | 9.4 | 45.6 | |
| 1–2 | 1,686 (30.0) | 4.8 | 36.8 | 8.4 | 50.0 | |
| ≥ 3 | 651 (11.6) | 3.7 | 31.8 | 9.1 | 55.4 | |
|
| ||||||
| Clinical tumor stage | 0.07 | |||||
|
| ||||||
| T1c | 4,655 (82.9) | 5.5 | 37.8 | 8.9 | 47.8 | |
|
| ||||||
| T2a | 961 (17.1) | 5.6 | 35.2 | 9.7 | 49.5 | |
|
| ||||||
| PSA (ng/mL, before cancer diagnosis) | <0.0001 | |||||
|
| ||||||
| < 4 | 884 (15.7) | 6.2 | 30.1 | 8.8 | 54.9 | |
| 4 to <10 | 4,732 (84.3) | 5.4 | 38.7 | 9.1 | 46.8 | |
| ≥ 10 | – | – | – | – | – | |
|
| ||||||
| Gleason score (biopsy)a | <0.0001 | |||||
|
| ||||||
| 2–6 | 5,616 (100.0) | 5.6 | 37.3 | 9.1 | 48.0 | |
| 7–10 | – | – | – | – | – | |
|
| ||||||
| Distance to diagnosing VA (miles)a | <0.0001 | |||||
|
| ||||||
| 0 to <10 | 3,020 (53.8) | 5.1 | 37.8 | 8.9 | 48.2 | |
| 10 to <50 | 2,503 (44.6) | 6.0 | 37.1 | 9.2 | 47.7 | |
| 50+ | 91 (1.6) | 7.7 | 29.7 | 9.9 | 52.7 | |
|
| ||||||
| Seen at community-based outpatient clinic within 1 year before/after cancer diagnosis | 0.003 | |||||
|
| ||||||
| No | 3,708 (66.0) | 6.2 | 37.8 | 9.3 | 46.7 | |
| Yes | 1,908 (34.0) | 4.3 | 36.3 | 8.7 | 50.7 | |
|
| ||||||
| Academic affiliationa | 0.30 | |||||
|
| ||||||
| No | 152 (2.8) | 7.2 | 34.9 | 12.5 | 45.4 | |
| Yes | 5,278 (97.2) | 5.3 | 37.3 | 8.9 | 48.5 | |
|
| ||||||
| Availability of urologists | <0.001 | |||||
|
| ||||||
| No | 263 (4.7) | 8.0 | 33.4 | 11.8 | 46.8 | |
| Urologists but no urology residents | 1,566 (27.9) | 5.6 | 42.2 | 12.9 | 39.3 | |
| Urologists and urology residents | 3,787 (67.4) | 5.4 | 35.6 | 7.3 | 51.7 | |
|
| ||||||
| Availability of radiation oncologists | <0.001 | |||||
|
| ||||||
| No | 2,486 (44.3) | 7.2 | 38.6 | 10.4 | 43.8 | |
| Yes | 3,130 (55.7) | 4.3 | 36.3 | 8.0 | 51.4 | |
|
| ||||||
| Year of cancer diagnosis | <0.001 | |||||
|
| ||||||
| 2003 | 839 (14.9) | 6.6 | 41.3 | 11.0 | 41.1 | |
| 2004 | 998 (17.8) | 6.4 | 39.3 | 12.0 | 42.3 | |
| 2005 | 870 (15.5) | 4.7 | 38.2 | 9.5 | 47.6 | |
| 2006 | 923 (16.4) | 3.8 | 40.2 | 9.0 | 47.0 | |
| 2007 | 1,034 (18.4) | 5.1 | 33.8 | 8.6 | 52.5 | |
| 2008 | 952 (17.0) | 6.7 | 32.1 | 4.4 | 56.8 | |
Missing values: race, 21; marital status, 47; college education, 185; VA distance, 2; academic affiliation, 186.
