Highlights
-
•
A quantitative assessment of bowel viability is desired in gastrointestinal surgery.
-
•
In pancreatectomy post gastrectomy, assessment of stomach viability is essential.
-
•
Near-infrared spectroscopy helped the surgeons to evaluate intestinal viability.
Abbreviations: PD, pancreaticoduodenectomy; DP, distal pancreatectomy; rSO2, regional saturation of oxygen; ICG, indocyanine green; CT, computed tomography; RGA, right gastric artery; RGEA, right gastroepiploic artery; PET, positron emission tomography; SUV, standardized uptake value; GDA, gastroduodenal artery; DG, distal gastrectomy; EUS, endoscopic ultrasonography; MRI, magnetic resonance imaging; ERCP, endoscopic retrograde cholangiopancreatography; CHA, common hepatic artery
Keywords: Near-infrared spectroscopy, Preservation of remnant stomach, Pancreatectomy
Abstract
Introduction
Objective and quantitative intraoperative methods of bowel viability assessment could decrease the risk of postoperative ischemic complications in gastrointestinal surgery. Because the remnant stomach and the pancreas share an arterial blood supply, it is often unclear whether the remnant stomach can be safely preserved when performing pancreaticoduodenectomy (PD) or distal pancreatectomy (DP) post gastrectomy. We herein report two cases in which the remnant stomach was safely preserved using near-infrared spectroscopy to assess the regional saturation of oxygen (rSO2) in the remnant stomach during operation.
Presentation of case
The first patient, a 68-year-old man, was diagnosed with cancer of the pancreatic head and underwent PD a year after proximal gastrectomy for gastric cancer. The remnant stomach was safely preserved by evaluation of the rSO2 before and after reconstruction of the arteries. The second patient, an 82-year-old woman with a history of distal gastrectomy for gastric cancer 40 years previously, was diagnosed with a main duct intraductal papillary mucinous neoplasm of the pancreatic body, requiring DP. As in the previous case, we could safely preserve the remnant stomach through assessing the intraoperative rSO2 of the remnant stomach.
Discussion
Through comparing changes in the rSO2 during surgery, near-infrared spectroscopy provides objective and quantitative assessments of intestinal viability to predict ischemic complications.
Conclusion
This method may be a viable option to evaluate the blood supply to the alimentary tract.
1. Introduction
Pancreatic cancer is the fourth leading cause cancer deaths [1] and has a poor prognosis; surgery is the only potentially curative treatment [2]. It has been reported that partial gastrectomy may be a risk factor for pancreatic cancer [3], [4]. In such cases, standard pancreatectomy is associated with potential loss of blood supply to the remnant stomach, which may lead to postoperative ischemic complications. However, there is no consensus on how to manage the remnant stomach most effectively when performing pancreatectomy in these patients. Intraoperative assessment of bowel viability has been performed using Doppler ultrasonography, indocyanine green (ICG) fluorescence angiography, and near-infrared spectroscopy [5], [6], [7], [8]. The In Vivo Optical Spectroscopy (INVOS) system (Covidien, JAPAN) allows real-time monitoring of regional saturation of oxygen (rSO2) in the brain or body tissue directly beneath the sensor through near-infrared spectroscopy [9], [10]. We herein report two cases of pancreatectomy in patients who had previously undergone gastrectomy, in which intestinal viability was objectively assessed and the remnant stomach was safely preserved using this system.
