Abstract
Dietary proteins are important modulators of glucose metabolism. However, few longitudinal studies have evaluated the associations between intake of protein and protein type and risk of type 2 diabetes (T2D). We investigated the associations between total, animal, and vegetable protein and incident T2D in 72,992 women from the Nurses' Health Study (1984–2008), 92,088 women from Nurses' Health Study II (1991–2009) and 40,722 men from the Health Professionals Follow-up Study (1986–2008). During 4,146,216 person-years of follow-up, we documented 15,580 cases of T2D. In pooled multivariate models including body mass index, participants in the highest quintiles of percentage of energy derived from total protein and animal protein had 7% (95% confidence interval (CI): 1, 17) and 13% (95% CI: 6, 21) increased risks of T2D compared with those in the lowest quintiles, respectively. Percentage of energy intake from vegetable protein was associated with a moderately decreased risk of T2D (comparing extreme quintiles, hazard ratio =0.91, 95% CI: 0.84, 0.98). Substituting 5% of energy intake from vegetable protein for animal protein was associated with a 23% (95% CI: 16, 30) reduced risk of T2D. In conclusion, higher intake of animal protein was associated with an increased risk of T2D, while higher intake of vegetable protein was associated with a modestly reduced risk.
Keywords: animal protein, diabetes mellitus, dietary protein, nuts, peanuts, type 2 diabetes, vegetable protein, whole grains
Diets high in protein have shown beneficial effects on glucose homeostasis in short-term trials (1, 2) and have thus been suggested as a potential strategy for type 2 diabetes (T2D) prevention. In contrast, findings from a few prospective cohort studies have shown positive associations between protein intake and risk of T2D that are driven by intake of animal protein (3, 4). However, because these studies used baseline intake only, they were not able to capture long-term intake patterns.
Findings from epidemiologic studies evaluating food sources of protein suggest divergent associations of animal and vegetable protein with T2D risk. Intake of red and processed meat has been positively associated with risk of T2D (5, 6), while intake of plant-based sources of protein, such as nuts (7, 8) and legumes (9), has been associated with decreased risk. It is unclear whether different types of protein are differentially associated with risk of T2D and whether it is the protein per se or other components of protein-rich foods that may account for observed associations. In this study, we aimed to investigate the associations between total protein intake and protein type (vegetable, animal) and risk of T2D in 3 large prospective cohort studies of US adults, using repeated measurements of protein intake taken over 18–24 years of follow-up. We also estimated the associations between substituting vegetable protein for animal protein, substituting protein for carbohydrates of differing quality, and substituting vegetable protein foods for animal protein and low-quality carbohydrate foods and T2D risk.
METHODS
Study population
Our analysis was conducted in 3 ongoing prospective cohort studies: the Nurses' Health Study (NHS), which consists of 121,700 female registered nurses who were aged 30–55 years at baseline in 1976; Nurses' Health Study II (NHS II), which consists of 116,671 female registered nurses aged 24–42 years at baseline in 1989; and the Health Professionals Follow-up Study (HPFS), which consists of 51,529 male health professionals aged 40–75 years at baseline in 1986. For each cohort, mailed questionnaires are administered biennially to collect data on lifestyle factors and health. Diet is assessed using a validated self-administered food frequency questionnaire (FFQ) every 4 years. For this analysis, we used 1984, 1991, and 1986 as the baseline years for the NHS, NHS II, and HPFS, respectively; those years respectively were then considered the year in which the FFQ was first administered in each cohort. We excluded men and women who had a diagnosis of diabetes, cardiovascular disease, or cancer at baseline, left more than 70 items on the baseline FFQ blank, reported implausible total energy intakes, or were missing baseline data on protein intake or follow-up information on date of diabetes diagnosis. After these exclusions, a total of 72,992 participants from the NHS, 92,088 participants from NHS II, and 40,722 participants from the HPFS remained. All 3 studies were approved by the institutional review boards of Brigham and Women's Hospital and the Harvard T.H. Chan School of Public Health (Boston, Massachusetts).
Assessment of dietary protein
Diet was assessed using a 131-item FFQ, administered at baseline and every 4 years. Each FFQ asked participants how often, on average, they consumed standard portions of foods and beverages, using 9 possible responses ranging from “never or less than once per month” to “6 or more times per day.” Nutrient and energy intakes were calculated by multiplying the frequency of consumption of each unit of food and beverage by nutrient and energy contents and summing across all items. Values were obtained using the US Department of Agriculture food composition database (10). Intakes of total, animal, and vegetable protein were calculated for each participant and expressed as a percentage of total energy by multiplying the grams of protein consumed per day by the number of kilocalories in 1 gram of protein (4 kcal/g) and then dividing by total caloric intake (11). The reproducibility and validity of these FFQs have been described in detail elsewhere (12–16). In a subsample of NHS participants, the coefficients for correlations between the FFQ and multiple dietary records were 0.50 for protein, 0.57 for fat, and 0.64 for carbohydrate (14, 16). Similar values were reported in a subsample of HPFS participants (13).
Assessment of T2D
Participants who reported a diagnosis of T2D on the biennial questionnaire were mailed a supplementary questionnaire about symptoms and treatment. In accordance with the National Diabetes Data Group criteria (17), a case of T2D was confirmed if at least 1 of the following was reported on the supplementary questionnaire: 1) 1 or more classic symptoms of T2D (excessive thirst, polyuria, weight loss, hunger) and fasting plasma glucose concentration of ≥11.1 mmol/L; 2) ≥2 elevated plasma glucose measurements taken on different occasions (fasting concentrations of ≥7.8 mmol/L, random plasma glucose concentrations of ≥11.1 mmol/L, and/or concentrations of ≥11.1 mmol/L after ≥2 hours shown by an oral glucose tolerance test) in the absence of symptoms; or 3) treatment with hypoglycemic medication (insulin or oral hypoglycemic agent). For cases identified after 1998, we applied the American Diabetes Association criteria (18), in which the threshold for diagnosis of diabetes changed from a fasting plasma glucose concentration of 7.8 mmol/L to a concentration of 7.0 mmol/L. The validity of the supplementary questionnaire has been documented through medical record review (19, 20).
Covariates
The biennial follow-up questionnaires collected updated information on lifestyle factors and medical history, including age, body weight, smoking status, physical activity, medication use, and history of chronic diseases. Family history of diabetes in first-degree relatives was assessed in 1982 and 1988 in the NHS; in 1989, 1997, 2001, and 2005 in NHS II; and in 1987 in the HPFS. Information on dietary factors was obtained from the FFQs.
