Abstract
It is unclear whether dyslipidemia is associated with risk of colorectal neoplasia. The incidence of both conditions is increasing in Asia, motivating a number of new studies from this region. We performed a systematic literature search of Asian colonoscopy-based studies that collected blood lipid concentrations at the time of endoscopy. Persons found to have colorectal adenoma were considered cases, and those found to be adenoma-free were considered controls. Seventeen studies published between 2000 and 2014 met inclusion criteria, collectively enrolling 17,387 cases and 30,427 controls. Mean differences and adjusted odds ratios were summarized with random-effects meta-analyses. Compared with controls, cases had higher total cholesterol (mean difference (MD) = 2.4 mg/dL, 95% confidence interval (CI): 0.2, 4.6), higher low-density lipoprotein cholesterol (MD = 1.3 mg/dL, 95% CI: 0.1, 2.6), higher triglyceride (MD = 16.4 mg/dL, 95% CI: 11.2, 21.5), and lower high-density lipoprotein (HDL) cholesterol (MD = −2.1 mg/dL, 95% CI: −2.7, −1.6) concentrations. Based on adjusted odds ratios, associations for 40-mg/dL-higher triglyceride levels (odds ratio = 1.13, 95% CI: 1.05, 1.21) and 10-mg/dL-higher HDL cholesterol levels (odds ratio = 0.96, 95% CI: 0.92, 1.00) achieved statistical significance. Persons with adenoma were more likely to have unfavorable cholesterol profiles at the time of colonoscopy than those without adenoma. The most convincing evidence for an association between dyslipidemia and colorectal neoplasia was observed for hypertriglyceridemia.
Keywords: cholesterol, colonoscopy, colorectal adenoma, triglycerides
Dyslipidemia is a hypothesized risk factor for colorectal adenomas (1, 2), common neoplastic lesions that can develop into colorectal cancer (3–5). Several endoscopy studies of adenomas have investigated, without complete consensus, possible associations with blood concentrations of total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, or triglycerides. In a meta-analysis of studies from the United States, Europe, and Asia, Tian et al. (6) reported that higher levels of LDL cholesterol, triglycerides, and total cholesterol were associated with higher adenoma prevalence, but with differences by geographic region.
Both colorectal cancer incidence and dyslipidemia incidence are increasing in Asia (7, 8), likely as a result of diet and lifestyle changes (9, 10). Consequently, the number of recent studies from Asia on this topic has also increased. In contrast, many of the early endoscopy studies from the 1980s and 1990s that focused on metabolic exposures were conducted in Europe (11–13) and the United States (14, 15). It has been challenging in more recent studies in Western countries to assess the association between cholesterol levels and adenoma, given the widespread use of lipid-controlling medications such as statins (3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors) (16). It remains unclear whether statin use is related to the risk of developing colorectal adenomas (17–19). Such evidence from Asian populations is lacking, primarily because statins are not as commonly prescribed in Asia, and when prescribed they are usually given at lower doses (20–22).
The relatively low prevalence of statin use in Asia makes this region particularly attractive for evaluations of cholesterol as an exposure. To quantify the strength of evidence for the association between cholesterol and adenoma in Asia, we conducted a systematic review and meta-analysis of colonoscopy-based case-control studies from this region published in the peer-reviewed literature between 2000 and 2014. By focusing attention on only the most recent investigations, with similar study designs, from a single geographic region, we attempted to limit between-study heterogeneity.
METHODS
Literature search
We searched PubMed (including MEDLINE and articles submitted to PubMed Central) for original research articles published between January 1, 2000, and December 31, 2014. The following search string was used: (adenoma or polyp or hyperplastic) and (cholesterol or lipid or triglyceride) and (plasma or serum or blood) and (colorectal or colon or rectal or rectum). The search was limited to studies of humans published in English, and did not include proceedings from scientific conferences.
Exclusion criteria
We employed a 2-stage study selection procedure involving an abstract review that screened for general exclusion criteria, followed by a full-text review that screened for more specific exclusion criteria. All abstracts from the search were screened. Studies were excluded if the abstract indicated that: 1) no primary data had been collected (e.g., review articles); 2) the study had not been conducted in humans; 3) colorectal adenoma was not a studied outcome; 4) controls had not also undergone colonoscopy; 5) the study setting was specified to be outside of East or Southeast Asia; or 6) no full-text article in English was available.
The full texts of articles passing the abstract assessment were re-screened in detail for the 6 exclusion criteria listed above. In addition, studies were excluded at the full-text review stage if: 1) lipid concentrations had not been measured directly from serum or plasma (e.g., abstracted from medical records or estimated from a food frequency questionnaire); 2) there was uncertainty about whether cholesterol was measured at the time of the study colonoscopy (fasting); 3) no estimated case- and control-group-specific mean lipid concentrations or odds ratios for adenoma prevalence were reported for at least one of HDL cholesterol, LDL cholesterol, triglycerides, or total cholesterol; or 4) sigmoidoscopy was conducted instead of full colonoscopy. Together, these criteria helped to ensure that all included studies would score ≥6 on the Newcastle-Ottawa Quality Assessment Scale for case-control studies (23). We also confirmed that there were not duplicate reports. If multiple publications based on the same data set were suspected, we included only the article with the earliest date of publication.
For studies that distinguished between different types of polyps, information on serrated adenomas, sessile serrated adenomas, or hyperplastic polyps was not considered. Data were abstracted from studies that identified pathology-confirmed tubular, villous, or tubulovillous adenomas of any size or grade. It was not required that studies distinguish advanced adenomas (≥1 mm in diameter, with ≥20% villous components, or high-grade dysplasia) from nonadvanced adenomas. Data abstraction was performed by the first author (M.N.P.).
