Abstract
Introduction
The mucopolysaccharidoses (MPSs) are a group of rare genetic lysosomal disorders with progressive multisystem involvement. An MPS-specific physical symptom scale was developed and introduced a Physical Symptom Score (PSS) to quantify the somatic disease burden across MPS I, II and VI.
Hypothesis
Somatic burden of disease in patients with attenuated MPS I, II and VI as measured by the PSS will be positively associated with age and negatively associated with neuropsychological functions [i.e. full scale intelligence quotient (FSIQ) and attention].
Materials and methods
Forty-eight patients with attenuated MPS I (n = 24), II (n = 14), and VI (n = 10) aged 6 to 32 years on enzyme replacement therapy who were enrolled in “Longitudinal Studies of Brain Structure and Functions in MPS Disorders” across seven centers. Somatic disease burden was measured by the PSS. Neuropsychological functions were measured by the Wechsler Abbreviated Scale of Intelligence (WASI) and Test of Variables of Attention (TOVA).
Results
PSS was positively associated with age in attenuated MPS I (P < 0.001), MPS II (P < 0.01) and MPS VI (P < 0.05). There was a negative association of PSS with FSIQ in attenuated MPS I (P < 0.001) and in MPS VI (P < 0.001) but not with MPS II. Although attention scores were below average in all groups, a significant negative association between PSS and one measures of sustained attention (TOVA d prime) was found only in MPS VI.
Conclusions
Physical Symptom Score increased with age in attenuated MPS I, II and VI, reflecting progressive somatic burden of disease despite treatment with enzyme replacement therapy. Furthermore, the association of increased somatic disease burden with decreased neurocognitive ability suggests that both measures reflect disease severity and are not independent.
Keywords: Mucopolysaccharidosis (MPS), Physical Symptom Score (PSS), Somatic disease burden, Neuropsychological measures, Enzyme replacement therapy
Highlights
-
•
MPS-specific physical symptom scale introduced a Physical Symptom Score (PSS) by quantifying the somatic disease burden
-
•
PSS was significantly positively associated with age in attenuated MPS I, MPS II and MPS VI
-
•
PSS was significantly negatively associated with Full Scale IQ (FSIQ) in attenuated MPS I and in MPS VI
-
•
PSS was not statistically significantly associated with FSIQ in MPS II, but had similar direction as other two groups
-
•
• There were no previous studies that examined the association of PSS in MPS disorders with neuropsychological functions
1. Introduction
The mucopolysaccharidoses (MPSs) are a group of rare genetic lysosomal disorders each caused by a specific enzyme deficiency, leading to progressively worsening multi-organ dysfunction.
Previously an MPS-specific physical symptom scale was developed and introduced a Physical Symptom Score (PSS) by quantifying the somatic disease burden in attenuated MPS I (Hurler-Scheie and Scheie syndromes) [1]. A relationship of PSS with age and Daily Living Skills (DLS) from the Vineland Adaptive Behavior Scales-Second Edition (VABS-II) [2] and the PhS (Physical Summary) measure of quality of life from the Child Health Questionnaire Parent Form 50 (CHQ-PF50) [3] has been found in attenuated MPS I, providing concurrent validation for the scale [1]. This study extends our prior work with the PSS to (i) examine the validity of this scale in attenuated MPS I, II and VI longitudinally, (ii) investigate whether associations of PSS with age are also seen in MPS II and VI, and (iii) examine its association with measures of neuropsychological functions (i.e. FSIQ and attention) in within and across these diagnostic groups.
We asked the question whether the PSS would prove valid with progression of the disease in attenuated MPS I, II and VI in longitudinal study, and whether it would be valid for other MPS disorders i.e. MPS II and VI. Further, we investigated whether Physical Symptom Score was associated with cognitive ability in MPS I, II, and VI.