Statistical analysis
We calculated person-time for each participant from the date of return of the baseline questionnaire to the date of diagnosis of T2D, death, loss to follow-up, or the end of the follow-up period, whichever occurred first. Cox proportional hazards regression was used to model the association between protein intake and risk of T2D. Protein intake was expressed as a percentage of total energy using the nutrient density method (16) and categorized into quintiles. Regression models included age in years as the time scale, stratified by calendar time in 2-year intervals. Multivariate models adjusted for race/ethnicity (white or nonwhite), family history of diabetes (yes/no), and various lifestyle factors, including smoking status (never smoker, past smoker, or current smoker of 1–14, 15–24, or ≥25 cigarettes/day), alcohol intake (0, 0.1–4.9, 5.0–14.9, or ≥15 g/day in women; 0, 0.1–4.9, 5.0–29.9, or ≥30 g/day in men), physical activity (3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, or ≥27.0 metabolic equivalent-hours/week), total energy intake (kcal/day; quintiles), and, for women, menopausal status, postmenopausal hormone use (NHS and NHS II; premenopausal, postmenopausal with no history of hormone replacement, or postmenopausal with current hormone replacement), and oral contraceptive use (NHS II only; never user, past user, or current user). Results were also adjusted for percentages of energy derived from trans- fat, saturated fat, monounsaturated fat, and polyunsaturated fat; dietary fiber; dietary cholesterol; and glycemic index, and included mutual adjustment for percentages of energy derived from animal protein and vegetable protein (quintiles). Since body weight may partly mediate the association between protein intake and risk of T2D (21), we subsequently adjusted for body mass index (BMI), defined as weight (kg)/height (m)2 (<23, 23–24.9, 25–29.9, 30–34.9 or ≥35). We also conducted sensitivity analyses by adding red and processed meat, heme iron, and branched chain and aromatic amino acids to multivariate models, since these factors may partially mediate associations. For dietary measures, we used the cumulative average of intakes recorded from baseline to the censoring event (22). We replaced missing values with the cumulative average from preceding FFQ cycles. Because a diagnosis of cardiovascular disease or cancer may lead to changes in diet that may confound the relationship between diet and T2D, we stopped updating dietary variables when participants reported a diagnosis of one of these conditions (22, 23). To test the robustness of our findings, we repeated the analysis while continuing to update diet after a report of an intermediate chronic disease and also by using baseline diet and using the most recent measure of diet instead of repeated measures. We conducted tests for linear trend by assigning the median value to each quintile and treating this as a continuous variable in the regression model. Potential effect modification by age, BMI, physical activity, diet quality as assessed by the Alternate Healthy Eating Index (24), and family history of diabetes was evaluated using cross-product terms based on median protein intake and respective binary stratification variables.
We also estimated the association between substituting vegetable protein for an equal exchange of animal protein and T2D risk and simulated the isocaloric substitution of dietary protein for total carbohydrate, carbohydrate from food sources with low or medium glycemic index values (including intact and milled whole grains), and carbohydrate from food sources with high glycemic index values (including refined grains, potatoes, and added sugar). To fit these models, we simultaneously included total energy, percentage of energy derived from protein, and the substitution macronutrient of interest as continuous variables along with the covariates listed above, except for total fiber and glycemic index. Coefficients were multiplied by 5 to estimate the association of substituting 5% of energy intake. We also investigated the association between substituting 1 serving of foods rich in vegetable protein (composite variable comprised of legumes, peanuts, peanut butter, other nuts, and whole grains) for 1 serving of major food sources of animal protein, refined grains, and potatoes and T2D risk by simultaneously modeling all terms as continuous variables (servings/day) in the same multivariate model, adjusted for the nondietary covariates listed above and intakes of total energy, alcohol, sugar-sweetened beverages, fruit, and vegetables (quintiles). For all substitution models, hazard ratios and 95% confidence intervals were estimated using the difference between coefficients for 2 foods or macronutrients of interest and their variance and covariance (6). All analyses were conducted separately in each cohort, and results were then combined using fixed-effects meta-analysis. All statistical tests were 2-sided and performed using SAS, version 9.2 for UNIX (SAS Institute, Inc., Cary, North Carolina).
RESULTS
During 4,146,216 person-years of follow-up among 205,802 participants (72,992 from NHS, 92,088 from NHS II, and 40,722 from HPFS), we documented 15,580 cases of T2D (7,214 in NHS, 5,032 in NHS II, and 3,334 in HPFS). Mean percentages of energy intake from total, animal, and vegetable protein were 18.1%, 15.1%, and 5%, respectively, in the NHS; 18.9%, 13.7%, and 7.3% in the NHS2; and 18.2%, 13.0%, and 5.1% in the HPFS. Intake of protein was fairly stable across FFQ cycles in the 3 cohorts, and animal protein contributed to the majority of total protein intake (see Web Figure 1, available at http://aje.oxfordjournals.org/). Major food sources of animal and vegetable protein are listed in Web Table 1. For all cohorts, participants who had a higher percentage of energy derived from protein as compared with a lower percentage were less likely to smoke, tended to have higher BMIs, and were more likely to be overweight, to be physically active, and to have a family history of diabetes (Tables 1–3). They also had a lower total energy intake, a lower percentage of energy from carbohydrate, lower intakes of sugar-sweetened beverages, processed meat, and nuts, and a lower glycemic index. Similar trends were noted for baseline characteristics according to quintile of percentage of energy from animal protein (Web Table 2). In contrast, persons with higher intakes of percentage of energy from vegetable protein tended to have a lower BMI and were less likely to be overweight. They also had higher glycemic index values and higher intakes of whole grains, legumes, peanuts, fruits, and vegetables (Web Table 3).
Table 1.
Age-Adjusted Characteristics of Participants According to Baseline (1984) Total Protein Intake Among 72,992 Women in the Nurses’ Health Study, 1984–2008
| Characteristic | Quintile of Protein Intake |
|||||
|---|---|---|---|---|---|---|
| 1 (n = 14,598) (14.8% of Energy)a |
3 (n = 14,598) (18.0% of Energy) |
5 (n = 14,598) (21.6% of Energy) |
||||
| % | Mean | % | Mean | % | Mean | |
| Demographic and lifestyle factors | ||||||
| Age, yearsb | 50.1 | 50.1 | 50.7 | |||
| White race/ethnicity | 97.5 | 98.1 | 97.6 | |||
| Body mass indexc | 24.3 | 24.8 | 25.7 | |||
| Body mass index ≥25 | 31.0 | 36.1 | 44.6 | |||
| Physical activity, MET-hours/week | 12.8 | 14.2 | 16.1 | |||
| Current smoker | 30.1 | 22.6 | 21.8 | |||
| Hypertension | 8.0 | 7.6 | 8.8 | |||
| High cholesterol | 3.1 | 3.2 | 4.2 | |||
| Family history of diabetesd | 26.8 | 28.1 | 31.1 | |||
| Postmenopausal hormone use | 22.6 | 25.0 | 26.2 | |||
| Multivitamin use | 33.6 | 37.0 | 40.8 | |||
| Alcohol, g/day | 10.2 | 6.8 | 4.2 | |||
| Dietary intake | ||||||
| Total energy, kcal/day | 1,880 | 1,772 | 1,550 | |||
| Carbohydrate, % of energy intake | 51.3 | 46.4 | 41.8 | |||
| Total fat, % of energy intake | 33.0 | 35.1 | 35.1 | |||
| Saturated fat, % of energy intake | 11.7 | 12.6 | 12.8 | |||
| Monounsaturated fat, % of energy intake | 12.0 | 12.9 | 12.8 | |||
| Polyunsaturated fat, % of energy intake | 6.8 | 6.7 | 6.4 | |||
| Trans- fat, % of energy intake | 2.0 | 2.0 | 1.7 | |||
| Dietary cholesterol, mg/day | 229.6 | 283.2 | 344.5 | |||
| Daily dietary glycemic loade | 111.7 | 99.3 | 86.5 | |||
| Daily dietary glycemic indexf | 54.8 | 53.5 | 51.3 | |||
| Total dietary fiber, g/day | 15.2 | 16.5 | 17.0 | |||
| Cereal fiber, g/day | 4.0 | 4.3 | 4.0 | |||
| Magnesium, mg/day | 252.8 | 287.3 | 326.1 | |||
| Heme iron, mg/day | 0.76 | 1.1 | 1.5 | |||
| Potassium, mg/day | 2,570 | 2,908 | 3,207 | |||
| Calcium, mg/day | 725.2 | 883.4 | 1,040 | |||
| Vitamin C, mg/day | 301.2 | 326.0 | 389.8 | |||
| Vitamin E, mg/day | 71.1 | 83.2 | 104.3 | |||
| Whole grains, servings/day | 1.0 | 1.2 | 1.2 | |||
| Fruit, servings/day | 1.3 | 1.4 | 1.4 | |||
| Vegetables, servings/day | 2.4 | 2.8 | 3.2 | |||
| Red meat, servings/day | 0.62 | 0.88 | 0.93 | |||
| Processed meat, servings/day | 0.35 | 0.32 | 0.23 | |||
| Fish, servings/day | 0.10 | 0.15 | 0.28 | |||
| Chicken, servings/day | 0.20 | 0.29 | 0.46 | |||
| Eggs, servings/day | 0.30 | 0.36 | 0.38 | |||
| Dairy foods, servings/day | 1.7 | 2.0 | 2.1 | |||
| Legumes, servings/day | 0.35 | 0.40 | 0.42 | |||
| Nuts, servings/day | 0.31 | 0.33 | 0.25 | |||
| Peanuts, servings/day | 0.12 | 0.12 | 0.09 | |||
| Peanut butter, servings/day | 0.20 | 0.21 | 0.16 | |||
| Potatoes, servings/day | 0.43 | 0.41 | 0.29 | |||
| Coffee, servings/day | 1.9 | 1.8 | 1.6 | |||
| Sugar-sweetened beverages, servings/day | 0.69 | 0.23 | 0.10 | |||
Abbreviations: GI, glycemic index; MET, metabolic equivalent of task.