States comprising census regions. Midwest: IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI; Northeast: CT, MA, ME, NJ, NY, PA, RI, VT; South: AR, AL, D.C., DE, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, WV; West: AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY.
Clinical tumor stage definitions: 1C, tumor identified by needle biopsy (e.g., because of elevated PSA); 2, tumor confined within prostate; 2A, tumor involves one half of one lobe or less; 2B, tumor involves more than one half of one lobe but not both lobes; 2C, tumor involves both lobes
PADT, primary androgen deprivation; PSA, prostate-specific antigen; VA, Veterans Affairs.
Treatment Selection
Tables 1 and 2 also show the unadjusted rates of primary treatments for men with localized and low-risk prostate cancers, respectively, according to patient-level and VA-facility-level characteristics and time period. Overall, radiotherapy was the most common active treatment and surgery was the least common treatment for men with localized or low-risk cancer. Nearly half of the men with a low-risk cancer received no active treatment. In bivariate analyses, almost all covariates were significantly associated with the type of primary treatment. From 2003 to 2008 the proportion of men with localized and low-risk cancers receiving no active treatment increased markedly, while fewer men received PADT. For men with localized cancers, the absolute increase in receiving no active treatment during this time period was 7 percentage points (P < 0.001) for trend: a relative increase of 22%. The absolute decrease in receiving PADT was 6 percentage points (P < 0.001): a relative decrease of 28%. For men with low-risk cancers, the absolute increase in receiving no active treatment was 16 percentage points (P < 0.001): a relative increase of 38%. The absolute decrease in receiving PADT was 7 percentage points (P < 0.001): a relative decrease of 60%.
Factors independently associated with treatment for localized prostate cancer
Results from multivariable analyses of factors associated with receiving individual active treatments (surgery, radiotherapy, PADT) for localized prostate cancer versus receiving no active treatment are shown in Table 3. Older men were significantly less likely than younger men to undergo either surgery or radiotherapy versus receiving no active treatment, but more likely to receive PADT. Black men were less likely than white men to receive surgery versus receiving no active treatment; we found no differences by race for receiving radiotherapy or PADT. Married men were more likely than unmarried men to undergo surgery versus receiving no active treatment. Men living in higher socioeconomic areas (based on educational achievement) were less likely than those living in lower socioeconomic areas to receive PADT versus receiving no active treatment.
Table 3.
Relative risk ratios (95%CI) for receiving specific active treatments versus no active treatment by baseline sociodemographic, clinical, tumor, and facility characteristics, and year of diagnosis among men with localized cancer (N = 17,206)
| Baseline characteristics | Surgery versus no active treatment | Radiotherapy versus no active treatment | PADT versus no active treatment |
