Abstract
Background and study aims: Despite a well-established tool for diagnosis of pancreatic masses, endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) studies have shown suboptimal diagnostic performance at divergent mass sizes. Since the impact of gold standard follow-up and presence of on-site evaluation on this observation is unknown, we aimed to study the performance characteristics of EUS-FNA under these strict conditions.
Patients and methods: EUS-FNA results from pancreatic mass lesions performed between July 2000 and March 2013 were evaluated. All patients with histological follow-up were then stratified into four groups: Group A ( ≤ 10 mm), Group B (11 – 20 mm), Group C (21 – 40 mm), and Group D (> 40 mm). Sensitivity and diagnostic accuracy were calculated for each group and compared.
Results: A total of 612 /3832 (16 %) patients with pancreatic masses who underwent EUS-FNA had histology confirmation. Of these, 81 were excluded due to unavailable lesion size, while the rest formed the study cohort. Mean age (SD) was 65.8 years (9.3) with 51.2 % female. The mean number of passes for the entire cohort was 2.9 (SD 1.9; range 1 – 12); patients in group D had a significantly higher number of passes for on-site diagnosis (P = 0.0124). There was no significant difference between the groups for sensitivity (P = 0.1134) or diagnostic accuracy (P = 0.2111). Proportional trend analysis revealed no significant correlation between size and sensitivity (P = 0.6192). The size of lesion measured by EUS was not associated with sensitivity or specificity after adjusting for age, sex, and pancreatic location.
Conclusion: In the presence of rapid on-site cytopathology and when final histology is taken as the gold standard, pancreatic mass size does not affect the performance characteristics of EUS-FNA.
Introduction
Endoscopic ultrasound (EUS) is a well-established, cost-effective procedure for investigating solid pancreatic mass lesions 1 2. While CT scan is the primary modality for investigating pancreatic disease, EUS is the preferred tool in highly suspicious circumstances due to its ability to reveal lesions in the absence of a mass on CT scan 3 and its superior performance characteristics for diagnosing lesions less than 30 mm in size 4. Between 2006 and 2010, the usage of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) increased by 69.3 %; percutaneous biopsy increased by 1.8 % compared to a corresponding decrease in open surgical biopsy by 41.7 % 1. With the growing utilization of EUS-FNA 5, there is a renewed focus to evaluate and target small lesions as early stage diagnosis leads to more curative resections and significantly impacts clinical outcomes.
Although EUS-FNA has a high sensitivity and specificity for diagnosis of pancreatic mass lesions, diagnostic accuracy of EUS-FNA is dependent on operator experience 6, presence of rapid on-site cytopathologist evaluation (ROSE) 7, needle size 8, and the presence of chronic pancreatitis 9. In the presence of a mass and high clinical suspicion but a negative EUS-FNA, there is also evidence that repeat EUS-FNA improves diagnostic accuracy 10. Even though a recent meta-analysis confirmed that diagnostic accuracy is superior in the presence of ROSE 7, suboptimal EUS-FNA performance was noted for pancreatic mass sizes at either end of the spectrum. A study from 1999 11 showed that the sensitivity of EUS-FNA for small lesions was modest while another study showed that diagnostic accuracy rises as the size of the mass increases with a reduction in accuracy for lesions more than 40 mm 12. While additional endoscopic ultrasound imaging features may aid in the differentiation between solid pancreatic mass lesions 13 and are helpful for predicting the possibility of adenocarcinoma 14, tissue diagnosis is essential for further clinical management.
Given the importance of pancreatic mass size for staging, increasing use of EUS-FNA for tissue acquisition and uncertainty with regard to its performance at different mass sizes, we sought to study the operating characteristics of EUS-FNA vis-à-vis pancreatic mass size under gold standard conditions.
Materials and methods
Patient population
This is a retrospective clinicopathologic correlation study of patients who underwent EUS-FNA of pancreatic masses from July 2000 to March 2013. Data including EUS findings, rapid on-site cytopathology, and final histology were documented in an institutional review board (IRB) approved database. After identifying all patients who had final histology as the gold standard for diagnosis, the available cohort was stratified into four groups based on longest dimension of cross-sectional pancreatic mass size – Group A (≤ 10 mm), Group B (11 – 20 mm), Group C (21 – 40 mm), and Group D (> 40 mm). These four groups were compared for the operating characteristics of EUS-FNA.
