Abstract
Reward sensitivity and possible alterations in the dopaminergic-reward system are associated with obesity. We therefore aimed to investigate the influence of dopamine depletion on food-reward processing. We investigated 34 female subjects in a randomized placebo-controlled, within-subject design (body mass index (BMI)=27.0 kg/m2 ±4.79 SD; age=28 years ±4.97 SD) using an acute phenylalanine/tyrosine depletion drink representing dopamine depletion and a balanced amino acid drink as the control condition. Brain activity was measured with functional magnetic resonance imaging during a ‘wanting' and ‘liking' rating of food items. Eating behavior-related traits and states were assessed on the basis of questionnaires. Dopamine depletion resulted in reduced activation in the striatum and higher activation in the superior frontal gyrus independent of BMI. Brain activity during the wanting task activated a more distributed network than during the liking task. This network included gustatory, memory, visual, reward, and frontal regions. An interaction effect of dopamine depletion and the wanting/liking task was observed in the hippocampus. The interaction with the covariate BMI was significant in motor and control regions but not in the striatum. Our results support the notion of altered brain activity in the reward and prefrontal network with blunted dopaminergic action during food-reward processing. This effect is, however, independent of BMI, which contradicts the reward-deficiency hypothesis. This hints to the hypothesis suggesting a different or more complex mechanism underlying the dopaminergic reward function in obesity.
INTRODUCTION
Obesity is associated with neuronal changes especially related to processing of food items. Several studies showed differential neuronal response in appetite-associated regions such as the anterior insula, orbitofrontal cortex, hippocampus, but also striatal reward regions and prefrontal areas (for review see Carnell et al (2012) and Dagher (2012)). Dopamine is one of the most important neurotransmitters involved in reward processing in general. Alterations in the dopamine system are therefore strongly associated with addictive behavior (Volkow et al, 2012). Over the last few decades, the importance of reward/hedonic processing in eating behavior has also been widely acknowledged. This shift in research from a pure homeostatic control of eating behavior to an interaction model of homeostatic and reward processes was mainly triggered by studies investigating brain processes related to eating behavior. The behavioral impact of food reward can be operationalized by subdividing the construct into ‘wanting' and ‘liking' (Berridge, 1996). While liking can be explained as the hedonic reaction to the pleasure of a reward, the wanting component can be described as the incentive salience linked with the motivation towards an item (Berridge, 2009). This approach, first applied in animal research, was also translated to human studies (Finlayson et al, 2007).
Thanks to numerous studies investigating neuronal correlates, dopamine is known to be one of the key agents for food reward and control of food intake (Kenny, 2011; Richard et al, 2012; Volkow et al, 2011). The mechanism of striatal dopaminergic hypofunctioning, as found in reduced striatal D2-receptor availability, ie, blunted striatal response to food, is believed to be a major contributor to overeating according to the reward-deficiency model of obesity (Blum et al, 2014; Wang et al, 2001). In addition, a blunted striatal response in obesity appears to be moderated by a Taq1A polymorphism that influences the dopamine D2-receptor binding (Stice et al, 2008). However, a causal interpretation of such findings has led to some controversy (Berridge, 2009). An alternative model leading to obesity is the reward surfeit theory which postulates that, in obese individuals, greater inborn-neuronal reward activity in response to food intake might trigger overeating (Stice et al, 2011). The incentive sensitization theory is based on an acquired higher neuronal reward activity in response to food items in individuals showing higher reward sensitivity (Davis et al, 2004).
To investigate such food-reward aspects as a function of central dopaminergic processes, it is necessary to compare brain functions during normal and experimentally reduced dopamine levels. The neuronal dopamine level can be manipulated by using amino acid drinks containing or lacking the precursors of dopamine. Acute phenylalanine/tyrosine depletion leads to a significant short-term reduction in the tyrosine (tyr) and phenylalanine (phe) concentrations (McTavish et al, 1999a) and, therefore, also in central dopamine level, as shown in positron-emission tomography studies (Leyton et al, 2004; Montgomery et al, 2003).
In previous studies using the depletion approach, dopamine depletion was observed to have an impact on behavioral reward processes (Bjork et al, 2013), perceptual timing (Coull et al, 2012), valuation processes (Medic et al, 2014), working memory (Ellis et al, 2007; Nagano-Saito et al, 2008), decision-making (Nagano-Saito et al, 2012), and addictive behavior (Leyton et al, 2000, 2002, 2005; Munafo et al, 2007; Venugopalan et al, 2011). As regards eating behavior, Hardman et al (2012) did not observe any compensatory food intake in the dopamine-depleted state in normal weight subjects. However, as assumed by the authors, only the normal weight subjects experience the depletion acutely and may not be comparable to the overweight subjects. In rodent studies, there is evidence for reduced-feeding behavior in dopamine-depleted rats (Ninan and Kulkarni, 1998; Tellez et al, 2013). Another study showed that dopamine depletion decreased the motivation to work for food but increased free-food consumption (Salamone et al, 1991).
In the current study, we therefore tested the acute effect of different neuronal dopamine levels, induced by nutritional dopamine depletion, on the wanting/liking evaluation of food items in a subject sample covering a wide body mass index (BMI) range. We used the dopamine depletion model to simulate the assumed hypofunction of the dopaminergic-reward system of obese individuals. On the basis of a generally high dopamine-receptor density in striatal regions (Montgomery et al, 2003), we hypothesized reduced activity in the striatum during depletion within the wanting and the liking task in general. Furthermore, we predicted that the effect of dopamine depletion in obese subjects would be reduced on account of their presumed predisposition to blunted dopaminergic activity.
MATERIALS AND METHODS
We conducted a randomized placebo-controlled functional magnetic resonance imaging (fMRI) study using a within-subject design in female participants.
Participants
In all, 36 female subjects participated in the study. As previous literature states that women are more sensitive to the depletion effect (de Wit et al, 2012; Munafo et al, 2007; Robinson et al, 2010), only females were recruited. Two subjects could not be included in the analyses owing to nausea during the measurements, resulting in a total of 34 participants. Because BMI was one of the main variables of the study, a wide BMI range was covered within this population (BMI: mean 27.0 kg/m2 ±4.79 SD, range 18.8–37.2 kg/m2; age: 28 years ±4.97 SD, range 21–44 years).