a Median percentage of energy intake in the quintile.
b Not adjusted for age.
c Weight (kg)/height (m)2.
d First-degree relatives.
e Average glycemic load was calculated by multiplying the amount of carbohydrates in the diet by the average glycemic index. For 1 serving of a food, a glycemic load of ≥20 is considered high, 11–19 is considered medium, and ≤10 is considered low. Among these 72,992 women from the Nurses’ Health Study, the mean glycemic load was 102.5 (range, 0–213).
f Average dietary glycemic index (GI) was calculated by summing the products of 1) the carbohydrate content of each food item per serving, 2) the average daily number of servings of that food, and 3) the food's GI value (derived from available databases and publications) and dividing by total daily carbohydrate content. Foods with a GI value of ≤55 are considered to have a low GI, foods with a value of 56–69 are considered to have a medium GI, and foods with a value of ≥70 are considered to have a high GI. Among these 72,992 women from the Nurses’ Health Study, the mean GI was 52.8 (range, 0.01–70.9).
Table 2.
Age-Adjusted Characteristics of Participants According to Baseline (1991) Total Protein Intake Among 92,088 Women in Nurses’ Health Study II, 1991–2009
| Characteristic | Quintile of Protein Intake |
|||||
|---|---|---|---|---|---|---|
| 1 (n = 18,417) (15.3% of Energy)a |
3 (n = 18,466) (18.8% of Energy) |
5 (n = 18,417) (22.6% of Energy) |
||||
| % | Mean | % | Mean | % | Mean | |
| Demographic and lifestyle factors | ||||||
| Age, yearsb | 35.9 | 36.0 | 36.4 | |||
| White race/ethnicity | 95.6 | 96.8 | 96.1 | |||
| Body mass indexc | 23.8 | 24.5 | 25.5 | |||
| Body mass index ≥25 | 31.0 | 36.1 | 44.6 | |||
| Physical activity, MET-hours/week | 20.7 | 20.3 | 22.4 | |||
| Current smoker | 14.6 | 11.8 | 11.7 | |||
| Hypertension | 5.8 | 5.9 | 7.3 | |||
| High cholesterol | 13.7 | 13.8 | 16.4 | |||
| Family history of diabetesd | 32.4 | 34.3 | 36.5 | |||
| Postmenopausal hormone use | 4.3 | 4.4 | 5.0 | |||
| Oral contraceptive use | 12.0 | 10.4 | 10.1 | |||
| Multivitamin use | 42.7 | 43.9 | 44.5 | |||
| Alcohol, g/day | 4.0 | 3.1 | 2.3 | |||
| Dietary intake | ||||||
| Total energy, kcal/day | 1,901 | 1,815 | 1,626 | |||
| Carbohydrate, % of energy intake | 55.8 | 49.4 | 44.6 | |||
| Total fat, % of energy intake | 30.4 | 32.1 | 31.5 | |||
| Saturated fat, % of energy intake | 10.7 | 11.4 | 11.2 | |||
| Monounsaturated fat, % of energy intake | 11.7 | 12.2 | 11.7 | |||
| Polyunsaturated fat, % of energy intake | 5.6 | 5.7 | 5.6 | |||
| Trans- fat, % of energy intake | 1.8 | 1.7 | 1.4 | |||
| Dietary cholesterol, mg/day | 187.1 | 241.7 | 297.6 | |||
| Daily dietary glycemic loade | 139.1 | 120.3 | 106.4 | |||
| Daily dietary glycemic indexf | 55.3 | 53.9 | 52.4 | |||
| Total dietary fiber, g/day | 17.7 | 18.3 | 18.6 | |||
| Cereal fiber, g/day | 5.6 | 5.7 | 5.4 | |||
| Magnesium, mg/day | 285.2 | 314.9 | 345.2 | |||
| Heme iron, mg/day | 0.75 | 1.1 | 1.5 | |||
| Potassium, mg/day | 2,649 | 2,944 | 3,189 | |||
| Calcium, mg/day | 857.7 | 1,028 | 1,145 | |||
| Vitamin C, mg/day | 264.5 | 246.5 | 268.3 | |||
| Vitamin E, mg/day | 47.1 | 41.2 | 48.6 | |||
| Whole grains, servings/day | 1.3 | 1.4 | 1.3 | |||
| Fruit, servings/day | 1.2 | 1.2 | 1.2 | |||
| Vegetables, servings/day | 3.0 | 3.3 | 3.6 | |||
| Red meat, servings/day | 0.57 | 0.77 | 0.75 | |||
| Processed meat, servings/day | 0.25 | 0.24 | 0.17 | |||
| Fish, servings/day | 0.18 | 0.27 | 0.42 | |||
| Chicken, servings/day | 0.39 | 0.66 | 1.1 | |||
| Eggs, servings/day | 0.17 | 0.19 | 0.17 | |||
| Dairy foods, servings/day | 2.2 | 2.5 | 2.4 | |||
| Legumes, servings/day | 0.35 | 0.38 | 0.40 | |||
| Nuts, servings/day | 0.29 | 0.26 | 0.18 | |||
| Peanuts, servings/day | 0.05 | 0.04 | 0.02 | |||
| Peanut butter, servings/day | 0.20 | 0.20 | 0.14 | |||
| Potatoes, servings/day | 0.39 | 0.39 | 0.31 | |||
| Coffee, servings/day | 1.5 | 1.6 | 1.5 | |||
| Sugar-sweetened beverages, servings/day | 1.1 | 0.36 | 0.13 | |||
Abbreviations: GI, glycemic index; MET, metabolic equivalent.
a Median percentage of energy intake in the quintile.
b Not adjusted for age.
c Weight (kg)/height (m)2.
d First-degree relatives.
e Average glycemic load was calculated by multiplying the amount of carbohydrates in the diet by the average glycemic index. For 1 serving of a food, a glycemic load of ≥20 is considered high, 11–19 is considered medium, and ≤10 is considered low. Among these 92,088 women from Nurses’ Health Study II, the mean glycemic load was 119.0 (range, 47.8–222.4).
f Average dietary glycemic index (GI) was calculated by summing the products of 1) the carbohydrate content of each food item per serving, 2) the average daily number of servings of that food, and 3) the food's GI value (derived from available databases and publications) and dividing by total daily carbohydrate content. Foods with a GI value of ≤55 are considered to have a low GI, foods with a value of 56–69 are considered to have a medium GI, and foods with a value of ≥70 are considered to have a high GI. Among these 92,088 women from Nurses’ Health Study II, the mean GI was 52.9 (range, 37.0–64.8).
