Abstract
Background and study aims: SmartPill® (Given Imaging Corp.,Yoqneam,Israel) is an ingestible, non-imaging capsule that records physiological data including contractions and pH throughout the gastrointestinal tract. There are scarce data looking at SmartPill® assessment of patients with known/suspected small-bowel Crohn’s Disease (CD). This pilot study aims to investigate feasibility and safety of SmartPill® to assess gut motility in this group.
Patients and methods: Over 1 year, patients with known/suspected CD, referred for small-bowel capsule endoscopy (SBCE), were invited to participate and 12 were recruited (7 female, 5 male, mean age 44.2 ± 16.6 years). They underwent hydrogen breath test to exclude small-bowel bacterial overgrowth, patency capsule (Agile®), and provided stool samples for fecal calprotectin (FC). Patients ingested PillCam®SB2 and SmartPill® 4 hours apart. Using unpublished data, 33 healthy controls also were identified for the study. P < 0.05 was considered statistically significant.
Results: Of the 12 patients enrolled, 10 underwent complete Smartpill® examination (1 stomach retention, 1 dropout). Pillcam® was complete in 10 (1 dropout, 1 stomach retention). Mean fecal calprotectin was 340 ± 307.71 mcg/g. The study group had longer transit times and lower gut motility index than did the controls. The difference in motility appears to be statistically significant (P < 0.05). Longer transit times for SmartPill® (not statistically significant) may have been due to different specifications between the capsules. Limitations included transient Smartpill® signal loss (5/10 studies).
Conclusions: This is the first pilot to attempt combining SBCE and SmartPill® to assess small-bowel CD. Data on motility in CD are scarce. Multimodal information can provide a clearer clinical picture. Despite concerns about capsule retention in CD patients, SmartPill® seems safe for use if a patency capsule is employed beforehand.
Introduction
The wireless motility capsule (WMC) (SmartPill®; Given Imaging Corp., Yoqneam, Israel) is a single-use, ingestible device 1 2. With dimensions 26.8 × 11.7 mm, it is slightly bulkier than its imaging counterpart (PillCam®SB Medtronic, Minnesota, USA). SmartPill® records intraluminal pH, pressure and temperature as it is propelled through the gastrointestinal tract. Hence, the WMC is capable of providing gut motility parameters (i. e. gastric transit time [GTT], small-bowel transit time [SBTT], colonic transit time [CTT] and whole gut transit time [WGTT]) noninvasively. The American and European Neurogastroenterology & Motility Societies recommend the use of WMC to assess suspected gastroparesis, suspected small-bowel (SB) dysmotility and/or CTT in chronic constipation 3.
Data are scarce on the motility patterns in patients with known or suspected Crohn’s disease (CD). Furthermore, the use and clinical validity of the WMC has not been evaluated in this patient group. It is envisaged that future wireless investigation platforms for the digestive tract will be multimodal and versatile, and therefore able to incorporate imaging information with physiological or biochemistry data such as fecal calprotectin (FC), hemoglobin and gas constituents of the gastrointestinal tract. Such combination data could be useful in the investigation and management of patients with CD. For instance, orocecal transit time has been found to be prolonged in CD patients for various reasons including SB bacterial overgrowth (SBBO) whereas SBTT may conversely be shortened in CD patients following ileocecal resection; this would affect absorption of medications and should ideally be taken into account during drug design 4. Therefore, we designed a pilot study to investigate whether WMC examination is feasible and safe in the assessment of gut motility in patients with known or suspected CD, and its utility compared to conventional video capsule endoscopy.
Patients and methods
Patient recruitment and study protocol
Consecutive patients with known or suspected CD (FC > 200 μg/g), referred for SB evaluation with small-bowel capsule endoscopy (SBCE), were invited to participate in this study. The inclusion & exclusion criteria of the study are summarized in Table 1. Patients who accepted the invitation and consented to participate were invited for a lactulose hydrogen breath test for exclusion of SB bacterial overgrowth (SBBO) and were provided with a kit for stool sample collection and FC measurement (CALPROLAB™ ELISA (ALP), Calpro AS, Lysaker, Norway; reference range < 50 μg/g). Those with a positive breath test, indicating SBBO, were excluded. Patients with negative SBBO breath test were invited to return a stool specimen and attend for a SB patency check with the AGILE® capsule (Given Imaging Corp., Yoqneam, Israel).