2. Presentation of case
2.1. Case 1
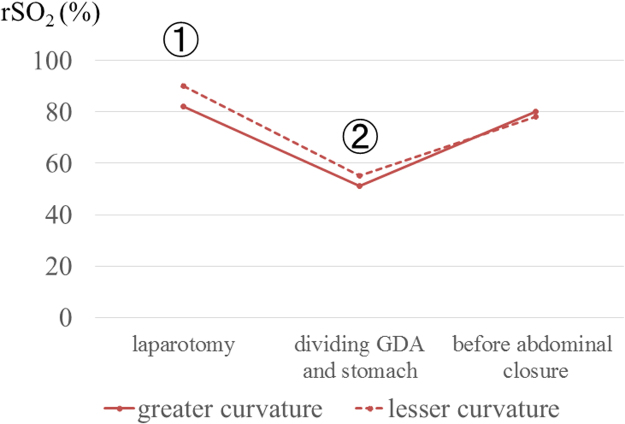
A 68-year-old man underwent proximal gastrectomy for additional resection following endoscopic submucosal dissection of gastric cardia cancer. A solid mass was detected at the head of the pancreas on follow-up computed tomography (CT) a year after the surgery. Serum biochemistry was as follows: aspartate aminotransferase (AST), 18 U/L; alanine aminotransferase (ALT), 15 U/L; total bilirubin, 0.9 mg/dL; amylase (AMY), 150 U/L; carcinoembryonic antigen (CEA), 5.6 ng/mL; cancer antigen 19-9 (CA 19-9), 143 U/mL; and s-pancreas-1 antigen (SPAN-1), 32 U/mL. CT imaging showed a low-enhanced mass with a diameter of 15 mm × 12 mm at the pancreatic head. Lymph node swelling and vascular invasion were not detected. The right gastric artery (RGA) and right gastroepiploic artery (RGEA) were preserved in the prior operation (Fig. 1). On positron emission tomography (PET)-CT, abnormal fludeoxyglucose uptake was seen (standardized uptake value (SUV)-max: 3.1–4.0) at the head of the pancreas. Endoscopic ultrasound-guided fine needle aspiration revealed adenocarcinoma. Therefore, pancreaticoduodenectomy (PD) with lymph node dissection was planned. During the PD procedure, we needed to divide the gastroduodenal artery (GDA). We needed to know whether the remnant stomach could be safely preserved by reconstructing the circulation between the GDA and RGEA (Fig. 2). The intraoperative rSO2 of the greater curvature of the remnant stomach was assessed using the INVOS system. At the time of laparotomy, the rSO2 of the remnant stomach was 82%. After dividing the GDA and stomach, it decreased to 51%. Reconstruction of the arteries was performed, and at the end of the operation, it increased to 80% (Fig. 3). We judged that the remnant stomach could be safely preserved. Pathological examination showed invasive ductal carcinoma of the pancreas, pathological stage T4N1M0 Stage IVa (TNM classification). The postoperative course was uneventful, and the patient was discharged on the 17th postoperative day.
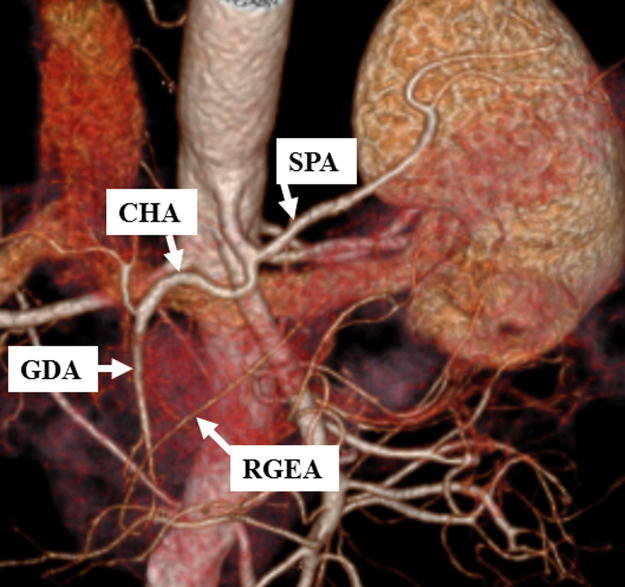
Fig. 1.
Preoperative 3D-CT (Case 1).
The right gastroepiploic artery (RGEA) was preserved in the prior surgery.
