Abstract
Statins are drugs that have been used for decades in humans for the treatment of hypercholesterolemia. More recently, several lines of evidence demonstrate that statins, in addition to their peripheral effects, produce a wide variety of effects in the brain and may be beneficial in neurological and psychiatric conditions. In this study, we allowed rats to self-administer cocaine for several weeks and, at the end of self-administration training, we treated them with low doses of statins daily for a 21-day period of abstinence. Chronic administration of brain-penetrating statins, simvastatin (1 mg/kg) and atorvastatin (1 mg/kg), reduced cocaine seeking compared with vehicle, whereas administration of pravastatin (2 mg/kg), a statin with low brain penetrability, did not. Importantly, the effects of brain-penetrating statins persisted even after discontinuation of the treatment and were specific for drug seeking because drug taking was not altered by simvastatin treatment. Finally, the effects of simvastatin were found to generalize to another drug of abuse such as nicotine, but not to food reward, and to reinstatement of cocaine seeking induced by stress. These results demonstrate that brain-penetrating statins can reduce risks of relapse to addiction. Given their well-known safety profile in humans, statins could be a novel effective treatment for relapse to cocaine and nicotine addiction and their use could be implemented in clinical settings without major health risks.
INTRODUCTION
One of the hallmarks of addiction, and one of its most troubling aspects, is the long-lasting risk of relapse. As a matter of fact, the rates of relapse are very high and can occur even after several months from the last drug-taking episodes when acute withdrawal symptoms are no more present (Kreek et al, 2002). Although a great deal of information has been collected on the psychological, neurobiological, cellular and molecular mechanisms of drug addiction, effective therapies for addiction are still very limited (O'Brien, 2005; Vocci et al, 2005). Thus, the discovery of new pharmacological agents, which can help people suffering from addictive disorders in their recovery efforts, is a pressing necessity.
Statins constitute a class of drugs that have been used for decades in humans for the treatment of hypercholesterolemia and they have been shown to have a relatively safe profile (Law and Rudnicka, 2006). These compounds reduce de novo synthesis of cholesterol by inhibiting 3-hydroxy-3methyl-glutaryl coenzymeA (HMG-Co) reductase enzymes (Mahley and Bersot, 2005). Importantly, a study in humans has found that increase in transcripts related to cholesterol biosynthesis and trafficking is a common feature of cocaine, cannabis, and phencyclidine abuse (Lehrmann et al, 2006). Accumulating evidence suggests that statins produce effects on the brain that are independent from cholesterol synthesis (Laufs and Liao, 2003; Rahola, 2012). For example, inhibition of HMG-Co reductase enzymes results in the blockade of synthesis of mevalonate and in the reduction of post-translational modifications of proteins such as isoprenylation of small GTP-binding proteins. These modifications may ultimately result in neuronal altered function at the levels of morphogenesis, cell signaling, synaptic plasticity, etc (Orban et al, 1999; Luo, 2000). Furthermore, statins can directly bind to histone deacetylation enzymes, key enzymes in promoting epigenetic modifications (Tsankova et al, 2007), and inhibit their function (Lin et al, 2008), an effect that has been shown to reduce addiction-related behaviors of cocaine (Romieu et al, 2008). In addition, chronic treatment with low-to-moderate doses of statins has been shown to upregulate the expression of vascular endothelial growth factor and brain-derived neurotropic factor (BDNF) to induce hippocampal neurogenesis (Lu et al, 2007; Wu et al, 2008), to interact with the PI3K/Akt and ras/ERK pathways (Wu et al, 2008), and to be beneficial in animal models of neurological conditions such as Alzheimer and Parkinson's diseases (Schuster et al, 2008; Malfitano et al, 2014). These positive effects of statins are reminiscent to those obtained with the enriched environment, an experimental paradigm that was also shown to be beneficial in reducing risks of relapse to cocaine addiction in models of relapse (Solinas et al, 2008; Chauvet et al, 2009; Thiel et al, 2009; Chauvet et al, 2012). Therefore, statins appear to act as ‘environmimetics' (Nithianantharajah and Hannan, 2006), ie, molecules that mimic the effects of environmental enrichment, and we hypothesized that chronic treatment with statins could reduce the risks of relapse to drug seeking.
To test this hypothesis, we designed a study to investigate whether (1) statins would reduce cocaine seeking in a rat model of relapse (Shaham et al, 2003); (2) the effects of statins would be long-lasting and whether they would persist after discontinuation of the treatment; (3) the effects would be generalized to different statins and whether differences in brain penetration would influence these effects; (4) active drug-taking would be reduced by statin treatment; (5) statins would be effective against another drug of abuse such as nicotine; (6) seeking for a natural reward such as food would be affected by statins; and (7) statins could reduce stress-induced reinstatement.
MATERIALS AND METHODS
For more details of experimental procedures see Supplementary Materials and Methods.
Subjects
Adult male Sprague Dawley rats were used in this study. All experiments were conducted in accordance to European Union directives (2010/63/EU) for the care of laboratory animals.