During a screening appointment, we ensured that all subjects were healthy and had no diagnosed psychiatric disorder.
The study protocol was approved by the ethics committee of the University of Tübingen and all subjects gave their written informed consent. Subjects were informed about the procedures and the protocol beforehand, the single-blinded design and possible side effects of the scanning procedure and the amino acid drinks (such as nausea). The study was registered at ClinicalTrials.gov, NCT01906411.
Acute Dopamine Depletion
To manipulate central dopamine levels simulating acute dopamine depletion (DOPD), a phe/tyr-depleted amino acid drink and a balanced amino acid drink as a control condition (CON) were used on two separate study days in randomized and single-blinded but balanced order. The study days were at least 2 weeks apart.
The amino acids composition of both drinks is based on a formula by McTavish et al (1999b) that has been used in several subsequent studies (Hardman et al, 2012; Hitsman et al, 2008; Munafo et al, 2007). In several of these studies the formula used for men was reduced by 20% for women to allow for differences in weight (de Wit et al, 2012; McTavish et al, 1999b; Munafo et al, 2007). On the basis of a rather wide BMI range in the current study, we used three formulas of the amino acid drink for the three weight categories. We applied the formula for male subjects by McTavish et al (1999b) for the heaviest group of women (>84 kg); for women in the weight range of 68–83 kg, we reduced the formula by 20%, and for women with a weight of 50–67 kg, by 40%. The resulting formulas are shown in the Supplementary Table S1.
Study Protocol
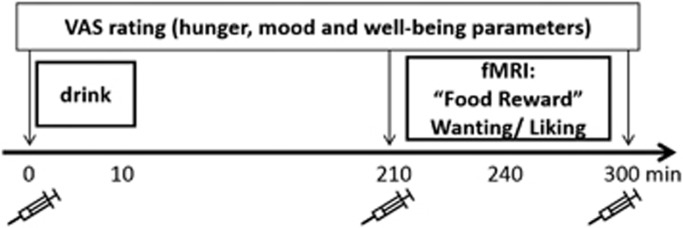
Dietary information was provided beforehand to all subjects, who were requested to keep a low protein diet the day before the measurements to ensure that the amino acid drinks provided on the study days could take full effect. On scanning days, subjects were requested not to eat or drink anything except water. The application of CON and DOPD was counterbalanced. Two subjects per scanning day were measured one after the other. The scheme of a scanning day is illustrated in Figure 1. Subjects arrived at the study site at 1030 hours and 1145 hours, respectively. After the ‘baseline' blood sample, subjects rated mood, hunger, and well-being parameters (hunger, satiety, appetite, good mood, sadness, anxiety, nausea, bloating, and urge to move) on a 100 mm visual analogue scale (VAS). Drink intake was considered as time point 0. As the peak effect of DOPD occurs between 3 and 5 h after intake, subjects were instructed to fill in several food-related trait questionnaires during the 3-h period prior to the scanning session (TFEQ: Three Factor Eating Questionnaire with the scales cognitive restraint, disinhibition, and experienced hunger (Pudel and Westenhöfer, 1989), PFS: Power of Food Scale investigating the power of available, present, or tasted food (Lowe and Butryn, 2007), and EDE: eating disorder examination with the scales restraint eating, eating concern, weight concern, and shape concern (Hilbert et al, 2007)). During this period, subjects were provided with water and protein-free snacks (100 g carrots, 100 g cucumber). After 210 min, a further blood sample was taken and participants were instructed to fill in the VAS a second time. Afterwards, subjects were positioned in the scanner. Within the first 30 min of scanning, both a reward paradigm and resting-state measurement were performed (data not included). Four hours after the drink, subjects were stimulated with 40 food pictures (20 high- and 20 low-caloric value) and performed a wanting/liking task. Each picture was presented for 3 s with an inter-stimulus interval of 1–12 s. The task was programmed with the presentation software (version 10.2, www.neurobs.com). In two runs, each lasting 5 min, subjects were first asked to rate each picture for wanting (‘how much do you want to eat this food now', run 1) and then for liking (‘how much do you like this food in general', run 2) on a 5-point Likert scale within the 3 s in which the picture was presented. Responses were given via an fMRI compatible button box. After the scanning session, the third blood sample was taken and subjects filled in the VAS a third time.
Figure 1.
Study design. Four hours after intake of the drink, subjects were scanned during a wanting and liking rating. At baseline (before drink intake) and pre- (210 min) and post- (300 min) fMRI measurement blood samples were taken as indicated by the syringe; whereas hunger, mood parameters, and well-being parameters were obtained by a VAS.
Blood Samples
Blood samples were taken at three time points (before drink intake as a baseline measurement, after 210 min (pre-scan) and after 300 min (post-scan)). In the DOPD condition, the 210 and 300 min samples are missing for one subject and the 300 min sample for another subject. In the CON condition, the 300 min sample is missing for one subject.
Phe and tyr, together with other large neutral amino acids (LNAA: valine, leucine isoleucine, and methionine) were analyzed by ion-exchange chromoatography (F. Gutjahr Chromatographie, Balingen, Germany). A proxy of neuronal dopamine synthesis is represented in the supply of its precursors (Fernstrom and Fernstrom, 1994). We therefore calculated the availability of the dopamine precursors tyr+phe by the formula tyr+phe/LNAA (Coull et al, 2012). To evaluate the change on dopamine precursor availability after drink intake, the 210 min and the 300 min value were standardized on the baseline value (210/baseline, 300/baseline, respectively).