Table 1. Inclusion and Exclusion Criteria.
| Inclusion criteria | Exclusion criteria |
|
|
Abbreviations: CD, Crohn’s disease; DM, diabetes mellitus; FC, fecal calprotectin; ICD, implantable cardioverter defibrillator; PC, patency capsule; SB, small-bowel; pts, patients
The detailed flowchart of the study design is presented in Fig. 1. Patients ingested the PillCam®SB, followed 4 hours later, by the SmartPill®. The technical characteristics of the two capsules used (PillCam®SB and SmartPill®) are detailed in Table 2.
Fig. 1.
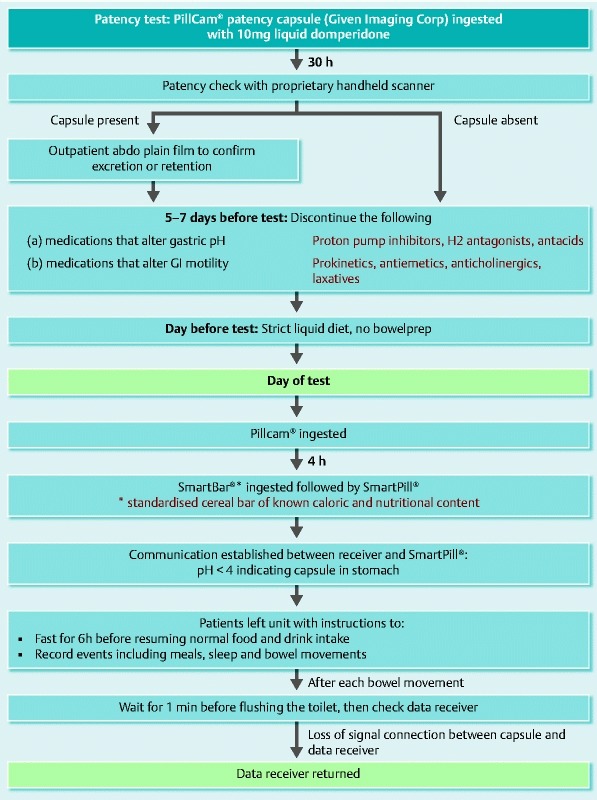
Summary of study protocol.
Table 2. Comparison between specifications of PillCam® SB2 and SmartPill®.
| Specifications | PillCam® SB2 | SmartPill® |
| Length (mm) | 26 | 26 |
| Diameter (mm) | 11 | 13 |
| Battery life | 8 h | 5 days |
| Mode of data transmission | Ultra-high frequency band radio telemetry | Radiofrequency-based |
Data collection
Data were downloaded from the recorders to the relevant workstations and analyzed using proprietary software (RAPID ® for PillCam®SB and semi-automated pressure analysis software, MotiliGI® [Given®Imaging Corp] for SmartPill®). For the latter, results are presented in both graphical and statistical forms. PillCam® data include gut transit times and SB findings. Inflammation levels were quantified using the Lewis score (LS), which has been devised to objectively report SB inflammation in SBCE. SmartPill® data examined in this study were pH, transit times (GTT, SBTT, CTT and WGTT) and motility index (MI) per segment, where MI = Ln (sum of pressure amplitudes × number of contractions + 1). The data acquired from the study group were compared to historical controls (healthy individuals with no known pathology obtained from unpublished data), used to establish the normal range for segmental and total gut transit times.
Statistical analysis
Microsoft Excel (© 2015 Microsoft) and StatsDirect (StatsDirect Ltd, Altrincham, UK) software were used for statistical analysis. A two-tailed Mann-Whitney U test was used for comparison of the study and control groups. Linear regression was used to establish any correlation between motility indices and FC or LS. P values < 0.05 were considered statistically significant.