Data abstraction
The 2 primary summary statistics abstracted from eligible studies were: 1) case- and control-group mean values and standard deviations for HDL cholesterol, LDL cholesterol, triglycerides, or total cholesterol (the standard deviation was calculated from the standard error of the mean if necessary); and 2) odds ratios for adenoma prevalence with 95% confidence intervals. If the reported means or odds ratios had been adjusted for potentially confounding factors, these variables were noted. Additionally, we abstracted the following data from eligible studies: 1) the numbers of cases and controls; 2) the range of calendar years of colonoscopy; 3) the proportion of men in the study; 4) the age range of participants (if not available, then the mean age of controls); and 5) whether the investigators had chosen to exclude participants who reported a previous diagnosis of any colorectal polyp or colorectal cancer (studies that did not specifically report on these criteria were assumed to have not made such exclusions).
Statistical analyses of mean values
The mean differences in blood concentration (mean for controls subtracted from the mean for cases) for each lipid component, in mg/dL, were pooled using DerSimonian-Laird random-effects meta-analysis with inverse variance weighting (24). The statistical significance of the pooled mean difference was quantified with a z statistic and a P value from a Wald test. Between-study heterogeneity was quantified using I2 (25). Separate analyses were performed for HDL cholesterol, LDL cholesterol, triglycerides, and total cholesterol. Cholesterol concentrations normalized by logarithmic transformation were back-transformed by exponentiation. HDL cholesterol and LDL cholesterol concentrations reported in mmol/L were converted to mg/dL by dividing by 0.02586. Triglyceride concentrations reported in mmol/L were converted to mg/dL by dividing by 0.01129. If only subgroup-specific mean values were reported, we derived a pooled mean by taking the weighted arithmetic average.
In secondary analyses, we calculated stratified mean difference estimates from random-effects meta-analysis according to 1) country, 2) year of colonoscopy (midpoint), 3) sample size, 4) whether the mean values were adjusted or crude, and 5) whether the study had excluded persons with a personal history of colorectal polyps or colorectal cancer. The robustness of the combined mean difference was evaluated using a leave-1-out approach. The possibility of publication bias was assessed by visual inspection of funnel plots and a nonparametric test for asymmetry (26).
Statistical analyses of odds ratios
We ensured that all odds ratios were in the direction of increasing values of the cholesterol measurement. If odds ratios from multiple regression models were available, we considered only those estimated from the model that included the most adjustment variables. If only subgroup-specific odds ratios were available, we derived a pooled odds ratio using fixed-effects meta-analysis.
The approach of Greenland and Longnecker (27) was used to calculate estimates and standard errors for a linear trend in log odds ratios for measures of association originally reported in categories. For this analysis, we assigned a cholesterol dose as the midpoint of closed categories (weighted average if the cutoff varied for subgroups) and, for open-ended categories, 25 mg/dL beyond the cutpoint for LDL cholesterol, triglycerides, and total cholesterol and 15 mg/dL beyond the cutpoint for HDL cholesterol. The trend-estimated odds ratios were scaled to reflect a 10-mg/dL increase in HDL cholesterol, a 20-mg/dL increase in LDL cholesterol and total cholesterol, and a 40-mg/dL increase in triglycerides. For each of the 4 measurements, trend-estimated odds ratios were combined using random-effects meta-analysis with inverse variance weighting. The statistical significance of the pooled odds ratio was quantified with a z statistic and a P value from a Wald test. Similar to the analyses of mean differences, stratified estimates were also calculated for trend-estimated odds ratios, and both leave-1-out estimates and funnel plots were assessed (26).
We visualized the odds ratios as reported in the eligible studies by plotting categorical odds ratios for each blood lipid measurement against the assigned dose using circles proportional to the precision of the estimate (larger circles indicated more precision) and squares to denote the reference group. Continuous odds ratios were included by plotting a single circle at the mean value among controls. All analyses were performed using the meta package for R 3.1.2 (R Foundation for Statistical Computing; Vienna, Austria). All statistical tests were 2-sided, with P ≤ 0.05 being considered statistically significant.
RESULTS
Description of included studies
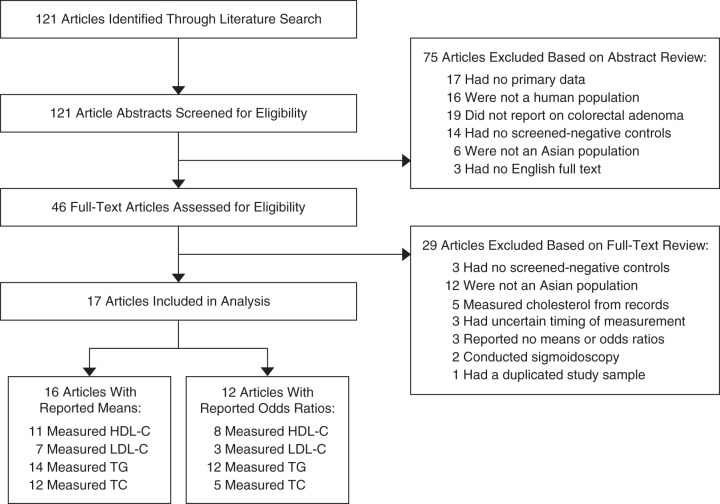
The literature search identified 121 potential articles (Figure 1). A total of 75 articles were determined to be ineligible based on abstract review. An additional 29 were determined to be ineligible after the full-text assessment. The 17 studies that met all inclusion criteria comprised 17,387 adenoma cases and 30,427 adenoma-free controls (Table 1) (28–44). All studies had been conducted in East Asia, including 8 studies from Japan, 7 from South Korea, and 1 each from China and Taiwan. No studies from Southeast Asia (e.g., Indonesia, Malaysia, Vietnam, Thailand) were identified.
Figure 1.
Selection of published (2000–2014) colonoscopy-based case-control studies from Asia for a systematic review and meta-analysis of blood lipid concentrations and colorectal adenoma. HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides.
Table 1.