2. Methods
2.1. Subjects
Forty-eight patients with MPS conditions [attenuated MPS I or MPS IA = 24 (including Hurler-Scheie and Scheie patients), MPS II = 14 (only the attenuated form) and MPS VI = 10 (excluding all transplanted patients)] from seven centers who were enrolled in LDN-6703 study “Longitudinal Studies of Brain Structure and Functions in MPS Disorders”, were included. Patients aged 6–32 years were evaluated annually over the course of 5 years; all were on disease-specific enzyme replacement therapy (ERT). Selection criteria were: i) documented MPS I, II and VI by enzyme assay or genotyping, ii) availability of medical data from case report forms and medical records review from each visit to obtain a PSS score, and iii) completion of either the Wechsler Abbreviated Scale of Intelligence (WASI) [4] or the Test of Variables of Attention (TOVA) [5] at each visit. Exclusion criteria were: i) age: too young (< 6 years) to complete the WASI or too old (> 32 years), and ii) absence of both IQ and attention measures. We excluded patients over 32 years to keep the mean age of patients were comparable across the three groups.
2.2. Procedures
2.2.1. MPS-specific Physical Symptom Score (PSS)
Medical and treatment histories were obtained by interview with the patient or parents. The same researcher then reviewed patients' medical records to corroborate information from the interview and obtain any additional information needed for every patient. Using this information, a PSS for each patient's visit was derived using a scoring system that was devised to quantify abnormalities across six domains and scored by a system of frequency and severity of symptom in relevant organ systems (Table 1). The overall summary score is based on scores from six domains, which were, skeletal/orthopedic, vision, hearing, and cardio-respiratory, the number of surgical procedures, and presence or absence of hydrocephalus with or without shunt placement. Each of the 6 domains was scored 0 (absent) to 3 (severe). Total summary scores were calculated by totaling each domain score. The range of total summary scores could be from 0 to 18. The specific criteria for coding each domain are provided in Table 1. Higher Physical Symptom Scores reflect greater disease burden. The reliability and concurrent validity of the PSS was demonstrated in previous publication [1].
Table 1.
MPS-specific physical symptom scale.
| Feature | Score | Description |
|---|---|---|
| Skeletal/orthopedic | 0 | No orthopedic symptom |
| 1 | 1–2 symptoms | |
| 2 | 3–4 symptoms | |
| 3 | 5–6 symptoms or cord compression | |
| Skeletal/orthopedic symptoms include limited range of motion, kyphosis, scoliosis, hip dysplasia, knock knee and high arch foot | ||
| Vision | 0 | No visual impairment or symptom |
| 1 | Mild corneal clouding or glaucoma or cataract | |
| 2 | Moderate corneal clouding or both glaucoma and cataract | |
| 3 | Severe corneal clouding or retinal degeneration | |
| Hearing | 0 | None |
| 1 | Mild hearing loss | |
| 2 | Moderate hearing loss | |
| 3 | Severe hearing loss | |
| Cardio-respiratory | 0 | None |
| 1 | 1–2 cardiac or respiratory symptoms | |
| 2 | 3–4 symptoms or presence of sleep apnea | |
| 3 | 5–6 symptoms or history of cardiac surgery | |
| Cardiac and respiratory symptoms include murmur, hypertension, valve disease, cardiac surgery, chronic nasal discharge/obstruction, tonsils/adenoids, respiratory infection/reactive airway disease and sleep apnea | ||
| Hydrocephalus | 0 | Absent |
| 1 | Hydrocephalus without shunt | |
| 2 | Hydrocephalus with shunt | |
| 3 | Revision of shunt | |
| Number of surgeries | 0 | No surgery |
| 1 | < 4 surgeries | |
| 2 | 4 to 8 surgeries | |
| 3 | > 8 surgeries | |
| Total score = 0–18 | ||
2.2.2. Cognitive measure
To measure neuropsychological functions, the Wechsler Abbreviated Scale of Intelligence (WASI) [4] was used to estimate Full Scale IQ. FSIQ provides a brief, reliable measure of cognitive ability for use in research settings. The Test of Variables of Attention (TOVA) [5] was used to assess attention. The TOVA yields 5 scores: d prime (measure of signal detection or how quickly performance worsens), omission errors (number of targets missed by a subject), commission errors (number of impulsive responses by a subject), reaction time (average time it takes the subject to respond correctly to a target), and variability of reaction time (measure of the variability in the subject's response time on accurate responses). Commission errors were not included in this study. IQ and TOVA scores are reported as standard scores with a mean of 100 and standard deviation of 15.
