Abstract
CC-486, the oral formulation of azacitidine (AZA), is an epigenetic modifier and DNA methyltransferase inhibitor in clinical development for treatment of hematologic malignancies. CC-486 administered for 7 days per 28-day treatment cycle was evaluated in a phase 1 dose-finding study. AZA has a short plasma half-life and DNA incorporation is S-phase-restricted; extending CC-486 exposure may increase the number of AZA-affected diseased target cells and maximize therapeutic effects. Patients with lower-risk myelodysplastic syndromes (MDS) received 300 mg CC-486 once daily for 14 days (n=28) or 21 days (n=27) of repeated 28-day cycles. Median patient age was 72 years (range 31–87) and 75% of patients had International Prognostic Scoring System Intermediate-1 risk MDS. Median number of CC-486 treatment cycles was 7 (range 2–24) for the 14-day dosing schedule and 6 (1–24) for the 21-day schedule. Overall response (complete or partial remission, red blood cell (RBC) or platelet transfusion independence (TI), or hematologic improvement) (International Working Group 2006) was attained by 36% of patients receiving 14-day dosing and 41% receiving 21-day dosing. RBC TI rates were similar with both dosing schedules (31% and 38%, respectively). CC-486 was generally well-tolerated. Extended dosing schedules of oral CC-486 may provide effective long-term treatment for patients with lower-risk MDS.
Introduction
Parenteral azacitidine (AZA) prolongs overall survival in patients with International Prognostic Scoring System (IPSS)1-defined higher-risk myelodysplastic syndromes (MDS) compared with conventional care regimens.2 In studies with patients with lower-risk MDS, subcutaneous (SC) AZA demonstrated promising rates of hematologic response and red blood cell (RBC) transfusion independence (TI).3, 4 An oral formulation of AZA in clinical development, CC-486, may provide a more convenient route of administration than SC injection and will eliminate injection-site reactions. In addition, because CC-486 is an oral dosage form, it could be easier to prolong administration to achieve and maintain hematologic response. Extended lower drug exposure may also improve tolerability by decreasing AZA-related exacerbations of existing cytopenias.
A two-part, multicenter phase 1/2 study was conducted to determine the biological activity, safety and efficacy of CC-486. Results of part 1, a dose-finding study in patients with MDS, acute myeloid leukemia (AML) or chronic myelomonocytic leukemia, have been reported.5 Part 1 showed once-daily CC-486 administered for the first 7 days of 28-day cycles was bioavailable, clinically active and well-tolerated and the maximally tolerated dose with the 7-day schedule was 480 mg/day. In part 2 of this study, extended CC-486 dosing was evaluated in a new cohort of patients with lower-risk MDS.
The rationale for extended CC-486 dosing schedules is based on the putative mechanism of AZA activity. AZA is a cytidine analog epigenetic modifier with a short plasma half-life,5 that is incorporated into DNA and RNA.6, 7, 8, 9 AZA is believed to exert its clinical efficacy through reduction of DNA hypermethylation and induction of cytotoxicity in abnormal hematopoietic cells.6, 10, 11, 12, 13, 14 Re-expression of aberrantly hypermethylated genes involved in normal cell cycle regulation, differentiation and apoptotic pathways may improve hematopoiesis and suppress the malignant clone.15, 16 Incorporation of AZA into DNA is S-phase-restricted.6, 12, 17 Once incorporated, AZA inactivates DNA methyltransferases.18, 19, 20, 21 DNA methylation reduction occurs during DNA replication in the absence of active DNA methyltransferases. Additional mechanisms of AZA activity may be mediated via incorporation into newly synthesized RNA, as well as having an effect on ribonucleotide reductase, thereby leading to a shift in nucleotide pools within cells.9, 22
Given its short plasma half-life and S-phase-restricted DNA incorporation, AZA exposure time could influence the number of diseased target cells acted upon.23 Extending CC-486 administration during the treatment cycle could increase the number of diseased progenitor cells exposed to AZA and maximize therapeutic effects. There is some evidence that extending AZA exposure with administration of lower dosages (<75 mg/m2/day) can enhance therapeutic effects.23 In a recent study, 10-day SC AZA administration at 50 mg/m2/day in patients with MDS or AML showed a slightly higher rate of hematologic response than that reported for the conventional 7-day 75 mg/m2/day SC schedule in MDS patients in the Cancer and Leukemia Group B 9221 trial.24, 25
Reported here are results from part 2 of the CC-486 study, in which patients received CC-486 300 mg once daily in extended treatment schedules (for 14 or 21 days of repeated 28-day cycles). This analysis was limited to the subgroup of patients in part 2 with lower-risk MDS to determine the safety and efficacy of CC-486 in this patient population, and to identify an effective dosing schedule for treatment of lower-risk MDS in future studies.
Study design and methods
This study is registered at ClinicalTrials.gov (NCT00528983). All procedures pertaining to study conduct, evaluation and documentation were in accordance with Good Clinical Practice, per the International Conference on Harmonization Guideline E6, and complied with ethical principles outlined in the Declaration of Helsinki. The study protocol was approved by relevant Institutional Review Boards or Independent Ethics Committees before commencement. All patients provided written, informed consent before participating. All authors had access to trial data. Statistical analyses were performed by Celgene Corporation.
Part 2 of this multicenter, open-label study began in 2009 and data cutoff for this analysis occurred in September, 2013. Eligible patients were aged ⩾18 years with Eastern Cooperative Oncology Group performance status 0–2 and IPSS-defined lower-risk MDS (Low or Intermediate (Int)-1) as diagnosed by the treating physician; and were RBC transfusion-dependent or had a hemoglobin (Hgb) level ⩽9 g/l, or were platelet transfusion-dependent or thrombocytopenic (platelet count ⩽50 × 109 g/l) within 56 days before screening. Patients must have had serum creatinine ⩽2.5 times the upper limit of normal, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ⩽2.5 times the upper limit of normal, and serum bilirubin levels ⩽1.5 times the upper limit of normal.
Exclusion criteria included non-MDS malignancy; use of anticancer or investigational treatments or incomplete recovery from toxicity from previous cancer treatments within 21 days before receiving study drug; and prior use of hypomethylating agents, including AZA.
Patients were sequentially assigned to receive CC-486 300 mg once daily for the first 14 or 21 days of repeated 28-day treatment cycles. After 6 cycles, patients not responding to study drug could discontinue, remain on-study or cross-over to receive SC AZA 75 mg/m2/day. All patients were followed for 28 days after the last CC-486 dose.
On days 1 through 14 or 21 of each 28-day cycle, patients took a 300 mg dose of CC-486 once daily. Antiemetic treatment with 5-HT3 serotonin-receptor antagonists 30 min before CC-486 dosing was recommended. Site visits occurred weekly during cycles 1 and 2, and on days 1, 14 and 28 in cycle 3 and beyond for clinical assessments, adverse event (AE) reporting, hematology (CBC and differential, platelets) and to return unused tablets and a medication diary card of CC-486 doses taken at home. A bone marrow aspirate sample was taken on day 21 of cycles 1 and 2, and on day 28 of cycle 3 and every three cycles thereafter.