Characteristics of 17 Colonoscopy-Based Case-Control Studies of the Association Between Blood Lipid Measurements and Adenoma Prevalence, Asia, 2000–2014
| First Author, Year (Reference No.) | Country | Year(s) of Colonoscopy | No. of Cases |
No. of Controls |
Blood Lipid Measurement(s) | % Men | Age Range,a years | Excluded Prior Polyps/CRCb | Reported Mean Values/ORs | Adjustmentc |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Values | ORs | ||||||||||
| Park, 2000 (28) | South Korea | 1997–1998 | 134 | 134 | HDL-C, LDL-C, TG, TC | 100 | NR | N/Y | Y/Y | Age, year, study site | Age, BMI, education, alcohol, family history |
| Shinomiya, 2001 (29) | Japan | 1995–1996 | 179 | 219 | HDL-C, LDL-C, TG, TC | 100 | 47–55 | Y/Y | Y/N | BMI, smoking, alcohol, physical activity, military rank, study site | NA |
| Fujimori, 2002 (30) | Japan | 1994–2001 | 817 | 350 | TG, TC | 100 | 40–80 | Y/N | Y/N | Age, alcohol | NA |
| Morita, 2005 (31) | Japan | 1995–2002 | 756 | 1,751 | HDL-C, TG | 100 | 44–59 | Y/Y | N/Y | NA | Age, study site, military rank |
| Chung, 2006 (32) | South Korea | 2002–2004 | 105d | 105 | HDL-C, LDL-C, TG, TC | 53 | 35–75 | N/Y | Y/Ye | Age, sex | Age, sex, BMI, glucose, TG, TC |
| Tabuchi, 2006 (33) | Japan | 1995–2003 | 3,821 | 958 | TG, TC | 61 | 10–94 | N/N | Y/Y | Unadjusted | Age, sex, TG, TC |
| Otani, 2006 (34) | Japan | 2004–2005 | 7820 | 738 | TG | 66 | 40–80 | Y/Y | Y/Y | Age, sex, year | Age, sex, year, BMI, smoking, alcohol, physical activity, family history, NSAIDs, fiber, folate, calcium, vitamin D, red meat |
| Lee, 2008 (35) | South Korea | 2005 | 689 | 1,209 | HDL-C, TG | 100 | 40–70 | Y/Y | Y/Y | Unadjusted | Age, BMI, education, income, smoking, alcohol, physical activity, medications, waist circumference |
| Kang, 2010 (36) | South Korea | 2006–2007 | 1,122 | 1,122 | HDL-C, TG, TC | 77 | 40–75 | Y/Y | Y/Ye | Age, sex | Age, sex, smoking, alcohol, family history, NSAIDs |
| Liu, 2010 (37) | China | 2006–2008 | 719 | 3,062 | HDL-C, TG, TC | 56 | >30 | Y/Y | Y/Ye | Unadjusted | Age, sex, smoking, alcohol |
| Sasaki, 2011 (38) | Japan | 2008 | 109 | 261 | HDL-C, LDL-C, TG | 74 | 48 | Yf/Y | Y/N | Unadjusted | NA |
| Sato, 2011 (39) | Japan | 2008–2010 | 261 | 702 | HDL-C, LDL-C, TG | 100 | 49 | Y/Y | Y/Ye | Unadjusted | Age, smoking, alcohol, family history |
| Choe, 2013 (40) | South Korea | 2004–2008 | 554 | 557 | TC | 67 | 59 | Y/Y | Y/N | Age, sex | NA |
| Yang, 2013 (41) | South Korea | 2006–2009 | 5,958 | 13,323 | HDL-C, LDL-C, TG, TC | 65 | 40–79 | N/Y | Y/Y | Unadjusted | Age, sex, BMI, smoking, alcohol, physical activity, family history, NSAIDs, previous polyp(s), waist circumference, diabetes, hypertension, heart disease, fiber, calories, fat, carbohydrates, protein, HDL-C, LDL-C, TG, TC |
| Huang, 2014 (42) | Taiwan | 2001–2009 | 684 | 5,074 | HDL-C, TG, TC | 58 | 50 | Y/Y | Y/Ye | Unadjusted | Age, sex, BMI, smoking, alcohol, physical activity, hypertension, TG, TC:HDL-C ratio |
| Hong, 2014 (43) | South Korea | 2011 | 246 | 494 | HDL-C, LDL-C, TG, TC | 58 | 30–75 | Y/Y | Y/Y | Unadjusted | Age, sex, BMI |
| Okuyama, 2014 (44) | Japan | 2001–2002 | 451 | 368 | TC | 50 | 35–80 | N/N | Y/N | Unadjusted | NA |
Abbreviations: BMI, body mass index; CRC, colorectal cancer; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NA, not applicable; NR, not reported; NSAID, nonsteroidal antiinflammatory drug; OR, odds ratio; TC, total cholesterol; TG, triglycerides.
a The mean age of controls is provided if age range was not reported in the article.
b Assumed to be “no” if not specifically reported.
c Including matching and/or statistical modeling.
d Advanced adenoma cases only.
e OR not reported for each lipoprotein fraction for which a mean was reported.
f Persons with a history of colorectal polyps were excluded only from the control group.
Overall, 16 of the 17 studies reported mean blood lipid concentrations for cases and controls (11 HDL cholesterol, 7 LDL cholesterol, 12 total cholesterol, and 14 triglycerides), and 12 of the 17 reported odds ratios (8 HDL cholesterol, 3 LDL cholesterol, 12 triglycerides, and 5 total cholesterol). Consistent with the expected prevalence of adenoma among average-risk individuals in unmatched studies, controls typically outnumbered adenoma cases 5-to-1. It was not always clear for some studies, however, how controls had been sampled (for example, in the study by Tabuchi et al. (33), over 80% of all participants were adenoma cases).