Table 3.
Age-Adjusted Characteristics of Participants According to Baseline (1986) Total Protein Intake Among 40,722 Men in the Health Professionals Follow-up Study, 1986–2008
| Characteristic | Quintile of Protein Intake |
|||||
|---|---|---|---|---|---|---|
| 1 (n = 7,921) (14.7% of Energy)a |
3 (n = 7,921) (18.0% of Energy) |
5 (n = 7,921) (21.9% of Energy) |
||||
| % | Mean | % | Mean | % | Mean | |
| Demographic and lifestyle factors | ||||||
| Age, yearsb | 52.9 | 52.9 | 53.1 | |||
| White race/ethnicity | 94.7 | 95.5 | 94.4 | |||
| Body mass indexc | 25.0 | 25.4 | 25.9 | |||
| Body mass index ≥25 | 47.0 | 52.1 | 57.7 | |||
| Physical activity, MET-hours/week | 20.8 | 21.0 | 21.5 | |||
| Current smoker | 12.3 | 9.1 | 8.3 | |||
| Hypertension | 18.1 | 19.3 | 21.4 | |||
| High cholesterol | 8.8 | 10.1 | 12.0 | |||
| Family history of diabetesd | 17.1 | 18.6 | 19.6 | |||
| Multivitamin use | 39.9 | 40.8 | 44.8 | |||
| Alcohol, g/day | 17.4 | 10.7 | 6.8 | |||
| Dietary intake | ||||||
| Total energy, kcal/day | 2,076 | 1,986 | 1,764 | |||
| Carbohydrate, % of energy intake | 50.9 | 46.9 | 42.7 | |||
| Total fat, % of energy intake | 30.7 | 32.5 | 32.4 | |||
| Saturated fat, % of energy intake | 10.5 | 11.2 | 11.1 | |||
| Monounsaturated fat, % of energy intake | 11.8 | 12.4 | 12.2 | |||
| Polyunsaturated fat, % of energy intake | 5.9 | 6.0 | 6.0 | |||
| Trans- fat, % of energy intake | 1.4 | 1.3 | 1.1 | |||
| Dietary cholesterol, mg/day | 236.2 | 300.2 | 374.2 | |||
| Daily dietary glycemic loade | 136.8 | 124.6 | 111.0 | |||
| Daily dietary glycemic indexf | 54.0 | 53.3 | 52.0 | |||
| Total dietary fiber, g/day | 19.9 | 21.1 | 21.4 | |||
| Cereal fiber, g/day | 5.6 | 6.0 | 5.8 | |||
| Magnesium, mg/day | 324.2 | 351.5 | 380.6 | |||
| Heme iron, mg/day | 0.93 | 1.3 | 1.7 | |||
| Potassium, mg/day | 3,084 | 3,426 | 3,708 | |||
| Calcium, mg/day | 767.3 | 906.3 | 1,002 | |||
| Vitamin C, mg/day | 389.9 | 426.0 | 493.1 | |||
| Vitamin E, mg/day | 82.5 | 91.2 | 116.9 | |||
| Whole grains, servings/day | 1.5 | 1.7 | 1.5 | |||
| Fruit, servings/day | 2.4 | 2.4 | 2.1 | |||
| Vegetables, servings/day | 2.7 | 3.1 | 3.3 | |||
| Red meat, servings/day | 0.62 | 0.82 | 0.78 | |||
| Processed meat, servings/day | 0.41 | 0.38 | 0.26 | |||
| Fish, servings/day | 0.24 | 0.38 | 0.66 | |||
| Chicken, servings/day | 0.33 | 0.54 | 0.87 | |||
| Eggs, servings/day | 0.29 | 0.34 | 0.34 | |||
| Dairy foods, servings/day | 1.7 | 2.0 | 1.9 | |||
| Legumes, servings/day | 0.37 | 0.43 | 0.45 | |||
| Nuts, servings/day | 0.51 | 0.49 | 0.34 | |||
| Peanuts, servings/day | 0.17 | 0.17 | 0.12 | |||
| Peanut butter, servings/day | 0.27 | 0.23 | 0.16 | |||
| Potatoes, servings/day | 0.43 | 0.43 | 0.35 | |||
| Coffee, servings/day | 2.0 | 2.0 | 1.8 | |||
| Sugar-sweetened beverages, servings/day | 0.68 | 0.30 | 0.14 | |||
Abbreviations: GI, glycemic index; MET, metabolic equivalent.
a Median percentage of energy intake in the quintile.
b Not adjusted for age.
c Weight (kg)/height (m)2.
d First-degree relatives.
e Average glycemic load was calculated by multiplying the amount of carbohydrates in the diet by the average glycemic index. For 1 serving of a food, a glycemic load of ≥20 is considered high, 11–19 is considered medium, and ≤10 is considered low. Among these 40,722 men from the Health Professionals Follow-up Study, the mean glycemic load was 128.3 (range, 6–263).
f Average dietary glycemic index (GI) was calculated by summing the products of 1) the carbohydrate content of each food item per serving, 2) the average daily number of servings of that food, and 3) the food's GI value (derived from available databases and publications) and dividing by total daily carbohydrate content. Foods with a GI value of ≤55 are considered to have a low GI, foods with a value of 56–69 are considered to have a medium GI, and foods with a value of ≥70 are considered to have a high GI. Among these 40,722 men from the Health Professionals Follow-up Study, the mean GI was 53.1 (range, 15.4–72.4).
A higher intake of percentage of energy from total protein was associated with a higher risk of T2D in age- and multivariate-adjusted models across all 3 cohorts (all P's for trend < 0.0001) (Table 4). After further adjustment for BMI, associations were attenuated and no longer statistically significant in the NHS (P for trend = 0.14) and NHS II (P for trend = 0.24) but remained statistically significant in the HPFS (P for trend = 0.001). In the pooled analysis of the 3 cohorts, persons in the highest quintile of intake compared with the lowest quintile had a 7% increased risk of T2D after adjustment for BMI (comparing extreme quintiles, hazard ratio (HR) = 1.07, 95% confidence interval (CI): 1.01, 1.17; P for trend = 0.001). Estimates were also attenuated but remained statistically significant after adjustment for red and processed meat and heme iron (Web Table 4). Adjusting for branched chain and aromatic amino acids also attenuated associations (Web Table 4).
Table 4.