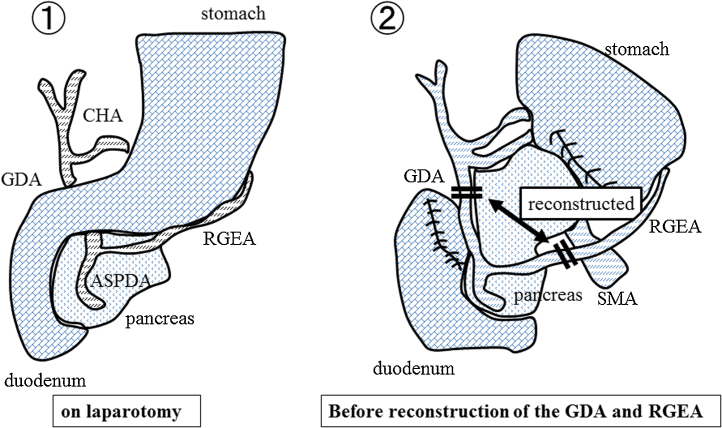
Fig. 2.
Scheme of the operative findings (Case 1).
Reconstruction between the GDA and RGEA was performed.
Fig. 3.
Intraoperative rSO2 (Case 1).
After the reconstruction of the arteries, rSO2 level improved.
2.2. Case 2
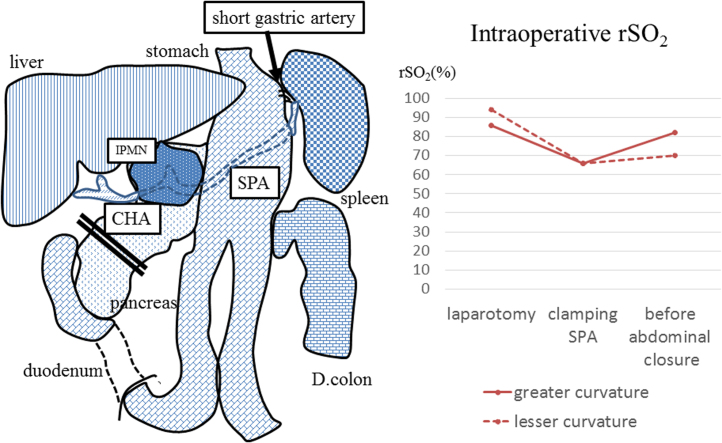
An 82-year-old woman was initially admitted to a nearby hospital for a periodical medical examination. Ultrasound examination revealed a cystic mass at the body of the pancreas, and she was referred to our hospital for further investigation. On admission, she had no specific symptoms, with poor performance status. Physical examination was within the normal limits. She had a history of distal gastrectomy (DG, Billroth-II) for gastric cancer 40 years ago. Serum biochemistry was as follows: AST, 27 U/L; ALT, 24 U/L; total bilirubin, 0.4 mg/dL; AMY, 79 U/L; CEA, 3.7 ng/mL; CA19-9, 13 U/mL; and SPAN-1, 8.3 U/mL. CT scan showed a cystic mass with a diameter of 50 mm × 30 mm in the pancreatic body. The cystic mass did not invade the splenic artery or vein, but had a connection with the main pancreatic duct. Lymph node swelling and metastases were not detected. Early phase imaging revealed that the left gastric, right gastric, and right gastroepiploic arteries and veins were cut in the prior operation (Fig. 4.). Endoscopic ultrasonography (EUS) and magnetic resonance imaging (MRI) also showed a connection between the cystic lesion and the main pancreatic duct. On PET-CT, abnormal fludeoxyglucose uptake was seen (SUV-max: 4.5–5.4) at the solitary lesion. From these examinations, the patient was preoperatively diagnosed with a main duct intraductal papillary mucinous neoplasm (MD-IPMN). Oncologically, pancreatic body/tail resection with lymph node dissection was required. However, we had to seek a less invasive approach for this patient because she was elderly, with early stage dementia and a poor performance status of 3. Although these co-morbidities increased the risk of the surgery, the operation was performed as the patient and her family were strongly in favor of the operative treatment. As the remnant stomach had an arterial supply from the splenic artery (SPA), we needed to assess whether the remnant stomach could be safely preserved during No. 10 lymph node resection. As in the previous case, we assessed the rSO2 of the greater curvature of the remnant stomach intraoperatively using the INVOS system. On laparotomy, the rSO2 of the stomach was 82%. On clamping the SPA, it decreased to 66% (Fig. 5). We realized that we could not preserve the remnant stomach if we performed splenectomy. The cystic lesion did not invade into the surrounding tissue, and we could not detect any lymph node enlargement. Taking into account the patient’s physical condition, we decided to preserve the spleen and remnant stomach. Pathological examination showed IPMN with severe dysplasia (WHO classification), TisN0M0 stage 0 (TNM classification). The postoperative course was uneventful, and she was transferred to a different hospital for further rehabilitation on the 14th postoperative day.