2.2.3. Ethical considerations
All patients and/or their legal guardians provided written informed consent to participate in the study. This study was approved by the University of Minnesota Institutional Review Board: Human Subjects Committee. The protocol was approved by each site's institutional review board or independent research ethics committee.
2.2.4. Statistical analysis
Descriptive statistics were tabulated separately for each MPS group. These included the mean and standard deviation for continuous variables and the frequency and percentage for categorical variables. The association of PSS with age and each neuropsychological measure with PSS was estimated for each MPS group with generalized estimating equations and a working independence correlation structure. The analyses of IQ and attention were adjusted for age at first treatment. Confidence intervals and P-values were based on robust variance estimation to account for the correlated nature of longitudinal measurements. All analyses were conducted using R v3.1.1 (R Core Team, 2014) [6].
3. Results
Patient characteristics and outcomes of interest are presented in Table 2. Here we can see that MPS II patients were treated earlier and younger age at 1st assessment with narrower age range than attenuated MPS I and MPS VI. Attenuated MPS I and MPS VI, they were almost similar in age at 1st assessment and age at 1st treatment. PSS average was lowest in MPS II and highest in MPS VI and IQ average was highest in MPS II and lowest in attenuated MPS I. Overall, attention scores were below average in TOVA-d prime, omission errors and variability for all 3 groups, only TOVA-reaction time scores were within the average range for all 3 MPS groups.
Table 2.
Patient characteristics and outcomes of interest; values presented as mean (SD) or N (%) where indicated.
| Patient characteristics | MPS IA (N = 24) | MPS II (N = 14) | MPS VI (N = 10) |
|---|---|---|---|
| Male | 11 (50.0%) | 14 (100.0%) | 3 (30.0%) |
| Age at first assessment (years) | 15.23 (6.11) | 10.27 (2.79) | 16.06 (5.58) |
| Number of visits | 2.38 (1.10) | 2.93 (0.83) | 2.10 (1.29) |
| Age at first treatment (years) | 8.98 (5.31) | 7.52 (2.97) | 9.76 (4.81) |
| Number of years on treatment (years) | 6.25 (3.39) | 2.75 (1.84) | 6.30 (2.52) |
| Outcomes of interest | |||
| PSS | 8.88 (2.98) | 8.07 (1.44) | 9.60 (2.84) |
| FSIQ | 93.46 (18.43) | 98.21 (15.46) | 96.30 (20.91) |
| TOVA: d prime | 81.91 (14.40) | 81.14 (13.89) | 77.10 (11.06) |
| TOVA: omission errors | 76.77 (25.50) | 76.07 (27.27) | 67.20 (29.20) |
| TOVA: reaction time | 93.64 (21.38) | 87.86 (22.23) | 100 (13.71) |
| TOVA: variability | 83.36 (22.67) | 75.64 (25.21) | 84.40 (21.51) |
Notes: Attenuated MPS I or MPS IA includes both Hurler-Scheie and Scheie patients. 2 MPS IA patients did not have TOVAs; n = 22 for the TOVA in this group.
Table 3 shows the association of PSS with age in each MPS group. A statistically significant positive association of PSS with age was found in MPS I (P < 0.001), MPS II (P < 0.01) and MPS VI (P < 0.05) (Table 3, Fig. 1) such that with increasing age, the disease burden was greater.
Table 3.
Association of PSS with age for each MPS group as obtained from regression analyses.
| Outcome | Covariate | Association with age (95% CI) | P-value |
|---|---|---|---|
| PSS | MPS IA (per 5 years) | 1.68 (1.10, 2.27) | < 0.001 |
| MPS II (per 5 years) | 1.30 (0.33, 2.27) | 0.008 | |
| MPS VI (per 5 years) | 1.43 (0.18, 2.68) | 0.025 |
Notes: Number of observations was 119. Attenuated MPS I or MPS IA includes both Hurler-Scheie and Scheie patients.