General Procedure and Statin Treatment
The general procedure used in this study was to allow animals to self-administer drugs or food for several weeks and then to treat them daily with statins during a 21-day period of abstinence except for experiment 3 in which rats were treated with statins shortly before self-administration sessions. Statins or vehicle were always injected between 10 AM and 12 AM and tests for relapse were conducted ~24 h after the last simvastatin administration under extinction condition, ie, in the absence of drug or food reinforcement. The number of active responses during these sessions was used as a measure of drug or food seeking.
Experiment 1a: Effects of Different Doses of Simvastatin on Cocaine-Seeking Behavior
Rats were allowed to self-administer cocaine for 20 experimental 3-h sessions using a fixed ratio 1 (FR1) schedule of reinforcement. During these sessions, a single response in the active nose-poke hole immediately delivered an i.v. injection of cocaine at a unitary dose of 0.6 mg/inj associated with activation of a light cue. During abstinence rats were treated with simvastatin (0.1 or 1 mg/kg i.p.) or its vehicle. Drug seeking was assessed the day following the last injection during 3 h.
Experiment 1b: Effects of Simvastatin After Discontinuation of the Treatment
The rats from experiment 1a treated with vehicle or 1 mg/kg of simvastatin were used in this experiment. After the first test for cocaine-seeking behavior, rats underwent a second 21-day period of abstinence and statin treatment. They were semi-randomly assigned to four groups: (a) a group received vehicle during the first and the second period of abstinence (Veh–Veh); (b) a group received vehicle during the first and simvastatin (1 mg/kg) during the second period of abstinence (Veh–Sim); (c) a group received simvastatin (1 mg/kg) during the first and vehicle during the second period of abstinence (Sim–Veh); and (d) a group received simvastatin (1 mg/kg) during the first and the second period of abstinence (Sim–Sim). Drug seeking was assessed the day following the last injection during 3 h.
Experiment 2: Effects of Other Statins on Cocaine-Seeking Behavior
Rats were allowed to self-administer cocaine for 20 experimental 3-h sessions as in experiment 1a. During abstinence rats were treated with atorvastatin (1 mg/kg i.p.), pravastatin (2 mg/kg i.p.), or vehicle daily. Doses of atorvastatin and pravastatin were chosen based on the relative doses used in humans (Mahley and Bersot, 2005). Drug seeking was assessed the day following the last injection during 3 h.
Experiment 3: Effects of Simvastatin on Cocaine-Taking Behavior
Rats were allowed to self-administer cocaine for 10 experimental 3-h sessions as in experiment 1a. Starting on the 11th session, rats were daily injected with simvastatin (1 mg/kg i.p.) or vehicle 15 min before the start of each of the following 20 self-administration sessions. Self-administration sessions were identical throughout the experiment. The number of cocaine injections was used as a measure of the reinforcing effects of cocaine.
Experiment 4: Effects of Simvastatin on Nicotine-Seeking Behavior
Rats were allowed to self-administer nicotine for a total of 24 experimental sessions. The duration of the session, the schedule of reinforcement and the dose of nicotine were changed over time to maximize rates of responding. During the first eight sessions of training, nicotine was available at a dose of 22 μg/inj for 3 h according to an FR1 schedule for session 1–6 and then to an FR3 schedule for session 7–8. From the 9th session, we reduced the dose to 7.5 μg/inj. In addition, we reduced the duration of the session to 1 h because analysis of pattern of self-administration suggested that rats self-administered most injections during the first 60 min of the session. During abstinence rats were treated with simvastatin (1 mg/kg) or its vehicle. Drug seeking was assessed the day following the last injection during 3 h.
Experiment 5: Effects of Simvastatin on Food-Seeking Behavior
Rats were allowed to self-administer 45 mg food pellets for a total of 15 experimental sessions according to a FR1 schedule. After five sessions, the duration of the session was changed from 1 to 4 h to maximize rates of responding. During abstinence rats were treated with simvastatin (1 mg/kg) or its vehicle. Food seeking was assessed the day following the last injection during 3 h.
Experiment 6: Effects of Simvastatin on Stress-Induced Reinstatement
Rats were allowed to self-administer cocaine for 20 experimental 3-h sessions using lever presses as operanda and were treated for 21 days with 1 mg/kg of simvastatin as in experiment 1a. The following day, rats underwent extinction of cocaine seeking in one single 6 h session and then were injected with yohimbine (2.5 mg/kg, 30 min before the session) to trigger stress-induced reinstatement, which was assessed for 1 h.
Statistical Analysis
Differences were assessed by one-way, two-way or three-way ANOVA. Results showing significant overall changes were subjected to Student–Newman–Keuls post hoc test. Differences were considered significant when P<0.05.