Laboratory assessments were performed at the end of each treatment cycle. To continue to the next cycle and to be evaluable for neutrophil and/or platelet toxicity, patients must have had an absolute neutrophil count (ANC) >0.5 × 109/l and platelet count >25 × 109/l, without evidence of hypocellular marrow (<5% cellularity). Patients who were not evaluable for neutrophil and/or platelet toxicity because of very low neutrophil counts (ANC<0.5 × 109/l) and/or platelet counts (<25 × 109/l) at baseline were treated as scheduled, regardless of neutrophil and/or platelet count recovery. Treatment was discontinued if abnormal renal, hepatic and/or hematologic function persisted for >21 days.
Pharmacokinetic assessments
Cumulative AZA exposure and pharmacokinetic (PK) parameters in a subset of patients with MDS, chronic myelomonocytic leukemia or AML in part 2 of this study who received extended CC-486 300 mg once daily for 14 or 21 days/28-day cycle were compared with those of SC AZA in patients from part 1 of this study5 who had received a single cycle of SC AZA 75 mg/m2/day for 7 days. PK assessments were performed on day 1 and on the last dosing day (day 7 for SC AZA and day 14 or 21 for CC-486, according to the assigned schedule). Samples were collected up to 8 h after drug administration and analyzed using a validated high-performance liquid chromatography/tandem mass spectrometric method. Parameters were calculated using noncompartmental methods, including maximum observed plasma concentration (Cmax), time of maximum observed plasma concentration (Tmax), area under the plasma concentration-time curve (AUC∞), apparent total clearance (CL/F), relative oral bioavailability (F) and apparent volume of distribution (Vz/F).
Pharmacodynamic assessments
To assess the pharmacodynamic activity of CC-486 dosing regimens, DNA methylation levels in whole blood were measured in a subgroup of lower-risk MDS patients (based on sample availability and DNA yield). Whole blood samples were collected at screening (baseline) and before drug administration in cycle 1 on days (±1) 1, 15, 22 and 28 (cycle end) to assess methylation changes over the cycle. Distributions of global DNA methylation profiles were examined by kernel density plot using genomic methylation profiles for each sampling time averaged across patients in each dosing schedule. Genomic DNA was purified from whole blood samples using the PAXgene Blood DNA System (Qiagen; Valencia, CA, USA). Methylation profiling was performed using the Infinium HumanMethylation27 BeadArray (Illumina; San Diego, CA, USA).
Clinical efficacy and safety
As this is a phase 1/2 study, no long-term follow-up to evaluate disease evolution or survival was prospectively planned. Hematologic response was assessed using International Working Group (IWG) 2006 MDS criteria,26 with modifications. Overall response rate was calculated as the proportion of patients who achieved complete remission (CR); partial remission (PR); any hematologic improvement (HI) in the erythroid (HI-E), platelet (HI-P) or neutrophil (HI-N) lineages; or RBC or platelet TI. To be evaluated for RBC or platelet TI, patients must have been transfusion-dependent at baseline; that is, received ⩾4 units of packed RBCs or ⩾2 platelet transfusions in the 56 days before first CC-486 dose (IWG 2006 modification). RBC TI was defined as an Hgb increase of 1.5 g/dl from baseline and no RBC transfusions during any consecutive 56-day period on-treatment. Only RBC transfusions given for Hgb ⩽9.0 g/dl were counted in RBC transfusion response evaluations. Baseline RBC transfusion-dependent patients who achieved ⩾50% reduction in transfusion requirements for 56 consecutive days, and patients not RBC transfusion-dependent at baseline who achieved a 1.5 g/dl Hgb increase for 56 consecutive days, were considered to have achieved HI-E (IWG 2006 modification). Patients who achieved ⩾50% reduction in platelet transfusion requirement for 56 consecutive days, but not platelet TI, were considered to have achieved HI-P (IWG 2006 modification). Marrow CR was assessed but not included in overall response. The potential correlation between overall response (any treatment cycle) and changes in DNA methylation during cycle 1 was evaluated.
Safety and tolerability assessments were based on reported AEs, coded by Medical Dictionary for Regulatory Activities and graded by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) v3.
Statistical analysis
Demographic, efficacy and safety outcomes are reported descriptively. No formal comparisons were made between the 14-day and 21-day CC-486 dosing regimens. Univariate logistic regression analysis of relationships between baseline characteristics and hematologic response was performed with age, gender, WHO27 MDS classification, IPSS risk category, cytogenetic risk and baseline ANC, platelet and Hgb counts, as variables in the model.
Global DNA methylation scores were assigned to whole blood samples by calculating the percentage of highly methylated (beta ⩾0.7) loci. The 0.7 cutoff was chosen because the overall distribution of DNA methylation levels for loci on the array was bimodal, with a peak centered at approximately beta 0.1 and another centered at approximately 0.85; a 0.7 cutoff value has also been used in other studies.28 Statistical analyses were performed using R statistical software (R Foundation for Statistical Computing; Vienna, Austria; http://www.R-project.org).
Results
In total, 55 patients received CC-486 300 mg once daily for 14 (n=28) or 21 (n=27) days per 28-day cycle. Baseline characteristics were similar between dosing schedules (Table 1). Overall, median age was 72 years (range 31–87), with most patients (71%) aged 65 years or older. Most patients had IPSS Int-1 MDS (75%). Twenty-five patients (45%) had IPSS-R low/very low-risk MDS, 14 (25%) had intermediate-risk MDS and 15 (27%) had high-risk MDS. The most common WHO-defined MDS classifications were refractory anemia (RA), RA with ringed sideroblasts and refractory cytopenia with multilineage dysplasia/multilineage dysplasia with or without ringed sideroblasts. Nine patients had RAEB (RA with excess blasts)-1 (n=8) or RAEB-2 (n=1) disease. Median (range) hematology counts at baseline were Hgb 8.7 g/dl (6.0–13.0), ANC 1.6 × 109/l (0–30.3) and platelets 65.0 × 109/l (6.0–564.0). The patient with RAEB-2 disease, and a different patient with ANC 30.3 × 109/l (who most likely had chronic myelomonocytic leukemia), were included based on diagnosis of lower-risk MDS by the treating physician. At study entry, 38% of patients had received no prior MDS treatment (except transfusions), 47% had received erythropoiesis-stimulating agents and 16% had received granulocyte colony-stimulating factors.
