Highlights
-
•
Spontaneous necrosis of hepatocellular carcinoma without any pretreatment or angiography is extremely rare.
-
•
Spontaneous necrosis of HCC was highly suspected given the history of alcoholic hepatitis, based on the elevation of AFP and the CT findings.
-
•
The mechanisms of spontaneous regression are still unclear.
-
•
Recurrence after regression or viable malignant cells in resected specimen are reported.
-
•
The ideal management strategy for this disease is surgical intervention if the liver function is acceptable.
Keywords: Hepatocellular carcinoma, Complete spontaneous necrosis, Alcoholic hepatitis, Case report
Abstract
Introduction
Complete spontaneous necrosis of hepatocellular carcinoma (HCC) without any pretreatment or angiography is rare. We present a rare case of spontaneous complete necrosis of HCC, as confirmed after hepatectomy.
Presentation of case
The patient, a 74-year-old man with a history of alcoholic hepatitis, was referred to our hospital for confirmation of suspected HCC. In March 2015, abdominal ultrasonography detected a low echoic mass in segment 8 (S8) of the liver. Contrast-enhanced computed tomography (CT) imaging revealed interval growth of this tumor and showed that the tumor was well enhanced in the arterial phase and washed out in the portal and delayed phases. The serum alpha-fetoprotein level was elevated at 30.8 ng/mL and the percentage of the L3 isoform was 25.5%. Two months later, CT imaging showed that the tumor was of low density and had decreased in size; no contrast enhancement of the tumor was seen. Spontaneous necrosis of the HCC was considered; however, as we could not exclude viable malignant cells in the tumor, we performed S8 segmentectomy of the liver. The resected tumor specimen had a thick fibrous capsule. Histopathological findings showed only granulation and necrotic tissue accompanied by bleeding and hemosiderosis. No viable tumor cells were observed. The serum alpha-fetoprotein level returned to the normal range one month after surgery.
Discussion
If spontaneous regression has occurred, there is a possibility of HCC recurrence and of remnant viable tumor cells.
Conclusion
We present a rare case of complete spontaneous necrosis of HCC and strongly recommended surgical intervention.
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent malignancies worldwide, and its incidence is increasing [1]. Despite the development of diagnostic techniques and surgical procedures, the postoperative outcomes for HCC remain unsatisfactory due to the high rate of recurrence [2]. In clinical situations, spontaneous regression of a malignant tumor is a rare phenomenon and is defined as the partial or complete disappearance of a malignant tumor without any treatment or with inadequate treatment for prevention of tumor growth [3]. To the best of our knowledge, only 11 cases of complete regression of HCC, confirmed by the resection of the tumor, have been reported in the English literature between 2000 and 2015 [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]; however, the mechanism of spontaneous regression is still unclear. Spontaneous regression could be associated with interventional radiology, alcohol cessation, the host’s immune system, and vessel thrombosis. Herein, we report a rare case of complete spontaneous regression of HCC following hepatectomy.