Hazard Ratios for the Association Between Protein Intake and Risk of Type 2 Diabetes in the Nurses’ Health Study (1984–2008), Nurses’ Health Study II (1991–2009), and the Health Professionals Follow-up Study (1986–2008)
| Cohort and Quintile of Protein Intake | Median Intake, % of Energy | No. of Cases | No. of Person-Years | Age-Adjusted HR | 95% CI | Multivariate- Adjusted HRa | 95% CI | Multivariate- and BMIb- Adjusted HRc |
95% CI |
|---|---|---|---|---|---|---|---|---|---|
| Total Protein | |||||||||
| NHS | |||||||||
| 1 | 14.8 | 1,220 | 309,146 | 1.00 | 1.00 | 1.00 | |||
| 2 | 16.7 | 1,248 | 309,248 | 1.02 | 0.94, 1.10 | 1.04 | 0.96, 1.13 | 0.99 | 0.91, 1.07 |
| 3 | 18.0 | 1,408 | 309,052 | 1.15 | 1.06, 1.24 | 1.16 | 1.06, 1.25 | 1.04 | 0.96, 1.13 |
| 4 | 19.4 | 1,565 | 308,986 | 1.27 | 1.18, 1.37 | 1.27 | 1.17, 1.39 | 1.08 | 0.99, 1.17 |
| 5 | 21.6 | 1,773 | 308,742 | 1.43 | 1.33, 1.54 | 1.38 | 1.26, 1.51 | 1.05 | 0.95, 1.15 |
| P for trend | <0.001 | <0.001 | 0.14 | ||||||
| NHS II | |||||||||
| 1 | 15.3 | 857 | 354,913 | 1.00 | 1.00 | 1.00 | |||
| 2 | 17.4 | 839 | 355,439 | 0.98 | 0.89, 1.08 | 0.99 | 0.89, 1.09 | 0.92 | 0.83, 1.02 |
| 3 | 18.8 | 927 | 355,299 | 1.08 | 0.99, 1.19 | 1.07 | 0.97, 1.19 | 0.91 | 0.82, 1.01 |
| 4 | 20.2 | 1,062 | 354,884 | 1.23 | 1.12, 1.34 | 1.19 | 1.07, 1.32 | 0.96 | 0.86, 1.07 |
| 5 | 22.6 | 1,347 | 353,537 | 1.53 | 1.41, 1.67 | 1.45 | 1.30, 1.62 | 1.03 | 0.92, 1.15 |
| P for trend | <0.001 | <0.001 | 0.24 | ||||||
| HPFS | |||||||||
| 1 | 14.7 | 596 | 165,260 | 1.00 | 1.00 | 1.00 | |||
| 2 | 16.7 | 567 | 165,866 | 0.95 | 0.84, 1.06 | 0.90 | 0.80, 1.01 | 0.87 | 0.77, 0.98 |
| 3 | 18.0 | 614 | 165,809 | 1.02 | 0.91, 1.14 | 0.95 | 0.84, 1.07 | 0.90 | 0.80, 1.02 |
| 4 | 19.5 | 679 | 165,606 | 1.12 | 1.00, 1.25 | 1.02 | 0.90, 1.15 | 0.93 | 0.83, 1.06 |
| 5 | 21.9 | 878 | 164,429 | 1.45 | 1.30, 1.61 | 1.35 | 1.19, 1.53 | 1.18 | 1.04, 1.34 |
| P for trend | <0.001 | <0.001 | 0.001 | ||||||
| Pooled | |||||||||
| 1 | 1.00 | 1.00 | |||||||
| 2 | 0.99 | 0.94, 1.05 | 0.94 | 0.89, 0.99 | |||||
| 3 | 1.08 | 1.02, 1.14 | 0.97 | 0.92, 1.03 | |||||
| 4 | 1.19 | 1.20, 1.26 | 1.01 | 0.95, 1.07 | |||||
| 5 | 1.39 | 1.31, 1.48 | 1.07 | 1.01, 1.17 | |||||
| P for trend | <0.001 | 0.001 | |||||||
| Animal Protein | |||||||||
| NHS | |||||||||
| 1 | 9.7 | 1,158 | 309,267 | 1.00 | 1.00 | 1.00 | |||
| 2 | 11.6 | 1,233 | 309,319 | 1.07 | 0.98, 1.15 | 1.06 | 0.97, 1.15 | 0.99 | 0.91, 1.08 |
| 3 | 12.9 | 1,383 | 309,141 | 1.19 | 1.10, 1.29 | 1.16 | 1.06, 1.26 | 1.03 | 0.95, 1.12 |
| 4 | 14.4 | 1,565 | 309,024 | 1.35 | 1.25, 1.46 | 1.27 | 1.17, 1.38 | 1.06 | 0.97, 1.16 |
| 5 | 16.8 | 1,875 | 308,423 | 1.61 | 1.50, 1.74 | 1.43 | 1.31, 1.57 | 1.08 | 0.99, 1.19 |
| P for trend | <0.001 | <0.001 | 0.04 | ||||||
| NHS II | |||||||||
| 1 | 9.9 | 722 | 355,405 | 1.00 | 1.00 | 1.00 | |||
| 2 | 12.0 | 876 | 355,377 | 1.23 | 1.12, 1.36 | 1.15 | 1.04, 1.28 | 1.03 | 0.92, 1.14 |
| 3 | 13.5 | 931 | 355,474 | 1.31 | 1.19, 1.44 | 1.17 | 1.05, 1.31 | 0.97 | 0.87, 1.09 |
| 4 | 15.1 | 1,106 | 354,678 | 1.55 | 1.41, 1.70 | 1.35 | 1.20, 1.51 | 1.03 | 0.92, 1.16 |
| 5 | 17.6 | 1,397 | 353,138 | 1.94 | 1.77, 2.12 | 1.62 | 1.44, 1.83 | 1.11 | 0.98, 1.25 |
| P for trend | <0.001 | <0.001 | 0.05 | ||||||
| HPFS | |||||||||
| 1 | 9.4 | 515 | 165,717 | 1.00 | 1.00 | 1.00 | |||
| 2 | 11.4 | 567 | 165,969 | 1.11 | 0.98, 1.25 | 1.00 | 0.88, 1.13 | 0.96 | 0.85, 1.09 |
| 3 | 12.8 | 610 | 165,831 | 1.19 | 1.05, 1.33 | 1.02 | 0.90, 1.16 | 0.96 | 0.85, 1.10 |
| 4 | 14.4 | 731 | 165,387 | 1.41 | 1.26, 1.58 | 1.18 | 1.03, 1.34 | 1.06 | 0.93, 1.21 |
| 5 | 17.0 | 911 | 164,067 | 1.77 | 1.59, 1.97 | 1.46 | 1.27, 1.68 | 1.27 | 1.11, 1.46 |
| P for trend | <0.001 | <0.001 | <0.001 | ||||||
| Pooled | |||||||||
| 1 | 1.00 | 1.00 | |||||||
| 2 | 1.07 | 1.01, 1.13 | 0.99 | 0.94, 1.05 | |||||
| 3 | 1.13 | 1.07, 1.20 | 1.00 | 0.94, 1.06 | |||||
| 4 | 1.27 | 1.20, 1.35 | 1.05 | 0.99, 1.12 | |||||
| 5 | 1.49 | 1.40, 1.59 | 1.13 | 1.06, 1.21 | |||||
| P for trend | <0.001 | <0.001 | |||||||
| Plant Protein | |||||||||
| NHS | |||||||||
| 1 | 3.9 | 1,698 | 308,253 | 1.00 | 1.00 | 1.00 | |||
| 2 | 4.6 | 1,551 | 308,928 | 0.91 | 0.85, 0.97 | 0.96 | 0.89, 1.04 | 0.95 | 0.88, 1.02 |
| 3 | 5.0 | 1,428 | 309,125 | 0.83 | 0.77, 0.89 | 0.94 | 0.86, 1.02 | 0.91 | 0.84, 0.99 |
| 4 | 5.4 | 1,335 | 309,343 | 0.77 | 0.72, 0.83 | 0.93 | 0.85, 1.02 | 0.89 | 0.81, 0.97 |
| 5 | 6.1 | 1,202 | 309,525 | 0.69 | 0.64, 0.74 | 0.96 | 0.86, 1.06 | 0.91 | 0.82, 1.02 |
| P for trend | <0.001 | 0.34 | 0.05 | ||||||
| NHS II | |||||||||
| 1 | 4.0 | 1,109 | 348,121 | 1.00 | 1.00 | 1.00 | |||
| 2 | 4.7 | 1,061 | 356,756 | 0.82 | 0.76, 0.89 | 0.92 | 0.84, 1.00 | 0.95 | 0.87, 1.04 |
| 3 | 5.1 | 941 | 358,050 | 0.73 | 0.68, 0.80 | 0.89 | 0.80, 0.99 | 0.92 | 0.92, 1.02 |
| 4 | 5.6 | 959 | 356,832 | 0.70 | 0.65, 0.76 | 0.93 | 0.83, 1.04 | 0.95 | 0.85, 1.06 |
| 5 | 6.6 | 962 | 354,313 | 0.53 | 0.48, 0.58 | 0.85 | 0.75, 0.98 | 0.90 | 0.79, 1.04 |
| P for trend | <0.001 | 0.03 | 0.12 | ||||||
| HPFS | |||||||||
| 1 | 3.9 | 833 | 163,820 | 1.00 | 1.00 | 1.00 | |||
| 2 | 4.6 | 710 | 165,172 | 0.85 | 0.77, 0.94 | 0.91 | 0.82, 1.02 | 0.93 | 0.93, 1.04 |
| 3 | 5.1 | 644 | 165,617 | 0.76 | 0.69, 0.84 | 0.87 | 0.77. 0.98 | 0.87 | 0.77, 0.99 |
| 4 | 5.6 | 637 | 166,037 | 0.74 | 0.67, 0.82 | 0.93 | 0.81, 1.07 | 0.96 | 0.84, 1.10 |