Potential duplication of data
There were 2 eligible publications from Sasaki et al. (38, 45) that included data from what appeared to be the same participants; we considered only data from the paper published first. An overlap in authorship was also noted in the studies by Sasaki et al. (38) and Sato et al. (39). Both studies enrolled colonoscopy patients from Tohoku Central Hospital in Japan, but Sasaki et al. (38) included examinations conducted between January and December of 2008, whereas Sato et al. (39) included examinations conducted between June 2008 and January 2010. Although we decided to retain both studies in the analysis, it was unclear whether data from the last half of 2008 were duplicative. Two authors from the study by Kang et al. (36) were also contributors to the study by Choe et al. (40). Both studies enrolled colonoscopy patients from Seoul National University Hospital in South Korea, but Kang et al. (36) included examinations conducted between January 2006 and December 2007, whereas Choe et al. (40) included examinations conducted between October 2004 and December 2008. However, despite the shorter recruitment window, the total sample size of the former study was more than twice that of the latter; thus, we decided that duplication was unlikely. Again, both studies were retained in the analysis.
Study-specific summarizations
Stratified results were reported in several studies. Tabuchi et al. (33) reported mean cholesterol levels for tubular, villous, and serrated adenomas separately, but because odds ratios were estimated only for tubular adenomas, we restricted attention to tubular adenomas. We used the weighted average mean cholesterol measurement among proximal and distal adenoma cases for Shinomiya et al. (29) and among nonadvanced and advanced adenoma cases for 3 studies (37, 41, 42). Because the magnitudes of the standard errors reported by Shinomiya et al. (29) were unrealistic, we assumed that what were reported as log-transformed standard errors had mistakenly been twice transformed. For Otani et al. (34), standard deviations were inferred from P values from 2-sample t tests, which were assumed to be calculated for equal variances.
Mean differences in blood lipid concentrations
Meta-analysis of mean differences suggested that adenoma cases generally had lower HDL cholesterol, higher LDL cholesterol, higher triglyceride, and higher total cholesterol concentrations (see Web Figures 1–4, available at http://aje.oxfordjournals.org/) at the time of colonoscopy than adenoma-free controls (Web Table 1). Only the associations for HDL cholesterol and triglycerides maintained statistical significance when we restricted attention to studies that reported adjusted (or matched) means or excluded those with previous polypectomy (Web Table 2). Substantial heterogeneity between studies (I2 >70%) was noted for triglycerides and total cholesterol. We did not find evidence of publication bias (Web Figure 5), and our conclusions were not altered with the exclusion of any particular study (Web Table 3).
Odds ratios for adenoma prevalence
Of the 12 studies that reported odds ratios, 11 used categories (7 binary, 2 ternary, and 2 quaternary), and Tabuchi et al. (33) used a continuously scaled odds ratio. The level of confounding control varied greatly among studies. Investigators in all 12 studies adjusted their estimates for, at minimum, age and sex (in studies that included both men and women), and 7 of the 12 studies adjusted for body mass index (Table 1).
The direction of associations based on combined odds ratios was the same as for the combined mean differences; however, only combined odds ratios for HDL cholesterol and triglycerides were statistically significant at the 0.05 level (Figures 2–5). Although odds ratios generally had less heterogeneity than mean differences, less precise odds ratios were of larger magnitude than more precise odds ratios (Web Figure 6). The magnitudes of associations tended to be larger in studies that excluded persons with previously detected colorectal polyps than in studies that did not (Web Table 4). There was no evidence of publication bias for these odds ratios (Web Figure 7), and estimates from leave-1-out analysis were similar (Web Table 5).
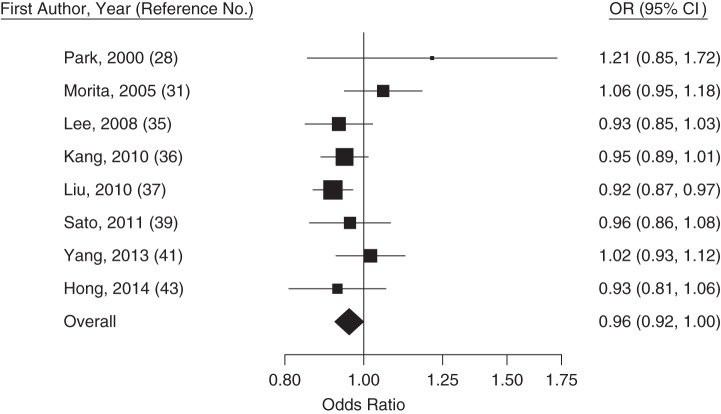
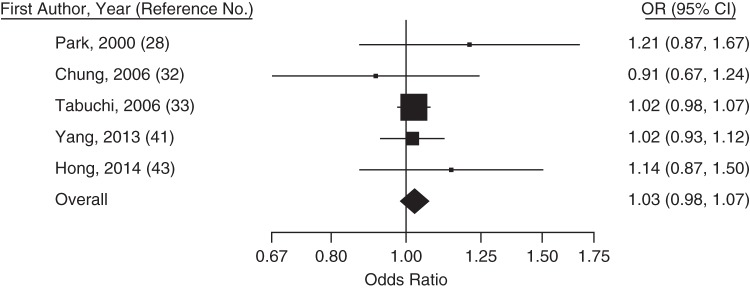
Figure 2.
Trend-estimated odds ratios (ORs) for colorectal adenoma for a 10-mg/dL increase in high-density lipoprotein cholesterol concentration, Asia, 2000–2014. Squares represent study-specific estimates and are proportional in size to the weight from random-effects meta-analysis (larger squares indicate more weight). For the overall estimate from random-effects meta-analysis, I2 = 25% (95% confidence interval (CI): 0, 67) and z = −2.94 (P = 0.04). Horizontal lines, 95% CIs.
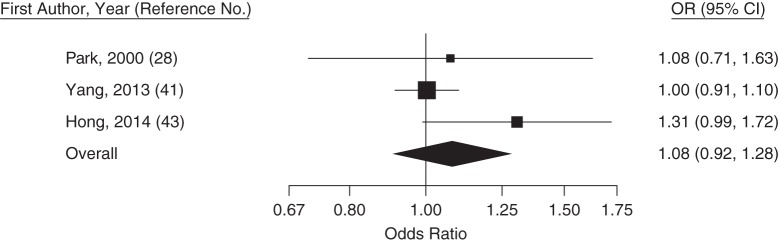
Figure 3.