|---|---|---|---|
|
| |||
| Age (years) | |||
| 65–69 (reference) | – | – | – |
| 70–74 | 0.47 (0.40–0.55) | 0.90 (0.83–0.98) | 1.29 (1.13–1.49) |
| 75+ | 0.13 (0.09–0.17) | 0.47 (0.41–0.54) | 2.25 (1.94–2.61) |
|
| |||
| Race | |||
| White (reference) | – | – | – |
| Black | 0.55 (0.42–0.73) | 1.04 (0.90–1.20) | 1.07 (0.91–1.27) |
| Other | 1.07 (0.70–1.63) | 1.06 (0.81–1.39) | 1.81 (1.38–2.38) |
|
| |||
| Marrieda | |||
| No (reference) | – | – | – |
| Yes | 1.47 (1.23–1.76) | 1.06 (0.98–1.16) | 0.91 (0.82–1.02) |
|
| |||
| Lived in an area in which ≥ 25% of adults had a college educationb | |||
| No (reference) | – | – | – |
| Yes | 0.96 (0.80–1.14) | 0.97 (0.88–1.08) | 0.84 (0.73–0.97) |
|
| |||
| Census region | |||
| Midwest (reference) | – | – | – |
| Northeast | 1.21 (0.78–1.88) | 1.13 (0.87–1.45) | 0.85 (0.57–1.27) |
| South | 0.95 (0.65–1.39) | 1.07 (0.81–1.42) | 1.49 (1.07–2.08) |
| West | 1.17 (0.80–1.71) | 0.79 (0.59–1.06) | 0.79 (0.53–1.19) |
|
| |||
| Charlson comorbidity score | |||
| 0 (best health) (reference) | – | – | – |
| 1–2 (average health) | 0.71 (0.61–0.83) | 0.96 (0.88–1.04) | 1.08 (0.95–1.22) |
| ≥ 3 (worst health) | 0.46 (0.34–0.63) | 0.86 (0.76–0.97) | 1.18 (1.04–1.33) |
|
| |||
| Clinical tumor stage | |||
| T1c (reference) | |||
| T2 | 1.38 (1.14–1.66) | 1.08 (0.98–1.18) | 1.34 (1.18–1.50) |
|
| |||
| PSA (proximal to cancer diagnosis) | |||
| < 4 ng/mL (reference) | – | – | – |
| 4 to < 10 ng/mL | 0.92 (0.72–1.17) | 1.33 (1.13–1.56) | 0.96 (0.77–1.19) |
| ≥ 10 ng/mL | 0.73 (0.55–0.95) | 1.28 (1.10–1.48) | 2.44 (1.93–3.09) |
|
| |||
| Gleason score | |||
| 2–6 (reference) | – | – | – |
| 7–10 | 2.67 (2.26–3.16) | 2.58 (2.30–2.89) | 3.35 (2.89–3.88) |
|
| |||
| Distance to diagnosing VA | |||
| 0 to <10 miles (reference) | – | – | – |
| 10 to <50 miles | 0.98 (0.84–1.14) | 0.95 (0.86–1.06) | 0.98 (0.85–1.13) |
| 50+ miles | 1.34 (0.72–2.49) | 1.17 (0.88–1.55) | 1.15 (0.75–1.76) |
|
| |||
| Seen at VA community-based outpatient clinic in 1 year on/after cancer diagnosis date | |||
| No (reference) | – | – | – |
| Yes | 0.62 (0.49–0.78) | 0.94 (0.82–1.08) | 1.04 (0.89–1.21) |
|
| |||
| VA with academic affiliation | |||
| No (reference) | – | – | – |
| Yes | 1.00 (0.54–1.86) | 1.02 (0.67–1.55) | 0.99 (0.59–1.67) |
|
| |||
| Availability of urologists | |||
| No (reference) | – | – | – |
| Urologists but no urology residents | 1.15 (0.50–2.61) | 1.28 (0.82–1.98) | 0.90 (0.50–1.65) |
| Urologists and urology residents | 1.35 (0.61–2.99) | 0.92 (0.61–1.39) | 0.58 (0.32–1.06) |
|
| |||
| Availability of radiation oncologists | |||
| No (reference) | – | – | – |
| Yes | 0.69 (0.51–0.93) | 0.95 (0.76–1.20) | 0.95 (0.71–1.27) |
|
| |||
| Year of cancer diagnosis | |||
| 2003 (reference) | – | – | – |
| 2004 | 1.05 (0.72–1.53) | 0.92 (0.74–1.14) | 0.96 (0.78–1.18) |
| 2005 | 0.83 (0.59–1.17) | 0.91 (0.71–1.17) | 0.77 (0.61–0.98) |
| 2006 | 0.78 (0.52–1.19) | 0.91 (0.72–1.16) | 0.78 (0.62–0.98) |
| 2007 | 0.85 (0.60–1.20) | 0.72 (0.56–0.92) | 0.74 (0.58–0.94) |
| 2008 | 1.01 (0.70–1.46) | 0.68 (0.53–0.88) | 0.47 (0.36–0.62) |
PADT, primary androgen deprivation; PSA, prostate-specific antigen; VA, Veterans Affairs.