2. Case report
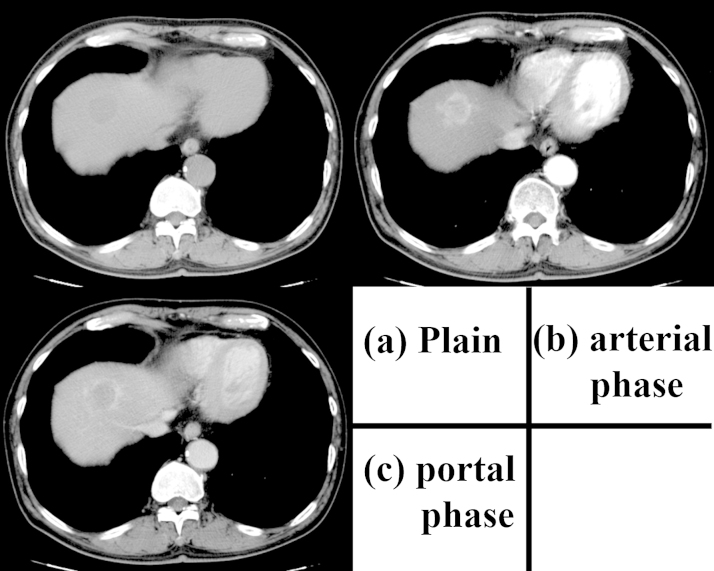
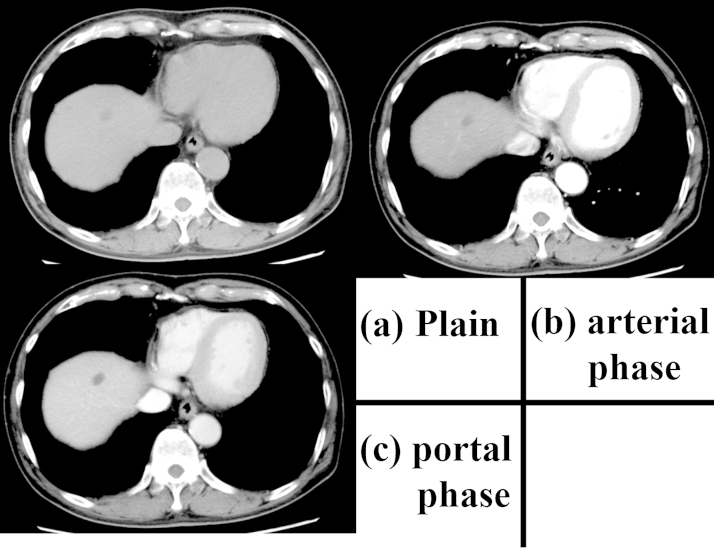
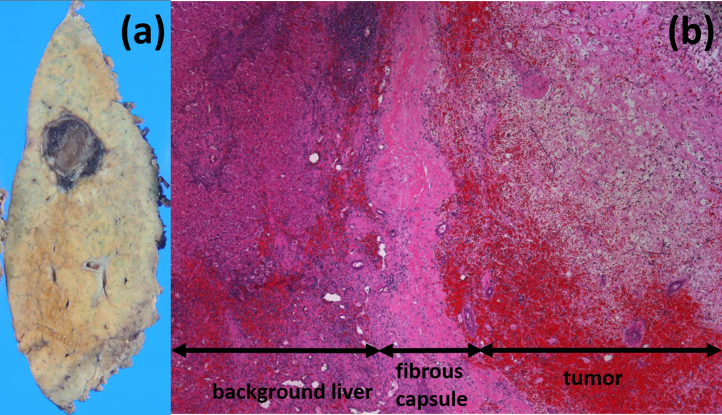
The patient was a 74-year-old man previously diagnosed with alcoholic hepatitis. He had consumed 480 mL of Japanese sake (60 g ethanol) per day for 50 years and stopped drinking one month prior to visiting our hospital. He had smoked 15 cigarettes per day for 50 years and had a medical history of diabetes mellitus treated with medication. In March 2015, abdominal ultrasonography (US) showed a low-echoic mass measured at 22 mm in segment 8 (S8) of the liver. Contrast-enhanced computed tomography (CT) performed in July 2015 revealed that the tumor had grown to a 36-mm maximum diameter and was well enhanced in the arterial phase and washed out in the portal and delayed phases (Fig. 1). Upper gastrointestinal endoscopy and colonoscopy revealed no specific findings. The patient was referred to our hospital for confirmation of the suspected HCC. The patient’s height was 168 cm and weight was 60 kg. The results of the preoperative blood tests were as follows: white blood cell count, 6520 cells/μL; red blood cell count, 480 × 104 cells/μL; serum hemoglobin concentration, 16.8 g/dL; serum platelet count, 15 × 104 platelets/μL; serum aspartate aminotransferase, 71 IU/L; serum alanine aminotransferase, 50 IU/L; serum alkaline phosphatase, 257 IU/L; serum gamma glutamic transpeptidase, 119 IU/L; total serum bilirubin, 1.08 mg/dL; serum albumin, 4.0 g/dL; and serum C-reactive protein, 0.08 mg/dL. The serum concentration of proteins induced by vitamin K absence or antagonist (PIVKA-II) was 17 mAU/mL, carbohydrate antigen 19–9 was 4.7 U/mL, and alpha-fetoprotein (AFP) was slightly elevated at 30.8 ng/mL. The percentage of the Lens culinaris agglutinin-reactive α-fetoprotein isoform 3 (L3) was 25.5% and the indocyanine green clearance rate at 15 min was 15.5%. Hepatitis B surface antigen and hepatitis C virus antibody test results were negative. Both Child-Pugh and Liver Damage classifications were categorized as A. CT imaging was performed two months later (Fig. 2). The tumor was noted to have decreased in size and appeared uniform with low density and a maximum diameter of 16 mm. In addition, contrast enhancement of the tumor had disappeared. Magnetic resonance imaging (MRI) was also performed. The lesion had high signal intensity on T2-weighted imaging, low signal intensity on T1-weighted imaging, and no contrast enhancement in the dynamic study. Spontaneous necrosis of the HCC was considered; however, we could not rule out the presence of viable malignant cells based on these findings. Thus, we performed segmentectomy of S8 of the liver. On inspection at the time of resection, the surface of the liver was rough; however, no ascites was found. The low-echoic lesion with clear borders was detected in S8, and segmentectomy was performed. The tumor was reddish and encapsulated with a thick fibrous capsule. Histopathological findings showed granulation tissue and necrotic tissue, accompanied by bleeding, hemosiderosis, and dense infiltrates of inflammatory cells (Fig. 3). No viable tumor cells were observed. Chronic hepatitis was observed in the background liver. The postoperative course was uneventful, and the serum concentration of AFP decreased to 3.4 ng/mL one month after surgery.