Imaging Procedures and Analyses
Whole-brain fMRI blood oxygen-level dependent data were obtained by a 3-T fMRI scanner (Siemens TimTrio, Erlangen, Germany) equipped with a 12-channel head coil. During the stimulation paradigm, each session consisted of 150 scans (repetition time=2 s, echo time=30 ms, matrix 64 × 64, flip angle 90°, voxel size 3.3 × 3.3 × 3.2 mm3, slice thickness 3.2 mm, 0.8 mm gap, 30 slices, images acquired in ascending order). On one of the measurement days, high-resolution T1-weighted anatomical images (MPRage: 160 slices, matrix: 256 × 224, 1 × 1 × 1 mm3) of the brain were obtained at the end of the scanning period.
FMRI data were analyzed with the Statistical Parametric Mapping 8 (SPM8) software (http://www.fil.ion.ucl.ac.uk/spm/). Data were preprocessed, beginning with slice timing and realignment of the images to the mean image. To allow for susceptibility by movement artifacts, unwarping of time series was performed. The anatomical T1-weighted image was co-registered to the mean functional image. Normalization into Montreal Neurological Institute space (3 mm iso-trop voxel size) and Gaussian spatial smoothing (FWHM: 6 mm) were then performed. A general linear model with the condition types wanting, liking, and high- and low-caloric food items for both the DOPD and the CON condition was applied to each subject. For each condition, a separate regressor was modeled using a canonical hemodynamic response function including time derivatives. Movement parameters were modeled as confounds. Data were high-pass filtered (cutoff: 128 s) and global AR(1) autocorrelation correction was performed.
Statistical Analyses
For the fMRI data, a repeated-measurement ANCOVA was performed including the factors ‘food' (high- or low-caloric), ‘task' (wanting, liking), and the repeated measure ‘dopamine' (DOPD, CON). Analyses were controlled for BMI, age, and hunger. The interaction effect of the covariate BMI with the factors ‘dopamine', ‘task', and ‘food' was modeled as an additional regressor on the basis of the hypothesis of impaired dopaminergic function with increasing BMI. Main effects and interactions were considered significant on a cluster level at P<0.05 family-wise error-corrected using an uncorrected primary threshold level of P<0.001 (Woo et al, 2014).
In addition to the whole-head analyses, a region of interest analyses with a priori defined masks of the nucleus caudatus and the putamen were performed, as those striatal regions are the main target of dopaminergic (food) reward activity and dopamine depletion (Wang et al, 2001). Masks were derived from the wake forest pick atlas (www.fmri.wfubmc.edu/software/pickatlas).
Behavioral data were analyzed using SPSS (IBM SPSS Statistics Version 22, Armonk, NY, USA). To investigate differences between the wanting and liking rating, an ANOVA was performed that included the factors ‘task' and ‘food' and the repeated-measurement factor ‘dopamine'. Behavioral analyses were controlled for age, BMI, and hunger. Results were considered significant at P<0.05.
RESULTS
Dopamine Precursor Availability
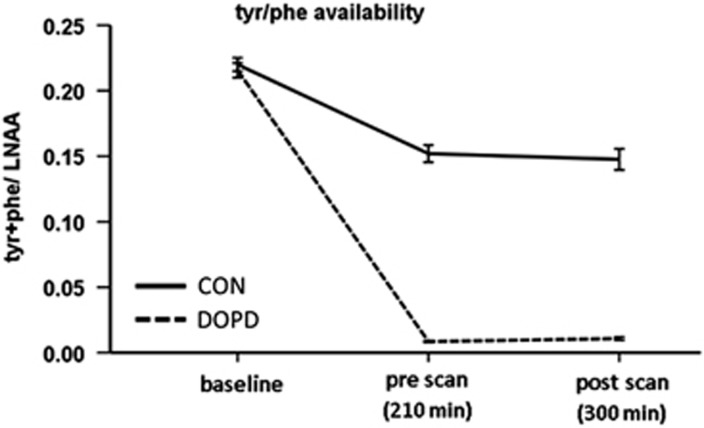
The plasma amino acid levels of all subjects confirmed a successful intervention, suggesting a depletion of neuronal dopamine level in the DOPD condition vs the CON (Supplementary Figure S1). As a consequence of the intake of the control drink, the tyr and phe levels increased. After intake of the DOPD drink, tyr and phe levels decreased. In accordance with previous literature (Coull et al, 2012), the tyr+phe availability decreased more radically in the DOPD condition than in the CON condition (Figure 2).
Figure 2.
Availability of tyr+phe, represented in the plasma tyr+phe/LNAA ratio.
At baseline (before drink intake) on both measurement days, the tyr+phe/LNAA quotient, as an approximation of the neuronal dopamine level, correlated positively with BMI, indicating higher baseline tyr and phe availability with increasing BMI (both scanning days r=0.33, P<0.05, Supplementary Figure S2). In addition, in the CON, the higher the BMI, the lower the decrease of tyr+phe availability after 210 min (r=−0.37, P<0.05) and 300 min (r=−0.44, P<0.05, Supplementary Figure S3).
Behavioral Results
Hunger, mood, and well-being parameters did not differ between the dopamine conditions. Significant increases in hunger towards the end of the measurement day were shown by significant time changes in the hunger, satiety, and appetite rating. In general, low nausea, bloating, anxiety, and sadness levels were observed, but medium-to-high good mood ratings showed a slight decrease as time went on in both measurement days. No time changes in sadness, anxiety, bloating, or urge to move were observed. Low nausea level showed a significant time change with a slight increase after 210 min and a decrease by 300 min (Supplementary Table S2).
For the analysis of the picture ratings, an ANOVA with the between factors ‘task' and ‘food' and the within factor ‘dopamine' revealed a significant effect for the factor task (P<0.001). The wanting scores were higher than the liking scores on both measurement days (mean wanting: 3.5±0.05 SEM, mean liking: 3.1±0.06 SEM, P<0.001). BMI did not show any significant correlation between the rating in the two tasks or on the two measurement days.
Descriptive data of eating behavior traits and snacking behavior between the drink and the fMRI measurement are shown in the Supplementary Tables S3 and S4.