Fig. 1.
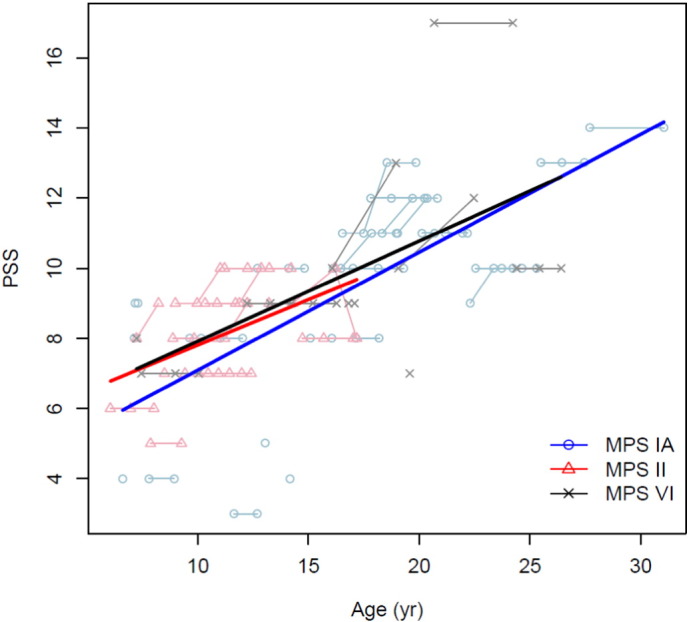
Association of PSS with age in MPS I, II and VI.
Table 4 shows the association of PSS with FSIQ in each MPS group. A statistically significant negative association of PSS occurred with FSIQ in MPS I (P < 0.001) and in MPS VI (P < 0.001) adjusting for age at first treatment (Table 4, Fig. 2) such that the greater the disease burden, the lower the IQ. The association was in similar direction for MPS II, though was not statistically significant.
Table 4.
Association of PSS with FSIQ for each MPS group as obtained from regression analyses.
| Outcome | Covariate | Association with FSIQ (95% CI) | P-value |
|---|---|---|---|
| PSS | MPS IA (per PSS point) | 1.68 (1.10, 2.27) | < 0.001 |
| MPS II (per PSS point) | 1.30 (0.33, 2.27) | 0.008 | |
| MPS VI (per PSS point) | 1.43 (0.18, 2.68) | 0.025 |
Notes: Number of observations 118 across 48 patients, one of the patients missing one visit. Attenuated MPS I or MPS IA includes both Hurler-Scheie and Scheie patients.
Fig. 2.
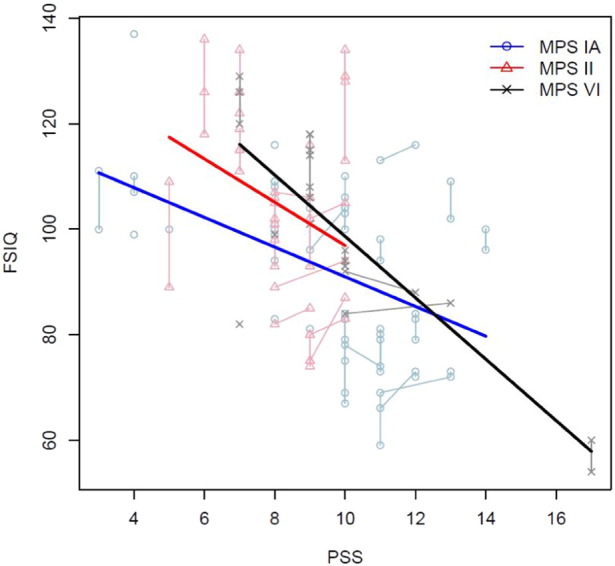
Association of PSS with FSIQ in MPS I, II and VI.