Table 1. Patient demographic and disease characteristics at baseline.
| Characteristic | CC-486 300 mg Once daily 14 days/cycle (n=28) | CC-486 300 mg Once daily 21 days/cycle (n=27) |
|---|---|---|
| Age (years), median (range) | 72.5 (51–85) | 70.0 (31–87) |
| RBC transfusion-dependent,a n (%) | 16 (57) | 16 (59) |
| Platelet transfusion-dependent,b n (%) | 4 (14) | 2 (7) |
| Hematology, median (range) | ||
| Hgb (g/dl) | 8.6 (6.4–13.0) | 8.7 (6.0–11.6) |
| ANC (109/l) | 1.3 (0–21.5) | 1.8 (0.4–30.3) |
| Platelets (109/l) | 69.0 (6.0–564.0) | 56.0 (8.0–362.0) |
| WBC (109/l) | 3.0 (0.9–26.2) | 3.5 (0.9–42.1) |
| MDS WHO classification, n (%) | ||
| RA/RARSc | 9 (32) | 9 (33) |
| RCMD/RCMD-RSc | 10 (36) | 7 (26) |
| RAEB-1 | 4 (14) | 4 (15) |
| RAEB-2 | 0 | 1d (4) |
| MDS-U | 3 (11) | 3 (11) |
| Del(5q) | 1 (4) | 1 (4) |
| Missing | 1 (4) | 2 (7) |
| IPSS risk classification, n (%) | ||
| Low | 6 (21) | 8 (30) |
| Intermediate-1 | 22 (79) | 19 (70) |
| IPSS-R risk classification, n (%) | ||
| Low/very low | 11 (39) | 14 (52) |
| Intermediate | 7 (25) | 7 (26) |
| High | 9 (32) | 6 (22) |
| Unknown | 1 (4) | 0 |
| Cytogenetics, n (%) | ||
| Normal/diploid | 12 (43) | 15 (56) |
| ⩾1 abnormality | 9 (32) | 10 (37) |
| Indeterminate | 6 (21) | 2 (7) |
| Prior treatment, n (%) | ||
| Erythropoiesis-stimulating agents | 16 (57) | 10 (37) |
| Granulocyte colony-stimulating factors | 5 (18) | 4 (15) |
| Other | 8 (29) | 5 (19) |
| Nonee | 9 (32) | 12 (44) |
Abbreviations: ANC, absolute neutrophil count; Hgb, hemoglobin; IPSS, International Prognostic Scoring System; MDS, myelodysplastic syndrome; MDS-U, myelodysplastic syndrome-unclassified; RA, refractory anemia; RAEB, refractory anemia with excess blasts; RARS, refractory anemia with ringed sideroblasts; RBC, red blood cell; RCMD, refractory cytopenia with multilineage dysplasia; RCMD-RS, refractory cytopenia with multilineage dysplasia and ringed sideroblasts; WBC, white blood cell.
Defined as receipt of ⩾4 units of packed RBC within 56 days of the first dose of CC-486.
Defined as receipt of ⩾2 platelet transfusions within 56 days of the first dose of CC-486.
Because of the limited number of patients in the study, these classifications were grouped prospectively.
Assessed as lower-risk MDS by the treating physician on the case report form.
Other than transfusions.
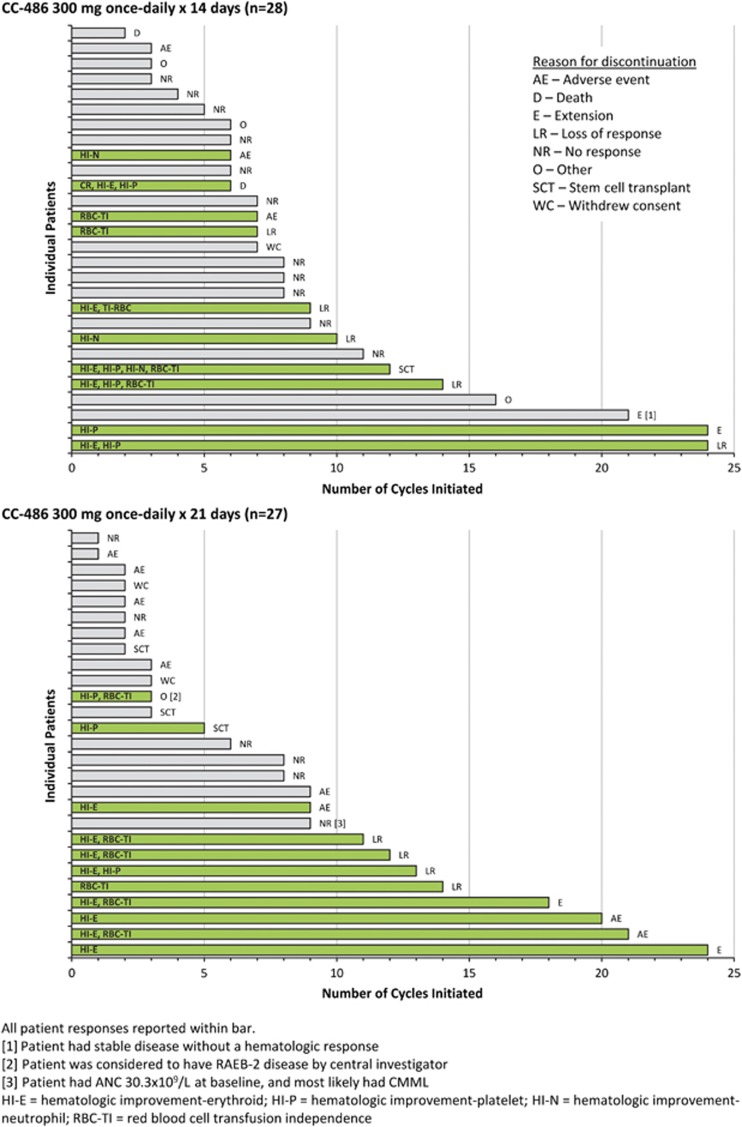
Median numbers of CC-486 cycles were 7 (range 2–24) and 6 (1–24) for the 14-day and 21-day schedules, respectively (Figure 1), and median cycle lengths were 31 (range 28–56) and 32 (18–64) days. Two patients in the 14-day and 5 in the 21-day schedule groups received reduced CC-486 doses (200 mg once daily) owing to cytopenias and/or gastrointestinal events. The most common reasons for discontinuations overall were no response (n=17), AEs (n=12) and loss of response (n=9) (Supplementary Table 1). Three patients died during the study: a 79-year-old male who received 6 CC-486 cycles for 14 days/cycle died due to septic shock leading to cardiac arrest; a 74-year-old female who received 2 CC-486 cycles for 14 days/cycle died owing to a systemic bacterial infection; and a 73-year-old male who received 10 CC-486 cycles for 14 days/cycle died from congestive heart failure 3 weeks after study discontinuation. No death was attributed to study drug.
Figure 1.
Duration of CC-486 treatment and response. Gray bars indicate no response and green bars indicate a response.
Four patients (one in the 14-day group and three in the 21-day group) proceeded to allogeneic hematopoietic stem cell transplant. Four patients continued to receive CC-486 on a compassionate-use basis at data cutoff (monitored for safety only).