Compared to men with no comorbidities, those with ≥ 3 comorbidities were less likely to undergo surgery or radiotherapy and more likely to receive PADT versus receiving no active treatment. Men with clinical stage T2 were more likely than those with clinical stage T1c to receive an active treatment versus receiving no active treatment. Men with PSA levels ≥ 10 ng/mL were more likely than those with PSA levels < 4.0 ng/mL to receive either radiotherapy or PADT (and less likely to undergo surgery) versus receiving no active treatment. Men with Gleason scores >6 were uniformly more likely than those with lower Gleason scores to receive an active treatment versus receiving no active treatment.
Availability of radiation oncologists was associated with a decreased likelihood of receiving surgery versus receiving no active treatment, though this was not associated with the likelihood of receiving radiation versus no active treatment. In more recent years, men with localized prostate cancer were significantly less likely to receive radiotherapy or PADT versus receiving no active treatment, though surgery rates remained stable relative to no active treatment.
Factors independently associated with treatment for low-risk localized prostate cancer
Table 4 shows results of multivariable analyses of factors associated with receiving aggressive treatment (surgery or radiotherapy) or receiving PADT versus receiving no active treatment for men with a low-risk prostate cancer. The factors associated with treatment were similar to those for men with localized cancer.
Table 4.
Relative risk ratios (95%CI) for receiving active treatments versus no active treatment by baseline sociodemographic, clinical, tumor, and facility characteristics, and year of diagnosis among men with low-risk localized cancers (N = 5,616)
| Baseline characteristics | Surgery or radiotherapy versus no active treatment | PADT versus no active treatment |
|---|---|---|
|
| ||
| Age (years) | ||
| 65–69 (reference) | – | – |
| 70–74 | 0.79 (0.70–0.90) | 1.38 (1.05–1.80) |
| 75+ | 0.36 (0.30–0.43) | 2.13 (1.50–3.02) |
|
| ||
| Race | ||
| White (reference) | – | – |
| Black | 1.11 (0.91–1.34) | 0.93 (0.70–1.25) |
| Other | 1.38 (0.88–2.15) | 1.83 (0.88–3.80) |
|
| ||
| Marrieda | ||
| No (reference) | – | – |
| Yes | 1.05 (0.92–1.20) | 0.91 (0.74–1.13) |
|
| ||
| Lived in an area in which ≥ 25% of adults had a college educationa | ||
| No (reference) | – | – |
| Yes | 0.97 (0.83–1.13) | 0.72 (0.56–0.93) |
|
| ||
| Census region | ||
| Midwest (reference) | – | – |
| Northeast | 1.19 (0.90–1.58) | 0.80 (0.43–1.50) |
| South | 1.17 (0.85–1.62) | 1.65 (1.00–2.73) |
| West | 0.73 (0.52–1.03) | 0.78 (0.45–1.37) |
|
| ||
| Charlson comorbidity score | ||
| 0 (best health) (reference) | – | – |
| 1–2 (average health) | 0.91 (0.80–1.04) | 0.83 (0.65–1.06) |
| ≥ 3 (worst health) | 0.75 (0.62–0.90) | 0.82 (0.62–1.10) |
|
| ||
| Clinical tumor stage | ||
| T1a (reference) | – | – |
| T2a | 1.06 (0.90–1.25) | 1.06 (0.81–1.40) |
|
| ||
| PSA (proximal to cancer diagnosis) | ||
| < 4 ng/mL (reference) | – | – |
| 4 to <10 ng/mL | 1.45 (1.15–1.82) | 1.29 (0.92–1.81) |
|
| ||
| Distance to diagnosing VA | ||
| 0 to <10 miles (reference) | – | – |
| 10 to <50 miles | 0.88 (0.77–1.01) | 0.87 (0.66–1.16) |
| 50+ miles | 0.74 (0.47–1.15) | 0.80 (0.30–2.10) |
|
| ||
| Seen at VA community–based outpatient clinic in 1 year on/after cancer diagnosis date | ||
| No | – | – |
| Yes | 0.98 (0.84–1.14) | 1.14 (0.88–1.48) |
|
| ||
| VA with academic affiliation | ||