Table 5 shows the association of PSS with FSIQ and TOVA domains, with and without measurements from a highly influential MPS VI patient. PSS was statistically significantly negatively associated with attention in MPS VI (in the domains of TOVA-d prime) but no significant association of PSS with attention was found in MPS I and MPS II (Table 5). However, one of the patient in the MPS VI group was highly influential. The influential patient was > 2 standard deviations (SDs) below the MPS VI group mean for both IQ and attention measures as well as being 2 SDs above the mean on the PSS. Here FSIQ was 54, TOVA: d prime 62, omission errors 40, reaction time 78, variability 60 and PSS 17. Given these outlying scores, we also evaluated the association of PSS with FSIQ and TOVA domains in MPS VI excluding the influential measurements from that patient. Results for PSS compared with IQ did not change meaningfully but results for PSS compared with attention changed markedly. The association of PSS and TOVA d prime was no longer significantly negatively associated in MPS VI, instead the estimate switched to a positive association and association of PSS with other 3 domains of TOVA, from no association, estimates turned to significant positive association (Table 5); which was not in accordance with one of our hypotheses.
Table 5.
Association of PSS with FSIQ and TOVA domains, with and without measurements from a highly influential MPS VI patient.
| Outcome | Covariate | Association with outcome (95% CI) with influential patient | P-value with influential patient | Association with outcome (95% CI) without influential patient | P-value without influential patient |
|---|---|---|---|---|---|
| FSIQ | MPS IA (per PSS point) | − 2.82 (− 4.46, − 1.18) | < 0.001 | − 2.81 (− 4.45, − 1.18) | < 0.001 |
| MPS II (per PSS point) | − 4.11 (− 11.17, 2.95) | 0.254 | − 4.11 (− 11.17, 2.95) | 0.254 | |
| MPS VI (per PSS point) | − 5.83 (− 7.82, − 3.83) | < 0.001 | − 5.52 (− 10.05, − 0.98) | 0.017 | |
| d prime | MPS IA (per PSS point) | 0.03 (− 1.99, 2.05) | 0.979 | 0.09 (− 1.92, 2.11) | 0.927 |
| MPS II (per PSS point) | 2.19 (− 3.11, 7.48) | 0.418 | 2.24 (− 3.08,7.55) | 0.409 | |
| MPS VI (per PSS point) | − 3.04 (− 5.64, − 0.43) | 0.022 | 1.11 (− 1.47, 3.68) | 0.400 | |
| Omission errors | MPS IA (per PSS point) | − 0.46 (− 3.73, 2.80) | 0.781 | − 0.33 (− 3.62, 2.96) | 0.843 |
| MPS II (per PSS point) | 0.29 (− 6.38, 6.95) | 0.933 | 0.40 (− 6.28, 7.08) | 0.906 | |
| MPS VI (per PSS point) | − 2.28 (− 7.29, 2.73) | 0.372 | 5.57(0.47, 10.68) | 0.032 | |
| Reaction time | MPS IA (per PSS point) | 0.73 (− 0.90, 2.36) | 0.380 | 0.85 (− 0.75, 2.44) | 0.297 |
| MPS II (per PSS point) | 0.16 (− 5.91, 6.22) | 0.960 | 0.26 (− 5.81, 6.32) | 0.933 | |
| MPS VI (per PSS point) | 0.50 (− 3.51, 4.52) | 0.806 | 7.51 (4.91, 10.12) | < 0.001 | |
| Variability | MPS IA (per PSS point) | 1.10 (− 1.58, 3.77) | 0.422 | 1.23 (− 1.49, 3.95) | 0.374 |
| MPS II (per PSS point) | 1.35 (− 6.57, 9.28) | 0.738 | 1.47 (− 6.45, 9.39) | 0.716 | |
| MPS VI (per PSS point) | − 0.38 (− 5.20, 4.45) | 0.878 | 7.72 (3.74, 11.70) | < 0.001 |
4. Discussion
The primary goal of this study was to evaluate the association of somatic disease burden with age and with neuropsychological functioning. Other chronic illnesses have been shown to have an association between duration of disease severity and neuropsychological impairment [7], [8], [9]. There were no previous studies that examined the association of somatic disease burden in MPS disorders with neuropsychological functions. This question is important for several reasons. First, does the severity of disease in MPS disorders extend to the neuropsychological domain? Whether patients with MPS diseases who have more severe physical disease also have more cognitive impairment has not been addressed through any empirical research. Second, if there is an association, then steps to intervene through rehabilitation therapies and educational interventions can be carried out earlier in the disease course with the knowledge that such children are at greater risk for cognitive impairment. The findings demonstrate that all three groups, attenuated MPS I, II, and VI, despite being on ERT, showed increasing somatic disease burden with age, with older patients showing more disease burden (Table 3, Fig. 1). Both MPS I and MPS VI showed significant association of PSS with FSIQ demonstrating that increased disease burden was related to lower FSIQ score. For MPS II, the association was not statistically significant, though we observed similar