Pharmacokinetics and pharmacodynamics
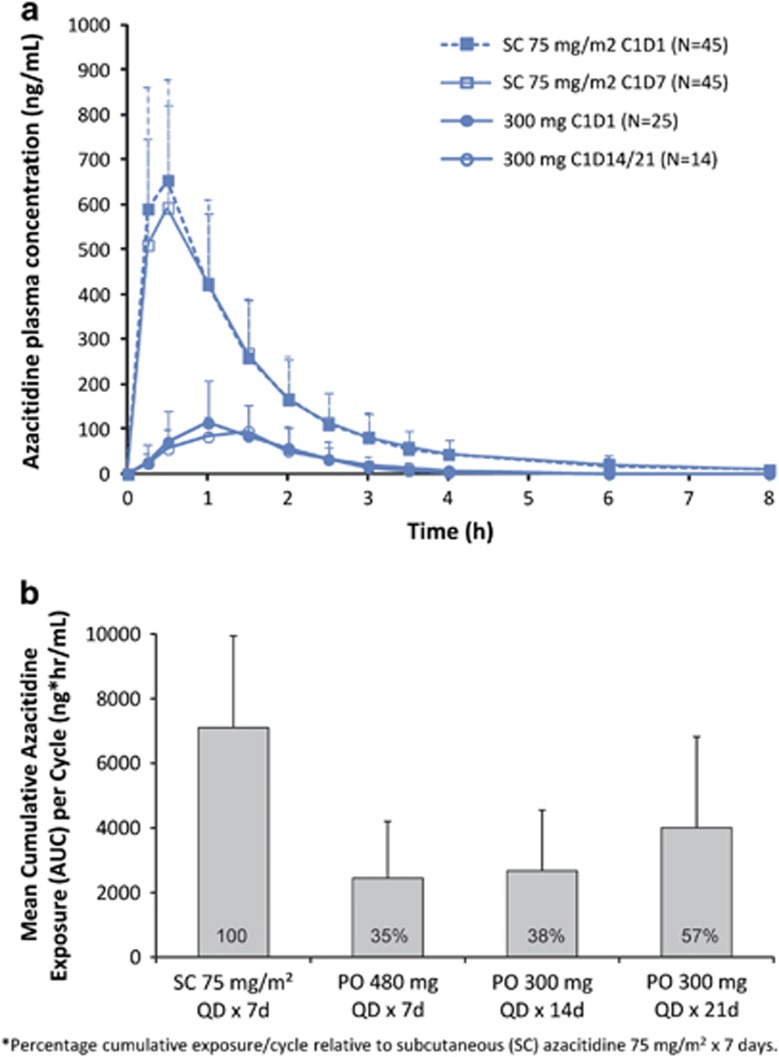
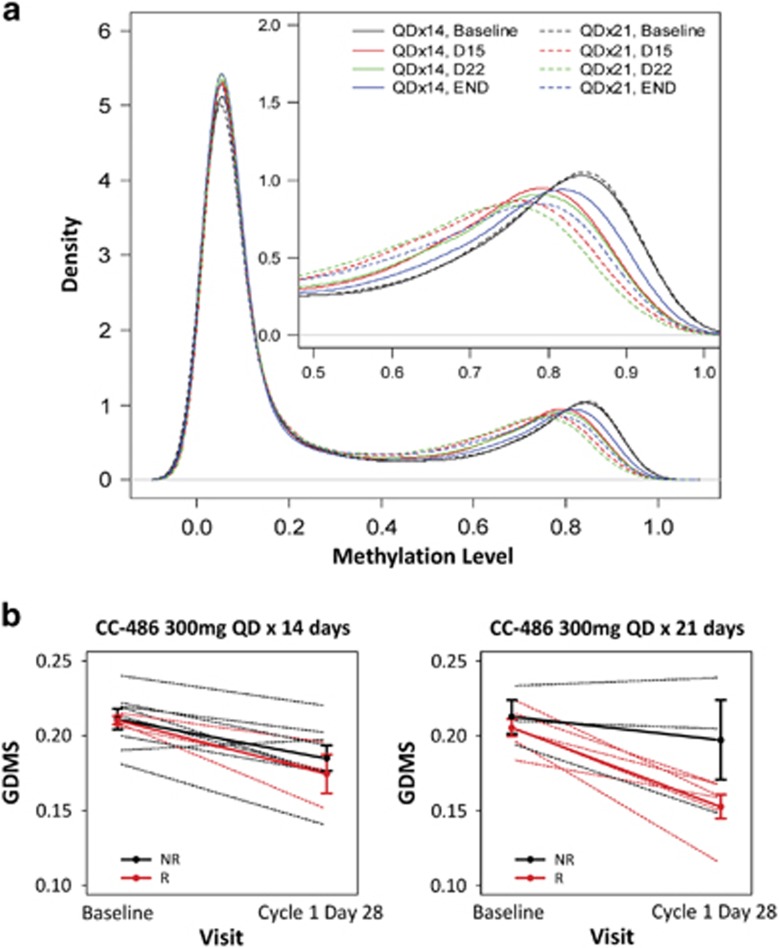
High interpatient variability was noted for all PK parameters (Supplementary Table 2). AZA was rapidly absorbed after SC and oral administration, reaching Cmax on day 1 within 0.5 h (range 0.2–1.1) and 1.0 h (0.47–2.00) post dose, respectively. As expected, AZA concentrations-versus-time profiles were similar in shape, with higher mean concentrations with SC dosing (Figure 2a). Cumulative AZA exposures per cycle with the CC-486 300 mg 14-day and 21-day schedules were 38% and 57%, respectively, of the cumulative exposure of SC AZA 75 mg/m2 administered for 7 days (Figure 2b). With both CC-486 schedules, global DNA methylation reductions from baseline were observed at days 15 and 22, and were sustained at cycle end (Figure 3a).
Figure 2.
(a) Mean (+s.d.) plasma concentration-vs-time profiles following SC azacitidine administration on days 1 and 7, and CC-486 300 mg once daily on days 1 and 14; and (b) Cumulative azacitidine exposure per cycle with extended CC-486 dosing regimens relative to azacitidine exposure with subcutaneous (SC) azacitidine 75 mg/m2 administered for 7 days. CC-486 480 mg/day was identified as the maximally tolerated dose (MTD).5
Figure 3.
(a) Kernel density plots of averaged genome-wide DNA methylation profiles across patients throughout the first treatment cycle. Shifts to the left from baseline (black line) represent overall reduction in methylation level. The x axis shows the percent of methylation on a locus and the y axis shows the density of loci at the different methylation levels; and (b) changes in global DNA methylation scores (GDMS) from baseline to day 28 (cycle end) in treatment cycle 1 vs clinical response. *Each dashed line represents one patient whereas the solid lines are the mean profiles of GDMS changes. The error bars represent one standard error away from the means. NR=no response; R=response. Response categories include overall response (complete remission (CR), any hematologic improvement (HI), RBC transfusion independence (TI) and platelet TI) and marrow CR (mCR). (*at any cycle of CC-486).