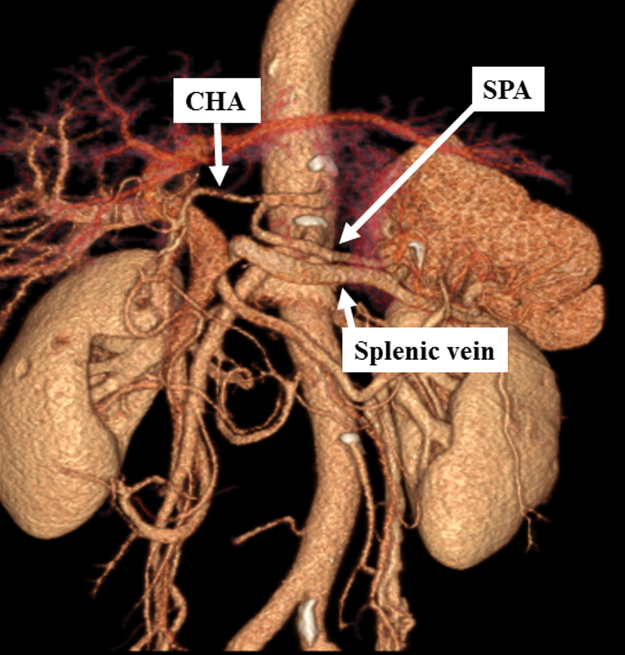
Fig. 4.
Preoperative 3D-CT (Case 2).
Left gastric, right gastric, and right gastroepiploic arteries and veins were cut in the prior surgery.
Fig. 5.
Scheme of the operative findings (Case 2).
On clamping the SPA, the rSO2 level decreased.
3. Discussion
In this case report, we have shown that intraoperative rSO2 measurement using the INVOS system can be used to objectively assess the viability of the remnant stomach. Pancreatectomy following partial gastrectomy has been reported to be a risk factor for ischemia of the remnant stomach [11]. Although it is important to evaluate bowel viability, there are no standard methods for doing this during the surgery. Intraoperative INVOS systems may be helpful for evaluating blood supply in the remnant stomach. Ischemic complications after pancreatectomy should be avoided in patients with a history of partial gastrectomy. The stomach has been thought to be resistant to post-operative ischemia due to its abundant blood supply. However, insufficient blood supply to the remnant stomach can cause complications such as leaks or disruption of the suture line [12]. Post-operative gastric ischemia could occur even after subtotal gastrectomy [13]. Other causes of gastric ischemia include the devascularization procedures used in portal hypertension, splenectomy, gastric operations for morbid obesity, and esophageal surgery [11]. Takahashi et al. reported that two of ten patients who underwent distal pancreatectomy after distal gastrectomy developed severe ischemic complications [7]. Furthermore, Reinhard Bittner et al. reported that mortality and morbidity rates after total gastrectomy for patients aged >70 years are higher than those for younger patients [14]. These reports, and our patients’ conditions, highlight the importance of preserving the remnant stomach to the greatest possible extent.