Brain Activation Pattern
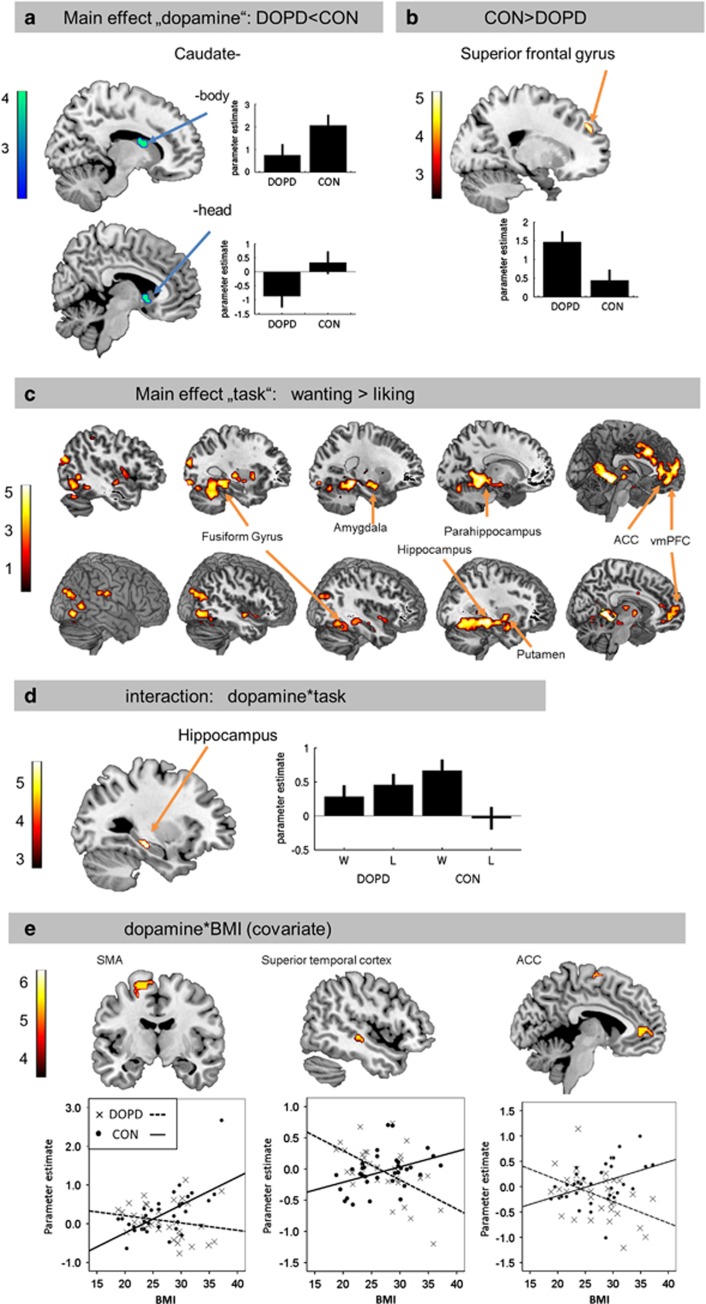
The main effect ‘dopamine' revealed reduced activation in the DOPD condition in two separate clusters within the striatum (caudate body and head, Figure 3a, Table 1). Higher activation was found in the superior frontal gyrus during depletion than in the CON (Figure 3b, Table 1).
Figure 3.
Brain activation pattern superimposed on an anatomical brain and thresholded with P<0.001 by way of illustration. (a) Lower activation in striatal regions (caudate head and body) in the depleted state. Results are significant with P<0.05 family-wise error small volume corrected. (b) Higher activation in the superior frontal gyrus in the depleted state. (c) Activation differences between the wanting and liking task in 5 consecutive sagittal slices for each hemisphere (upper row: left hemisphere, lower row: right hemisphere). Each brain region that is more activated in the wanting than in the liking task is labeled one-time exemplary. (d) Interaction-effect dopamine × task in the hippocampus. (e) Interaction-effect dopamine × BMI (covariate) in the supplementary motor area, superior temporal gyrus, and the anterior cingulate cortex. The color bars represent T-values. If not otherwise stated, results are significant with P<0.05 family-wise error-corrected. L, liking; W, wanting.
Table 1. Significant Differences in Brain Activation of the Repeated-Measurement Analyses with the Factor ‘Dopamine', ‘Task' and ‘Food'.
| Effect | Brain region |
MNI coordinates |
Cluster size (in voxels)a | T-value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Main ‘dopamine': DOPD<CON | Caudate (head) | 6 | 11 | −2 | 7 | 3.99b |
| Caudate (body) | 12 | 5 | 16 | 10 | 3.76b | |
| Main ‘dopamine': DOPD>CON | Superior frontal gyrus | 18 | 47 | 31 | 23 | 4.84 |
| Main ‘task': W>L | Parahippocampus/hippocampus/amygdala/putamen/fusiform gyrus | −24 | −37 | −11 | 603 | 5.58 |
| Anterior/middle cingulate cortex | −3 | 17 | 37 | 128 | 5.63 | |
| Parahippocampus/hippocampus/amygdala/putamen/fusiform gyrus | 30 | −28 | −11 | 615 | 5.41 | |
| Ventromedial prefrontal cortex | 0 | 47 | −8 | 276 | 4.88 | |
| Dopamine × task | Hippocampus | −30 | −25 | −11 | 15 | 5.09 |
| Dopamine × BMI (CON>DOPD) | Supplementary motor region/postcentral cortex | −18 | −7 | 64 | 306 | 5.77 |
| Superior temporal cortex | 48 | −22 | −5 | 16 | 5.56 | |
| Anterior cingulate cortex | −6 | 44 | 4 | 101 | 5.54 | |
Abbreviations: CON, control; DOPD, dopamine depletion; L, liking; W, wanting.
All results are family-wise error-corrected for multiple comparison.
The cluster size of FWE-corrected clusters, based on a primary uncorrected-threshold level of P<0.001.
ROI analyses, results are significant with P<0.05 at cluster and peak level.
For the factor ‘task' we found higher activity for wanting than for liking in middle frontal (ventromedial prefrontal cortex (vmPFC), anterior cingulate cortex (ACC)), hippocampal/parahippocampal and amygdalar, as well as in striatal regions (putamen) and the fusiform gyrus (Figure 3c, Table 1).