Efficacy
Treatment with CC-486 was associated with an overall response rate of 38% (95% confidence interval: 25%, 51%), with generally comparable efficacy between dosing regimens: 36% (95% confidence interval: 18%, 53%) with the 14-day schedule and 41% (95% confidence interval: 22%, 59%) with the 21-day schedule (Table 2). Three patients in the 21-day group achieved marrow CR. Mean (±s.d.) number of CC-486 treatment cycles to first hematologic response was 3.6 (±2.8). Of the 21 patients who had a response, 15 (71%) had a first response by treatment cycle 3, and approximately one-fourth (n=5, 24%) had a first response after receiving >6 CC-486 cycles. On average, patients who responded to CC-486 showed larger reductions in global methylation levels between baseline and cycle 1 end than non-responders (Figure 3b), with a trend for more pronounced methylation reduction in responding patients who received the 21-day CC-486 schedule. Approximately one-third of patients (11/32) who were RBC transfusion-dependent at baseline attained RBC TI. Median duration of RBC TI was 197 days (range 57–381). With the 14-day dosing schedule, 5 of 16 patients (31%) attained RBC TI, 2 of whom maintained RBC TI for ⩾84 days. With the 21-day dosing schedule, 6 patients (38%) attained RBC TI for 56 days and 5 of them (83%) maintained TI for ⩾84 days. Six patients (19%) experienced sustained RBC TI for ⩾6 months (two patients in the 14-day group and four in the 21-day group) and one patient had sustained RBC TI for ⩾12 months. Of the 11 patients who achieved RBC TI on-treatment, 5 (14-day n=2, 21-day n=3) had received erythropoiesis-stimulating agents prior to study entry. Six patients were platelet transfusion-dependent at baseline (14-day n=4; 21-day n=2); none achieved platelet TI on-study. Of patients who were not RBC transfusion-dependent at baseline, 8/12 patients (67%) on the 14-day schedule and 9/11 (82%) on the 21-day schedule remained TI on-study. Of patients who were platelet TI at baseline, 23/24 patients (96%) in the 14-day group and 21/25 (84%) in the 21-day group remained platelet TI.
Table 2. Hematologic response and transfusion independence.
| Parameter |
Treatment schedule n responders/N evaluable (%) |
||
|---|---|---|---|
| CC-486 300 mg once daily 14 days/cycle (n=28) | CC-486 300 mg once daily 21 days/cycle (n=27) | Total (N=55) | |
| Overall response (CR, PR, any HI, TI)a | 10/28 (36) | 11/27 (41) | 21/55 (38) |
| CRb | 1/7(14) | 0/5 | 1/12 (8.3) |
| PR | 0/5 | 0/3 | 0/7 |
| Any HI | 7/28 (25) | 10/27 (37) | 17/55 (31) |
| HI-E | 4/25 (16) | 8/25 (32) | 12/50 (24) |
| HI-P | 4/18 (22) | 3/15 (20) | 7/33 (21) |
| HI-N | 3/10 (30) | 0/6 | 3/16 (19) |
| Marrow CR | 0/7 | 3/5 (60) | 3/12 (25) |
| RBC TIc | |||
| Sustained for 56 days | 5/16 (31) | 6/16 (38) | 11/32 (34) |
| Sustained for 84 days | 2/16 (13) | 5/16 (31) | 7/32 (22) |
| Platelet TId | 0/4 | 0/2 | 0/6 |
Abbreviations: CR=complete remission; HI=hematologic improvement; HI-E=hematologic improvement-erythroid; HI-P=hematologic improvement-platelet; HI-N=hematologic improvement-neutrophil; IWG=International Working Group; PR=partial remission; TI=transfusion independence. IWG 2006 criteria.28
Patients are counted only once for overall response, but may be counted more than once in individual response categories. Marrow CR was not included in overall response. Lower-risk patients with <5% bone marrow blasts at baseline were not evaluable for CR or PR.
Subjects who had a CR are not counted for PR, any HI or marrow CR.
To be evaluated for RBC TI, patients must have been RBC transfusion-dependent at baseline and been on-study at least 56 days. RBC transfusion dependence at baseline was defined as receipt of ⩾4 units of packed RBC within 56 days of the first dose of CC-486. To be evaluated for platelet TI, patients must have been platelet transfusion-dependent at baseline and been on-study at least 56 days. Platelet transfusion dependence at baseline was defined as receipt of ⩾2 platelet transfusions within 56 days of the first dose of CC-486.
To be evaluated for platelet TI, patients must have been platelet transfusion-dependent at baseline and been on-study at least 56 days. Platelet transfusion dependence at baseline was defined as receipt of ⩾2 platelet transfusions within 56 days of the first dose of CC-486.
Of 12 evaluable patients, 1 patient (8%) in the 14-day schedule group achieved CR. Across treatment groups, 17 patients (31%) achieved any HI as best response (Figure 1, Table 2). Median duration of any response was 270 days with the 14-day schedule and 467 days with the 21-day schedule. Baseline demographic and disease characteristics based on treatment response are shown in Supplementary Table 3. In univariate analysis, only baseline platelet count was a significant predictor of response (P=0.0401): patients with higher platelet counts at baseline were more likely to respond to treatment. Hematologic response rate by IPSS-R risk category was 44% in patients with low/very low-risk, 36% in patients with intermediate-risk and 33% in patients with high-risk disease (Supplementary Table 4). Rates of RBC TI in patients with IPSS-R-defined low/very low-, intermediate-and high-risk disease were 54%, 30% and 13%, respectively.
Tolerability and safety
CC-486 was generally well-tolerated. Twelve patients (22%) discontinued owing to an AE, three from the 14-day group (n=1 each of angioedema, syncope and lower GI hemorrhage with associated thrombocytopenia) and nine from the 21-day group (thrombocytopenia n=3; and n=1 each of diarrhea, interstitial lung disease, progression to AML, cellulitis, nausea and pancytopenia). The most common AEs were gastrointestinal (Table 3). Frequency of gastrointestinal events was similar between dosing schedules, but fewer infections occurred in patients receiving the CC-486 21-day schedule.
Table 3. Adverse eventsa of interest.
| CC-486 300 mg once daily 14 days/cycle (n=28) | CC-486 300 mg once daily 21 days/cycle (n=27) | |
|---|---|---|
| Adverse events (any grade) | ||
| Gastrointestinal disorders, n (%) | ||
| All | 28 (100) | 27 (100) |
| Diarrhea | 22 (79) | 20 (74) |
| Nausea | 17 (61) | 14 (52) |
| Vomiting | 13 (46) | 16 (59) |
| Infections, n (%) | ||
| All | 18 (64) | 12 (44) |
| Pneumonia | 4 (14) | 1 (4) |
| Cellulitis | 8 (29) | 1 (4) |
| Grade 3–4 adverse events of interest | ||
| All | 19 (68) | 19 (70) |
| Hematologic adverse events, n (%) | ||
| Neutropenia | 2 (7) | 7 (26) |
| Febrile neutropenia | 1 (4) | 3 (11) |
| Thrombocytopenia | 3 (11) | 4 (15) |
| Anemia | 4 (14) | 4 (15) |
| Non-hematologic adverse events, n (%) | ||
| Diarrhea | 2 (7) | 4 (15) |
| Vomiting | 2 (7) | 2 (7) |
| Pneumonia | 4 (14) | 1 (4) |
| Cellulitis | 3 (11) | 1 (4) |
Patient exposures ranged from 1 to 24 CC-486 treatment cycles.
Adverse events graded by NCI-CTCAE v3.0.