Recently, several studies have reported intraoperative assessments of intestinal viability. Doppler ultrasonography was trialed for the assessment of vascularization of the intestinal edges during colorectal anastomosis [15]. However, the sensitivity of Doppler ultrasound was reported to be lower than that of other methods due to the lack of oxygen delivery data [16]. Intraoperative ICG fluorescence angiography was used to visualize the blood flow of the remnant stomach in distal pancreatectomy after distal gastrectomy [7], [17]. However, this did not provide an objective measurement. Compared to these methods, near-infrared spectroscopy such as INVOS should be useful in assessing intestinal viability during surgery [5], [18], [19]. This method provides objective and quantitative assessments of intestinal viability to help avoid ischemic complications by comparing changes in the rSO2 during the surgery. In animal models, intestinal ischemia resulted in approximately 20% reduction in rSO2 on near-infrared spectroscopy [20], [21]. In humans, low rSO2 (less than 60%) on both sides of the anastomosis may indicate an increased risk of anastomotic complications [5]. We believe that further investigation is needed to determine the criteria that suggest a high chance of intestinal viability.
4. Conclusion
We saw two cases of patients who underwent pancreatectomy after gastrectomy, in which the intestinal viability was objectively assessed and the remnant stomach was safely preserved using near-infrared spectroscopy. The INVOS system with near-infrared spectroscopy might be one option to evaluate the blood flow of the alimentary tract.
Conflicts of interest
None.
Funding
None.
Ethical approval
All procedures used in this research were approved by the Ethical Committee of Hiroshima University Hospital.
Consent
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Author contribution
Akabane, Ohira, and Ohdan ware responsible for the conception and design of this study. Tahara, Kuroda, Tanimine, and Shimizu participated in the data acquisition. Akabane, Ohira, Ishiyama, Kobayashi, Ide, and Tanabe performed the analysis and interpretation of the data. Ohira and Kobayashi helped to draft the manuscript. Ohira and Ohdan coordinated the study and critically revised the manuscript. All of the authors read and approved the final manuscript.
Guarantor
Masahiro Ohira has accepted full responsibility for this work and the decision to publish it.
Acknowledgment
None.
Contributor Information
Shintaro Akabane, Email: red_wing412@yahoo.co.jp.
Masahiro Ohira, Email: mohira@hiroshima-u.ac.jp.
Kohei Ishiyama, Email: ishiyama@hiroshima-u.ac.jp.
Tsuyoshi Kobayashi, Email: tsukoba@hiroshima-u.ac.jp.
Kentaro Ide, Email: ideken@hiroshima-u.ac.jp.
Hiroyuki Tahara, Email: htahara@hiroshima-u.ac.jp.
Shintaro Kuroda, Email: df26@smn.enjoy.ne.jp.
Naoki Tanimine, Email: tanimine1217@gmail.com.
Seiichi Shimizu, Email: s.kiyomizu@hotmail.co.jp.
Kazuaki Tanabe, Email: ktanabe2@hiroshima-u.ac.jp.
Hideki Ohdan, Email: hohdan@hiroshima-u.ac.jp.
References
- 1.Siegel R., Ma J., Zou Z. Cancer statistics, 2014. CA: Cancer J. Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Hartwig W., Hackert T., Hinz U. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann. Surg. 2011;254(2):311–319. doi: 10.1097/SLA.0b013e31821fd334. [DOI] [PubMed] [Google Scholar]
- 3.Maringhini A., Thiruvengadam R., Melton L.J. Pancreatic cancer risk following gastric surgery. Cancer. 1987;60(2):245–247. doi: 10.1002/1097-0142(19870715)60:2<245::aid-cncr2820600222>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Tascilar M., van Rees B.P., Sturm P.D. Pancreatic cancer after remote peptic ulcer surgery. J. Clin. Pathol. 2002;55(5):340–345. doi: 10.1136/jcp.55.5.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirano Y., Omura K., Tatsuzawa Y. Tissue oxygen saturation during colorectal surgery measured by near-infrared spectroscopy: pilot study to predict anastomotic complications. World J. Surg. 2006;30(3):457–461. doi: 10.1007/s00268-005-0271-y. [DOI] [PubMed] [Google Scholar]