Fig. 1.
The contrast-enhanced computed tomography scan obtained on admission.
The image shows that the tumor is 36 mm in size, well-contrasted in the arterial phase, and washed out in the portal and delayed phases. (a) Plain; (b) arterial phase; (c) portal phase.
Fig. 2.
The contrast-enhanced computed tomography scan obtained approximately two months after the first examination.
The tumor is uniform with low-density and has decreased in size. The diameter is 16 mm and no contrast enhancement can be seen. (a) Plain; (b) arterial phase; (c) portal phase.
Fig. 3.
The macroscopic and histological images of the resected specimen. (a) The tumor is reddish and encapsulated with a thick fibrous capsule. (b) Hematoxylin-eosin stain, magnification ×50.
Histopathological findings show only granulation and necrotic tissue, accompanied by bleeding, hemosiderosis, and dense infiltrates of inflammatory cells.
3. Discussion
The reported rate of spontaneous partial regression of HCCs is 0.4% and complete regression is extremely rare [14]. To our knowledge, only 11 cases, including our case, of complete regression of HCC confirmed by hepatectomy were reported in the English literature from 2000 to 2015 [4], [5], [6], [7], [8], [9], [10], [11], [12], [13] (Table 1). The mean age of the patients was 68.2 years, ranging from 50 to 78 years, and most patients were male. The mean size of the tumor was 45.5 mm, ranging from 30 to 100 mm. The cause of hepatitis was alcohol in five patients, hepatitis B virus in three patients, and hepatitis C virus in four patients. Chronic hepatitis was observed in nine patients and background liver cirrhosis in one patient. The cause of the necrosis was related to capsule formation in nine patients, vessel thrombosis in 4 patients, and an immunological reaction in 5 patients (this includes the overlapping cases).
Table 1.
Clinical features describing reported cases of complete spontaneous regression of hepatocellular carcinomas resected by surgery.
| No. | Author | Year | Age | Sex | Etiology | Background liver | Size (Mm) | Capsule formation | Number of tumors | Location of tumor | AFP (ng/mL) |
PIVKA-2 (mAU/mL) |
Preoperative angiography |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Our case | 2015 | 74 | M | A | CH | 36 | positive | solitary | S8 | 30.8 | 17 | none |
| 2 | Takeda et al. [4] | 2015 | 68 | M | A | CH | 30 | positive | solitary | S4 | 1.5 | 427 | none |
| 3 | Arakawa et al. [5] | 2008 | 78 | F | HBV | LC | 30 | positive | solitary | S2/3 | 1041 | 92 | none |
| 4 | Ohtani et al. [6] | 2005 | 69 | M | HCV and A | CH | 40 | positive | solitary | S4 | 5 | 1773 | done |
| 5 | Ohta et al. [7] | 2005 | 74 | M | unknown | mild liver dysfunction |
60 | positive | multiple | S2/3 | 2.7 | 8450 | done |
| 6 | Li et al. [8] | 2003 | 53 | M | HBV | CH | 30 | positive | solitary | S6 | normal | N/A | none |
| 7 | Iiai et al. [9] | 2003 | 69 | M | HCV | CH | 70 | N/A | solitary | S6/7 | 3476 | 6190 | done |
| 8 | Morimoto et al. [10] | 2002 | 73 | M | A | CH | 100 | positive | solitary | S2/3 | 55 | 62300 | done |
| 9 | Lee et al. [11] | 2002 | 70 | M | HBV and A | CH | 35 | positive | solitary | S2/3 | 1.47 | N/A | none |
| 10 | Matsuo et al. [12] | 2001 | 72 | M | HCV | CH | 35 | none | solitary | S5 | 1000 | 2000 | done |
| 11 | Izuishi et al. [13] | 2000 | 50 | M | HCV | CH | 35 | positive | solitary | S2/3 | 16.4 | N/A | done |
Abbreviations: A, Alcohol; AFP, alpha-fetoprotein; CH, chronic hepatitis; F, female; LC, liver cirrhosis; M, male; N/A, not addressed; PIVKA-2, proteins induced by vitamin K absence or antagonist.