The study was supported by a defined grant by Given®Imaging Ltd (ESGE- Given®Imaging Research grant 2011) and approved by the local ethics committee (ref. 12/SS/0013).
Results
Over a 12-month period (2012), 19 patients were recruited. Three patients were excluded because their previous history included a known strong functional component to their symptoms that could affect gut motility independently of CD, including irritable bowel syndrome, chronic idiopathic intestinal pseudo-obstruction and cyclic vomiting. Another four patients, referred for SBCE on suspicion of CD, were also excluded because their FC levels were < 200 μg/g. Twelve patients completed the study (7 female/5 male; mean age 44.2 ± 16.6 years). Fig. 2 shows the number of patients recruited, dropouts, and complete/incomplete data sets obtained. Clinical characteristics and per patient study results are tabulated in detail in Table 3. The differences in the motility of the study group vs. the control group are depicted in Table 4. Patients in our study had longer transit times and significantly lower gut motility when compared to the control group, Fig. 3 and Fig. 4.
Fig. 2.
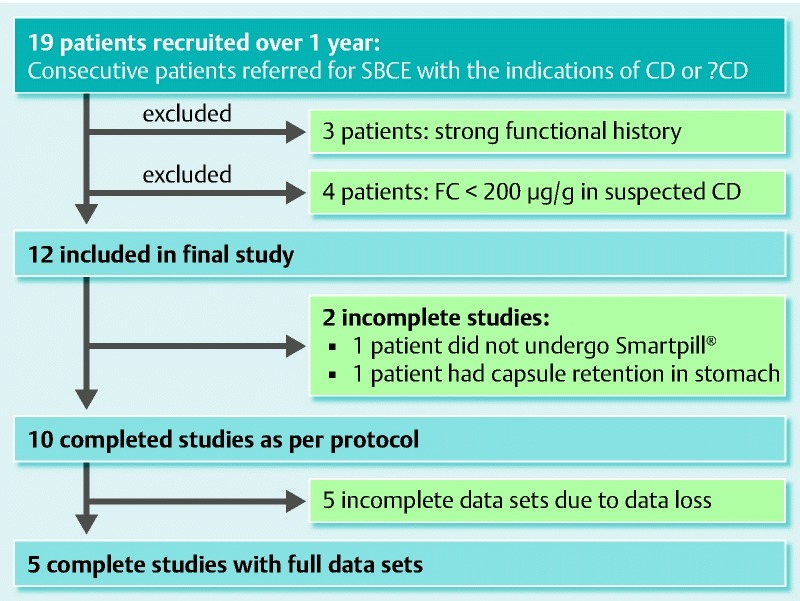
Recruitment process for this study.
Table 3. Summary of clinical characteristics and findings of patients in our study.