RESULTS
Simvastatin Decreases Cocaine Seeking After Abstinence
First, we investigated whether chronic treatment with simvastatin during withdrawal would reduce cocaine-seeking behavior (see Figure 1a for schematic representation of the experimental protocol). During self-administration training, rats increased the number of cocaine injections from 20 to 26 injections per session (Figure 1b; F(19,684)=14.66, P<0.0001). Active nose-pokes also increased, whereas inactive nose-pokes rapidly decreased (Figure 1b; Active/inactive device effect: F(1,19)=938.67, P<0.0001; time effect: F(19,684)=2.92, P<0.0001; time × active/inactive interaction: F(19,684)=19.11, P<0.0001). During the 21-day withdrawal period animals were injected daily with vehicle or two doses of simvastatin: a 1 mg/kg dose that has been shown to have significant neurobiological and behavioral effects in rats (Chen et al, 2003; Lu et al, 2007; Wu et al, 2008) and a 10-fold lower dose. Only the 1 mg/kg dose of simvastatin was effective in decreasing the number of active responses in rats compared with vehicle, without affecting inactive nose-poke responses (Figure 1c; dose: F(2,32)=3.60, P<0.05; active–inactive device: F(1,32)=112.80, P<0.001, dose × active–inactive interaction, F(2,32)=8.0, P<0.01). Therefore, the dose of 1 mg/kg was selected as reference for subsequent experiments.
Figure 1.
Chronic administration of simvastatin (Sim) during the withdrawal period reduces cocaine-seeking behavior. (a) Timeline of the experiment. (b) Cocaine self-administration training. Data are expressed as mean±SEM of cocaine injections (left) and active and inactive nose-pokes (right) during the 3 h daily self-administration sessions (N=37). During training, active nose-pokes were reinforced according to an FR1 schedule. (c) Extinction test after a chronic treatment with Sim during the 21 days of withdrawal. Tests were performed 22–24 h after the last injection of Sim or vehicle (veh). Data are expressed as mean±SEM of responses on the active or inactive nose-pokes during the 3 h extinction test. Rats were injected i.p. daily with veh (N=13), Sim 0.1 mg/kg (N=7) or Sim 1 mg/kg (N=17). Two-way ANOVA followed by Student–Neuman–Keuls post hoc test: $$, P<0.01 Sim-treated different from veh-treated control; **P<0.01 active different from inactive nose-pokes.
The Effects of Simvastatin are Long Lasting
We then tested whether the effects of simvastatin would persist after discontinuation of the treatment. For this, after the first test for relapse, rats from experiment 1a underwent a second period of 21 day of withdrawal in which they either received simvastatin or vehicle according to a crossover design and were tested again for drug-seeking (Figure 2a). Rats that received statin during both abstinence periods (Sim–Sim) showed significant reduction in drug-seeking behavior compared with control rats (Veh–Veh), suggesting that longer treatment with simvastatin does not provide further benefit and that the effects of simvastatin do not undergo tolerance (Figure 2b). Rats that received simvastatin at the second treatment period only (Veh–Sim) showed significant reduction in drug-seeking behavior compared with Veh–Veh, suggesting that the treatment with simvastatin is effective even when it is implemented at late stages of withdrawal (Figure 2b). Most importantly, rats that received simvastatin during the first period but were off statin during the second withdrawal period (Sim–Veh) also showed significant reduction of cocaine seeking, indicating that the effects of statin are long-lasting (Figure 2b). Statistical analysis revealed a significant effect of active/inactive device (F(1,52)=43.72, P<0.0001) and significant interaction between the first and the second treatment period treatment F(19,608)=2.04, P<0.01; time × active/inactive interaction: F(1,52)=5.01, P<0.05).
Figure 2.
The effects of simvastatin (Sim) on cocaine seeking last after discontinuation of the treatment. (a) Timeline of the experiment. After a first test for cocaine seeking shown in Figure 1 (gray lines), rats underwent a second abstinence period (black lines) in which they were treated with Veh or Sim (1 mg/kg) obtaining four groups: (1) Veh–Veh (N=6); (2) Veh–Sim, (N=7); (3) Sim–Veh (N=10); Sim–Sim (N=7). (b) Second extinction test after 21 additional days of withdrawal and of treatment with Sim or vehicle (veh). Tests were performed 22–24 h after the last injection of Sim or veh. Data are expressed as mean±SEM of responses on the active or inactive nose-pokes during the 3 h extinction test. Two-way ANOVA followed by Student–Neuman–Keuls post hoc test: $ and $$P<0.05 and P<0.01 Sim-treated different from veh-treated control; **P<0.01 active different from inactive nose-pokes.
Only Statins that Pass the Blood–Brain Barrier (BBB) Reduce Cocaine-Seeking Behavior
We then investigated whether the effects of simvastatin could be generalized to other molecules of the same pharmacological class (see Figure 3a for schematic representation of the experimental protocol). Importantly, statins commonly used in humans to reduce blood cholesterol vary considerably in their ability to pass the BBB (Saheki et al, 1994; Sierra et al, 2011). Therefore, we hypothesized that only brain-penetrating statins would be effective in reducing drug-seeking behavior. For this, we selected two statins, atorvastatin because it is the most used statin in humans and has brain penetration similar to simvastatin (White, 2002), and pravastatin because it has low brain penetration (Tsuji et al, 1993).
Figure 3.