Trend-estimated odds ratios (ORs) for colorectal adenoma for a 20-mg/dL increase in low-density lipoprotein cholesterol concentration, Asia, 2000–2014. Squares represent study-specific estimates and are proportional in size to the weight from random-effects meta-analysis (larger squares indicate more weight). For the overall estimate from random-effects meta-analysis, I2 = 0% (95% confidence interval (CI): 0, 80) and z = 0.91 (P = 0.36). Horizontal lines, 95% CIs.
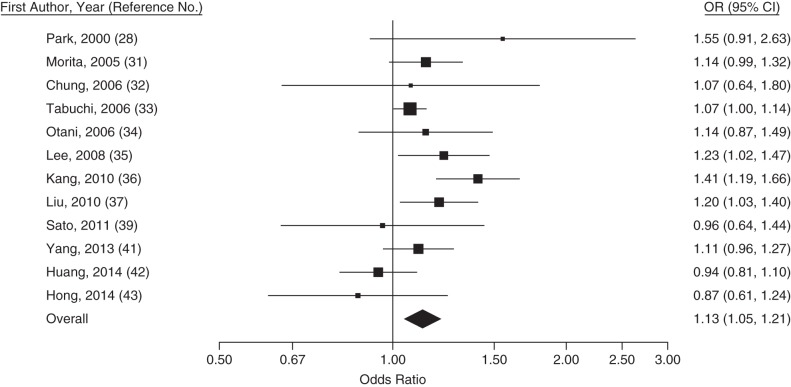
Figure 4.
Trend-estimated odds ratios (ORs) for colorectal adenoma for a 40-mg/dL increase in triglyceride concentration, Asia, 2000–2014. Squares represent study-specific estimates and are proportional in size to the weight from random-effects meta-analysis (larger squares indicate more weight). For the overall estimate from random-effects meta-analysis, I2 = 44% (95% confidence interval (CI): 0, 71) and z = 3.29 (P = 0.001). Horizontal lines, 95% CIs.
Figure 5.
Trend-estimated odds ratios (ORs) for colorectal adenoma for a 20-mg/dL increase in total cholesterol concentration, Asia, 2000–2014. Squares represent study-specific estimates and are proportional in size to the weight from random-effects meta-analysis (larger squares indicate more weight). For the overall estimate from random-effects meta-analysis, I2 = 0% (95% confidence interval (CI): 0, 61) and z = 1.20 (P = 0.23). Horizontal lines, 95% CIs.
DISCUSSION
This systematic review and meta-analysis showed that recent studies from East Asia have reported, on average, a 13% higher (95% confidence interval: 5, 21) prevalence of colorectal adenomas per 40-mg/dL increase in blood triglyceride levels at colonoscopy and a 4% lower (95% confidence interval: 0, 8) prevalence of colorectal adenomas per 10-mg/dL increase in HDL cholesterol. Colonoscopy recipients determined to have adenoma had, on average, similar blood concentrations of LDL cholesterol and total cholesterol as those without adenomas.
Whether dyslipidemia is associated with colorectal cancer risk has been debated for decades (46, 47). It is hypothesized that persons with unfavorable lipid profiles may be susceptible to chronic inflammation in the gut, possibly related to mechanisms involving bile acid malabsorption or butyrate suppression (48, 49), which can create a microenvironment that promotes DNA damage, cellular proliferation, and angiogenesis (50). High circulating cholesterol levels may also be related to increased exposure to insulin-like growth factors and estrogens, which can increase cellular proliferation (51, 52).
The majority of recent studies of cholesterol and colorectal adenomas have been conducted exclusively among East Asian populations. Of the 18 studies that failed to pass our abstract screening or full-text review because the study population was not Asian, only 5 studies published between 2000 and 2014 would have met all other eligibility criteria, including 1 study from the United States (53) and 4 from Europe (54–57). Seven of these 18 studies did not measure cholesterol, and the 6 studies that did (4 studies from the United States (17, 58–60), 1 from Europe (61), and 1 from the Middle East (62)) used medical records, used blood not drawn at the time of colonoscopy, or did not specify when the blood test had occurred.
Levels of lipoprotein fractions, most prominently HDL cholesterol and triglycerides (63), are correlated, making it difficult to determine which cholesterol component is driving associations with adenoma in these studies. Surprisingly, LDL cholesterol was the least often measured lipoprotein fraction in eligible studies, despite being the primary target of therapy (64, 65). None of the studies reported on the prevalence of use of medications such as statins, which probably reflects the fact that increasing cholesterol levels remain mostly medically uncontrolled in China (66), Japan (67), and South Korea (68). As a result, statin use is unlikely to have been a major confounder in the studies included in our meta-analysis.
The degree of control for potentially confounding variables varied greatly across included studies. Mean values were often reported for descriptive purposes and were usually unadjusted. In addition to mean differences, we summarized odds ratios, all of which were estimated from logistic regression and adjusted for at least age and sex. Many odds ratios had also been adjusted for body mass index, smoking status, and alcohol consumption, but only a few accounted for dietary exposures or other cardiovascular disease risk factors. We cannot be certain whether the observed consistency between unadjusted mean differences and adjusted odds ratios is indicative of a true association or of incomplete confounding control.
Given the inconsistent parameterization of odds ratios in eligible studies, trend estimation permitted a common scale upon which to combine adjusted estimates (27). Trend estimation, however, is not a remedy for dissimilarities that arise from biased exposure assessments. Because these calculations rely on adjusted log odds ratios but unadjusted counts, this approach involves assumptions that may not be valid when there is strong confounding. We made cholesterol dose definitions a priori, and altering the assigned dose symmetrically beyond the cutpoints for open-ended categories resulted in minimal changes (data not shown).