direction as the other groups. The lack of statistical significance may be due to the slightly higher IQ and lower variability of IQ in this group. It has been previously noted that attenuated MPS II patients present with symptoms and complications that usually lead to progressive physical disability retaining normal or nearly normal intelligence [10], [11], [12]. It also may be due to the younger age of the MPS II patients at both evaluation and start of treatment compared to MPS I and VI. One should note that neurological factors such as hydrocephalus and abnormalities on MRI are equally common in MPS II as in MPS I [13] or MPS VI [14]. Attention was chosen as previous studies have found abnormal attentional function in MPS disorders [15], [16], [17]. It has been hypothesized that attention is related to white matter abnormalities in MPS conditions [18]. Overall, attention scores were below the average range for the d prime, omission errors, and variability for all three groups. Reaction time was within the average range for all three groups. However, for all three groups no significant association was found with PSS. Although it was hypothesized that attention would be negatively associated with somatic disease burden, the associations were not as strong as with IQ and were not statistically significant for MPS I and II. This is the first such study that quantified severity of somatic disease and then associated it with neuropsychological functions in MPS disorders. There is surprisingly little information on the relationship between severity of disease burden and neuropsychological functioning in children with chronic medical conditions. Although Suris et al. [19] state that it is unlikely that chronic illness “affects the basic neuronal maturational mechanisms that underlie the development of abstract thinking capabilities in adolescence,” with the exception of inherited metabolic diseases, evidence for neuropsychological effects of disease processes in type I diabetes [7], [8] sickle cell disease (without cerebrovascular accidents) [20] congenital heart disease [9] and multiple sclerosis [21] have been found. These reports indicate that these diseases most often affect visual spatial ability but often only tests of cognitive ability have been given without more specialized testing, limiting the conclusions that have been made. In these selected reports, quantification of the disease burden was disease-specific and included congenital heart disease severity which was coded as complex, moderate and simple, in diabetes age of onset/duration, severity of hypo/hyper-glycemic events were examined, but in multiple sclerosis cognitive impairment was related to physical disability (which was measured by a disability scale) not related to duration of the disease. In the case of the MPS disorders, multiple body systems are involved and the effects of multiple surgeries, as well as sensory and orthopedic and cardio-respiratory abnormalities on cognitive ability are for the first time quantified and associated. Our findings suggest that as individuals with MPS I, II and VI age, they appear to be at greater risk for worsening physical symptoms. It will be important to pursue treatment that will both ameliorate as many physical symptoms as possible while also recognizing that early interventions for cognitive abnormalities will be necessary for improving long term outcomes and quality of life in MPS disorders. While problems in cognition among MPS I patients have been well documented [15], the abnormalities in MPS VI have only been alluded to in the literature and not well described. It should be noted that the PSS score for MPS VI is higher than the others, and the patients started treatment later. For these patients, earlier treatment, as is seen in the MPS II group, may have positive benefits for both somatic and neuropsychological function.
5. Conclusions
We found that a standardized method for measuring MPS-specific Physical Symptom Score (PSS) increased with age in attenuated MPS I, II and VI, reflecting progressive somatic burden of disease despite treatment with enzyme replacement therapy. Furthermore the PSS was negatively associated with FSIQ in both MPS I and MPS VI. In MPS II, who were treated earlier, had younger and narrower age range, a significant association with FSIQ was not found though results showed a similar direction as the other groups. The association of increased somatic disease burden with decreased neurocognitive ability suggests that both measures reflect disease severity and are not independent.