Procedure
EUS was performed under moderate sedation or anesthesia with a linear array echoendoscope (Olympus UCT140, Olympus America Corp, Center Valley, PA, United States) utilizing the standard station approach by four experienced endosonographers. When a mass was identified, cross-sectional size measurements were undertaken along with echo features, surrounding vasculature, evaluation for peripancreatic lymph nodes, and examination of the liver. Fine needle aspiration (FNA) of the mass was performed with a standard FNA needle (Echotip, Cook Endoscopy, Winston-Salem, NC, United States; Expect™, Boston Scientific Corporation, Natick, MA, United States); the size of the needle (25G, 22G, and 19G) used was determined by individual choice of the endosonographer. Patients then recovered as per standard procedure in the endoscopy unit and were discharged when stable.
Preparation of specimen for on-site analysis
Predominantly air-dried and a few alcohol-stained smears were prepared on-site after individual passes. Air-dried smears were stained with Diff-Quick stain (Baxter, McGraw Park, IL, United States) for immediate review by a cytopathologist to ascertain sample adequacy and provide a preliminary diagnosis. Number of passes made was dependent on on-site evaluation for adequacy and diagnosis. In the cytopathology laboratory, alcohol-stained smears were prepared using Papanicolaou’s stain; cell block pellets were prepared, sectioned, and stained with routine hematoxylin and eosin. The cytopathologist then characterized the diagnosis into previously described 15 cytopathologic categories: positive for malignancy; negative for malignancy; atypical; suspicious; benign and non-diagnostic after additional review of slides.
Preparation of cellblock for histological analysis
The EUS-FNA specimen was placed in Cytolyte and taken to the laboratory where it was spun in the centrifuge. After decanting the supernatant, the sediment was made into a pellet and placed in a Tissue-Loc HistoScreen cassette (Microm International, Walldorf, Germany) and fixed in formalin. Thereafter, it was embedded in paraffin and sections made for hematoxylin and eosin (H&E) staining to examine for the presence of a histological core. Only the diagnostic specimens with definable histological core were included.
Surgical pathology specimens
Once surgery was performed, surgical pathology specimens were analyzed and the final diagnosis recorded in the database. Patients who had histological core tissue acquired at the time of surgical exploration, but were deemed inoperable were also included in the database. Surgically obtained specimens were sectioned and placed in formalin. These were further processed, embedded in paraffin, and stained with hematoxylin and eosin.
Statistical analysis
Lesions defined as “malignant” and “suspicious” by EUS-FNA, with a final pathology diagnosis of malignancy, were categorized as True Positive (TP) for applying the Multinominal Logistic Regression (MLR) model; those with benign final diagnosis were categorized as False Positive (FP). Similarly, patients diagnosed as “negative” and “benign” by histology were considered True Negative (TN), whereas those “negative” or “atypical” on EUS-FNA but malignant on surgical pathology were categorized as False Negative (FN). In all of these, only the final cytologic diagnosis was considered in the analysis. Chi-squared tests were used for each categorical variable (sex, location of the mass, final diagnosis, sensitivity) to test their association among the four groups. Analysis of variance (ANOVA) was conducted to compare means of age and the number of FNA passes across all groups. Cochran-Armitage trend tests were performed to examine for increasing or decreasing trends in diagnostic accuracy and sensitivity with tumor size. Pairwise comparisons of absolute difference among the groups were done by Chi-squared tests with Bonferroni adjustment.
As suggested by Dwivedi et al. 16, we fitted multinomial logistic regression models for the four outcomes (TP, FP, TN, and FN) with covariates of age, sex, diameter of lesion as measured by EUS, and pancreatic location. We considered two separate models with maximum and minimum diameter of lesion per outcome category. The sensitivity model used false negative as reference value and the specificity model used false positive as reference value. Two-sided P values < 0.05 were considered statistically significant. Analysis was done using SAS software, version 9.3 (SAS Institute Inc., Cary, NC, United States), as well as IBM-SPSS version 22 (Armonk, New York, United States).
Results
A total of 3832 EUS-FNAs were performed during the study period. Of these, 612 patients had histological follow-up. Accurate information with regard to mass size was lacking in 81 cases and these were excluded. The rest (531/3832; 13.9 %, 95 %CI: 12.8 – 15.0) formed the study cohort (Fig. 1).
Fig. 1.