Chronic administration of atorvastatin (Ator), but not pravastatin (Prava), reduces cocaine-seeking behavior. (a) Timeline of the experiment. (b) Cocaine self-administration training. Data are expressed as mean±SEM of cocaine injections (left) and active and inactive nose-pokes (right) during the 3 h daily self-administration sessions (N=30). During training, active nose-pokes were reinforced according to an FR1 schedule. (c) Extinction tests after a chronic treatment with Ator, Prava, or vehicle (veh) during the 21 days of withdrawal. Tests were performed 22–24 h after the last injection of Ator, Prava, or veh. Data are expressed as mean±SEM of responses on the active or inactive nose-pokes during the 3 h extinction test. Rats were injected i.p. daily with veh (N=7 and N=8 for Ator and Prava experiments, respectively), Ator 1 mg/kg (N=7) or Prava 2 mg/kg (N=8). Two-way ANOVA followed by Student–Neuman–Keuls post hoc test: $, P<0.05 Atorvastatin-treated different from vehicle-treated control; **P<0.01 active different from inactive nose-pokes.
During self-administration training, rats increased the number of cocaine injections (Figure 3b; F(19,589)=14.01, P<0.0001) and showed a preference for the active nose-pokes (Figure 3b; active/inactive device effect: F(1,32)=764.75, P<0.0001; time effect: F(19,608)=2.04, P<0.01; time × active/inactive interaction: F(19,608)=19.29, P<0.0001). Chronic treatment with atorvastatin (1 mg/kg/day i.p.) produced a significant reduction (~50%) in cocaine-seeking behavior (Figure 3c; treatment: F(1,24)=10.40, P<0.01; active–inactive: F(1,24)=54.27, P<0.0001). In contrast, pravastatin (2 mg/kg/day i.p.) did not alter cocaine-seeking behavior compared with vehicle-treated rats (Figure 3c; active–inactive: F(1,26)=32.95, P<0.0001).
Simvastatin Does not Affect Cocaine-Taking Behavior
From a translational point of view, it is important to determine whether simvastatin, in addition to blocking drug seeking after abstinence, could facilitate quitting drug-taking habits. For this, we tested whether chronic administration of simvastatin (1 mg/kg, 15 min before each self-administration session) would reduce active cocaine self-administration (see Figure 4a for schematic representation of the experimental protocol). During self-administration training, rats rapidly learned to self administer cocaine and showed a preference for the active over the inactive nose-pokes (Figure 4b; active/inactive device effect: F(1,6)=34.18, P<0.01).
Figure 4.
Chronic administration of simvastatin (Sim) does not reduce active cocaine-taking behavior. (a) Timeline of the experiment. (b) Cocaine self-administration training. Data are expressed as mean±SEM of cocaine injections (left) and active and inactive nose-pokes (right) during the 3 h daily self-administration sessions (N=7). In all sessions, active nose-pokes were reinforced according to an FR1 schedule. After a 10-day training period, rats were injected daily with veh (N=4) or sim 1 mg/kg (N=3) 15 min before the beginning of the self-administration sessions.
We found that treatment with simvastatin did not alter active cocaine taking in any of the 20 self-administration sessions (Figure 4b; treatment: F(1,145)=0.45, P=0.49; sessions: F(1,145)=1.94, P<0.01, treatment × active–inactive interaction, F(1,145)=0.93, P=0.57) with no change compared with basal levels of self-administration (Figure 4b; treatment: F(1,95)=0.008, P=0.93; sessions: F(1,95)=0.43, P=0.43, treatment × active–inactive interaction, F(1,95)=0.60, P=0.90). This suggests that the primary reinforcing effects of cocaine are not affected by simvastatin and that statins may not be effective in reducing active drug-taking habits.
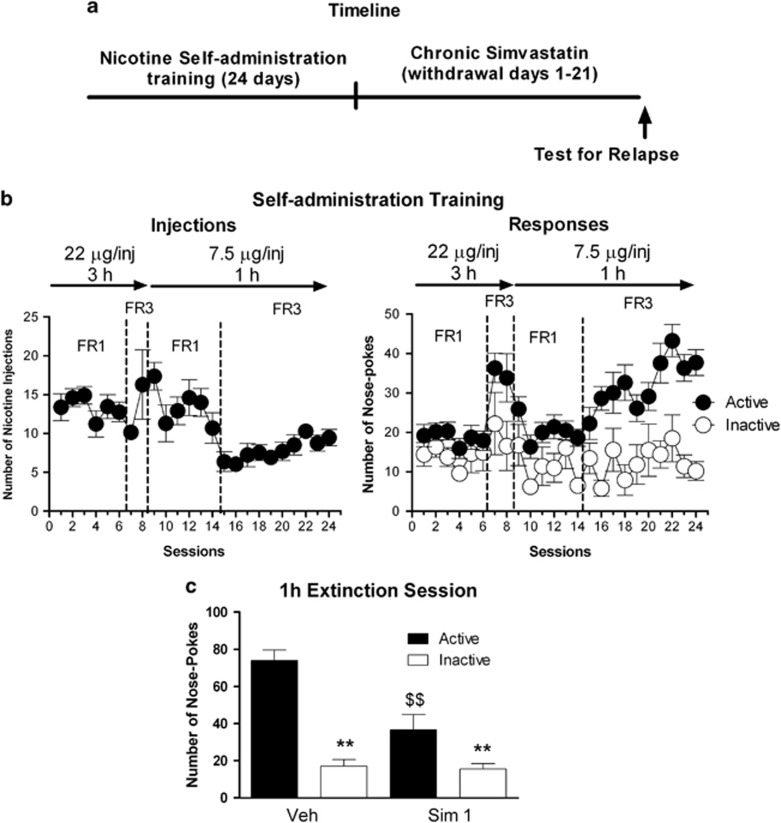
Simvastatin Decreases Relapse to Nicotine
We next investigated whether simvastatin would be effective also in reducing risks of relapse to nicotine, a drug whose use causes millions of deaths each year (see Figure 5a for schematic representation of the experimental protocol). During the self-administration training, the dose, the duration, and the schedule of reinforcement were adapted to maximize rates of responses. During the last 10 days of self-administration in which the animals could self administer nicotine at a dose of 7.5 μg/inj for 1 h according to an FR3 schedule, rats increased the number of nicotine injections (Figure 5b; F(9,108)=3.08, P<0.01) and showed a clear preference for the active nose-poke (Figure 5b; active/inactive device effect: F(1,12)=39.12, P<0.0001; time effect: F(9,108)=2.66, P<0.01; time × active/inactive interaction: F(9,108)=4.08, P<0.01). Chronic administration of simvastatin (1 mg/kg) during abstinence from nicotine self-administration significantly reduced nicotine-seeking behavior (~50%). Simvastatin produced a significant decrease in the number of active responses in rats without affecting inactive nose-poke responses (Figure 5c; treatment: F(1,22)=13.10, P<0.01; active–inactive: F(1,22)=52.52, P<0.0001, treatment × active–inactive interaction, F(1,22)=8.0, P<0.01).