- 6.Karliczek A., Benaron D.A., Baas P.C. Intraoperative assessment of microperfusion with visible light spectroscopy for prediction of anastomotic leakage in colorectal anastomoses. Colorectal Dis. 2010;12(10):1018–1025. doi: 10.1111/j.1463-1318.2009.01944.x. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi H., Nara S., Ohigashi H. Is preservation of the remnant stomach safe during distal pancreatectomy in patients who have undergone distal gastrectomy? World J. Surg. 2013;37(2):430–436. doi: 10.1007/s00268-012-1860-1. [DOI] [PubMed] [Google Scholar]
- 8.Cooperman M., Martin E.W., Jr., Carey L.C. Evaluation of ischemic intestine by Doppler ultrasound. Am. J. Surg. 1980;139(1):73–77. doi: 10.1016/0002-9610(80)90232-9. [DOI] [PubMed] [Google Scholar]
- 9.Casati A., Fanelli G., Pietropaoli P. Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesth. Analg. 2005;101(3):740–747. doi: 10.1213/01.ane.0000166974.96219.cd. (Table of contents) [DOI] [PubMed] [Google Scholar]
- 10.Murkin J.M., Adams S.J., Novick R.J. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth. Analg. 2007;104(1):51–58. doi: 10.1213/01.ane.0000246814.29362.f4. [DOI] [PubMed] [Google Scholar]
- 11.Schein M., Saadia R. Postoperative gastric ischaemia. Br J. Surg. 1989;76(8):844–848. doi: 10.1002/bjs.1800760828. [DOI] [PubMed] [Google Scholar]
- 12.Isabella V., Marotta E., Bianchi F. Ischemic necrosis of proximal gastric remnant following subtotal gastrectomy with splenectomy. J. Surg. Oncol. 1984;25(2):124–132. doi: 10.1002/jso.2930250215. [DOI] [PubMed] [Google Scholar]
- 13.Rutter A.G. Ischaemic necrosis of the stomach following subtotal gastrectomy. Lancet. 1953;265(6794):1021–1022. doi: 10.1016/s0140-6736(53)91310-5. [DOI] [PubMed] [Google Scholar]
- 14.Bittner R., Butters M., Ulrich M. Total gastrectomy: updated operative mortality and long-term survival with particular reference to patients older than 70 years of age. Ann. Surg. 1996;224(1):37–42. doi: 10.1097/00000658-199607000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrosetti P., Robert J., Mathey P. Left-sided colon and colorectal anastomoses: doppler ultrasound as an aid to assess bowel vascularization. A prospective evaluation of 200 consecutive elective cases. Int. J. Colorectal Dis. 1994;9(4):211–214. doi: 10.1007/BF00292253. [DOI] [PubMed] [Google Scholar]
- 16.Urbanavicius L., Pattyn P., de Putte D.V. How to assess intestinal viability during surgery: a review of techniques. World J. Gastrointest. Surg. 2011;3(5):59–69. doi: 10.4240/wjgs.v3.i5.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita T., Sakaguchi T., Unno N. Intraoperative indocyanine green fluorography is useful in evaluating the blood flow of remnant stomach in distal pancreatectomy post distal gastrectomy. Jpn. J. Gastroenterol. Surg. 2014;47(12):762–767. [Google Scholar]
- 18.Hirano Y., Omura K., Yoshiba H. Near-infrared spectroscopy for assessment of tissue oxygen saturation of transplanted jejunal autografts in cervical esophageal reconstruction. Surg. Today. 2005;35(1):67–72. doi: 10.1007/s00595-004-2897-z. [DOI] [PubMed] [Google Scholar]
- 19.La Hei E.R., Shun A. Intra-operative pulse oximetry can help determine intestinal viability. Pediatr. Surg. Int. 2001;17(2-3):120–121. doi: 10.1007/s003830000484. [DOI] [PubMed] [Google Scholar]
- 20.Servais E.L., Rizk N.P., Oliveira L. Real-time intraoperative detection of tissue hypoxia in gastrointestinal surgery by wireless pulse oximetry. Surg. Endosc. 2011;25(5):1383–1389. doi: 10.1007/s00464-010-1372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohlenberg E., Payette J.R., Sowa M.G. Determining intestinal viability by near infrared spectroscopy: a veterinary application. Vib. Spectrosc. 2005;38(1):223–228. [Google Scholar]