| 5 | 6.6 | 510 | 166,324 | 0.59 | 0.53, 0.66 | 0.88 | 0.75, 1.04 | 0.91 | 0.77, 1.07 |
| P for trend | <0.001 | 0.20 | 0.34 | ||||||
| Pooled | |||||||||
| 1 | 1.00 | 1.00 | |||||||
| 2 | 0.94 | 0.89, 0.99 | 0.94 | 0.90, 0.99 | |||||
| 3 | 0.91 | 0.86, 0.96 | 0.91 | 0.85, 0.96 | |||||
| 4 | 0.93 | 0.88, 0.99 | 0.92 | 0.86, 0.98 | |||||
| 5 | 0.91 | 0.85, 0.98 | 0.91 | 0.84, 0.98 | |||||
| P for trend | 0.01 | 0.01 | |||||||
Abbreviations: CI, confidence interval; BMI, body mass index; HPFS, Health Professionals Follow-up Study; HR, hazard ratio; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II.
a Adjusted for family history of diabetes, smoking, alcohol intake, physical activity, race/ethnicity, total energy intake, postmenopausal hormone use (NHS, NHS II), oral contraceptive use (NHS II), percentages of energy from trans- fat, saturated fat, monounsaturated fat, and polyunsaturated fat, dietary cholesterol, dietary fiber, and glycemic index. Results were mutually adjusted for percentage of energy derived from animal protein and vegetable protein.
b Weight (kg)/height (m)2.
c Adjusted for family history of diabetes, smoking, alcohol intake, physical activity, race/ethnicity, total energy intake, postmenopausal hormone use (NHS, NHS II), oral contraceptive use (NHS II), percentages of energy from trans- fat, saturated fat, monounsaturated fat, and polyunsaturated fat, dietary cholesterol, dietary fiber, and glycemic index. Results were mutually adjusted for percentage of energy derived from animal protein and vegetable protein + BMI.
Associations with percentage of energy derived from animal protein were stronger than those observed for total protein and persisted after further adjustment for BMI across all cohorts (all P's for trend < 0.05). Comparing extreme quintiles from the pooled analysis, the hazard ratio was 1.13 (95% CI: 1.06, 1.21; P for trend < 0.0001). Percentage of energy from vegetable protein was associated with decreased risk of T2D in age-adjusted models (P for trend < 0.0001), but associations were attenuated after further adjustment for lifestyle and dietary factors. In the pooled analyses, the association persisted (comparing extreme quintiles from the fully adjusted model including BMI, HR = 0.91, 95% CI: 0.84, 0.98; P for trend = 0.01).
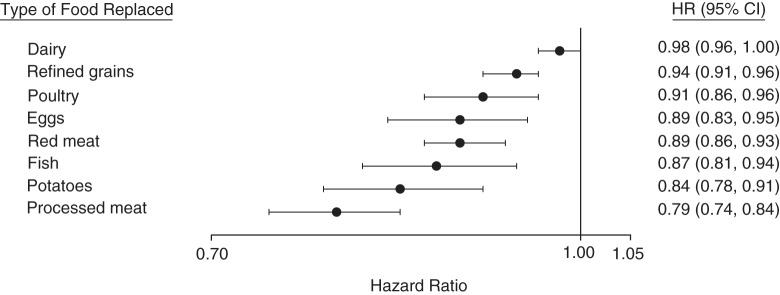
Substituting 5% of energy intake from total and animal protein for an equal exchange of total carbohydrate was not associated with risk of T2D, while substitution with vegetable protein was associated with reduced risk (HR = 0.78, 95% CI: 0.71, 0.86; P < 0.001) (Table 5). Similar estimates were observed after making substitutions for carbohydrate from refined grains, potatoes, and added sugar. In contrast, substituting total and animal protein for carbohydrate from whole grains was associated with 20% (95% CI: 14, 28) and 18% (95% CI: 11, 25) increased risks of T2D, respectively (P <0.001), while substitution with vegetable protein was not associated with risk of T2D. Substituting vegetable protein for animal protein was associated with a 23% reduced risk of T2D (HR = 0.77, 95% CI: 0.70, 0.84; P < 0.001). Substituting 1 serving per day of vegetable protein foods for an equal exchange of animal protein foods, refined grains, or potatoes was associated with reduced T2D risks ranging from 6% for refined grains to 21% for processed meat (Figure 1). The substitution for dairy foods was not statistically significant. In our cohorts, whole grains and peanuts and peanut butter were the most commonly consumed major food sources of vegetable protein. Substituting these individual vegetable protein foods for animal protein foods, refined grains, and potatoes yielded similar reductions in T2D as those reported above (Web Figure 2).
Table 5.
Hazard Ratios for the Association Between Protein Intake and Risk of Type 2 Diabetes (Pooled Estimates) After Substitution of 5% of Energy From Protein for Equal Exchanges of Total and Different-Quality Carbohydrates and Substitution of Vegetable Protein for Animal Protein, Nurses’ Health Study (1984–2008), Nurses’ Health Study II (1991–2009), and Health Professionals Follow-up Study (1986–2008)
| Substitution | Hazard Ratioa | 95% Confidence Interval | P Value |
|---|---|---|---|
| Substitution for total carbohydrate | |||
| Total protein | 0.99 | 0.95, 1.02 | 0.37 |
| Animal protein | 0.99 | 0.96, 1.03 | 0.76 |
| Vegetable protein | 0.78 | 0.71, 0.86 | <0.001 |
| Substitution for carbohydrate from intact and milled whole grains | |||
| Total protein | 1.20 | 1.14, 1.28 | <0.001 |
| Animal protein | 1.18 | 1.11, 1.25 | <0.001 |
| Vegetable protein | 1.02 | 0.90, 1.16 | 0.76 |
| Substitution for carbohydrate from refined grains, potatoes, and added sugar | |||
| Total protein | 1.00 | 0.97, 1.04 | 0.90 |
| Animal protein | 1.01 | 0.98, 1.05 | 0.49 |
| Vegetable protein | 0.81 | 0.73, 0.89 | <0.001 |
| Substitution of vegetable protein for animal protein | 0.77 | 0.70, 0.84 | <0.001 |
a Adjusted for age, family history of diabetes, smoking, alcohol intake, physical activity, race/ethnicity, postmenopausal hormone use (Nurses’ Health Study, Nurses’ Health Study II), oral contraceptive use (Nurses’ Health Study II), total energy intake, percentage of energy from fat, dietary cholesterol, and body mass index.