Figure 5.
Chronic administration of simvastatin (Sim) during the withdrawal phase reduces nicotine-seeking behavior. (a) Timeline of the experiment. (b) Nicotine self-administration training. Data are expressed as mean±SEM of nicotine injections (left) and active and inactive nose-pokes (right) during daily self-administration sessions (N=13). The first eight sessions lasted 3 h and nicotine was available at a 22 μg/inj dose, whereas the last 16 sessions lasted 1 h and nicotine was available at a 7.5 μg/inj dose. For each dose, rats were initially trained according to an FR1 schedule and then the FR requirement was increased to three. (c) Extinction test after a chronic treatment with Sim (1 mg/kg) during the 21 days of withdrawal. Tests were performed 22–24 h after the last injection of Sim (N=6) or veh (N=7). Data are expressed as mean±SEM of responses on the active or inactive nose-pokes during the 1 h extinction test. Two-way ANOVA followed by Student–Neuman–Keuls post hoc test: $$, P<0.01 Sim-treated different from veh-treated control; **P<0.01 active different from inactive nose-pokes.
Simvastatin Does not Alter Food Seeking
Next, we investigated whether the effects of statins were specific to drug seeking or whether they were general for natural rewards such as food (see Supplementary Figure S1A for schematic representation of the experimental protocol). Rats had access to food pellets for 1 h (session 1–5) and 4 h (session 6–15) according to an FR1 schedule. During these last 10 days of training, rats increased the number of pellets obtained (Supplementary Figure S1B; F(9,99)=12.57, P<0.0001) and showed a clear preference for the active nose-poke (Supplementary Figure S1B; active/inactive device effect: F(1,10)=114.78, P<0.0001). When tested for food-seeking behavior after the 21-day period of withdrawal and simvastatin treatment, all animals showed high levels of food-seeking behavior and a preference for the active over the inactive nose-poke. However, the chronic treatment with simvastatin did not reduce food-seeking behavior (Supplementary Figure S1C; treatment: F(1,16)=0.49, P=0.50; active–inactive: F(1,16)=20.41, P<0.01, treatment × active–inactive interaction, F(1,16)=0.002, P=0.96).
Simvastatin Decreases Stress-Induced Reinstatement
Finally, we investigated whether simvastatin could reduce reinstatement triggered by stress (see Supplementary Figure S2A for schematic representation of the experimental protocol). During self-administration training, rats increased the number of cocaine injections (Supplementary Figure S2B; F(15,285)=33.08, P<0.0001) and showed a preference for the active lever (Supplementary Figure S2B; Active/inactive device effect: F(1,15)=403.16, P<0.0001; time effect: F(19,285)=10.90, P<0.0001; time × active/inactive interaction: F(19,285)=40.88, P<0.0001). Chronic treatment with simvastatin (1 mg/kg/day i.p.) reduced cocaine-seeking behavior during extinction of cocaine-seeking behavior (Supplementary Figure S2C; active–inactive: F(1,14)=67.37, P<0.0001; treatment × active/inactive interaction: F(1,14)=6.10, P<0.05). Most importantly, treatment with simvastatin significantly decreased reinstatement induced by the pharmacological stressor, yohimbine (2.5 mg/kg i.p.) (Supplementary Figure S2D; treatment: F(1,14)=5.07, P<0.05 active–inactive: F(1,14)=41.00, P<0.0001; treatment × active/inactive interaction: F(1,14)=8.27, P<0.05).