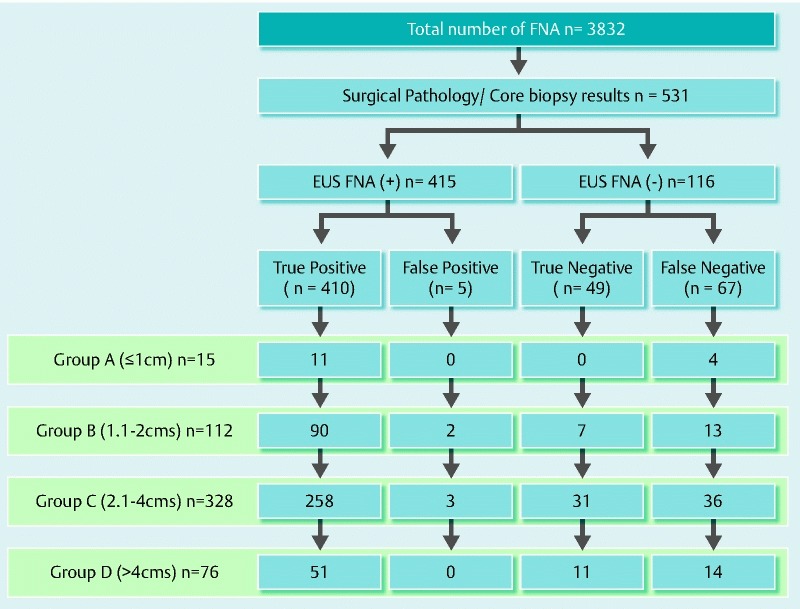
Flow chart showing the included study subjects.
Demographics
The groups were evenly matched for age and gender, while there was a significant difference in the location of the mass with predominant lesions noted in the head of pancreas (P = 0.0002). Final diagnosis revealed a significant proportion of ductal adenocarcinoma (P < 0.0001). The results are shown in Table 1.
Table 1. Patient characteristics and EUS findings for the entire cohort.
| Group A (n = 15) | Group B (n = 112) | Group C (n = 328) | Group D (n = 76) | P value | |
| Age, mean (SD), years | 65 (4) | 67 (7) | 67 (9) | 64 (12) | 0.0564 |
| Sex, n (%) | 0.8811 | ||||
| Male | 6 (40) | 56 (50) | 165 (50) | 37 (49) | |
| Female | 9 (60) | 56 (50) | 163 (50) | 39 (51) | |
| Location, n (%) | 0.0002 | ||||
| Uncinate | – | 14 (13) | 37 (11) | 5 (7) | |
| Head | 9 (60) | 70 (62) | 196 (60) | 32 (42) | |
| Body | 4 (27) | 20 (18) | 39 (12) | 12 (16) | |
| Tail | 2 (13) | 8 (7) | 56 (17) | 27 (35) | |
| Number of passes, mean (SD) | 2.2 (1.7) | 3 (2.1) | 2.8 (1.8) | 3.5 (1.9) | 0.0124 |
| Final diagnosis, n (%) | < 0.0001 | ||||
| Adenocarcinoma | 7 (47) | 79 (70) | 225 (69) | 28 (37) | |
| Neuroendocrine tumor | 4 (27) | 15 (13) | 24 (7) | 6 (8) | |
| Lymphoma | – | 1 (1) | 9 (3) | 8 (10.5) | |
| Chronic pancreatitis | – | 6 (5) | 31 (9) | 8 (10.5) | |
| Others | 4 (26) | 11 (11) | 39 (12) | 26 (34) |
Operating characteristics of EUS-FNA
The overall sensitivity was 85.95 %, diagnostic accuracy was 86.44 % and with a positive predictive value of 98.8 % (Table 2). The mean number passes of 2.2 (SD 1.7), 3 (SD 2.1), 2.8 (SD 1.8), and 3.5 (SD 1.9) required for on-site diagnosis (Fig. 2) between the four groups was statistically significantly different (P = 0.0124). There was no significant relationship between the four groups and sensitivity (P = 0.1134). The Cochran-Armitage trend test did not reveal an increasing or decreasing trend in diagnostic accuracy (P = 0.9923) or the probability of finding a true positive result in relation to tumor size (P = 0.6192).
Table 2. Operating characteristics of EUS-FNA between the groups.
| Group A | Group B | Group C | Group D | Total | |
| Sensitivity | 0.7333 | 0.8738 | 0.8776 | 0.7846 | 0.8595 |
| Diagnostic accuracy | 0.7333 | 0.8661 | 0.8811 | 0.8158 | 0.8644 |
Fig. 2.