| No. | Age (years) | Gender | Indication | MS (if known CD) | FC (μg/g) | SBCE findingsTotal time; GTT; SBTT (min) Findings | LS | MotilGI® report | TT (min)WBTT; GTT; SBTT | pH | MI (segmental) |
| 1 | 49 | M | Known CD | A2L1B1 | 60 | 546; 125; 205Single aphtha, poor views | 135 | Signal loss, long GTT of SBCE but not WMCs | 1667; 226; 141 | n/a | n/a |
| 2 | 37 | M | Known CD | A1 /2L1B1 | – | 516; 36; 242Blood in stomach,no mucosal inflammation | 0 | Generally prolonged transit times, poor motility | 6620; 2577; 288 | Gastric 1.4SB 7.2 | Gastric 16.75Duo 11.60SB 15.18Caecum 12.19 |
| 3 | 58 | F | Known CD | A3L1B1 | 590 | 683; 28; 552Gastric residue + + + , lymphangiectasias, mucosal erythema, ?stenosis x 2 | 3810 | Prolonged transit time | 7161; 1096; 638 | n/a | Gastric – Duo 12.51SB – Caecum 14.17 |
| 4 | 34 | F | Known CD | A2L3B1 p | Insuff | n/a | n/a | High gastric pH, ?pt on PPI | 2686; 867; 240 | Gastric 5.4SB 7.1 | Gastric 16.3Duo 9.89SB 16.24Caecum 14.72 |
| 5 | 72 | F | Known CD | A?L1B1 | Insuff | 857; 77; 252Distortion of folds,Lymphangiectasias, mucosal erythema, multiple aphthae | 5160 | Generally low motility | 1956; 798; 447 | Gastric 1.1SB 7.2 | Gastric 14.65Duo 9.19SB 14.16Caecum 12.00 |
| 6 | 51 | M | Known CD | A2L3B1 | 80 | 436; 65; 342aphtha x1, reticulonodular mucosal pattern | 450 | Signal loss | 1609; n/a; n/a | n/a | n/a |
| 7 | 37 | F | Known CDColectomy + ileoanal pouch | A2L3B1 | 290 | 384; 19; n/aNormal to pouch | 0 | WMC not done – dropout | n/a | n/a | n/a |
| 8 | 40 | F | Known CDPancolectomy + ileostomy | A2L3B1 | – | 410; 10; 254Gastritis, poor views | 0 | Signal loss, rapid transit time | 808*; 233; n/a | n/a | n/a |
| 9 | 66 | F | ?CD | NA | 970 | 369; n/a; n/aGastric retention, pyloric stenosis | n/a | Data loss, CR | n/a | n/a | n/a |
| 10 | 58 | M | ?CD | – | 320 | 517; 31; 169Mucosal oedema & denudation, ? enteropathy | 280 | Low motility, acidic SB | 1312; 167; 192 | Gastric 1.2SB 6.4 | Gastric 11.58Duo 11.41SB 15.98Caecum 14.61 |
| 11 | 36 | M | ?CD | – | 110 | 234; 33; 188Mucosal cobblestone, Several aphthae | 450 | Signal loss but normal transit of WMC | n/a | n/a | n/a |
| 12 | 23 | F | ?CD | – | 300 | 439; 14; 327Aphthae x 2 | 450 | High gastric pH, very long colon transit | 6650; 142; 252 | Gastric 3.7SB 6.6 | Gastric 10.26Duo 11.31SB 15.77Caecum 11.97 |
Abbreviations: CD, Crohn’s Disease; CR, capsule retention; Duo, duodenum; FC, fecal calprotectin; GTT, gastric transit time; LS, Lewis score; MI, motility index; MS, Montreal score; PPI, proton pump inhibitor; SB, small bowel; SBCE, small-bowel capsule endoscopy; SBTT, small-bowel transit time; TT, transit times; WBTT, whole-bowel transit time; WMC, wireless motility capsule
In the case of patient 8, WBTT was taken as time to excretion of capsule in ileostomy.
Table 4. Comparison of results from our patients vs controls. For our patients, some results were not available for all patients, therefore N is given where n = number of patients for whom results were available.
| Patients | Controls | P values | |
| Number | 12 | 33 | |
| Gender | 7 F, 5 M | 15 F, 18 M | |
| Average Age ±SD | 44.25 ±16.66 years | 40.85 ±16.28 years | |
| FC (μg/g) | 340 ±307.71 (n = 8) | n/a | |
| LS | 1073.5 ±1835.5 (n = 10) | n/a | |
| GTT (min) | 763.25 ±821.47 (n = 8) | 249.61 ±167.47 | 0.09 |
| SBTT (min) | 314 ±171.99 (n = 7) | 288.81 ±107.74 | 0.89 |
| WBTT (min) | 3385.44 ±2621.03 (n = 9) | 1988.67 ±972.99 | 0.82 |
| Gastric pH | 2.56 ±1.92 (n = 5) | 1.64 ±0.89 | 0.35 |
| SB pH | 6.9 ±0.37 (n = 5) | 7.16 ±0.45 | 0.17 |
| Gastric MI | 13.91 ±2.88 (n = 5) | 52.00 ±32.68 | 0.002 |
| Duodenal MI | 10.99 ±1.22 (n = 6) | 90.27 ±76.50 | 0.0001 |
| SB MI | 14.55 ±1.92 (n = 5) | 122.48 ±65.90 | 0.0004 |
| Cecal MI | 13.28 ±1.35 (n = 6) | 108.58 ±121.10 | 0.0006 |
Abbreviations: FC, fecal calprotectin; GTT, gastric transit time; LS, Lewis score; MI, motility index; SB, small bowel; SBTT, small-bowel transit time; WBTT, whole-bowel transit time
Fig. 3.