Selected subgroup analyses were performed, albeit with limited statistical power. We did not stratify our analyses by pathology or anatomical location, since few studies presented results according to adenoma characteristics. Although they were not formally tested, Liu et al. (37) and Huang et al. (42) reported similar-magnitude associations when comparing adenoma cases with controls and hyperplastic polyp cases with controls, whereas the LDL cholesterol and inverse HDL cholesterol associations reported by Yang et al. (41) were slightly more prominent when comparing advanced adenomas with controls than when comparing nonadvanced adenomas with controls. The latter was also the only study that provided estimates with and without adjustment for other lipoprotein fractions, noting minimal changes to odds ratios for total cholesterol, triglycerides, and LDL cholesterol but some differences for HDL cholesterol (a stronger inverse association for HDL cholesterol after adjustment for other fractions than not for nonadvanced adenomas, but a weaker association for HDL cholesterol after adjustment for other fractions than not for advanced adenomas). Lastly, the mean values reported in the single study that stratified by proximal and distal location did not differ meaningfully (stratified odds ratios were not included in this study) (29).
Our findings can be compared with those of a recent meta-analysis covering cohort and case-control studies of both colorectal adenomas and colorectal cancer conducted between 1990 and 2013, in which Tian et al. (6) reported associations between higher levels of LDL cholesterol, triglycerides, and total cholesterol and higher adenoma prevalence, but no association for HDL cholesterol. Unlike the study selection criteria of Tian et al., our criteria required 1) an Asian study population, 2) controls known to be without polyps, and 3) blood lipid evaluations made at the same time as the determination of adenoma diagnosis. In total, we included 10 studies not represented in the meta-analysis of Tian et al., and, unique to our analyses, we accounted for the fact that parameterizations of exposure differed by study.
Translating our results to non-Asian populations should be done with caution. Tian et al. noted differences in the magnitudes of associations and the degree of heterogeneity when comparing North American, European, and Asian studies (6). We aimed to limit the influence of secular trends by considering recent case-control studies from a region where confounding by statin use may be less prominent. Despite potential differences between studies from Eastern and Western countries, those studies included in our meta-analysis had average cholesterol ranges among controls that were not very different from nationally representative averages seen in the United States from 2007 to 2010 (69). Consistent with the findings from our meta-analysis, recent US studies that accounted for statin use identified higher triglyceride and lower HDL cholesterol levels to be the most strongly associated with adenoma prevalence, with little evidence of an association for LDL cholesterol and total cholesterol (70, 71).
In summary, based on evidence from recent colonoscopy studies conducted in Asia, high triglyceride and low HDL cholesterol concentrations were associated with higher prevalence of colorectal adenoma. Results from long-term longitudinal studies with repeated cholesterol measurements and routine colonoscopy screening are lacking, but our findings suggest that prevalent dyslipidemia may be a risk factor to consider when making clinical decisions regarding colorectal cancer screening and surveillance. These results suggest that future biological studies of adenomatous lesions in the colon and rectum may benefit from consideration of mechanisms for hypertriglyceridemia and impaired cholesterol efflux capacity (72).
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology and Biostatistics, School of Medicine, University of California, San Francisco, San Francisco, California (Michael N. Passarelli); Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington (Polly A. Newcomb); and Department of Epidemiology, School of Public Health, University of Washington, Seattle, Washington (Polly A. Newcomb).
This work was supported by grants (R03CA171014, R01CA097325, T32CA009168, K05CA152715, and R25CA112355) from the National Cancer Institute.
We thank Drs. Michael E. Rosenfeld, Carolyn M. Rutter, Stephen M. Schwartz, and Fredric M. Wolf for their guidance and comments on drafts of this article.
Conflict of interest: none declared.
REFERENCES
- 1.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;863:s836–s842. [DOI] [PubMed] [Google Scholar]
- 2.Kim JH, Lim YJ, Kim YH et al. Is metabolic syndrome a risk factor for colorectal adenoma? Cancer Epidemiol Biomarkers Prev. 2007;168:1543–1546. [DOI] [PubMed] [Google Scholar]
- 3.Potter JD. Colorectal cancer: molecules and populations. J Natl Cancer Inst. 1999;9111:916–932. [DOI] [PubMed] [Google Scholar]
- 4.Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;1386:2029–2043.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishihara R, Wu K, Lochhead P et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;36912:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian Y, Wang K, Li J et al. The association between serum lipids and colorectal neoplasm: a systemic review and meta-analysis. Public Health Nutr. 2015;1818:3355–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yee YK, Tan VP, Chan P et al. Epidemiology of colorectal cancer in Asia. J Gastroenterol Hepatol. 2009;2412:1810–1816. [DOI] [PubMed] [Google Scholar]
- 8.Farzadfar F, Finucane MM, Danaei G et al. National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3.0 million participants. Lancet. 2011;3779765:578–586. [DOI] [PubMed] [Google Scholar]
- 9.Pan WH, Yeh WT, Weng LC. Epidemiology of metabolic syndrome in Asia. Asia Pac J Clin Nutr. 2008;17(suppl 1):37–42. [PubMed] [Google Scholar]