| No (reference) | – | – |
| Yes | 1.21 (0.84–1.76) | 0.93 (0.32–2.73) |
|
| ||
| Availability of urologists | ||
| None (reference) | – | – |
| Urologists but no urology residents | 1.32 (0.82–2.13) | 1.09 (0.50–2.37) |
| Urologists and urology residents | 0.93 (0.60–1.45) | 0.51 (0.24–1.10) |
|
| ||
| Availability of radiation oncologists | ||
| No (reference) | – | – |
| Yes | 0.78 (0.60–1.02) | 0.86 (0.49–1.53) |
|
| ||
| Year of cancer diagnosis | ||
| 2003 (reference) | – | – |
| 2004 | 0.98 (0.75–1.29) | 1.05 (0.70–1.56) |
| 2005 | 0.86 (0.66–1.11) | 0.78 (0.53–1.15) |
| 2006 | 0.93 (0.73–1.19) | 0.75 (0.51–1.12) |
| 2007 | 0.71 (0.55–0.93) | 0.65 (0.43–0.99) |
| 2008 | 0.61 (0.46–0.81) | 0.30 (0.19–0.46) |
PADT, primary androgen deprivation; PSA, prostate-specific antigen; VA, Veterans Affairs.
Discussion
We found that nearly two thirds of the veterans diagnosed with localized prostate cancer from 2003 through 2008 received active treatment, with nearly 40% receiving radiotherapy, about 6% undergoing radical prostatectomy, and 20% receiving PADT. Among those with low-risk cancers, nearly half received no active treatment while 37% received radiotherapy. Treatment selection was associated with patient and tumor characteristics and year of diagnosis. Year of diagnosis was strongly correlated with the likelihood of receiving no active treatment for men with low-risk cancer, increasing from 41% in 2003 to 57% in 2008.
Increasing age and comorbidity scores were inversely associated with veterans receiving surgery or radiotherapy for localized cancer versus receiving no active treatment, but positively associated with receiving PADT. These findings are similar to results reported for SEER-Medicare[11] and CaPSURE[2] cohorts, except that older and sicker men in the latter cohorts were more likely to receive radiotherapy. The VA practice pattern is more consistent with guidelines suggesting that aggressive treatment will benefit only men with at least a 10-year life expectancy.[7] Guideline recommendations are based on the lengthy lead time associated with PSA-detected cancers[3] as well as clinical trial data showing a treatment survival benefit only for men diagnosed before the age of 65.[4]
We observed that veterans older than 75 were more likely to receive PADT than to receive no active treatment, although use of PADT declined over time. PADT is no longer considered a treatment option for localized prostate cancer[7] based on lack of efficacy[16] and potential harms, including increased risks for osteoporotic fractures[17], diabetes and cardiovascular disease.[18]
Higher-risk tumor features (classified by clinical stage, PSA level, and Gleason score) were associated with receipt of an active treatment. SEER–Medicare[11] and CaPSURE[2] data show similar treatment patterns, even among men over the age of 75.[10] Observational data have suggested that high-risk tumor features predict an increased likelihood of prostate cancer mortality across all age ranges.[19] In PIVOT, post-hoc analyses suggested that prostatectomy was associated with decreased prostate cancer mortality among men with PSA > 10 ng/mL and possibly among those with intermediate or high tumor risk scores.[5] Prostatectomy was also associated with decreased prostate cancer mortality in the SPCG-4, where most subjects had clinically detected tumors.[4] However, neither of these trials enrolled men aged 75 and older, so the benefit of attempting curative therapy among older men, even those with a high-risk cancer, is uncertain.