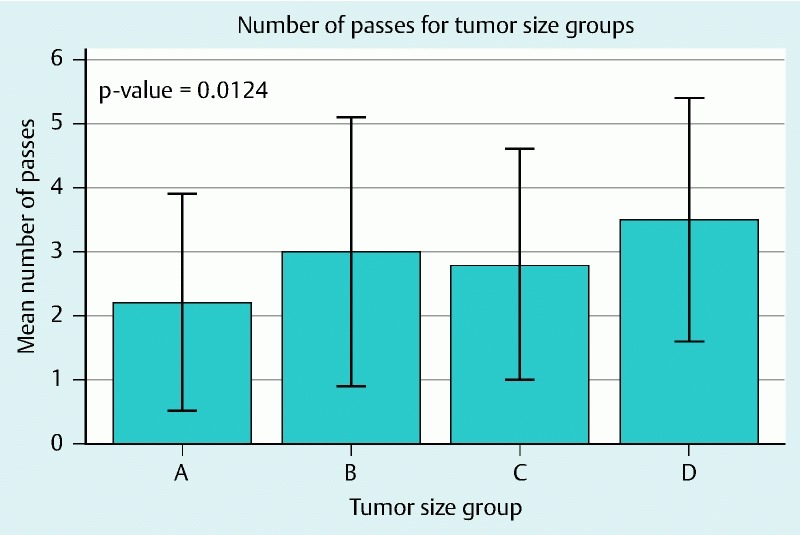
Bar chart depicting the mean number of passes required for on-site diagnosis between the four groups.
As shown in Table 3 and Table 4, the maximum diameter of lesion measured by EUS was not associated with sensitivity or specificity after adjusting for age, sex, and pancreatic location. We observed similar results from the models with the minimum diameter of lesion (not presented). The models for positive predictive value (PPV) and negative predictive value (NPV) showed similar results in terms of significance of the size of lesion (not presented).
Table 3. Parameter estimates for sensitivity model with maximum diameter of lesion.
| Adjusted odds ratio (OR) | P value | 95 %CI for adjusted OR | |
| Age | 1.01 | 0.543 | 0.97 – 1.03 |
| Maximum diameter of lesion | 0.94 | 0.546 | 0.78 – 1.14 |
| Female | 0.96 | 0.890 | 0.57 – 1.64 |
| Pancreatic location | |||
| Uncinate | 1 | ||
| Head | 0.72 | 0.509 | 0.27 – 1.93 |
| Body | 0.61 | 0.419 | 0.19 – 2.00 |
| Tail | 1.27 | 0.698 | 0.38 – 4.26 |
Table 4. Parameter estimates for specificity model with maximum diameter of lesion.
| Adjusted odds ratio (OR) | P value | 95 %CI for adjusted OR | |
| Age | 0.97 | 0.401 | 0.89 – 1.05 |
| Maximum diameter of lesion | 2.01 | 0.187 | 0.70 – 6.14 |
| Female | 0.64 | 0.649 | 0.09 – 4.43 |
| Pancreatic location* | |||
| Body | 0.66 | 0.771 | 0.04 – 11.06 |
| Head | 3.36 | 0.370 | 0.24 – 47.56 |
| Uncinate | 1 |
Categories of tail in pancreatic location were dropped because the frequency was very small or zero.
Discussion
This study determined that, under controlled, gold standard conditions, the performance of EUS-FNA is unaffected by pancreatic mass size and confirmed that, to obtain an on-site diagnosis, an increasing number of passes is required for larger lesions.
Siddiqui et al. 12 showed that diagnostic accuracy and sensitivity were strongly correlated with tumor size with a significant reduction for tumors less than 10 mm; however, in that study, the number of cases in each group was not reported, on-site assessment was rendered by a cytotechnician, and follow-up criteria included clinical review. In another multicenter study, Sahai et al. 11 concluded that the image quality and/or depth of penetration were insufficient to permit successful FNA of smaller lesions. However, that study was performed with a mechanical sector-scanning transducer without Doppler capability, not all centers that participated in the study had rapid on-site evaluation, and FNA passes were restricted to less than three. In contrast, our decade long experience is with curvilinear array echoendoscopes in the presence of ROSE, the number of passes made was smaller, and we used histological follow-up exclusively. In the last decade, advancements in echoendoscope technology and needle designs have allowed the endosonographer to accurately target even deeply placed small lesions with greater efficiency and accuracy. Since a small mass is a concentrated, dense area of abnormal cells with no necrosis, suitably targeted FNA often provides “excellent” material with minimal contamination. Sample adequacy is tested immediately by ROSE and, if negative, care is taken to ensure suitable needle placement inside the lesion for subsequent passes to achieve an accurate diagnosis.