DISCUSSION
Simvastatin decreased both cocaine- and nicotine-seeking behavior triggered by contextual and discrete drug-associated stimuli as well as reinstatement of cocaine-seeking behavior triggered by stress. The effects of simvastatin were dose dependent with doses of 1 mg/kg, but not 0.1 mg/kg, being effective in decreasing drug seeking. In rats, this dose has been previously shown to produce both neurobiological and behavioral effects after brain lesions with oral administration (Chen et al, 2003; Lu et al, 2007; Wu et al, 2008) or in animal models of anxiety with intraperitoneal administration (Segatto et al, 2014). If doses are compared taking into consideration body surface area (Reagan-Shaw et al, 2008), this effective dose would be roughly equivalent to 0.16 mg/kg in humans, which is in the low range of the doses normally prescribed (10–40 mg/day, 0.1–1 mg/kg) (Mahley and Bersot, 2005). Higher doses, from 10 to 80 mg/kg of simvastatin (corresponding to 1.6–12.8 mg/kg in humans), have also been shown to produce neurobiological and behavioral effects without major adverse effect in rodents (Cibickova et al, 2009; Wang et al, 2009; Kumar et al, 2012) but the translational interest of these doses is dubious given that they are considerably higher than those used in humans.
Our experiments demonstrate that not all statins reduce drug seeking in relapse models. In fact, only lipophilic statins that pass the BBB such as simvastatin and atorvastatin reduce drug-seeking behavior, whereas hydrophilic statins such as pravastatin had no effect. This demonstrates that the effects of statins on drug seeking are centrally mediated. Importantly, statins have been shown to produce a variety of effects in the brain such as increases in the expression of BDNF, interaction with the PI3K/Akt and ERK pathways (Wu et al, 2008; Yang et al, 2012), interaction with small G proteins such as Rho and Rack1 (Laufs and Liao, 2003) and stimulation of hippocampal neurogenesis (Lu et al, 2007; Wu et al, 2008). These mechanisms have been shown to have a role in addiction. For example, neurotrophic factors, via activation of PI3K/Akt and ras/ERK pathways, appear to have a major role in drug-induced plasticity, which is believed to be responsible for long-term risks of relapse (Russo et al, 2009; Li and Wolf, 2015). In addition, the ERK pathway can participate in addiction-related neuroadaptation independently from neurotrophic factors via the activation of dopamine and glutamate receptors (Lu et al, 2006; Girault et al, 2007). Finally, deficits in hippocampal neurogenesis appear to facilitate the development and increase the risks of relapse to addiction (Kempermann et al, 2008; Mandyam and Koob, 2012). Although we did not directly investigate the mechanisms underlying statins' effects in this study, it could be speculated that statins interact with these mechanisms and normalize or counteract drug-induced neuroadaptations. Further studies are necessary to determine the neurobiological mechanisms underlying the effects of statins and to determine whether statins would also be effective in reducing drug seeking after acute administration.
Whereas simvastatin was effective in reducing drug seeking in relapse procedures, it did not reduce drug-taking behavior. From a translational point of view, these data suggest that statins may not be effective in facilitating quitting active drug habits but instead that they can help reducing the risks of relapse in abstinent individuals. This dichotomy between effects on active taking phase and relapse measure is not uncommon. For example, the fatty-acid amide hydrolase inhibitor URB597 does not alter cocaine taking (Adamczyk et al, 2009) but its acute or chronic administration reduces cocaine seeking in relapse procedures (Adamczyk et al, 2009; Chauvet et al, 2014). These results also highlight the fact that different neurobiological processes appear to be involved in the ‘intoxication', the ‘withdrawal' and ‘craving' stages of the addiction cycle (Koob and Volkow, 2010). Therefore, it appears that statins do not interact directly with the main mechanisms that underlie the reinforcing effects of drugs such as cocaine but they rather produce neuroadaptations that may help restoring brain normal function and consequently reduce relapse. Importantly, the effects of simvastatin lasted even after discontinuation of its administration, which suggests that this restoration of brain functioning is long lasting. From a translational point of view, this indicates that statin treatment does not need to be a life-long treatment and that it may be interrupted after a certain treatment periods with no increase in the risk of relapse. This appears to be a major advantage because despite the good safety profile of statins, the risks to develop side effects with these drugs seem to increase over time (Huddy et al, 2013).
The effects of statins were not related to motor impairment or to general decrease in motivation because food-seeking behavior and active cocaine-taking behavior were not affected by a chronic treatment with simvastatin. The lack of effect on food seeking must be considered as a positive aspect because it would suggest that motivation for natural rewards are spared and individuals receiving statin treatment during the period of drug abstinence would show normal behavior toward natural reward. This is consistent with human data showing no reduction in food intake in people treated with statins (Kaestner et al, 2014; Sugiyama et al, 2014).