Future research is needed to provide a better understanding of the relationships between somatic burden of disease with attention and other neuropsychological measures in these patients, with more longitudinal data as well as brain imaging.
Funding sources
The Lysosomal Disease Network (LDN) U54-NS065768, is a part of the National Institute of Health (NIH) Rare Diseases Clinical Research Network, supported through collaboration between the NIH Office of Rare Diseases Research at the National Center for Advancing Translational Science, the National Institute of Neurological Disorders and Stroke (NINDS), and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This project was supported in part (Dr. Rudser) by the National Center for Advancing Translational Sciences, National Institutes of Health, through University of Minnesota-CTSI Grant Number NCATS UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or CTSI.
Acknowledgments
We are grateful to all of the families at the participating sites, as well as the following principal investigators, Dr. Paul Harmatz at Children's Hospital and Research Center, Oakland, California, Dr. Julian Raiman, formerly at the Hospital for Sick Children, Toronto, Canada, Dr. Morton Cowan at University of California, San Francisco, Dr. Suma Shankar at Emory University, Decatur, Georgia, Dr. Heather Lau at New York University, New York and Dr. Robert Steiner, formerly at Oregon Health & Science University, Portland for their contributions.
We are thankful to Kathleen Delaney and Brianna Yund for their assistance with this research. We are very thankful for the support provided by the National MPS Society, Ryan Foundation for Orphan Disease Research and Center for Neurobehavioral Development (CNBD).
References
- 1.Ahmed A., Rudser K., Kunin-Batson A., Delaney K., Whitley C., Shapiro E. Mucopolysaccharidosis (MPS) physical symptom score: development, reliability, and validity. J. Inherit. Metab. Dis. 2015;1-8 doi: 10.1007/8904_2015_485. PMID: 26303610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sparrow S.S., Cicchetti D.V., Balla D.A. second ed. Psychological Corporation; San Antonio, TX: 2005. Vineland Adaptive Behavior Scales. [Google Scholar]
- 3.Landgraf J.M., Abetz L., Ware J.E. first ed. The Health Institute; New England Medical Center, Boston: 1996. The CHQ User's Manual. [Google Scholar]
- 4.Wechsler D. Psychological Corporation; San Antonio TX: 1999. Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- 5.Greenberg L.M. The TOVA Company; Los Alamitos, CA: 2007. The Test of Variables of Attention (Version 7.3) [Computer software] [Google Scholar]
- 6.R Core Team, R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, 2014. http://www.R-project.org/.
- 7.Desrocher M., Rovet J. Neurocognitive correlates of type 1 diabetes mellitus in childhood. Child Neuropsychol. 2004;10(1):36–52. doi: 10.1076/chin.10.1.36.26241. [DOI] [PubMed] [Google Scholar]
- 8.Naguib J.M., Kulinskaya E., Lomax C.L., Garralda M.E. Neuro-cognitive performance in children with type 1 diabetes—a meta-analysis. J. Pediatr. Psychol. 2009;34(3):271–282. doi: 10.1093/jpepsy/jsn074. [DOI] [PubMed] [Google Scholar]
- 9.Karsdorp P.A., Everaerd W., Kindt M. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta-analysis. J. Pediatr. Psychol. 2007;32(5):527–541. doi: 10.1093/jpepsy/jsl047. [DOI] [PubMed] [Google Scholar]