Erickson et al. reported that the presence of a cytopathologist during EUS-FNA improves diagnostic yield, decreases the number of unsatisfactory samples, reduces the need for more passes, and consequently, the procedural duration 17. Our experience reflects that study as the mean number of passes for on-site diagnosis for the whole cohort was 2.6. In a recent meta-analysis, Hebert-Magee et al. showed that the presence of ROSE increases the diagnostic accuracy of EUS-FNA for pancreatic adenocarcinoma but lower performance was seen when histology alone was taken as the gold standard while this was higher for studies with clinical follow-up 7. This may explain the low rates noted in our data in comparison to other studies. ROSE is a vital feature as good communication, team work, and interdisciplinary collaboration are crucial for obtaining an on-site diagnosis. The presence of ROSE helps not only in reducing the number of passes, but also improving overall diagnostic accuracy 17; that study also showed that EUS-FNA performance is unaffected by pancreatic mass size.
In a randomized trial of 54 patients with solid pancreatic mass lesions, the fanning technique established a significantly higher first pass diagnosis in 85.7 % of patients compared to only 57.7 % with the standard technique 18. That study highlighted the importance of technique as a key factor for successful tissue procurement. It is well documented that larger lesions tend to have necrotic material in the center of the lesion and targeting the periphery yields adequate tissue for diagnosis 19. Our data reflect other previously noted observations 12 20 that multiple passes are required for larger lesions to make a diagnosis. However, randomized data in the presence of ROSE and the fanning technique indicate that on-site diagnosis can be achieved in a majority with a single pass 17 21 22. Another randomized trial comparing 19G vs. 25G FNA needles also showed that on-site diagnostic adequacy can be achieved in more than 97 % with a single pass even in large pancreatic lesions 23. These studies also showed that, in the presence of ROSE, when a structured fanning technique is adopted, diagnosis can be achieved in a significant majority with a single pass independent of needle or pancreatic mass sizes.
Our study had several limitations. First, the number of pancreatic masses in group A was small which may have had an impact on the results; this, however, reflects the strict histopathology criteria adopted in this study. Second, the procedure was performed by experienced endosonographers, without trainee involvement, with proficient and expert on-site cytopathologists in attendance; therefore, the results may not be applicable to all units and if trainees were involved. Third, only patients who underwent index FNA were included in the analysis while patients who underwent repeat EUS-FNA for high clinical suspicion were excluded; the results may be vastly different if these were included in the analysis. Fourth, there is a likelihood of verification bias within the dataset that precluded complete specificity analysis. Fifth, needle size and pathology subcategory stratification analysis could not be performed due to small size and inconsistent data availability. Finally, this is a retrospective study and has its attendant inadequacies; however, unlike other studies of EUS-FNA, the strength of this study lies in the stringent criteria used for follow-up.
In conclusion, this study shows that in the presence of ROSE, pancreatic mass size does not affect the performance of EUS-FNA, even when final histology is taken as gold standard. This further emphasizes the effectiveness of obtaining an on-site diagnosis.