A significant interaction between ‘dopamine' and ‘task' was found in the hippocampus. No differential effect for wanting and liking was observed for the DOPD condition, whereas hippocampal activation increased during the wanting task in the CON only (Figure 3d, Table 1).
No significant differences were observed for the factor ‘food' nor for any other interaction.
To further investigate the possible effects of the covariate BMI, we analyzed the corresponding interaction effects. For the factor ‘dopamine' (contrast CON>DOPD) we observed a significant interaction resulting in higher activation differences in the supplementary motor area, superior temporal regions, and the ACC with increasing BMI. Brain acitivity was positively associated with the BMI in the CON but negatively (or around zero) in the DOPD condition (Figure 3e, Table 1). However, no interaction effect of the BMI in any reward area was observed. No differential interaction was found between the wanting and liking task or between the high- and low-caloric pictures in association with BMI.
DISCUSSION
In our study, we investigated neuronal and behavioral correlates of DOPD. With the model of nutrition-based alterations of the dopamine level, we intended to introduce an acute hypofunction of the reward system which is proposed to lead to overeating in obese individuals within the reward-deficiency model of obesity (Blum et al, 2014; Wang et al, 2001). We were able to show that reduced-dopaminergic action affects reward processing of food items. However, the data provide no strong support for the aforementioned model, as several findings especially the independence of the effect on BMI are not consistent with the reward-deficiency model.
The depletion protocol was successful and dopamine availability was significantly reduced in the depletion state for all subjects as assessed by reduced-relative concentrations of the dopamine precursors tyr and phe.
As anticipated, the DOPD condition resulted in lower caudate activation than the CON condition, which tallies with previous studies (Leyton et al, 2004; Nagano-Saito et al, 2012). This effect was independent of BMI. However, we found that the baseline tyr+phe availability were positively correlated with BMI. Such findings can be discussed from the perspective of opposing theories. Proposing lower striatal dopamine-receptor availability in obese, this result suggests that there is a compensatory higher dopamine level in the obese population. A general compensatory mechanism of a higher endogenous dopamine synthesis rate with lower-binding potential of dopamine D2 receptors was also suggested independent of BMI differences (Ito et al, 2011). Furthermore, the tyr+phe availability decrease after meal intake in the CON of our study was more pronounced for obese than for normal weight subjects. The proposed compensatory mechanism of higher baseline tyr+phe availability and consequently higher neuronal dopamine level after meal intake might therefore not be sufficient to compensate for the reduced striatal dopamine-receptor availability. This finding supports the theory that striatal dopamine availability is reduced in obese subjects after meal consumption, thereby possibly resulting in increased food intake achieving the same relative dopamine response as lean subjects. On the other hand, our results can be interpreted from opposing models of higher reward activity after food consumption in obese, with might be inborn (reward surfeit model) or acquired (incentive sensitization model). There is evidence that obese individuals experience higher neuronal reward activity during visual food processing (Stoeckel et al, 2008), as well as during the anticipation of palatable food (Burger and Stice, 2013). Furthermore, normal weight adolescents with an increased risk for developing obesity show greater reward responsivity to food compared with adolescents at low risk (Stice et al, 2011). Dopaminergic food-reward variations are furthermore moderated by dopamine-related genetic variations (Stice et al, 2012, 2015).
These contradicting models are, however, not necessarily exclusive. Burger and Stice (2011) offered a possible combination of the two models by introducing the dynamic vulnerability model of obesity. This model describes an increased risk for obesity in individuals initially hyper-reward responsive to food intake which leads to overeating and weight gain. Through chronic overeating, however, striatal dopamine-receptor availability reduces and simultaneously a hyper-responsivity of regions encoding the incentive salience of food emerges induced by the conditioning processes during chronic overeating. This again leads to further overeating (Alonso-Alonso et al, 2015; Burger and Stice, 2011).
Besides striatal differential activity in the BAL and DOPD condition, we furthermore observed higher activity in the superior frontal gyrus during depletion. The superior frontal gyrus is involved in self-awareness (Goldberg et al, 2006; Sui et al, 2012) and inhibitory control (Batterink et al, 2010; Dambacher et al, 2014). In several studies, activity in frontal control regions was increased in obese subjects, particularly when the latter were confronted with tasty and high-caloric food (Scharmuller et al, 2012; Stoeckel et al, 2008). In a food-related go/no-go paradigm, the activity in the superior frontal cortex was increased during the no-go compared with the go trials (Batterink et al, 2010).
In the light of the hypothesis of a blunted dopaminergic-reward system in the obese, we expected to find BMI-related differential effect in the CON and DOPD condition. However, no association of the BMI within reward regions was observed and so such results do not support the dopamine reward-deficiency hypothesis in obese subjects. However, motor control for voluntary movements (SMA), as well as cognitive control (ACC) regions showed differential interaction with the BMI during the dopamine conditions. Thus, the BMI was positively associated with motor- and cognitive-control areas during the CON, but negatively or not at all during depletion. Therefore, in the CON condition, the higher the BMI, the higher the necessity to recruit motor and cognitive control during the wanting/liking task (which included button presses). This reflects the BMI effect after a ‘normal' meal intake. Considering that dopamine availability is a major mediator of this effect, we can assume that we observed the lower BMI associations in the DOPD condition, as the depleted state simulates a hypofunctioning reward system equally in all subjects independent of BMI.
Changes in cognitive-control regions in association with BMI in cognitive tasks has been reported (Bosak and Martin, 2014; Carnell et al, 2012; Hege et al, 2013; Kullmann et al, 2013) and dopamine has a crucial role in cognitive-control processes (Cools and D'Esposito, 2011). Furthermore, there is evidence of reduced executive function in obese (Smith et al, 2011; Stingl et al, 2012), mainly in decision-making, planning, and problem-solving tasks (Fitzpatrick et al, 2013). This might lead to increased recruitment of motor and cognitive regions also during our food-related rating task. Yet, the task in our study was not cognitively or motorically challenging and our results therefore do only allow for a careful speculation. In addition, dopaminergic signaling in relation to the performance of voluntary exercise is described in rodents (Garland et al, 2011). DOPD, thus, leads to reduced exploratory, open-field, spontaneous and locomotor activity in rats. Furthermore, dopamine has an impact on general energy balance by mediating the effects of leptin and ghrelin on eating behavior (van Zessen et al, 2012). Yet, studies investigating the cost of food in relation to dopamine showed no changed energy balance on hyperdopaminergic mice (Beeler et al, 2012).