Given the often indolent course of localized prostate cancer, treatment guidelines recommend observation for men with low-risk prostate cancer.[7, 20] This strategy minimizes intervention-related complications that can adversely affect quality of life, including erectile dysfunction, urinary incontinence, and bowel problems.[21] About half of the men in our cohort with low-risk cancers did not receive any active treatment during the study period; this proportion increased with time. Our overall treatment data reflect the national trends towards higher proportions of men with low-risk cancers receiving no active treatment.[22] However, among veterans with a low-risk cancer, 43% received surgery or radiation therapy. In contrast, SEER data from 2004–2006 showed that 75% of men (mean age 63.9 years) with PSA levels < 4.0 ng/mL and Gleason scores < 7 received primary surgery or radiotherapy.[9]
We found that black men with localized prostate cancer were less likely to undergo surgery than whites, although equally likely to receive radiotherapy or PADT. Similar racial differences in receiving surgery have been reported by investigators using data from SEER population-based registries[24] and CaPSURE.[25] Differences in these settings have been partly attributed to difficulties in accessing care related to socioeconomic status. However, finding differences in equal-access systems such as the Veterans Health Administration and Department of Defense[26] suggests more complex explanations. Lack of trust in the healthcare system[27] and less informed decision-making processes[28] could be factors, as could differences in comorbidity. The population-based Prostate Cancer Outcomes Study (PCOS) found that high comorbidity scores were inversely associated with undergoing surgery, and that racial differences in receipt of aggressive treatment occurred only among men over 60.[24] Medicare data show that older black men with prostate cancer have higher comorbidity than whites.[29] We did adjust for comorbidity, but black men could have had more unobserved comorbidity or more severe comorbidity than white men.
Distance from treatment facility has been associated with lower likelihood of receiving radiotherapy than surgery for breast cancer given the need for prolonged courses of radiation therapy.[30] However, on multivariate analysis we did not find distance from the diagnosing facility to be associated with treatment selection. Availability of radiation oncologists was inversely associated with men receiving surgery for localized cancer, possibly reflecting physician attitudes towards the treatment they deliver.[31] Alternatively, urologists are referring patients to radiation oncologists for second opinions, particularly when considering active surveillance. Academic medical centers have been among the strongest proponents of active surveillance,[32–34] and we found that facilities with urology residents had the highest proportion of men receiving no treatment, although this finding did not reach statistical significance on multivariate analysis.
Our study had some potential limitations. We categorized treatment selection according to treatment received within 6 months of diagnosis. We might have misclassified patients as having no active treatment if they subsequently received treatment after 6 months. While we could classify low-risk patients on the basis of clinical tumor stage (though the AJCC definition of stage T2a changed slightly from 2003 to 2004, from being unilateral tumor to unilateral tumor comprising ≤ 50% of the gland), PSA level, and Gleason score, we did not have information on biopsy results (number of positive cores, tumor volume) that are also used to identify men eligible for active surveillance.[7, 8] We also could not determine whether the category of no active treatment reflected active surveillance or watchful waiting. SEER data suggest that active surveillance was increasing during the study time period.[35] While we adjusted for comorbidity in evaluating treatment patterns, veterans generally have poorer health than civilians, and this could bias comparisons with population-based data.[36] Nonetheless, SEER population-based data show high proportions of active treatment among men with low-risk cancers and/or limited life expectancy.[9–11] Finally, we did not have information about patient preferences or decision-making processes which could help address racial differences in treatment selection.
Conclusions
VA practice patterns reflect concerns about over-treating localized prostate cancers, particularly those with low-risk characteristics. Older and sicker men were less likely to undergo surgery and radiotherapy. Use of PADT appropriately declined during the study period, while the proportion of men who received no treatment increased dramatically. VA facilities appeared less likely than other settings (based on nearly contemporaneous data) to provide treatments that were not supported by clinical evidence or recommended by guidelines. Our findings suggest the potential value of an integrated health-care system in reducing unnecessary utilization, though there is still considerable room for improvement.