- 10.Wraith J.E., Scarpa M., Beck M., Bodamer O.A., De Meirleir L., Guffon N., Lund A.M., Malm G., Van der Ploeg A.T., Zeman J. Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur. J. Pediatr. 2008;167:267–277. doi: 10.1007/s00431-007-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muenzer J., Beck M., Eng C.M., Escolar M.L., Giugliani R., Guffon N.H., Harmatz P., Kamin W., Kampmann C., Koseoglu S.T., Link B., Martin R.A., Molter D.W., Muñoz Rojas M.V., Ogilvie J.W., Parini R., Ramaswami U., Scarpa M., Schwartz I.V., Wood R.E., Wraith E. Multidisciplinary management of Hunter syndrome. Pediatrics. 2008;124(6):1228–1239. doi: 10.1542/peds.2008-0999. [DOI] [PubMed] [Google Scholar]
- 12.Martin R., Beck M., Eng C., Giugliani R., Harmatz P., Muñoz V., Muenzer J. Recognition and diagnosis of mucopolysaccharidosis II (Hunter syndrome) Pediatrics. 2008;121(2):377–386. doi: 10.1542/peds.2007-1350. [DOI] [PubMed] [Google Scholar]
- 13.Matheus M.G., Castillo M., Smith J.K., Armao D., Towle D., Muenzer J. Brain MRI findings in patients with mucopolysaccharidosis types I and II and mild clinical presentation. Neuroradiology. 2004;46(8):666–672. doi: 10.1007/s00234-004-1215-1. [DOI] [PubMed] [Google Scholar]
- 14.Vedolin L., Schwartz I.V.D., Komlos M., Schuch A., Azevedo A.C., Vieira T., Maeda F.K., Marques da Silva A.M., Giugliani R. Brain MRI in mucopolysaccharidosis Effect of aging and correlation with biochemical findings. Neurology. 2007;69(9):917–924. doi: 10.1212/01.wnl.0000269782.80107.fe. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro E.G., Nestrasil I., Rudser K., Delaney K., Kovac V., Ahmed A., Yund B., Orchard P.J., Eisengart J., Niklason G.R., Raiman J., Mamak E., Cowan M.J., Bailey-Olson M., Harmatz P., Shankar S.P., Cagle S., Ali N., Steiner R.D., Wozniak J.R., Lim K.O., Whitley C.B. Neurocognition across the spectrum of mucopolysaccharidosis type I: age, severity, and treatment. Mol. Genet. Metab. 2015;116(1–2):61–68. doi: 10.1016/j.ymgme.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yund B., Rudser K., Ahmed A., Kovac V., Nestrasil I., Raiman J., Mamak E., Harmatz P., Steiner R., Lau H., Vekaria P., Wozniak J.R., Lim K.O., Delaney K., Whitley C.B., Shapiro E.G. Cognitive, medical, and neuroimaging characteristics of attenuated mucopolysaccharidosis type II. Mol. Genet. Metab. 2015;114(2):170–177. doi: 10.1016/j.ymgme.2014.12.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro E.G., Guler O.E., Rudser K., Delaney K., Bjoraker K., Whitley C.B., Tolar J., Orchard P.J., Provenzale J., Thomas K.M. An exploratory study of brain function and structure in mucopolysaccharidosis type I: long term observations following hematopoietic cell transplantation (HCT) Mol. Genet. Metab. 2012;107(1):116–121. doi: 10.1016/j.ymgme.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Provenzale J.M., Nestrasil I., Chen S., Kan S.H., Le S.Q., Jens J.K., Snella E.M., Vondrak K.M., Yee J.K., Vite C.H., Elashoff D., Duan L., Wang R.Y., Ellinwood N.M., Guzman M.A., Shapiro E.G., Dickson P.I. Diffusion tensor imaging and myelin composition analysis reveal abnormal myelination in corpus callosum of canine mucopolysaccharidosis I. Exp. Neurol. 2015;273:1–10. doi: 10.1016/j.expneurol.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suris J.C., Michaud P.A., Viner R. The adolescent with a chronic condition. Part I: developmental issues. Arch. Dis. Child. 2004;89(10):938–942. doi: 10.1136/adc.2003.045369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schatz J., Finke R.L., Kellet J.M., Krammer J.H. Cognitive functioning in children with sickle cell disease: a meta-analysis. J. Pediatr. Psychol. 2002;27(8):739–748. doi: 10.1093/jpepsy/27.8.739. [DOI] [PubMed] [Google Scholar]
- 21.Lynch S.G., Parmenter B.A., Denney D.R. The association between cognitive impairment and physical disability in multiple sclerosis. Mult. Scler. 2005;11:469–476. doi: 10.1191/1352458505ms1182oa. [DOI] [PubMed] [Google Scholar]