The causes of spontaneous necrosis are still unclear. Possible mechanisms, reported in the literature, are as follows: drinking cessation, use of herbal medicine [15], high fever due to cholangitis [7], immunological reactions, deficiency of blood supply, androgen therapy [16], vascular injury due to angiography, and rapid tumor growth. Huz et al. identified systematic inflammatory response and tumor hypoxia as common mechanisms of regression [17]. In our case, it is conjectured that insufficiency of blood supply, the thick overlying capsule, and cessation of drinking and smoking were the reasons for the complete spontaneous regression. In the resected specimen, inflammatory cells, including acquired and naïve immune cells, were detected in the necrotic tissue. Clarifying the relationship between the immunological reaction and spontaneous regression was difficult; activation of host immune cells could be the most important factor for the spontaneous regression.
One limitation of our case study is that we could not prove the presence of malignant cells histologically before regression. Nevertheless, HCC was highly suspected given the history of alcoholic hepatitis and based on the elevation of AFP and the isoform of L3 and the CT findings that were typical of HCC. In light of the clinical and diagnostic findings, the final diagnosis of HCC with complete spontaneous necrosis was made.
Sasaki et al. reported a case in which preoperative imaging findings suggested the complete necrosis of HCC; however, a small number of viable tumor cells remained and were confirmed on immunohistochemical staining [18]. Ohtani et al. reported that 5 of 40 cases had a recurrence of HCC after spontaneous regression [6]. Establishing accurate preoperative diagnosis of complete regression is difficult because of the discrepancy between the preoperative radiological and pathological findings. In 9 of 11 cases listed in Table 1, no or partial regression was observed on preoperative radiological findings; these were finally diagnosed as complete regression. There are several local intervention techniques targeting HCC, including surgical modalities. Recent advances in surgical modalities have improved the prognosis of HCC patients [19], [20]. Therefore, we suggest that if liver function is acceptable, the ideal management strategy for this disease is surgical intervention.
4. Conclusion
In conclusion, we present a rare case of complete spontaneous necrosis of HCC. Although the mechanism of spontaneous regression of HCC remains unclear, the accumulation of such cases will help improve the treatment of HCC in the future.
Conflicts of interest
We have no financial and personal relationships with other people or organisations.
Funding
We have no sponsors involving this paper.
Ethical approval
Not involving patient’s ethical approval.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Author contribution
Ryusuke Saito helped with date collection and writing paper.
Tomoyuki Abe and Nobuaki Fujikuni contributed review of the final manuscript.
Hironobu Amano and Masahiro Nakahara contributed to study concept, and review of the final manuscript and submission of the paper.
Shuji Yonehara is the pathologist and diagnosed the disease.
Kazushi Teramen first diagnosed the HCC and introduced the patient to our hospital.
Toshio Noriyuki gave the final approval of the article.
Guarantor
Ryusuke Saito.