- 10.Yusof AS, Isa ZM, Shah SA. Dietary patterns and risk of colorectal cancer: a systematic review of cohort studies (2000–2011). Asian Pac J Cancer Prev. 2012;139:4713–4717. [DOI] [PubMed] [Google Scholar]
- 11.Mannes GA, Maier A, Thieme C et al. Relation between the frequency of colorectal adenoma and the serum cholesterol level. N Engl J Med. 1986;31526:1634–1638. [DOI] [PubMed] [Google Scholar]
- 12.Bayerdörffer E, Mannes GA, Richter WO et al. Decreased high-density lipoprotein cholesterol and increased low-density cholesterol levels in patients with colorectal adenomas. Ann Intern Med. 1993;1187:481–487. [DOI] [PubMed] [Google Scholar]
- 13.Bayerdörffer E, Mannes GA, Ochsenkühn T et al. Increased risk of ‘high-risk’ colorectal adenomas in overweight men. Gastroenterology. 1993;1041:137–144. [DOI] [PubMed] [Google Scholar]
- 14.Demers RY, Neale AV, Demers P et al. Serum cholesterol and colorectal polyps. J Clin Epidemiol. 1988;411:9–13. [DOI] [PubMed] [Google Scholar]
- 15.Bird CL, Ingles SA, Frankl HD et al. Serum lipids and adenomas of the left colon and rectum. Cancer Epidemiol Biomarkers Prev. 1996;58:607–612. [PubMed] [Google Scholar]
- 16.Lochhead P, Chan AT. Statins and colorectal cancer. Clin Gastroenterol Hepatol. 2013;112:109–118; quiz e13–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddiqui A, Nazario HE, Patel M et al. Reduction in low-density lipoprotein cholesterol levels during statin therapy is associated with a reduced incidence of advanced colon polyps. Am J Med Sci. 2009;3385:378–381. [DOI] [PubMed] [Google Scholar]
- 18.Bertagnolli MM, Hsu M, Hawk ET et al. Statin use and colorectal adenoma risk: results from the adenoma prevention with celecoxib trial. Cancer Prev Res (Phila). 2010;35:588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broughton T, Sington J, Beales ILP. Statin use is associated with a reduced incidence of colorectal adenomatous polyps. Int J Colorectal Dis. 2013;284:469–476. [DOI] [PubMed] [Google Scholar]
- 20.Hata Y, Mabuchi H, Saito Y et al. Report of the Japan Atherosclerosis Society (JAS) guideline for diagnosis and treatment of hyperlipidemia in Japanese adults. J Atheroscler Thromb. 2002;91:1–27. [DOI] [PubMed] [Google Scholar]
- 21.Tan C-E, Ma S, Wai D et al. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care. 2004;275:1182–1186. [DOI] [PubMed] [Google Scholar]
- 22.Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;993:410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells GA, Shea B, O'Connell D et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, Ontario, Canada: Ottawa Hospital Research Institute; 2014. http://www.ohri.ca/programs/clinical_epidemiology/oxford.Asp. Accessed April 1, 2015. [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;73:177–188. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta-analyses. BMJ. 2003;3277414:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;504:1088–1101. [PubMed] [Google Scholar]
- 27.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;13511:1301–1309. [DOI] [PubMed] [Google Scholar]
- 28.Park SK, Joo JS, Kim DH et al. Association of serum lipids and glucose with the risk of colorectal adenomatous polyp in men: a case-control study in Korea. J Korean Med Sci. 2000;156:690–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinomiya S, Sasaki J, Kiyohara C et al. Apolipoprotein E genotype, serum lipids, and colorectal adenomas in Japanese men. Cancer Lett. 2001;1641:33–40. [DOI] [PubMed] [Google Scholar]
- 30.Fujimori S, Kishida T, Mitsui K et al. Influence of alcohol consumption on the association between serum lipids and colorectal adenomas. Scand J Gastroenterol. 2002;3711:1309–1312. [DOI] [PubMed] [Google Scholar]
- 31.Morita T, Tabata S, Mineshita M et al. The metabolic syndrome is associated with increased risk of colorectal adenoma development: the Self-Defense Forces Health Study. Asian Pac J Cancer Prev. 2005;64:485–489. [PubMed] [Google Scholar]
- 32.Chung YW, Han DS, Park YK et al. Association of obesity, serum glucose and lipids with the risk of advanced colorectal adenoma and cancer: a case-control study in Korea. Dig Liver Dis. 2006;389:668–672. [DOI] [PubMed] [Google Scholar]
- 33.Tabuchi M, Kitayama J, Nagawa H. Hypertriglyceridemia is positively correlated with the development of colorectal tubular adenoma in Japanese men. World J Gastroenterol. 2006;128:1261–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otani T, Iwasaki M, Ikeda S et al. Serum triglycerides and colorectal adenoma in a case-control study among cancer screening examinees (Japan). Cancer Causes Control. 2006;1710:1245–1252. [DOI] [PubMed] [Google Scholar]
- 35.Lee GE, Park HS, Yun KE et al. Association between BMI and metabolic syndrome and adenomatous colonic polyps in Korean men. Obesity (Silver Spring). 2008;166:1434–1439. [DOI] [PubMed] [Google Scholar]
- 36.Kang HW, Kim D, Kim HJ et al. Visceral obesity and insulin resistance as risk factors for colorectal adenoma: a cross-sectional, case-control study. Am J Gastroenterol. 2010;1051:178–187. [DOI] [PubMed] [Google Scholar]
- 37.Liu CS, Hsu HS, Li CI et al. Central obesity and atherogenic dyslipidemia in metabolic syndrome are associated with increased risk for colorectal adenoma in a Chinese population. BMC Gastroenterol. 2010;10:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki Y, Takeda H, Sato T et al. Increased levels of serum glucose-dependent insulinotropic polypeptide as a novel risk factor for human colorectal adenoma. Metabolism. 2011;609:1253–1258. [DOI] [PubMed] [Google Scholar]
- 39.Sato T, Takeda H, Sasaki Y et al. Increased homeostasis model assessment-insulin resistance is a risk factor for colorectal adenoma in Japanese males. Tohoku J Exp Med. 2011;2234:297–303. [DOI] [PubMed] [Google Scholar]
- 40.Choe EK, Kim D, Kim HJ et al. Association of visceral obesity and early colorectal neoplasia. World J Gastroenterol. 2013;1945:8349–8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang MH, Rampal S, Sung J et al. The association of serum lipids with colorectal adenomas. Am J Gastroenterol. 2013;1085:833–841. [DOI] [PubMed] [Google Scholar]