For the task conditions, we found distinct activation differences between the wanting and liking task, with higher activation during the wanting task in a large brain network. It is important to note that we specifically asked for the current state (wanting) and the general trait (liking) of the food items. Here, the wanting component of food reward seems to recruit brain circuits involved in memory function (para-/hippocampus), food processing (fusiform gyrus), emotion (amygdala), reward (putamen), and hedonic evaluation (vmPFC), but also frontal control (ACC). This might be represented in higher wanting compared with liking ratings of the subjects on both scanning days, indicating a much higher neuronal impact of the current ‘wanting' than for the general ‘liking' when subjects are stimulated with food items. This lines up with higher striatal activity during anticipation of high vs low reward (which rather reflects the wanting condition) but not in the receipt phase (rather reflecting the liking condition) of food-related reward (Simon et al, 2014). In addition, a higher predictive value of reward-related neuronal activity for weight gain was found for the anticipation compared with the receipt of palatable food (Burger and Stice, 2013). Such results imply that wanting may have a larger role in individual food choice compared with liking. Furthermore, this neuronal effect is not independent of the dopaminergic state. We detected an interaction of the dopamine and the task variable in the left hippocampus. As shown in Figure 3e, the higher activation for ‘wanting' was visible in the CON only. We saw no difference in behavior between the wanting and liking rating for the two drinks. In our fMRI data, however, the hippocampal activation is lower for the liking task in the CON only. This might indicate that there is a higher need for memory processes in the depleted condition in order to ‘remember' what food is actually liked, which is not necessary in the CON. There is evidence for dopamine as a crucial modulator of hippocampal memory processes, especially in terms of long-term memories (Shohamy and Adcock, 2010). Furthermore, the dopaminergic system was found to be involved in the consolidation for reward-related memories during sleep (Feld et al, 2014). As the liking rating rather recruit long-term memory functions, this is in line with our finding of dopaminergic interaction in the hippocampus mainly based on the liking condition.
Because we limited our study to female subjects, we cannot draw any conclusions for the general population. Furthermore, the order of the task condition was not counterbalanced, which might have an influence on the task effect. However, no hunger or tiredness-related differences between the task conditions are to be expected as one run lasted for only 5 min. In general, DOPD was shown to be a valid method to induce reduced-dopamine levels. However, it may not exclusively affect dopamine synthesis, but consecutively also norepinephrine and epinephrine which are synthesized from dopamine. Therefore, the depletion of dopamine successors might also mediate the results. Furthermore, the depletion is not exclusive to the striatum, which opens the possibility that influenced neurotransmitters such as norepinephrine binding on adrenergic receptors might have an unknown influence. However, the acute phenylalanine/tyrosine depletion method (used in our study) is at-large specific to induce mainly dopamine changes (Booij et al, 2003).
CONCLUSION
In summary our study addresses the role of the dopaminergic-reward system in obese individuals. According to the hypothesis of a hypofunction in the dopaminergic-reward system in obesity, we simulated different neuronal dopamine levels by a DOPD approach. We observed differential BMI-related associations in motor- and cognitive-control areas in the two conditions, but not in reward-related regions, thereby contradicting the aforementioned hypothesis. This points to other hormonal or neuronal mechanisms associated with obesity that influence the dopaminergic system. In a recent study, the altered dopamine-receptor availability in obese subjects was reported to have a minor role only and μ-opioid receptors were believed to be the more important target for the association with obesity. The authors observed that obese subjects presented lower μ-opioid receptor availability than lean subjects, whereas dopamine-receptor availability was similar for both the groups (Karlsson et al, 2015). Therefore, the focus on altered dopaminergic-reward system in obesity might be extended to also include additional mechanisms as the μ-opioid system.
Disturbed dopaminergic-reward pathways might therefore be one of several contributors to overeating and obesity. Hence, it is still vital that we gain even deeper insight into the neurochemical alterations involved in obesity.
Funding and Disclosure
This work was supported by the ‘Kompetenznetz Adipositas (Competence Network for Adiposity)' funded by the German Federal Ministry of Education and Research (FKZ: 01GI1122F and 01GI1332), the German Diabetes Association (334/02/13), the German Center for Diabetes Research and by the European Union Seventh Framework Program (FP7/2007–2013) under Grant Agreement 607310 (Nudge-it). The authors declare no conflict of interest.
Acknowledgments
We thank Maike Borutta very much for her assistance during the measurements, Hubert Kalbacher for his advice regarding the amino acid analyses and Shirley Würth for language editing and proofreading of the manuscript.