Grade 3–4 AEs were reported in 12 patients (43%) in the 14-day dosing group and 13 patients (48%) in the 21-day group. The most frequent grade 3–4 non-hematologic AEs were pneumonia in the 14-day group (n=4 (14%)) and diarrhea in the 21-day group (n=4 (15%)). The most frequent (⩾5% of patients) grade 3–4 hematologic AEs were anemia in the 14-day dosing group (n=4 (14%)) and neutropenia in the 21-day group (n=7 (26%)). Grade 3–4 neutropenia was reported more frequently in the 21-day dosing group (26%) than in the 14-day group (Table 3). Grade 3–4 hematologic AEs decreased in frequency after the first 2 CC-486 treatment cycles in both dosing groups (Supplementary Table 5).
Of seven patients with ANC <0.5 × 109/l at baseline, most (n=5, 71%) recovered to ANC >0.5 × 109/l within two treatment cycles (Table 4); however, ANC recovery was not necessarily sustained over subsequent cycles. Twenty-eight of 48 patients (58%) with baseline ANC ⩾0.5 × 109/l had an ANC drop to <0.5 × 109/l on-study, 25 (89%) of whom then recovered during treatment to >0.5 × 109/l, with median time-to-recovery within the first treatment cycle. Platelet levels over the first three treatment cycles were generally stable. Of five patients with baseline platelets <20 × 109/l, platelet counts increased to ⩾20 × 109/l in 3 (60%), with median time-to-recovery within two cycles (Table 4). Similarly, of 50 patients with baseline platelet counts ⩾20 × 109/l, platelet counts dropped to <20 × 109/l in 19 patients (38%) but recovered under continued treatment in 17 of those patients (89%) with median time-to-recovery in the same CC-486 treatment cycle.
Table 4. ANC and platelet count changes during CC-486 treatment.
| CC-486 300 mg once daily: 14 days/cycle (n=28) | CC-486 300 mg once daily: 21 days/cycle (n=27) | |
|---|---|---|
| Neutrophils | ||
| ANC <0.5 × 109/l at baseline, n (%) | 4 (14) | 3 (11) |
| Recovered to ANC⩾0.5 × 109/l on study, n (%) | 2/4 (50) | 3/3 (100) |
| Study day of recovery, median (range) | 30.5 (8–53) | 15 (8–16) |
| ANC ⩾0.5 × 109/l at baseline, n (%) | 24 (86) | 24 (89) |
| ANC decrease to <0.5 × 109/l on study, n (%) | 13/24 (54) | 15/24 (63) |
| Recovered to ANC ⩾0.5 × 109/l on study, n (%) | 12/13 (92) | 13/15 (87) |
| Time to recoverya (days), median (range) | 14 (5–73) | 20 (6–37) |
| Platelets | ||
| Plt count <20 × 109/l at baseline, n (%) | 4 (14) | 1 (4) |
| Recovered to Plt count ⩾20 × 109/l on study, n (%) | 2/4 (50) | 1/1 (100) |
| Study day of recovery, median (range) | 8 (8–8) | 48 |
| Plt count ⩾20 × 109/l at baseline, n (%) | 24 (86) | 26 (96) |
| Plt count decrease to <20 × 109/l on study, n (%) | 7/24 (29) | 12/26 (46) |
| Recovered to Plt count ⩾20 × 109/l on study, n (%) | 7/7 (100) | 10/12 (83) |
| Time to recoverya (days), median (range) | 8 (1–21) | 13 (2–85) |
Abbreviations: ANC=absolute neutrophil count; Plt=platelet.
From the first recorded decrease below ANC or platelet threshold to first recorded increase above the respective threshold.
Discussion
Approximately three-fourths of all newly diagnosed patients with MDS have lower-risk disease.29 Reducing disease-related complications, improving cytopenias and decreasing transfusion requirements are essential treatment goals in this population.30, 31 Accordingly, 38% of patients with lower-risk MDS in this study attained a hematologic response with once daily 300 mg oral CC-486, administered over extended dosing schedules of 14 or 21 days per 28-day treatment cycle.
The natural history of lower-risk MDS can vary considerably and there is growing awareness that a patient subgroup with poorer prognosis exists within this patient population. A prognostic scoring system was developed to address disease heterogeneity in lower-risk MDS to aid therapeutic decision making.31, 32 This tool assigns points for specific risk factors, with higher scores indicating poorer prognosis. Using this validated scoring system, patients in the current study would have an intermediate prognostic score because of older age (>60 years), and low Hgb and/or platelet counts at study entry. Approximately one-quarter (27%) of the patients in this study were considered high-risk according to IPSS-R score. This phase 1/2 study did not evaluate overall survival; therefore, no definitive statement can be made regarding this outcome for study participants. Nevertheless, the expected median overall survival of these patients using the new prognostic scoring system ranges from ~1.8 to 3 years compared with 3.5–5.7 years expected survival for all IPSS lower-risk MDS patients,1 suggesting that these patients comprise a lower-risk MDS subgroup with poorer prognoses. An ~40% response rate with 300 mg once daily CC-486 is promising in this poorer prognosis group.
Lower doses of SC AZA administered over longer periods can be more effective than high doses administered less frequently.23 Despite lower cumulative AZA exposures with the extended CC-486 schedules (38–57% per cycle of cumulative exposure with SC AZA 75 mg/m2/day × 7 days), response rates with the oral regimens were generally comparable to those reported in a study by Musto et al.4 in lower-risk MDS patients treated with SC AZA 75 mg/m2 or a 100-mg fixed dose × 7 days (46%) when marrow CR is included in overall response in this study (44%), as was done in the Musto study.4 However, median duration of any response in the current study was at minimum 50% longer than that reported by Musto: 9 months with the 14-day CC-486 schedule and 15.6 months with the 21-day schedule, compared with 6 months in the earlier study. Only 1 patient attained CR in this study; however, only 12 patients had a bone marrow blast count high enough to be eligible to attain CR.
Extending CC-486 dosing to 14 or 21 days sustains methylation reductions over the entire treatment cycle, whereas, with 7-day CC-486 dosing global methylation reductions are greatest at approximately day 15 of the treatment cycle, after which global methylation levels rise to near-baseline levels by cycle end.5, 33 In this study, reduced global DNA methylation levels were associated with a higher rate of hematologic response to CC-486; however, further investigation is needed to confirm the relationship between demethylation and response. Differences in patient populations, receipt of SC AZA treatment and different CC-486 dosing between part 1 and part 2 of this study prevent efficacy comparisons between 7-day CC-486 administration and extended dosing schedules. In the univariate logistic regression analysis, only baseline platelet count was predictive of attaining a response (higher platelet count increased the probability of any response). Baseline platelet count was not an independent variable, as it was a criterion for an HI-P response; however, despite increasing the chance of attaining an HI-P, thrombocytopenia at baseline was significantly associated with no hematologic response, consistent with evidence that thrombocytopenia in lower-risk MDS is predictive of poorer survival31, 32 and increased risk of evolution to AML.34 No platelet transfusion-dependent patient (n=6) achieved platelet TI, although 21% of thrombocytopenic patients eligible for a platelet response achieved HI-P (per IWG 2006 criteria26 or a ⩾50% reduction in baseline transfusion requirements sustained for 56 days). Platelet count reductions during treatment were transient; for 89% of patients whose platelet counts dropped to <20 × 109/l on study, recovery occurred during the same treatment cycle in which platelets fell.