Highlights.
We evaluated treatment patterns for older veterans with localized prostate cancer
Nearly half of the men with low-risk prostate cancer received no active treatment
The use of primary androgen deprivation decreased over time
Increasing age and comorbidity were inversely associated with active treatment
Treatment patterns followed guidelines for conservatively managing localized cancer
Acknowledgments
Grant R01 CA134425 from the National Cancer Institute at the National Institutes of Health (LCW, SJF, RMH), grant K24AG041180 from the National Institute on Aging at the National Institutes of Health (LCW), the New Mexico Veterans Affairs Health Care System (RMH), and the Prostate Cancer Foundation (NLK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Hoffman has received partial salary support from the non-profit Foundation for Informed Medical Decisions to edit a decision aid on prostate cancer treatment.
Conflicts of interest
None of the other authors have any conflicts for this work.
Contributor Information
Richard M. Hoffman, Email: richard-m-hoffman@uoiwa.edu.
Ying Shi, Email: Ying.Shi2@va.gov.
Stephen J. Freedland, Email: stephen.freedland@cshs.org.
Nancy L. Keating, Email: keating@hcp.med.harvard.edu.
Louise C. Walter, Email: louise.walter@ucsf.edu.
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute; p. 2104. [Google Scholar]
- 2.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374–83. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932–42. doi: 10.1056/NEJMoa1311593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–13. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al. Prostate Testing for Cancer and Treatment (ProtecT) feasibility study. Health Technol Assess. 2003;7:1–88. doi: 10.3310/hta7140. [DOI] [PubMed] [Google Scholar]
- 7.Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–31. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Mohler J, Bahnson RR, Boston B, Busby JE, D’Amico A, Eastham JA, et al. NCCN clinical practice guidelines in oncology: prostate cancer. Journal of the National Comprehensive Cancer Network: JNCCN. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 9.Shao YH, Albertsen PC, Roberts CB, Lin Y, Mehta AR, Stein MN, et al. Risk profiles and treatment patterns among men diagnosed as having prostate cancer and a prostate-specific antigen level below 4.0 ng/ml. Arch Intern Med. 2010;170:1256–61. doi: 10.1001/archinternmed.2010.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts CB, Albertsen PC, Shao YH, Moore DF, Mehta AR, Stein MN, et al. Patterns and correlates of prostate cancer treatment in older men. The American journal of medicine. 2011;124:235–43. doi: 10.1016/j.amjmed.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs BL, Zhang Y, Schroeck FR, Skolarus TA, Wei JT, Montie JE, et al. Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA. 2013;309:2587–95. doi: 10.1001/jama.2013.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nambudiri VE, Landrum MB, Lamont EB, McNeil BJ, Bozeman SR, Freedland SJ, et al. Understanding variation in primary prostate cancer treatment within the Veterans Health Administration. Urology. 2012;79:537–45. doi: 10.1016/j.urology.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th. New York, NY: Springer; 2010. [Google Scholar]
- 14.US Census Bureau. Census regions and divisions of the United States/prepared by the Geography Division [Google Scholar]
- 15.US Census Bureau. Census 2000 summary file 3-United States/prepared by the US Census Bureau. 2002 [Google Scholar]
- 16.Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–81. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 18.Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2009;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293:2095–101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. The NCCN Clinical Practice Guidelines in Oncology Prostate Cancer (Version 1.2010) 2010 doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 21.Chou R, Dana T, Bougatsos C, Fu R, Blazina I, Gleitsmann K, et al. Treatments for Localized Prostate Cancer: Systematic Review to Update the 2002 US Preventive Services Task Force Recommendation. Rockville (MD): 2011. [PubMed] [Google Scholar]