- 42.Huang HE, Yang YC, Wu JS et al. The relationship between different glycemic statuses and colon polyps in a Taiwanese population. J Gastroenterol. 2014;497:1145–1151. [DOI] [PubMed] [Google Scholar]
- 43.Hong SH, Cha JM, Lee JI et al. Association of hyper-LDL cholesterolemia with increased risk of colorectal adenoma. Hepatogastroenterology. 2014;61134:1588–1594. [PubMed] [Google Scholar]
- 44.Okuyama Y, Ozasa K, Oki K et al. Inverse associations between serum concentrations of zeaxanthin and other carotenoids and colorectal neoplasm in Japanese. Int J Clin Oncol. 2014;191:87–97. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki Y, Takeda H, Sato T et al. Serum interleukin-6, insulin, and HOMA-IR in male individuals with colorectal adenoma. Clin Cancer Res. 2012;182:392–399. [DOI] [PubMed] [Google Scholar]
- 46.Wynder EL, Reddy BS. Metabolic epidemiology of colorectal cancer. Cancer. 1974;34(Suppl 3):801–806. [DOI] [PubMed] [Google Scholar]
- 47.McKeown-Eyssen G. Epidemiology of colorectal cancer revisited: are serum triglycerides and/or plasma glucose associated with risk? Cancer Epidemiol Biomarkers Prev. 1994;38:687–695. [PubMed] [Google Scholar]
- 48.McMichael AJ, Potter JD. Host factors in carcinogenesis: certain bile-acid metabolic profiles that selectively increase the risk of proximal colon cancer. J Natl Cancer Inst. 1985;752:185–191. [PubMed] [Google Scholar]
- 49.Clausen MR, Bonnén H, Mortensen PB. Colonic fermentation of dietary fibre to short chain fatty acids in patients with adenomatous polyps and colonic cancer. Gut. 1991;328:923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;4206917:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;62:164–179. [DOI] [PubMed] [Google Scholar]
- 52.Potter JD. Hormones and colon cancer. J Natl Cancer Inst. 1995;8714:1039–1040. [DOI] [PubMed] [Google Scholar]
- 53.Ashbeck EL, Jacobs ET, Martínez ME et al. Components of metabolic syndrome and metachronous colorectal neoplasia. Cancer Epidemiol Biomarkers Prev. 2009;184:1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meance S, Boutron-Ruault MC, Myara A et al. Fecal primary bile acids and serum cholesterol are associated with colorectal adenomas. Dig Dis Sci. 2003;489:1751–1757. [DOI] [PubMed] [Google Scholar]
- 55.Misciagna G, De Michele G, Guerra V et al. Serum fructosamine and colorectal adenomas. Eur J Epidemiol. 2004;195:425–432. [DOI] [PubMed] [Google Scholar]
- 56.Boutron-Ruault MC, Marteau P, Lavergne-Slove A et al. Effects of a 3-mo consumption of short-chain fructo-oligosaccharides on parameters of colorectal carcinogenesis in patients with or without small or large colorectal adenomas. Nutr Cancer. 2005;532:160–168. [DOI] [PubMed] [Google Scholar]
- 57.Danese E, Minicozzi AM, Montagnana M et al. Lack of an association between circulating adiponectin levels and risk of colorectal adenoma. Clin Lab. 2013;59(1-2):211–214. [DOI] [PubMed] [Google Scholar]
- 58.Lieberman DA, Holub JL, Moravec MD et al. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA. 2008;30012:1417–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsilidis KK, Brancati FL, Pollak MN et al. Metabolic syndrome components and colorectal adenoma in the CLUE II cohort. Cancer Causes Control. 2010;211:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson JC, Rangasamy P, Rustagi T et al. Risk factors for sessile serrated adenomas. J Clin Gastroenterol. 2011;458:694–699. [DOI] [PubMed] [Google Scholar]
- 61.Pot GK, Geelen A, van Heijningen EM et al. Opposing associations of serum n-3 and n-6 polyunsaturated fatty acids with colorectal adenoma risk: an endoscopy-based case-control study. Int J Cancer. 2008;1238:1974–1977. [DOI] [PubMed] [Google Scholar]
- 62.Tal S, Melzer E, Chsherbakov T et al. Metabolic syndrome is associated with increased prevalence of advanced colorectal polyps. J Nutr Health Aging. 2014;181:22–25. [DOI] [PubMed] [Google Scholar]
- 63.Davis CE, Gordon D, LaRosa J et al. Correlations of plasma high-density lipoprotein cholesterol levels with other plasma lipid and lipoprotein concentrations. Circulation. 1980;624:IV24–IV30. [PubMed] [Google Scholar]
- 64.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;28519:2486–2497. [DOI] [PubMed] [Google Scholar]
- 65.Goff DC Jr, Lloyd-Jones DM, Bennett G et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S49–S73. [DOI] [PubMed] [Google Scholar]
- 66.Yang W, Xiao J, Yang Z et al. Serum lipids and lipoproteins in Chinese men and women. Circulation. 2012;12518:2212–2221. [DOI] [PubMed] [Google Scholar]
- 67.Sekikawa A, Miyamoto Y, Miura K et al. Continuous decline in mortality from coronary heart disease in Japan despite a continuous and marked rise in total cholesterol: Japanese experience after the Seven Countries Study. Int J Epidemiol. 2015;445:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee YH, Lee SG, Lee MH et al. Serum cholesterol concentration and prevalence, awareness, treatment, and control of high low-density lipoprotein cholesterol in the Korea National Health and Nutrition Examination Surveys 2008–2010: beyond the tip of the iceberg. J Am Heart Assoc. 2014;31:e000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carroll MD, Kit BK, Lacher DA et al. Trends in lipids and lipoproteins in US adults, 1988–2010. JAMA. 2012;30815:1545–1554. [DOI] [PubMed] [Google Scholar]
- 70.Passarelli MN, Newcomb PA, Makar KW et al. Blood lipids and colorectal polyps: testing an etiologic hypothesis using phenotypic measurements and Mendelian randomization. Cancer Causes Control. 2015;263:467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coppola JA, Shrubsole MJ, Cai Q et al. Plasma lipid levels and colorectal adenoma risk. Cancer Causes Control. 2015;264:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khera AV, Cuchel M, de la Llera-Moya M et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;3642:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.