Extended CC-486 dosing regimens were generally well-tolerated, with no unexpected safety outcomes. Temporary exacerbation of cytopenias is a known feature of many MDS treatments, including AZA.35 In this study, rates of grade 3–4 anemia and thrombocytopenia with extended CC-486 dosing schedules (15% and 13%, respectively) were comparable to rates previously observed with parenteral AZA (16% and 18%3) and parenteral decitabine (15% each36) in similar patient populations. However, rate of grade 3–4 neutropenia with CC-486 (16%) was notably lower than that reported for SC AZA and decitabine (32% and 29%, respectively) in those studies.3, 36 The lower incidence of neutropenia may reflect lower AZA Cmax attained with CC-486. As observed with parenteral AZA,35, 37 the incidence of grade 3–4 hematologic AEs declined as treatment continued.
The most frequent non-hematologic AEs with CC-486 were gastrointestinal and were manageable. Similar rates of gastrointestinal events were reported in part 1 of this study with the 7-day CC-486 dosing schedule,5 suggesting these effects occur soon after drug initiation, and extending the dosing schedule generally does not worsen them. Adverse gastrointestinal events accompany most anticancer agents. Up to 55% of cancer patients use acid-reducing drugs, though increasing gastric pH can significantly alter the PK of some anticancer drugs.38 Modulation of gastric pH with the proton-pump inhibitor, omeprazole, during CC-486 administration had no effect on AZA absorption.39
Most cancer patients prefer oral over injectable drug formulations.40 Oral anticancer agents can improve patient quality of life by making therapy more convenient and eliminating complications of parenteral therapy, such as injection-site reactions, thrombosis, thrombophlebitis and bloodstream infections related to catheterization with a central line. In addition, oral AZA should reduce the need for repeated clinic visits for drug administration. A concern with oral formulations is the effect of food on drug absorption. Food restrictions can complicate dosing of anticancer drugs and increase the likelihood of non-adherence, especially when therapy is chronic.41, 42 CC-486 is rapidly absorbed and can be taken with or without food,39 which can benefit older patients (the largest population with MDS43, 44) who are more likely to have comorbidities that require concomitant medications and for whom restricted dosing could be a challenge. Compared with parenteral administration, oral AZA dosing will allow clinicians more flexibility to alter treatment schedules if tolerability is a problem.
Once-daily CC-486 administered in extended dosing schedules was generally well-tolerated and effective in these patients with lower-risk MDS and poor prognostic features. Flexible dosing schedules may allow optimization of CC-486 treatment to enhance patient response and improve tolerability. It remains unknown whether extended CC-486 dosing will delay transformation to AML or improve overall survival. However, based on these data, further investigation of 300 mg CC-486 administered once daily for 21 days per cycle in patients with lower-risk MDS is underway in a large, randomized, controlled phase 3 trial (ClinicalTrials.gov NCT01566695) that does evaluate these outcomes.
Acknowledgments
This study was supported by Celgene Corporation, Summit, NJ. The authors received editorial support in the preparation of this manuscript from Neil Malone of Celgene Corporation, Summit, NJ and Brian Kaiser and Sheila Truten of MC2 Inc., Wynnewood, PA, who were funded by Celgene Corporation. The authors were fully responsible for all content and editorial decisions for this paper.
Author contributions
GG-M, SDG, SK, B Scott, AT, CRC, WJE, KK, EL, TS, KJM and B Skikne designed the study and collected and analyzed data. JH analyzed the data and provided statistical support. All authors participated in manuscript development and revision. CRC contributed to study design, recruited study participants, clinically managed study participants, collected data and edited the manuscript. The primary author (GG-M) is responsible for manuscript content and approval to submit the manuscript.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
GG-M has received research support from Celgene Corporation, and is a consultant for Celgene; SDG receives research support from and consults for the Celgene; SK declares no relevant conflicts of interest; B Scott has received research support from Celgene, and is a consultant and speaker for Celgene; AT declares no relevant conflicts of interest; CRC serves on the Scientific Advisory Board for the Connect MDS/AML Disease Registry study, which is sponsored by Celgene; WJE declares no relevant conflicts of interest; JH, KK, EL, TS, KJM and B Skikne are employees of, and own stock in, Celgene Corporation, Summit, NJ, USA.
Supplementary Material
References
- Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997; 89: 2079–2088. [PubMed] [Google Scholar]
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009; 10: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons RM, Cosgriff TM, Modi SS, Gersh RH, Hainsworth JD, Cohn AL et al. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol 2009; 27: 1850–1856. [DOI] [PubMed] [Google Scholar]
- Musto P, Maurillo L, Spagnoli A, Gozzini A, Rivellini F, Lunghi M et al. Azacitidine for the treatment of lower risk myelodysplastic syndromes: a retrospective study of 74 patients enrolled in an Italian named patient program. Cancer 2010; 116: 1485–1494. [DOI] [PubMed] [Google Scholar]
- Garcia-Manero G, Gore SD, Cogle C, Ward R, Shi T, Macbeth KJ et al. Phase I study of oral azacitidine in myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J Clin Oncol 2011; 29: 2521–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LH, Olin EJ, Buskirk HH, Reineke LM. Cytotoxicity and mode of action of 5-azacytidine on L1210 leukemia. Cancer Res 1970; 30: 2760–2769. [PubMed] [Google Scholar]
- Glover AB, Leyland-Jones B. Biochemistry of azacitidine: a review. Cancer Treat Rep 1987; 71: 959–964. [PubMed] [Google Scholar]
- Stresemann C, Bokelmann I, Mahlknecht U, Lyko F. Azacytidine causes complex DNA methylation responses in myeloid leukemia. Mol Cancer Ther 2008; 7: 2998–3005. [DOI] [PubMed] [Google Scholar]
- Hollenbach PW, Nguyen AN, Brady H, Williams M, Ning Y, Richard N et al. A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines. Plos One 2010; 5: e9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann PG, Behrens M, Abraham D. Metabolism and cytotoxicity of 5-azacytidine in cultured Novikoff rat hepatoma and P388 mouse leukemia cells and their enhancement by preincubation with pyrazofurin. Cancer Res 1978; 38: 2458–2466. [PubMed] [Google Scholar]