Author contributions
Study design: AF, H-CF, HP, HS, PE, and SF; material/test preparation: HS, RV, PE, SF, TU, and U-MB; pilot study: AF, HP, HS, KL, MH, RV, SF, and TU; data acquisition: KL, MH, RV, SF, TU, and U-MB; blood sampling and sample preparation: KL, MH, TU, and U-MB; data analyses: KL, MH, RV, and SF; manuscript preparation: RV and SF; interpretation of the results: AF, H-CF, HP, MH, PE, RV, and SF; and manuscript revision and approval: all authors.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Alonso-Alonso M, Woods SC, Pelchat M, Grigson PS, Stice E, Farooqi S et al (2015). Food reward system: current perspectives and future research needs. Nutr Rev 73: 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterink L, Yokum S, Stice E (2010). Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage 52: 1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, Frazier CR, Zhuang X (2012). Dopaminergic enhancement of local food-seeking is under global homeostatic control. Eur J Neurosci 35: 146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (1996). Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev 20: 1–25. [DOI] [PubMed] [Google Scholar]
- Berridge KC (2009). 'Liking' and 'wanting' food rewards: brain substrates and roles in eating disorders. Physiol Behav 97: 537–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Grant SJ, Chen G, Hommer DW (2013). Dietary tyrosine/phenylalanine depletion effects on behavioral and brain signatures of human motivational processing. Neuropsychopharmacology 39: 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Thanos PK, Gold MS (2014). Dopamine and glucose, obesity, and reward deficiency syndrome. Front Psychol 5: 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij L, Van der Does AJ, Riedel WJ (2003). Monoamine depletion in psychiatric and healthy populations: review. Mol Psychiatry 8: 951–973. [DOI] [PubMed] [Google Scholar]
- Bosak K, Martin L (2014). Neuroimaging of goal-directed behavior in midlife women. Nurs Res 63: 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger KS, Stice E (2011). Variability in reward responsivity and obesity: evidence from brain imaging studies. Curr Drug Abuse Rev 4: 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger KS, Stice E (2013). Elevated energy intake is correlated with hyperresponsivity in attentional, gustatory, and reward brain regions while anticipating palatable food receipt. Am J Clin Nutr 97: 1188–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A (2012). Neuroimaging and obesity: current knowledge and future directions. Obes Rev 13: 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, D'Esposito M (2011). Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69: e113–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Hwang HJ, Leyton M, Dagher A (2012). Dopamine precursor depletion impairs timing in healthy volunteers by attenuating activity in putamen and supplementary motor area. J Neurosci 32: 16704–16715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher A (2012). Functional brain imaging of appetite. Trends Endocrinol Metab 23: 250–260. [DOI] [PubMed] [Google Scholar]
- Dambacher F, Sack AT, Lobbestael J, Arntz A, Brugmann S, Schuhmann T (2014). The role of right prefrontal and medial cortex in response inhibition: interfering with action restraint and action cancellation using transcranial magnetic brain stimulation. J Cogn Neurosci 26: 1775–1784. [DOI] [PubMed] [Google Scholar]
- Davis C, Strachan S, Berkson M (2004). Sensitivity to reward: implications for overeating and overweight. Appetite 42: 131–138. [DOI] [PubMed] [Google Scholar]
- de Wit S, Standing HR, Devito EE, Robinson OJ, Ridderinkhof KR, Robbins TW et al (2012). Reliance on habits at the expense of goal-directed control following dopamine precursor depletion. Psychopharmacology 219: 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis KA, Mehta MA, Naga Venkatesha Murthy PJ, McTavish SF, Nathan PJ, Grasby PM (2007). Tyrosine depletion alters cortical and limbic blood flow but does not modulate spatial working memory performance or task-related blood flow in humans. Hum Brain Mapp 28: 1136–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld GB, Besedovsky L, Kaida K, Munte TF, Born J (2014). Dopamine D2-like receptor activation wipes out preferential consolidation of high over low reward memories during human sleep. J Cogn Neurosci 26: 2310–2320. [DOI] [PubMed] [Google Scholar]
- Fernstrom JD, Fernstrom MH (1994). Dietary effects on tyrosine availability and catecholamine synthesis in the central nervous system: possible relevance to the control of protein intake. Proc Nutr Soc 53: 419–429. [DOI] [PubMed] [Google Scholar]
- Finlayson G, King N, Blundell JE (2007). Liking vs. wanting food: importance for human appetite control and weight regulation. Neurosci Biobehav Rev 31: 987–1002. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick S, Gilbert S, Serpell L (2013). Systematic review: are overweight and obese individuals impaired on behavioural tasks of executive functioning? Neuropsychol Rev 23: 138–156. [DOI] [PubMed] [Google Scholar]
- Garland T Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE et al (2011). The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol 214(Pt 2): 206–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg II, Harel M, Malach R (2006). When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron 50: 329–339. [DOI] [PubMed] [Google Scholar]
- Hardman CA, Herbert VM, Brunstrom JM, Munafo MR, Rogers PJ (2012). Dopamine and food reward: effects of acute tyrosine/phenylalanine depletion on appetite. Physiol Behav 105: 1202–1207. [DOI] [PubMed] [Google Scholar]
- Hege MA, Stingl KT, Ketterer C, Haring HU, Heni M, Fritsche A et al (2013). Working memory-related brain activity is associated with outcome of lifestyle intervention. Obesity 21: 2488–2494. [DOI] [PubMed] [Google Scholar]
- Hilbert A, Tuschen-Caffier B, Karwautz A, Niederhofer H, Munsch S (2007). Eating disorder examination-questionnaire: psychometric properties of the German version. Diagnostica 53: 144–154. [Google Scholar]
- Hitsman B, MacKillop J, Lingford-Hughes A, Williams TM, Ahmad F, Adams S et al (2008). Effects of acute tyrosine/phenylalanine depletion on the selective processing of smoking-related cues and the relative value of cigarettes in smokers. Psychopharmacology 196: 611–621. [DOI] [PubMed] [Google Scholar]
- Ito H, Kodaka F, Takahashi H, Takano H, Arakawa R, Shimada H et al (2011). Relation between presynaptic and postsynaptic dopaminergic functions measured by positron emission tomography: implication of dopaminergic tone. J Neurosci 31: 7886–7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson HK, Tuominen L, Tuulari JJ, Hirvonen J, Parkkola R, Helin S et al (2015). Obesity is associated with decreased mu-opioid but unaltered dopamine D2 receptor availability in the brain. J Neurosci 35: 3959–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ (2011). Reward mechanisms in obesity: new insights and future directions. Neuron 69: 664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Pape AA, Heni M, Ketterer C, Schick F, Haring HU et al (2013). Functional network connectivity underlying food processing: disturbed salience and visual processing in overweight and obese adults. Cereb Cortex 23: 1247–1256. [DOI] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A (2002). Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology 27: 1027–1035. [DOI] [PubMed] [Google Scholar]