Footnotes
Competing interests: None
References
- 1.Roy A K, Kim M, Hawes R. et al. 196. Changing trends in tissue acquisition in pancreatic diseases. Gastrointest Endosc. 2013;77:AB134. [Google Scholar]
- 2.Hewitt M J, McPhail M J, Possamai L. et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319–331. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Shpaner A, Krishna S G. et al. Use of EUS-FNA in diagnosing pancreatic neoplasm without a definitive mass on CT. Gastrointest Endosc. 2013;78:73–80. doi: 10.1016/j.gie.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Muller M F, Meyenberger C, Bertschinger P. et al. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology. 1994;190:745–751. doi: 10.1148/radiology.190.3.8115622. [DOI] [PubMed] [Google Scholar]
- 5.Eltoum I A, Alston E A, Roberson J. Trends in pancreatic pathology practice before and after implementation of endoscopic ultrasound-guided fine-needle aspiration: an example of disruptive innovation effect? Arch Pathol Lab Med. 2012;136:447–453. doi: 10.5858/arpa.2011-0218-OA. [DOI] [PubMed] [Google Scholar]
- 6.Eloubeidi M A, Tamhane A. EUS-guided FNA of solid pancreatic masses: a learning curve with 300 consecutive procedures. Gastrointest Endosc. 2005;61:700–708. doi: 10.1016/s0016-5107(05)00363-9. [DOI] [PubMed] [Google Scholar]
- 7.Hebert-Magee S, Bae S, Varadarajulu S. et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology. 2013;24:159–171. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madhoun M F, Wani S B, Rastogi A. et al. The diagnostic accuracy of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of solid pancreatic lesions: a meta-analysis. Endoscopy. 2013;45:86–92. doi: 10.1055/s-0032-1325992. [DOI] [PubMed] [Google Scholar]
- 9.Varadarajulu S, Tamhane A, Eloubeidi M A. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728–736. doi: 10.1016/j.gie.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki R, Lee J H, Krishna S G. et al. Repeat endoscopic ultrasound-guided fine needle aspiration for solid pancreatic lesions at a tertiary referral center will alter the initial inconclusive result. J Gastrointest Liver Dis. 2013;22:183–187. [PubMed] [Google Scholar]
- 11.Sahai A V, Schembre D, Stevens P D. et al. A multicenter U.S. experience with EUS-guided fine-needle aspiration using the Olympus GF-UM30P echoendoscope: safety and effectiveness. Gastrointest Endosc. 1999;50:792–796. doi: 10.1016/s0016-5107(99)70160-4. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui A A, Brown L J, Hong S K. et al. Relationship of pancreatic mass size and diagnostic yield of endoscopic ultrasound-guided fine needle aspiration. Dig Dis Sci. 2011;56:3370–3375. doi: 10.1007/s10620-011-1782-z. [DOI] [PubMed] [Google Scholar]
- 13.Aso A, Ihara E, Osoegawa T. et al. Key endoscopic ultrasound features of pancreatic ductal adenocarcinoma smaller than 20 mm. Scand J Gastroenterol. 2014;49:332–338. doi: 10.3109/00365521.2013.878745. [DOI] [PubMed] [Google Scholar]
- 14.Eloubeidi M A, Luz L P, Tamhane A. et al. Ratio of pancreatic duct caliber to width of pancreatic gland by endosonography is predictive of pancreatic cancer. Pancreas. 2013;42:670–679. doi: 10.1097/MPA.0b013e31827305b8. [DOI] [PubMed] [Google Scholar]
- 15.Eloubeidi M A, Tamhane A, Jhala N. et al. Agreement between rapid onsite and final cytologic interpretations of EUS-guided FNA specimens: implications for the endosonographer and patient management. Am J Gastroenterol. 2006;101:2841–2847. doi: 10.1111/j.1572-0241.2006.00852.x. [DOI] [PubMed] [Google Scholar]
- 16.Dwivedi A K, Mallawaarachchi I, Figueroa-Casas J. et al. Multinomial logistic regression approach to the evaluation of binary diagnostic test in medical research. Stat Transit New Ser. 2015;16:1–20. [Google Scholar]
- 17.Erickson R A, Sayage-Rabie L, Beissner R S. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–190. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 18.Bang J Y, Magee S H, Ramesh J. et al. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445–450. doi: 10.1055/s-0032-1326268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekberg O, Bergenfeldt M, Aspelin P. et al. Reliability of ultrasound-guided fine-needle biopsy of pancreatic masses. Acta Radiol. 1988;29:535–539. [PubMed] [Google Scholar]
- 20.Ranney N, Phadnis M, Trevino J. et al. Impact of biliary stents on EUS-guided FNA of pancreatic mass lesions. Gastrointest Endosc. 2012;76:76–83. doi: 10.1016/j.gie.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bang J Y, Hebert-Magee S, Trevino J. et al. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012;76:321–327. doi: 10.1016/j.gie.2012.03.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramesh J, Bang J Y, Hébert-Magee S. et al. 1022. Multi-center randomized trial comparing the 19G and 25G needles for EUS-guided FNA of solid pancreatic mass lesions. Gastrointest Endosc. 2013;77:AB179–AB180. doi: 10.1016/j.gie.2012.03.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasan M, Ramesh J, Bang J Y. et al. 100. Multi-center randomized trial comparing the 19G and 25G needles for EUS-guided FNA of large solid pancreatic mass lesions. Gastrointest Endosc. 2014;79:AB112. doi: 10.1016/j.gie.2012.03.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]