Figure 1.
Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) for type 2 diabetes associated with replacement of 1 serving of individual animal protein foods (dairy foods, poultry, eggs, red meat, and processed meat), refined grains, and potatoes with 1 serving of vegetable protein foods (composite variable comprised of whole grains, legumes, peanuts, peanut butter, and other nuts) in the Nurses’ Health Study (1984–2008), Nurses’ Health Study II (1991–2009), and the Health Professionals Follow-up Study (1986–2008). The models adjusted for age, family history of diabetes, smoking, alcohol intake, physical activity, race/ethnicity, total energy intake, postmenopausal hormone use (Nurses’ Health Study, Nurses’ Health Study II), oral contraceptive use (Nurses’ Health Study II), intakes of sugar-sweetened beverages, fruit, and vegetables, and body mass index. Results were mutually adjusted for other food sources of animal protein, refined grains, and potatoes.
In stratified analysis, associations between total and animal protein and T2D were greater among participants with BMI <30 compared with those with BMI ≥30 (P for interaction < 0.001) (Web Table 5). No significant effect modification by age, physical activity, Alternate Healthy Eating Index score, or family history of T2D was observed. When we used baseline diet and most recent diet as our exposure to examine associations between protein intake and risk of T2D, our results were similar, and results remained largely unchanged when we continued to update diet after the occurrence of coronary heart disease, stroke, or cancer (not shown).
DISCUSSION
In these 3 large prospective cohort studies of US adults, we found that total protein intake was positively associated with risk of T2D, largely due to intake of animal protein. In contrast, intake of vegetable protein was moderately inversely associated with risk of T2D. Substitution of 5% of energy intake from vegetable protein for an equal exchange of animal protein and carbohydrate from refined grains, potatoes, and added sugar was associated with decreased risk of T2D. These findings suggest a benefit of replacing animal protein and low-quality carbohydrates with vegetable protein in regard to T2D risk, which was corroborated in our food substitution models. To our knowledge, this is the first study that has examined long-term intake of protein in relation to T2D risk using repeated measurements taken over many years of follow-up and that has examined the role of substitution of protein and protein type by carbohydrate type in T2D risk. This has important public health implications, since protein and carbohydrate are often exchanged for one another in the diet, and both type of protein and type of carbohydrate have been associated with T2D risk.
Our results support those of previous studies that have found positive associations between total and animal protein and risk of T2D. In the European Prospective Investigation into Cancer and Nutrition (EPIC)-InterAct Case-Cohort Study, there were 17% and 22% increased risks of T2D when comparing extreme quintiles of total protein intake and animal protein intake, respectively (3). Similar associations were reported in the Dutch cohort of EPIC-InterAct, although estimates were not statistically significant after adjustment for BMI and waist circumference (4). However, both of these studies used baseline protein intake only, which may underestimate associations, and were not able to assess longitudinal intake. Other epidemiologic studies (25–28) have also found positive associations between total or animal protein and risk of T2D, which is consistent with findings from mid- (29) and long-term (30) trials.
Similar to previous observational studies (3, 4, 25), in our analysis, estimates were attenuated after adjustment for BMI, and this was more evident in the NHS and NHS II, suggesting that body weight may partly mediate the association between total and animal protein and risk of T2D, particularly among women (21). However, BMI could be both a confounder and an intermediate factor, and it is difficult to know which is driving the attenuation. In contrast to our findings, van Nielen et al. (3) reported greater attenuation of estimates in men compared with women after adjusting for BMI and waist circumference. They also found that associations were stronger among women who were obese (3). In our analysis, associations between total and animal protein and T2D were stronger among participants who had a BMI less than 30. Similarly, Sluijs et al. (4) reported weaker associations with increasing BMI. It is unclear why associations were stronger in nonobese participants than in obese participants, but it is possible that the positive associations between protein and T2D risk are more pronounced among persons who are presumably more insulin-sensitive (1). Further studies are warranted to evaluate the role of BMI in this association.
Unlike previous studies that did not observe associations between vegetable protein intake and risk of T2D, we found a modest inverse association that remained statistically significant in the pooled analysis. This discrepancy may be due to differences in sources of vegetable protein across study populations. In our cohorts, the main sources of vegetable protein intake included whole grains, nuts, peanut butter, and beans, whereas in EPIC-InterAct the main sources of vegetable protein intake were bread, pasta, rice, and potatoes (3), which may contribute to a high dietary glycemic load. Diets high in glycemic load have been shown to increase risk of T2D (31).
Potential biological mechanisms supporting divergent associations of animal and vegetable protein with risk of T2D are unknown but may relate to different protein-rich food sources, co-occurrence of other nutrients in protein-rich foods, and variations in the amino acid composition of these foods. In our cohorts, intake of red and processed meat has been positively associated with weight gain (32) and with risk of T2D (6), coronary heart disease (33), stroke (34), and mortality (35). Various nutrients in red and processed meat, including heme iron, advanced glycation end products, and nitrites, are thought to mediate the association between meat intake and risk of T2D (6). In our analysis, adjusting for red and processed meat and heme iron attenuated the estimates, although they remained statistically significant, suggesting that they are partial mediators. Inconsistent findings have been reported for consumption of fish (36, 37), while intake of low-fat and fermented dairy products may be beneficial (38). In contrast, plant-based sources of protein, such as nuts (8), legumes (9), and whole grains (39), have been associated with a decreased risk of T2D. These foods have healthful nutritional profiles characterized by low glycemic index values and a high content of fiber and micronutrients. Nuts are also rich in monounsaturated and polyunsaturated fatty acids.
In a metabolomics study, Wang et al. (40) found strong positive associations between branched chain and aromatic amino acids and incident T2D. These amino acids have also been found to be associated with increased T2D risk (41) and represent the majority of amino acids entering circulation after consumption of red meat (42). In our analysis, adjustment for these amino acids attenuated associations between protein intake and risk of T2D, suggesting that they may be partial mediators.
Our study had important strengths and limitations. The large sample size, long duration of follow-up, and high response rate provided us with the statistical power to detect meaningful differences in estimates. We also used repeated measurements of diet, which better represents long-term dietary intake. Because diet was assessed using FFQs, some measurement error in assessment of protein intake was inevitable. However, given the prospective study design, any measurement error in protein intake was independent of outcome assessment and thus was more likely to attenuate associations. Although we adjusted for a number of potential confounders, the possibility of residual confounding cannot be dismissed and thus precludes inference of causation. Our study population primarily consisted of white health-care professionals, which may have helped reduce confounding by socioeconomic status but also limits the generalizability of these associations to other populations.