- 22.Weiner AB, Patel SG, Etzioni R, Eggener SE. National trends in the management of low and intermediate risk prostate cancer in the United States. J Urol. 2015;193:95–102. doi: 10.1016/j.juro.2014.07.111. [DOI] [PubMed] [Google Scholar]
- 23.Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Annals of internal medicine. 2008;149:185–91. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 24.Harlan LC, Potosky A, Gilliland FD, Hoffman R, Albertsen PC, Hamilton AS, et al. Factors associated with initial therapy for clinically localized prostate cancer: prostate cancer outcomes study. J Natl Cancer Inst. 2001;93:1864–71. doi: 10.1093/jnci/93.24.1864. [DOI] [PubMed] [Google Scholar]
- 25.Moses KA, Paciorek AT, Penson DF, Carroll PR, Master VA. Impact of ethnicity on primary treatment choice and mortality in men with prostate cancer: data from CaPSURE. J Clin Oncol. 2010;28:1069–74. doi: 10.1200/JCO.2009.26.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moul JW, Wu H, Sun L, McLeod DG, Amling C, Lance R, et al. Epidemiology of radical prostatectomy for localized prostate cancer in the era of prostate-specific antigen: an overview of the Department of Defense Center for Prostate Disease Research national database. Surgery. 2002;132:213–9. doi: 10.1067/msy.2002.125315. [DOI] [PubMed] [Google Scholar]
- 27.Corbie-Smith G, Thomas SB, St George DM. Distrust, race, and research. Arch Intern Med. 2002;162:2458–63. doi: 10.1001/archinte.162.21.2458. [DOI] [PubMed] [Google Scholar]
- 28.Zeliadt SB, Ramsey SD, Penson DF, Hall IJ, Ekwueme DU, Stroud L, et al. Why do men choose one treatment over another?: a review of patient decision making for localized prostate cancer. Cancer. 2006;106:1865–74. doi: 10.1002/cncr.21822. [DOI] [PubMed] [Google Scholar]
- 29.Putt M, Long JA, Montagnet C, Silber JH, Chang VW, Kaijun L, et al. Racial differences in the impact of comorbidities on survival among elderly men with prostate cancer. Medical care research and review: MCRR. 2009;66:409–35. doi: 10.1177/1077558709333996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nattinger AB, Kneusel RT, Hoffmann RG, Gilligan MA. Relationship of distance from a radiotherapy facility and initial breast cancer treatment. J Natl Cancer Inst. 2001;93:1344–6. doi: 10.1093/jnci/93.17.1344. [DOI] [PubMed] [Google Scholar]
- 31.Fowler FJ, Jr, McNaughton Collins M, Albertsen PC, Zietman A, Elliott DB, Barry MJ. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. JAMA. 2000;283:3217–22. doi: 10.1001/jama.283.24.3217. [DOI] [PubMed] [Google Scholar]
- 32.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–31. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 33.Tosoian JJ, Trock BJ, Landis P, Feng Z, Epstein JI, Partin AW, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185–90. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 34.Cooperberg MR, Cowan JE, Hilton JF, Reese AC, Zaid HB, Porten SP, et al. Outcomes of active surveillance for men with intermediate-risk prostate cancer. J Clin Oncol. 2011;29:228–34. doi: 10.1200/JCO.2010.31.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filson CP, Schroeck FR, Ye Z, Wei JT, Hollenbeck BK, Miller DC. Variation in Use of Active Surveillance among Men Undergoing Expectant Treatment for Early Stage Prostate Cancer. J Urol. 2014 doi: 10.1016/j.juro.2014.01.105. [DOI] [PubMed] [Google Scholar]
- 36.Hoerster KD, Lehavot K, Simpson T, McFall M, Reiber G, Nelson KM. Health and health behavior differences: U.S. Military, veteran, and civilian men. American journal of preventive medicine. 2012;43:483–9. doi: 10.1016/j.amepre.2012.07.029. [DOI] [PubMed] [Google Scholar]