Although a study suggests a link between alteration in cholesterol homeostasis and addiction (Lehrmann et al, 2006), to the best of our knowledge no previous study has directly investigated the effects of statins on addiction in humans. On the other hand, because hypercholesterolemia and tobacco smoking are two of the main risk factors for cardiovascular disorders, statins are often prescribed to people that also smoke tobacco. One recent study using longitudinal self-report data from a cohort of ~2000 people in the USA has found that statins do not decrease significantly the number of smokers and heavy smokers in that population (Kaestner et al, 2014). Our data are not necessarily at odds with this finding and can instead help explaining the lack of significant effects found in that study. In fact, several factors could have contributed to masking or preventing the effect of statins. First of all, it should be considered that these studies pooled together data obtained with different statins. Therefore, negative results with statins that have low brain penetration could have masked the positive effects of brain-penetrating statins. Most importantly and in relation with the present study, statins were not specifically used in the context of smoking cessation attempts and therefore, people were not instructed to stop smoking and remain abstinent during the treatment. Our experiments on active self-administration behavior show that statins are not effective in reducing on-going drug taking. Therefore, a reason for the failure to find significant effects of statin treatment may be related to the timing and the protocol of the administration of statins. Future translational studies to test the efficacy of statins should be designed to avoid any of these possible confounding factors.
In conclusion, this study shows that a chronic treatment with brain-penetrating statins decreases the risk of relapse to cocaine and nicotine without affecting motivation for natural rewards. These results suggest that brain-penetrating statins such as simvastatin and atorvastatin could be useful pharmacological tools for the treatment of addiction. The considerable experience in the effects of chronic treatment with statins in humans and the vast knowledge about their safety profile make the translation from preclinical to clinical settings straightforward. On the other hand, the results of this study together with data from clinical studies, suggest that not all statins may be effective and that statin treatment may be effective only in reducing relapse but not in facilitating quitting. Therefore, our results call for the necessity of clinical trials specifically designed to evaluate the effect of brain-penetrating statins to fight relapse to drug addiction.
FUNDING AND DISCLOSURE
CC, MJ, and MS are inventors in the patent ‘The use of statins for the treatment of addiction' (WO2011128810A1) by the CNRS/University of Poitiers. CN, CLC, NT declare no competing financial interests.
Acknowledgments
We thank PO Fernagut and M Mameli for helpful comments on a previous version of the manuscript. We thank Ranbaxy Laboratories Limited for generously providing simvastatin. This work was supported by the Institute National de la Santé et de la Recherche Medical, le Centre National pour la Recherche Scientifique, University of Poitiers, and the Contrat de Projet Etat Region (CPER) and the Fonds Européens de Développement Régional (FEDER) programs. This study was supported by a CNRS PEPS grant to MS. CC was recipient of a PhD fellowship from the French Minister of research; CN was recipient of a PhD fellowship from the Poitou-Charentes region.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Adamczyk P, McCreary AC, Przegalinski E, Mierzejewski P, Bienkowski P, Filip M (2009). The effects of fatty acid amide hydrolase inhibitors on maintenance of cocaine and food self-administration and on reinstatement of cocaine-seeking and food-taking behavior in rats. J Physiol Pharmacol 60: 119–125. [PubMed] [Google Scholar]
- Chauvet C, Goldberg SR, Jaber M, Solinas M (2012). Effects of environmental enrichment on the incubation of cocaine craving. Neuropharmacology 63: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M (2009). Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology 34: 2767–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C, Nicolas C, Thiriet N, Lardeux MV, Duranti A, Solinas M (2014). Chronic stimulation of the tone of endogenous anandamide reduces cue- and stress-induced relapse in rats. Int J Neuropsychopharmacol 18: pyu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H et al (2003). Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol 53: 743–751. [DOI] [PubMed] [Google Scholar]
- Cibickova L, Hyspler R, Micuda S, Cibicek N, Zivna H, Jun D et al (2009). The influence of simvastatin, atorvastatin and high-cholesterol diet on acetylcholinesterase activity, amyloid beta and cholesterol synthesis in rat brain. Steroids 74: 13–19. [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D (2007). ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol 7: 77–85. [DOI] [PubMed] [Google Scholar]
- Huddy K, Dhesi P, Thompson PD (2013). Do the frequencies of adverse events increase, decrease, or stay the same with long-term use of statins? Curr Atheroscler Rep 15: 301. [DOI] [PubMed] [Google Scholar]
- Kaestner R, Darden M, Lakdawalla D (2014). Are investments in disease prevention complements? The case of statins and health behaviors. J Health Econ 36: 151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Krebs J, Fabel K (2008). The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr Opin Psychiatry 21: 290–295. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS, Butelman E (2002). Pharmacotherapy of addictions. Nat Rev Drug Discov 1: 710–726. [DOI] [PubMed] [Google Scholar]
- Kumar A, Sharma N, Gupta A, Kalonia H, Mishra J (2012). Neuroprotective potential of atorvastatin and simvastatin (HMG-CoA reductase inhibitors) against 6-hydroxydopamine (6-OHDA) induced Parkinson-like symptoms. Brain Res 1471: 13–22. [DOI] [PubMed] [Google Scholar]