In conclusion, we found that greater intakes of total and animal protein were associated with a higher risk of T2D, while intake of vegetable protein had a modest inverse association. Substituting vegetable protein for animal protein and low-quality carbohydrates was associated with reduced risk of T2D. These data suggest that adopting a diet rich in plant-based proteins should be considered for T2D prevention. Confirmatory results from dietary intervention studies are warranted and will provide further support for dietary recommendations to increase intake of vegetable protein in place of animal protein.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Nutrition, Harvard T.H Chan School of Public Health, Boston, Massachusetts (Vasanti S. Malik, Yanping Li, Frank B. Hu); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Frank B. Hu); Division of Preventive Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Deirdre K. Tobias); Department of Epidemiology and Biostatistics, MOE Key Lab of Environment and Health, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China (An Pan); and Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Frank B. Hu).
This study was supported by National Institutes of Health grants U54CA155626, P30DK46200, DK58845, UM1CA186107, UM1CA176726, and UM1CA167552. V.S.M. has received research support from the Peanut Institute (Albany, Georgia).
Conflict of interest: none declared.
REFERENCES
- 1.Rietman A, Schwarz J, Tomé D et al. High dietary protein intake, reducing or eliciting insulin resistance? Eur J Clin Nutr. 2014;689:973–979. [DOI] [PubMed] [Google Scholar]
- 2.Promintzer M, Krebs M. Effects of dietary protein on glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2006;94:463–468. [DOI] [PubMed] [Google Scholar]
- 3.van Nielen M, Feskens EJ, Mensink M et al. Dietary protein intake and incidence of type 2 diabetes in Europe: the EPIC-InterAct Case-Cohort Study. Diabetes Care. 2014;377:1854–1862. [DOI] [PubMed] [Google Scholar]
- 4.Sluijs I, Beulens JW, van der AD et al. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL Study. Diabetes Care. 2010;331:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.InterAct Consortium; Bendinelli B, Palli D et al. Association between dietary meat consumption and incident type 2 diabetes: the EPIC-InterAct Study. Diabetologia. 2013;561:47–59. [DOI] [PubMed] [Google Scholar]
- 6.Pan A, Sun Q, Bernstein AM et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011;944:1088–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan A, Sun Q, Manson JE et al. Walnut consumption is associated with lower risk of type 2 diabetes in women. J Nutr. 2013;1434:512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo C, Zhang Y, Ding Y et al. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a systematic review and meta-analysis. Am J Clin Nutr. 2014;1001:256–269. [DOI] [PubMed] [Google Scholar]
- 9.Villegas R, Gao YT, Yang G et al. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women's Health Study. Am J Clin Nutr. 2008;871:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agricultural Research Service, US Department of Agriculture. Welcome to the USDA National Nutrient Database for Standard Reference. https://ndb.nal.usda.gov/. Modified November 30, 2015. Accessed June 4, 2015.
- 11.Whitney E, Rolfes S. Understanding Nutrition. Belmont, CA: Wadsworth Publishing; 2004. [Google Scholar]
- 12.Feskanich D, Rimm EB, Giovannucci EL et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;937:790–796. [DOI] [PubMed] [Google Scholar]
- 13.Rimm EB, Giovannucci EL, Stampfer MJ et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;13510:1114–1126. [DOI] [PubMed] [Google Scholar]
- 14.Willett WC, Sampson L, Stampfer MJ et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;1221:51–65. [DOI] [PubMed] [Google Scholar]
- 15.Salvini S, Hunter DJ, Sampson L et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;184:858–867. [DOI] [PubMed] [Google Scholar]
- 16.Willett WC. Nutritional Epidemiology. 2nd ed New York, NY: Oxford University Press; 1998. [Google Scholar]
- 17.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;2812:1039–1057. [DOI] [PubMed] [Google Scholar]
- 18.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;207:1183–1197. [DOI] [PubMed] [Google Scholar]
- 19.Manson JE, Rimm EB, Stampfer MJ et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;3388770:774–778. [DOI] [PubMed] [Google Scholar]
- 20.Hu FB, Stampfer MJ, Solomon C et al. Physical activity and risk for cardiovascular events in diabetic women. Ann Intern Med. 2001;1342:96–105. [DOI] [PubMed] [Google Scholar]
- 21.Vergnaud AC, Norat T, Mouw T et al. Macronutrient composition of the diet and prospective weight change in participants of the EPIC-PANACEA Study. PLoS One. 2013;83:e57300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu FB, Stampfer MJ, Rimm E et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;1496:531–540. [DOI] [PubMed] [Google Scholar]
- 23.Hu FB, Stampfer MJ, Manson JE et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;33721:1491–1499. [DOI] [PubMed] [Google Scholar]
- 24.Fung TT, McCullough M, van Dam RM et al. A prospective study of overall diet quality and risk of type 2 diabetes in women. Diabetes Care. 2007;307:1753–1757. [DOI] [PubMed] [Google Scholar]
- 25.Tinker LF, Sarto GE, Howard BV et al. Biomarker-calibrated dietary energy and protein intake associations with diabetes risk among postmenopausal women from the Women's Health Initiative. Am J Clin Nutr. 2011;946:1600–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duc Son le NT, Hanh TT, Kusama K et al. Anthropometric characteristics, dietary patterns and risk of type 2 diabetes mellitus in Vietnam. J Am Coll Nutr. 2005;244:229–234. [DOI] [PubMed] [Google Scholar]
- 27.Wang ET, de Koning L, Kanaya AM. Higher protein intake is associated with diabetes risk in South Asian Indians: the Metabolic Syndrome and Atherosclerosis in South Asians Living in America (MASALA) Study. J Am Coll Nutr. 2010;292:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pounis GD, Tyrovolas S, Antonopoulou M et al. Long-term animal-protein consumption is associated with an increased prevalence of diabetes among the elderly: the Mediterranean Islands (MEDIS) Study. Diabetes Metab. 2010;366:484–490. [DOI] [PubMed] [Google Scholar]
- 29.Weickert MO, Roden M, Isken F et al. Effects of supplemented isoenergetic diets differing in cereal fiber and protein content on insulin sensitivity in overweight humans. Am J Clin Nutr. 2011;942:459–471. [DOI] [PubMed] [Google Scholar]
- 30.Linn T, Santosa B, Grönemeyer D et al. Effect of long-term dietary protein intake on glucose metabolism in humans. Diabetologia. 2000;4310:1257–1265. [DOI] [PubMed] [Google Scholar]
- 31.Bhupathiraju SN, Tobias DK, Malik VS et al. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr. 2014;1001:218–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mozaffarian D, Hao T, Rimm EB et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;36425:2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernstein AM, Sun Q, Hu FB et al. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;1229:876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernstein AM, Pan A, Rexrode KM et al. Dietary protein sources and the risk of stroke in men and women. Stroke. 2012;433:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan A, Sun Q, Bernstein AM et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;1727:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaushik M, Mozaffarian D, Spiegelman D et al. Long-chain omega-3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. Am J Clin Nutr. 2009;903:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallin A, Di Giuseppe D, Orsini N et al. Fish consumption, dietary long-chain n-3 fatty acids, and risk of type 2 diabetes: systematic review and meta-analysis of prospective studies. Diabetes Care. 2012;354:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirahatake KM, Slavin JL, Maki KC et al. Associations between dairy foods, diabetes, and metabolic health: potential mechanisms and future directions. Metabolism. 2014;635:618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Munter JS, Hu FB, Spiegelman D et al. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med. 2007;48:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang TJ, Larson MG, Vasan RS et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;174:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Floegel A, Stefan N, Yu Z et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;622:639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adeva MM, Calviño J, Souto G et al. Insulin resistance and the metabolism of branched-chain amino acids in humans. Amino Acids. 2012;431:171–181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.