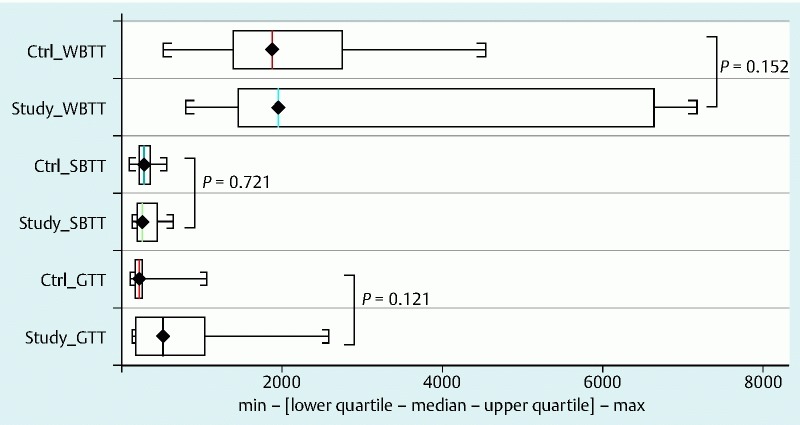
Comparison of transit times between study group and controls. Abbreviations: Ctrl, controls; GTT, gastric transit time; SBTT, small-bowel transit time; WBTT, whole bowel transit time
Fig. 5.

Floating characteristics of Pillcam SB2 (left) and Smartpill (right) submerged in 400 mL sterile water for irrigation.
Fig. 4.
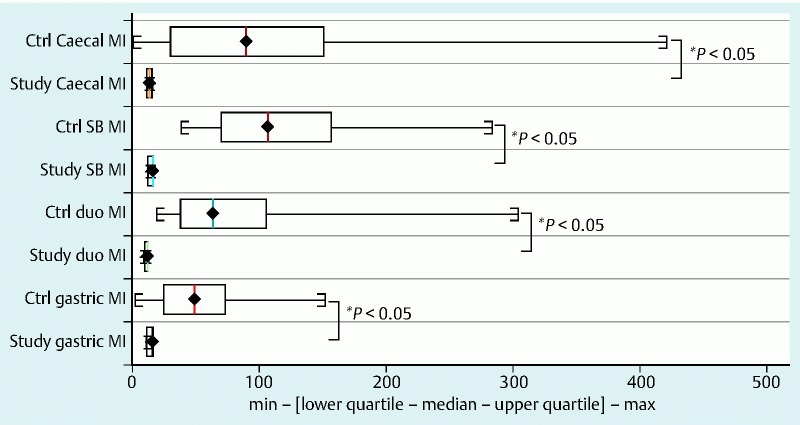
Comparison of motility index between study group and controls. Abbreviations: Ctrl, controls; duo, duodenum; MI, motility index; SB, small bowel
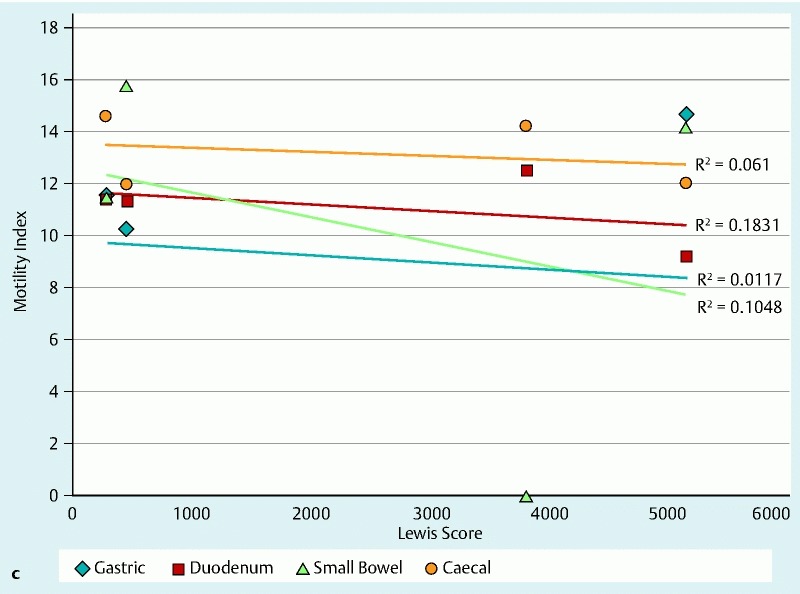
The motility index (MI) in the stomach, SB and colon was significantly lower in patients with CD, as compared with controls, and this was statistically significant (P < 0.05) for all motility indices measured throughout the gut. The total transit time for the WMC was longer compared with the SBCE, which could be attributed to the differences in the capsules’ specifications as detailed in Table 2 1 5 6 and the difference in capsule density, Fig. 5 7 8. The distribution of WGTT, FC and LS for those study subjects for whom the data were available is presented in Fig. 6a. Figs. 6b and 6c show the linear regression between MI/FC and MI/LS, respectively.
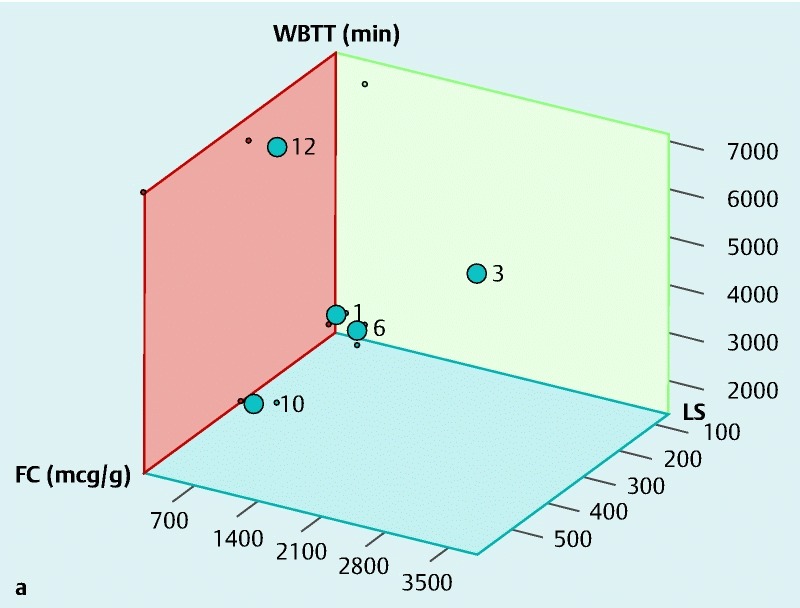
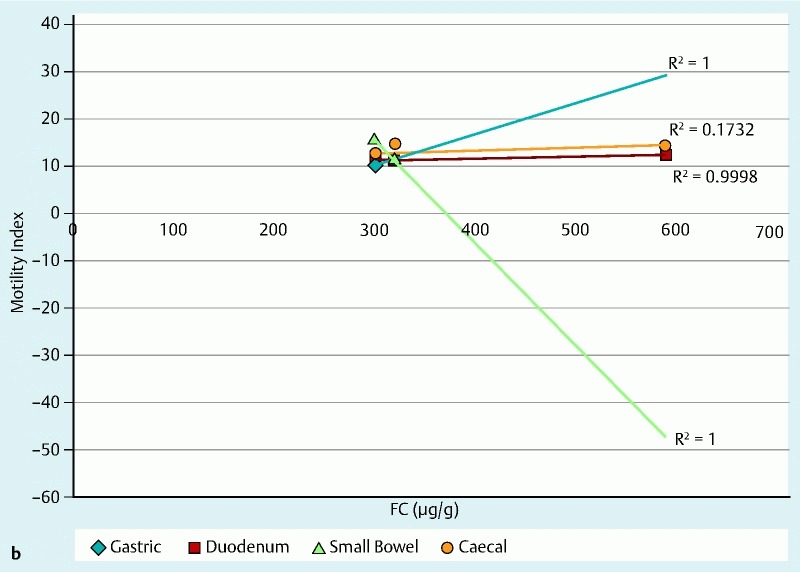
Fig. 6a (see above) Distribution of WBTT, FC and LS for patients in our study for whom the relevant data sets were available. Each plot point represents a patient in our study with the numbers corresponding to patient numbers in Table 3. Abbreviations: FC, fecal calprotectin; LS, Lewis score; WBTT, whole bowel transit time b Linear regression of FC against motility indices for patients in our study for whom the relevant data sets were available. c Linear regression of LS against motility indices for patients in our study for whom the relevant data sets were available.