- Leyton M, Casey KF, Delaney JS, Kolivakis T, Benkelfat C (2005). Cocaine craving, euphoria, and self-administration: a preliminary study of the effect of catecholamine precursor depletion. Behav Neurosci 119: 1619–1627. [DOI] [PubMed] [Google Scholar]
- Leyton M, Dagher A, Boileau I, Casey K, Baker GB, Diksic M et al (2004). Decreasing amphetamine-induced dopamine release by acute phenylalanine/tyrosine depletion: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology 29: 427–432. [DOI] [PubMed] [Google Scholar]
- Leyton M, Young SN, Blier P, Baker GB, Pihl RO, Benkelfat C (2000). Acute tyrosine depletion and alcohol ingestion in healthy women. Alcohol Clin Exp Res 24: 459–464. [PubMed] [Google Scholar]
- Lowe MR, Butryn ML (2007). Hedonic hunger: a new dimension of appetite? Physiol Behav 91: 432–439. [DOI] [PubMed] [Google Scholar]
- McTavish SF, Cowen PJ, Sharp T (1999. a). Effect of a tyrosine-free amino acid mixture on regional brain catecholamine synthesis and release. Psychopharmacology 141: 182–188. [DOI] [PubMed] [Google Scholar]
- McTavish SF, McPherson MH, Sharp T, Cowen PJ (1999. b). Attenuation of some subjective effects of amphetamine following tyrosine depletion. J Psychopharmacol 13: 144–147. [DOI] [PubMed] [Google Scholar]
- Medic N, Ziauddeen H, Vestergaard MD, Henning E, Schultz W, Farooqi IS et al (2014). Dopamine modulates the neural representation of subjective value of food in hungry subjects. J Neurosci 34: 16856–16864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery AJ, McTavish SF, Cowen PJ, Grasby PM (2003). Reduction of brain dopamine concentration with dietary tyrosine plus phenylalanine depletion: an [11C]raclopride PET study. Am J Psychiatry 160: 1887–1889. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Mannie ZN, Cowen PJ, Harmer CJ, McTavish SB (2007). Effects of acute tyrosine depletion on subjective craving and selective processing of smoking-related cues in abstinent cigarette smokers. J Psychopharmacol 21: 805–814. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A, Cisek P, Perna AS, Shirdel FZ, Benkelfat C, Leyton M et al (2012). From anticipation to action, the role of dopamine in perceptual decision making: an fMRI-tyrosine depletion study. J Neurophysiol 108: 501–512. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A (2008). Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J Neurosci 28: 3697–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninan I, Kulkarni SK (1998). Dopamine receptor sensitive effect of dizocilpine on feeding behaviour. Brain Res 812: 157–163. [DOI] [PubMed] [Google Scholar]
- Pudel D, Westenhöfer J (1989) Fragebogen zum Eßverhalten (FEV). Handanweisung. Hogrefe: Göttingen. [Google Scholar]
- Richard JM, Castro DC, Difeliceantonio AG, Robinson MJ, Berridge KC (2012). Mapping brain circuits of reward and motivation: in the footsteps of Ann Kelley. Neurosci Biobehav Rev 37(9 Pt A): 1919–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Standing HR, DeVito EE, Cools R, Sahakian BJ (2010). Dopamine precursor depletion improves punishment prediction during reversal learning in healthy females but not males. Psychopharmacology 211: 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K (1991). Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology 104: 515–521. [DOI] [PubMed] [Google Scholar]
- Scharmuller W, Ubel S, Ebner F, Schienle A (2012). Appetite regulation during food cue exposure: a comparison of normal-weight and obese women. Neurosci Lett 518: 106–110. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Adcock RA (2010). Dopamine and adaptive memory. Trends Cogn Sci 14: 464–472. [DOI] [PubMed] [Google Scholar]
- Simon JJ, Skunde M, Wu M, Schnell K, Herpertz SC, Bendszus M et al (2014). Neural dissociation of food- and money-related reward processing using an abstract incentive delay task. Soc Cogn Affect Neurosci 10: 1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, Hay P, Campbell L, Trollor JN (2011). A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes Rev 12: 740–755. [DOI] [PubMed] [Google Scholar]
- Stice E, Burger KS, Yokum S (2015). Reward region responsivity predicts future weight gain and moderating effects of the TaqIA allele. J Neurosci 35: 10316–10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM (2008). Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 322: 449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger K, Epstein L, Smolen A (2012). Multilocus genetic composite reflecting dopamine signaling capacity predicts reward circuitry responsivity. J Neurosci 32: 10093–10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, Epstein LH, Small DM (2011). Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci 31: 4360–4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl KT, Kullmann S, Ketterer C, Heni M, Haring HU, Fritsche A et al (2012). Neuronal correlates of reduced memory performance in overweight subjects. Neuroimage 60: 362–369. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW III, Twieg DB, Knowlton RC, Cox JE (2008). Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 41: 636–647. [DOI] [PubMed] [Google Scholar]
- Sui J, Chechlacz M, Humphreys GW (2012). Dividing the self: distinct neural substrates of task-based and automatic self-prioritization after brain damage. Cognition 122: 150–162. [DOI] [PubMed] [Google Scholar]
- Tellez LA, Medina S, Han W, Ferreira JG, Licona-Limon P, Ren X et al (2013). A gut lipid messenger links excess dietary fat to dopamine deficiency. Science 341: 800–802. [DOI] [PubMed] [Google Scholar]
- van Zessen R, van der Plasse G, Adan RA (2012). Contribution of the mesolimbic dopamine system in mediating the effects of leptin and ghrelin on feeding. Proc Nutr Soc 71: 435–445. [DOI] [PubMed] [Google Scholar]
- Venugopalan VV, Casey KF, O'Hara C, O'Loughlin J, Benkelfat C, Fellows LK et al (2011). Acute phenylalanine/tyrosine depletion reduces motivation to smoke cigarettes across stages of addiction. Neuropsychopharmacology 36: 2469–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Baler RD (2011). Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci 15: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD (2012). Obesity and addiction: neurobiological overlaps. Obes Rev 14: 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W et al (2001). Brain dopamine and obesity. Lancet 357: 354–357. [DOI] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, Wager TD (2014). Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 91: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.