- Juttermann R, Li E, Jaenisch R. Toxicity of 5-aza-2'-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc Natl Acad Sci USA 1994; 91: 11797–11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LH, Olin EJ, Fraser TJ, Bhuyan BK. Phase specificity of 5-azacytidine against mammalian cells in tissue culture. Cancer Res 1970; 30: 2770–2775. [PubMed] [Google Scholar]
- Jones PA, Taylor SM, Wilson V. DNA modification, differentiation, and transformation. J Exp Zool 1983; 228: 287–295. [DOI] [PubMed] [Google Scholar]
- Leone G, Teofili L, Voso MT, Lubbert M. DNA methylation and demethylating drugs in myelodysplastic syndromes and secondary leukemias. Haematologica 2002; 87: 1324–1341. [PubMed] [Google Scholar]
- Stresemann C, Brueckner B, Musch T, Stopper H, Lyko F. Functional diversity of DNA methyltransferase inhibitors in human cancer cell lines. Cancer Res 2006; 66: 2794–2800. [DOI] [PubMed] [Google Scholar]
- Flotho C, Claus R, Batz C, Schneider M, Sandrock I, Ihde S et al. The DNA methyltransferase inhibitors azacitidine, decitabine and zebularine exert differential effects on cancer gene expression in acute myeloid leukemia cells. Leukemia 2009; 23: 1019–1028. [DOI] [PubMed] [Google Scholar]
- Momparler RL, Goodman J. In vitro cytotoxic and biochemical effects of 5-aza-2'-deoxycytidine. Cancer Res 1977; 37: 1636–1639. [PubMed] [Google Scholar]
- Ghoshal K, Datta J, Majumder S, Bai S, Kutay H, Motiwala T et al. 5-Aza-deoxycytidine induces selective degradation of DNA methyltransferase 1 by a proteasomal pathway that requires the KEN box, bromo-adjacent homology domain, and nuclear localization signal. Mol Cell Biol 2005; 25: 4727–4741. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Santi DV, Garrett CE, Barr PJ. On the mechanism of inhibition of DNA-cytosine methyltransferases by cytosine analogs. Cell 1983; 33: 9–10. [DOI] [PubMed] [Google Scholar]
- Friedman S, Cheong LC. Effect of 5-azacytidine on deoxyribonucleic acid methylation in Escherichia coli K12. Biochem Pharmacol 1984; 33: 2675–2679. [DOI] [PubMed] [Google Scholar]
- Jones PA. Effects of 5-azacytidine and its 2'-deoxyderivative on cell differentiation and DNA methylation. Pharmacol Ther 1985; 28: 17–27. [DOI] [PubMed] [Google Scholar]
- Aimiuwu J, Wang H, Chen P, Xie Z, Wang J, Liu S et al. RNA-dependent inhibition of ribonucleotide reductase is a major pathway for 5-azacytidine activity in acute myeloid leukemia. Blood 2012; 119: 5229–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunthararajah Y. Key clinical observations after 5-azacytidine and decitabine treatment of myelodysplastic syndromes suggest practical solutions for better outcomes. Hematology Am Soc Hematol Educ Program; 2013: 511–521. [DOI] [PubMed] [Google Scholar]
- Prebet T, Sun Z, Figueroa ME, Ketterling R, Melnick A, Greenberg P et al. Prolonged administration of azacitidine with or without entinostat for myelodysplastic syndrome and acute myeloid leukemia with myelodysplasia-related changes: results of the US Leukemia Intergroup Trial E1905. J Clin Oncol 2014; 32: 1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the Cancer and Leukemia Group B. J Clin Oncol 2002; 20: 2429–2440. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006; 108: 419–425. [DOI] [PubMed] [Google Scholar]
- Bennett JM. World Health Organization classification of the acute leukemias and myelodysplastic syndrome. Int J Hematol 2000; 72: 131–133. [PubMed] [Google Scholar]
- Sproul D, Nestor C, Culley J, Dickson JH, Dixon JM, Harrison DJ et al. Transcriptionally repressed genes become aberrantly methylated and distinguish tumors of different lineages in breast cancer. Proc Natl Acad Sci USA 2011; 108: 4364–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekeres MA. How to manage lower-risk myelodysplastic syndromes. Leukemia 2012; 26: 390–394. [DOI] [PubMed] [Google Scholar]
- Fenaux P. Response assessments in low-risk and high-risk myelodysplastic syndromes (MDS). Semin Oncol 2005; 32: S11–S15. [DOI] [PubMed] [Google Scholar]
- Garcia-Manero G. Myelodysplastic syndromes: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol 2014; 89: 97–108. [DOI] [PubMed] [Google Scholar]
- Garcia-Manero G, Shan J, Faderl S, Cortes J, Ravandi F, Borthakur G et al. A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia 2008; 22: 538–543. [DOI] [PubMed] [Google Scholar]
- Laille E, Shi T, Garcia-Manero G, Cogle CR, Gore SD, Hetzer J et al. Pharmacokinetics and pharmacodynamics with extended dosing of CC-486 in patients with hematologic malignancies. Plos One 2015; 10: e0135520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Porras JR, Cordoba I, Such E, Nomdedeu B, Vallespi T, Carbonell F et al. Prognostic impact of severe thrombocytopenia in low-risk myelodysplastic syndrome. Cancer 2011; 117: 5529–5537. [DOI] [PubMed] [Google Scholar]
- Santini V, Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Silverman LR, List A et al. Management and supportive care measures for adverse events in patients with myelodysplastic syndromes treated with azacitidine. Eur J Haematol 2010; 85: 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Manero G, Jabbour E, Borthakur G, Faderl S, Estrov Z, Yang H et al. Randomized open-label phase II study of decitabine in patients with low- or intermediate-risk myelodysplastic syndromes. J Clin Oncol 2013; 31: 2548–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour JF, Fenaux P, Silverman LR, Mufti GJ, Hellstrom-Lindberg E, Santini V et al. Effects of azacitidine compared with conventional care regimens in elderly (⩾75 years) patients with higher-risk myelodysplastic syndromes. Crit Rev Oncol Hematol 2010; 76: 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol-Myers Squibb Company, Princeton NJ, USA, Rev 10/2010. [Google Scholar]
- Laille E, Savona MR, Scott BL, Boyd TE, Dong Q, Skikne B. Pharmacokinetics of different formulations of oral azacitidine (CC-486) and the effect of food and modified gastric pH on pharmacokinetics in subjects with hematologic malignancies. J Clin Pharmacol 2014; 54: 630–639. [DOI] [PubMed] [Google Scholar]
- Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol 1997; 15: 110–115. [DOI] [PubMed] [Google Scholar]
- Kang SP, Ratain MJ. Inconsistent labeling of food effect for oral agents across therapeutic areas: differences between oncology and non-oncology products. Clin Cancer Res 2010; 16: 4446–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KM, Reddy NJ, Cohen RB, Lewis NL, Whitehead B, Mackay K et al. Effects of food on the relative bioavailability of lapatinib in cancer patients. J Clin Oncol 2009; 27: 1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PL, Attar E, Bennett JM, Bloomfield CD, De Castro CM, Deeg HJ et al. NCCN Clinical Practice Guidelines in Oncology: myelodysplastic syndromes. J Natl Compr Canc Netw 2011; 9: 30–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogle CR, Craig BM, Rollison DE, List AF. Incidence of the myelodysplastic syndromes using a novel claims-based algorithm: high number of uncaptured cases by cancer registries. Blood 2011; 117: 7121–7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.