Discussion
This pilot study is the first to attempt dual use of SBCE and WMC in assessment of patients with known or suspected CD. Currently, diagnosing CD requires a clinical evaluation and a combination of endoscopic, histological, radiological, and/or biochemical investigations 9. To date, the value of SBCE in the investigation of CD has already been established 10. A previous study 11, in which cine magnetic resonance enterography (MRE) was employed in addition to the regular MRI protocol, found that imaging areas of altered gut motility helped to detect more CD-specific findings. Other studies have shown that CD is associated with delayed gastric emptying, possibly due to inflammation 12.
Therefore, addition of motility data in this setting could be of use 2 13, especially when first-line investigations are inconclusive. Compared to the traditional method of assessing gastrointestinal motility with scintigraphy/radio-opaque markers, WMC is not associated with any radiation exposure. Concurrent use of SBCE and WMC shows how multimodal information can provide information not only on the mucosal appearances of patients with CD but also physiological motility data. However, that needs to be balanced against the risk of capsule retention, a feared complication in patients with CD. In our study, there was one case of stomach retention of the capsule, which occurred despite patency check with follow-up plain abdominal x-ray where the patency capsule had been reported to be in the large bowel. Limited CT scanning post-patency may be more useful in these patients 14.
Our patients had significantly longer transit times compared to the controls (P < 0.05 for all parameters measured) (Table 4). However, statistical significance should be interpreted with caution given the small sample size. Other limitations of this pilot study include potential selection bias, as patients with significant SB inflammation were excluded due to fear of capsule retention, and the SmartPill® signal loss (resulting in incomplete data sets in 5 /10 completed WMC examinations). It is not clear if this is due to technological limitations or whether the concurrent use (4 hours apart) of two capsules caused some radiofrequency interference 1 5 6. Furthermore, the complexity of the WMC data did not allow meaningful correlation with other parameters such as FC and LS. This can be seen in other studies that have tried to explore the relationship between LS and FC in patients with SB CD 15.
Take home messages
Physiological data obtained from the use of the SmartPill® could be of value in conjunction with ‘conventional’ SBCE to shed more light in the pathophysiology of CD and perhaps assist in patient management. However, to better help clinicians to understand and maximize use of the motility information, the development of a simplified interpretation system is necessary.
Despite concerns about capsule retention in patients with CD, our study suggests that the SmartPill® seems generally safe for use in these patients, although use of a patency capsule is recommended beforehand.
Acknowledgements
The study was supported by a defined grant by Given®Imaging Ltd (ESGE- Given®Imaging Research grant 2011).