- Laufs U, Liao JK (2003). Rapid effects of statins: from prophylaxis to therapy for ischemic stroke. Arterioscler Thromb Vasc Biol 23: 156–157. [DOI] [PubMed] [Google Scholar]
- Law M, Rudnicka AR (2006). Statin safety: a systematic review. Am J Cardiol 97: 52C–60C. [DOI] [PubMed] [Google Scholar]
- Lehrmann E, Colantuoni C, Deep-Soboslay A, Becker KG, Lowe R, Huestis MA et al (2006). Transcriptional changes common to human cocaine, cannabis and phencyclidine abuse. PLoS One 1: e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wolf ME (2015). Multiple faces of BDNF in cocaine addiction. Behav Brain Res 279: 240–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Lin JH, Chou CW, Chang YF, Yeh SH, Chen CC (2008). Statins increase p21 through inhibition of histone deacetylase activity and release of promoter-associated HDAC1/2. Cancer Res 68: 2375–2383. [DOI] [PubMed] [Google Scholar]
- Lu D, Qu C, Goussev A, Jiang H, Lu C, Schallert T et al (2007). Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma 24: 1132–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y (2006). Role of ERK in cocaine addiction. Trends Neurosci 29: 695–703. [DOI] [PubMed] [Google Scholar]
- Luo L (2000). Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci 1: 173–180. [DOI] [PubMed] [Google Scholar]
- Mahley R, Bersot T (2005) Drug therapy for hypercholesterolemia and dyslipidemia In: Brunton L, Lazo JS, Parker KL (eds). Goodman & Gilman's The Pharmacological Basis of Therapeutics. McGraw-Hill: New York, NY, USA, pp 933–966. [Google Scholar]
- Malfitano AM, Marasco G, Proto MC, Laezza C, Gazzerro P, Bifulco M (2014). Statins in neurological disorders: an overview and update. Pharmacol Res 88C: 74–83. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Koob GF (2012). The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends Neurosci 35: 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ (2006). Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 7: 697–709. [DOI] [PubMed] [Google Scholar]
- O'Brien CP (2005). Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry 162: 1423–1431. [DOI] [PubMed] [Google Scholar]
- Orban PC, Chapman PF, Brambilla R (1999). Is the Ras-MAPK signalling pathway necessary for long-term memory formation? Trends Neurosci 22: 38–44. [DOI] [PubMed] [Google Scholar]
- Rahola JG (2012). Somatic drugs for psychiatric diseases: aspirin or simvastatin for depression? Curr Neuropharmacol 10: 139–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N (2008). Dose translation from animal to human studies revisited. Faseb J 22: 659–661. [DOI] [PubMed] [Google Scholar]
- Romieu P, Host L, Gobaille S, Sandner G, Aunis D, Zwiller J (2008). Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in rats. J Neurosci 28: 9342–9348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ (2009). Neurotrophic factors and structural plasticity in addiction. Neuropharmacology 56(Suppl 1): 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki A, Terasaki T, Tamai I, Tsuji A (1994). In vivo and in vitro blood-brain barrier transport of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors. Pharm Res 11: 305–311. [DOI] [PubMed] [Google Scholar]
- Schuster S, Nadjar A, Guo JT, Li Q, Ittrich C, Hengerer B et al (2008). The 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor lovastatin reduces severity of L-DOPA-induced abnormal involuntary movements in experimental Parkinson's disease. J Neurosci 28: 4311–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segatto M, Manduca A, Lecis C, Rosso P, Jozwiak A, Swiezewska E et al (2014). Simvastatin treatment highlights a new role for the isoprenoid/cholesterol biosynthetic pathway in the modulation of emotional reactivity and cognitive performance in rats. Neuropsychopharmacology 39: 841–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003). The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 168: 3–20. [DOI] [PubMed] [Google Scholar]
- Sierra S, Ramos MC, Molina P, Esteo C, Vazquez JA, Burgos JS (2011). Statins as neuroprotectants: a comparative in vitro study of lipophilicity, blood-brain-barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death. J Alzheimers Dis 23: 307–318. [DOI] [PubMed] [Google Scholar]
- Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M (2008). Reversal of cocaine addiction by environmental enrichment. Proc Natl Acad Sci USA 105: 17145–17150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Tsugawa Y, Tseng CH, Kobayashi Y, Shapiro MF (2014). Different time trends of caloric and fat intake between statin users and nonusers among US adults: gluttony in the time of statins? JAMA Intern Med 174: 1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL (2009). Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacol 12: 1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ (2007). Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci 8: 355–367. [DOI] [PubMed] [Google Scholar]
- Tsuji A, Saheki A, Tamai I, Terasaki T (1993). Transport mechanism of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors at the blood-brain barrier. J Pharmacol Exp Ther 267: 1085–1090. [PubMed] [Google Scholar]
- Vocci FJ, Acri J, Elkashef A (2005). Medication development for addictive disorders: the state of the science. Am J Psychiatry 162: 1432–1440. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zengin A, Deng C, Li Y, Newell KA, Yang GY et al (2009). High dose of simvastatin induces hyperlocomotive and anxiolytic-like activities: the association with the up-regulation of NMDA receptor binding in the rat brain. Exp Neurol 216: 132–138. [DOI] [PubMed] [Google Scholar]
- White CM (2002). A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol 42: 963–970. [PubMed] [Google Scholar]
- Wu H, Lu D, Jiang H, Xiong Y, Qu C, Li B et al (2008). Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J Neurotrauma 25: 130–139. [DOI] [PubMed] [Google Scholar]
- Yang D, Han Y, Zhang J, Chopp M, Seyfried DM (2012). Statins enhance expression of growth factors and activate the PI3K/Akt-mediated signaling pathway after experimental intracerebral hemorrhage. World J Neurosci 2: 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.