References
- 1.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Liver Cancer Study Group of Japan Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann. Surg. 1990;211:277–287. [PMC free article] [PubMed] [Google Scholar]
- 3.Everson T.C., Cole W.H. Spontaneous regression of malignant disease. J. Am. Med. Assoc. 1959;169:1758–1759. doi: 10.1001/jama.1959.03000320060014. [DOI] [PubMed] [Google Scholar]
- 4.Takeda Y., Wakui N., Asai Y. Spontaneous complete necrosis of hepatocellular carcinoma: a case report and review of the literature. Oncol. Lett. 2015;9:1520–1526. doi: 10.3892/ol.2015.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arakawa Y., Mori H., Ikegami T. Hepatocellular carcinoma with spontaneous regression: report of the rare case. Hepatogastroenterology. 2008;55:1770–1772. [PubMed] [Google Scholar]
- 6.Ohtani H., Yamazaki O., Matsuyama M. Spontaneous regression of hepatocellular carcinoma: report of a case. Surg. Today. 2005;35:1081–1086. doi: 10.1007/s00595-005-3066-8. [DOI] [PubMed] [Google Scholar]
- 7.Ohta H., Sakamoto Y., Ojima H. Spontaneous regression of hepatocellular carcinoma with complete necrosis: case report. Abdom. Imag. 2005;30:734–737. doi: 10.1007/s00261-005-0313-9. [DOI] [PubMed] [Google Scholar]
- 8.Li A.J., Wu M.C., Cong W.M., Shen F., Yi B. Spontaneous complete necrosis of hepatocellular carcinoma: a case report. Hepatobiliary Pancreat. Dis. Int. 2003;2:152–154. [PubMed] [Google Scholar]
- 9.Iiai T., Sato Y., Nabatame N., Yamamoto S., Makino S., Hatakeyama K. Spontaneous complete regression of hepatocellular carcinoma with portal vein tumor thrombus. Hepatogastroenterology. 2003;50:1628–1630. [PubMed] [Google Scholar]
- 10.Morimoto Y., Tanaka Y., Itoh T., Yamamoto S., Mizuno H., Fushimi H. Spontaneous necrosis of hepatocellular carcinoma: a case report. Dig. Surg. 2002;19:413–418. doi: 10.1159/000065822. [DOI] [PubMed] [Google Scholar]
- 11.Lee S.C., Chung H.W., Chung J.B. Total necrosis of hepatocellular carcinoma due to spontaneous occlusion of feeding artery. Yonsei Med. J. 2002;43:123–127. doi: 10.3349/ymj.2002.43.1.123. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo R., Ogata H., Tsuji H. Spontaneous regression of hepatocellular carcinoma—a case report. Hepatogastroenterology. 2001;48:1740–1742. [PubMed] [Google Scholar]
- 13.Izuishi K., Ryu M., Hasebe T., Kinoshita T., Konishi M., Inoue K. Spontaneous total necrosis of hepatocellular carcinoma: report of a case. Hepatogastroenterology. 2000;47:1122–1124. [PubMed] [Google Scholar]
- 14.Oquinena S., Guillen-Grima F., Inarrairaegui M., Zozaya J.M., Sangro B. Spontaneous regression of hepatocellular carcinoma: a systematic review. Eur. J. Gastroenterol. Hepatol. 2009;21:254–257. doi: 10.1097/MEG.0b013e328324b6a2. [DOI] [PubMed] [Google Scholar]
- 15.Nam S.W., Han J.Y., Kim J.I. Spontaneous regression of a large hepatocellular carcinoma with skull metastasis. J. Gastroenterol. Hepatol. 2005;20:488–492. doi: 10.1111/j.1440-1746.2005.03243.x. [DOI] [PubMed] [Google Scholar]
- 16.McCaughan G.W., Bilous M.J., Gallagher N.D. Long-term survival with tumor regression in androgen-induced liver tumors. Cancer. 1985;56:2622–2626. doi: 10.1002/1097-0142(19851201)56:11<2622::aid-cncr2820561115>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Huz J.I., Melis M., Sarpel U. Spontaneous regression of hepatocellular carcinoma is most often associated with tumour hypoxia or a systemic inflammatory response. HPB (Oxford) 2012;14:500–505. doi: 10.1111/j.1477-2574.2012.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki T., Fukumori D., Yamamoto K., Yamamoto F., Igimi H., Yamashita Y. Management considerations for purported spontaneous regression of hepatocellular carcinoma: a case report. Case Rep. Gastroenterol. 2013;7:147–152. doi: 10.1159/000350501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto M., Takasaki K., Ohtsubo T., Katsuragawa H., Fukuda C., Katagiri S. Effectiveness of systematized hepatectomy with Glisson’s pedicle transection at the hepatic hilus for small nodular hepatocellular carcinoma: retrospective analysis. Surgery. 2001;130:443–448. doi: 10.1067/msy.2001.116406. [DOI] [PubMed] [Google Scholar]
- 20.Eguchi S., Kanematsu T., Arii S. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery. 2008;143:469–475. doi: 10.1016/j.surg.2007.12.003. [DOI] [PubMed] [Google Scholar]