Footnotes
Competing interests: Dr. Koulaouzidis has received a research grant from ESGE Given Imaging.
References
- 1.Tran K, Brun R, Kuo B. Evaluation of regional and whole gut motility using the wireless motility capsule: relevance in clinical practice. Therap Adv Gastroenterol. 2012;5:249–560. doi: 10.1177/1756283X12437874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y T, Mohammed S D, Farmer A D. et al. Regional gastrointestinal transit and pH studied in 215 healthy volunteers using the wireless motility capsule: influence of age, gender, study country and testing protocol. Aliment Pharmacol Ther. 2015;42:761–772. doi: 10.1111/apt.13329. [DOI] [PubMed] [Google Scholar]
- 3.Rao S SC, Camilleri M, Haler W L. et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil. 2011;23:8–23. doi: 10.1111/j.1365-2982.2010.01612.x. [DOI] [PubMed] [Google Scholar]
- 4.Bai J P, Burckart G J, Mulberg A E. Literature Review of Gastrointestinal Physiology in the Elderly, in Pediatric Patients, and in Patients with Gastrointestinal Diseases. J Pharm Sci. 2015 doi: 10.1002/jps.24696. [DOI] [PubMed] [Google Scholar]
- 5.Wang A, Banerjee S, Barth B A. et al. Wireless capsule endoscopy. Gastrointest Endosc. 2013;78:805–15. doi: 10.1016/j.gie.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Koulaouzidis A, Plevris J N. Investigating the small-bowel: a brief and concise update. Glob J Gastroenterol Hepatol. 2013;1:18–28. [Google Scholar]
- 7.Kopylov U, Papageorgiou N P, Nadler M. et al. Head or tail: the orientation of the small bowel capsule endoscope movement in the small bowel. Dig Dis Sci. 2012;57:694–698. doi: 10.1007/s10620-011-1913-6. [DOI] [PubMed] [Google Scholar]
- 8.Koulaouzidis A, Douglas S, Plevris J N. Heads or tail orientation in small-bowel capsule endoscopy: 2 capsule models with 2 reviewers. Dig Dis Sci. 2012;57:1102–1104. doi: 10.1007/s10620-012-2062-2. [DOI] [PubMed] [Google Scholar]
- 9.Van Assche G, Dignass A, Panes J. et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Fireman Z, Mahajna E, Broide E. et al. Diagnosing small bowel Crohn’s disease with wireless capsule endoscopy. Gut. 2003;52:390–392. doi: 10.1136/gut.52.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Froehlich J M, Waldherr C, Stoupis C. et al. MR motility imaging in Crohn’s disease improves lesion detection compared with standard MR imaging. Eur Radiol. 2010;20:1945–1951. doi: 10.1007/s00330-010-1759-x. [DOI] [PubMed] [Google Scholar]
- 12.Nobrega A C, Ferreira B R, Oliveira G J. et al. Dyspeptic symptoms and delayed gastric emptying of solids in patients with inactive Crohn’s Disease. BMC Gastroenterol. 2012;7:175. doi: 10.1186/1471-230X-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones M P, Bratten J R. Small intestinal motility. Curr Opin Gastroenterol. 2008;24:164–172. doi: 10.1097/MOG.0b013e3282f33f5c. [DOI] [PubMed] [Google Scholar]
- 14.Assadsangabi A, Blakeborough A, Drew K. et al. Small bowel patency assessment using the patency device and a novel targeted (limited radiation) computed tomography-based protocol. J Gastroenterol Hepatol. 2015;30:984–989. doi: 10.1111/jgh.12891. [DOI] [PubMed] [Google Scholar]
- 15.Koulaouzidis A, Douglas S, Plevris J N. Lewis score correlates more closely with fecal calprotectin than Capsule Endoscopy Crohn's Disease Activity Index. Dig Dis Sci. 2012;57:987–993. doi: 10.1007/s10620-011-1956-8. [DOI] [PubMed] [Google Scholar]


