Abstract
Holocarboxylase synthetase deficiency is an autosomal recessive disorder of biotin metabolism resulting in multiple carboxylase deficiency. The typical presentation described in the medical literature is of neonatal onset within hours to weeks of birth with emesis, hypotonia, lethargy, seizures, metabolic ketolactic acidosis, hyperammonemia, developmental delay, skin rash and alopecia. The condition is screened for by newborn screening (NBS) tandem mass spectroscopy by elevated hydroxypentanoylcarnitine on dried blood spots. Urine organic acid profile may demonstrate elevated lactic, 3-OH isovaleric, 3-OH propionic, 3-MCC, methylcitric acids, and tiglylglycine consistent with loss of function of the above carboxylases. Here we describe a cohort of patients, 2 diagnosed pre-NBS and 3 post-NBS with broad differences in initial presentation and phenotype. In addition, prior to the advent of NBS, there are isolated reports of late-onset holocarboxylase synthetase deficiency in the medical literature, which describe patients diagnosed between 1 and 8 years of life, however to our knowledge there are no reports of late-onset HCLS being missed by NBS. Also we report two cases, each with novel pathogenic variants HCLS, diagnosed at age 3 years and 21 months respectively. The first patient had a normal newborn screen whilst the second had an abnormal newborn screen but was misdiagnosed as 3-methylcrotonylcarboxylase (3-MCC) deficiency and subsequently lost to follow-up until they presented again with severe metabolic acidosis.
Abbreviations: 3-MCC, 3-methylcrotonyl-CoA carboxylase; ACP, acylcarnitine profile; DOL, day of life; HCLS, holocarboxylase synthetase; NBS, newborn screen
Keywords: Holocarboxylase synthetase deficiency, Metabolic acidosis
1. Introduction
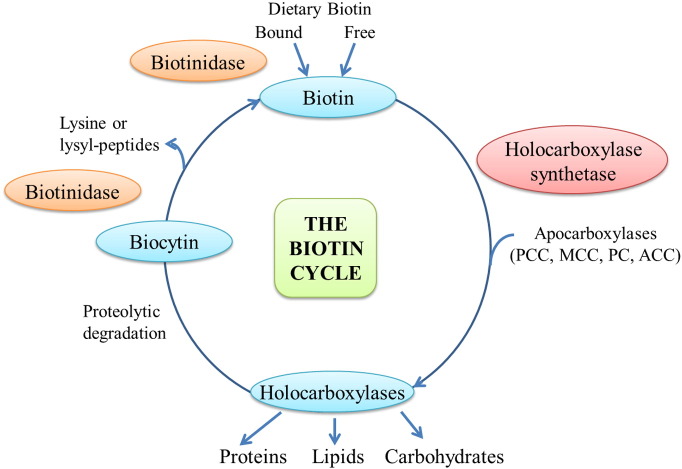
Holocarboxylase synthetase (HCLS) deficiency is an autosomal recessive disorder of biotin metabolism resulting in multiple carboxylase deficiency. It has an incidence of around 1/200,000 live births. HCLS is responsible for covalently linking biotin to propionyl-CoA carboxylase, 3-methylcrotonyl-CoA carboxylase (3-MCC), pyruvate carboxylase and acetyl-CoA carboxylase (see Fig. 1) [1], [2]. Failure to attach biotin causes reduced activity of these biotin-dependent carboxylases and results in multiple carboxylase deficiency. The typical presentation described in the medical literature is of neonatal onset within hours to weeks of birth with emesis, hypotonia, lethargy, seizures, metabolic ketolactic acidosis, hyperammonemia, developmental delay, skin rash and alopecia [1]. Left untreated, infants will progress to profound metabolic acidosis, cerebral edema, coma and death. Prior to the advent of universal newborn screening (NBS) in the United States, age of onset was used to differentiate between HCLS deficiency and biotinidase deficiency, with biotinidase deficiency generally presenting after 3 months [3]. The mainstay of treatment for HCLS deficiency is free biotin supplementation in an effort to alleviate the symptoms of the enzyme deficiency or prevent symptoms from developing in asymptomatic individuals.
Fig. 1.
The biotin cycle. Holocarboxylase synthetase covalently links biotin to the biotin-dependent carboxylases, propionyl-CoA carboxylase (PCC), 3-methylcrotonyl-CoA carboxylase (MCC), pyruvate carboxylase (PC), and acetyl-CoA carboxylase (ACC).
NBS in the United States is a public health initiative which includes screening, diagnosis, follow-up and management of inborn errors of metabolism identified at birth. The mainstay of testing for inborn errors of metabolism, (as opposed to other genetic conditions such as cystic fibrosis or congenital heart disease), involves tandem mass spectroscopy analysis of analytes such as amino acids, acylcarnitines and multiplex enzyme analysis on dried blood spots. Abnormal levels will flag newborns for subsequent confirmatory testing and referral to a metabolic specialist. HCLS deficiency is screened for through the detection of elevated hydroxypentanoylcarnitine (C5-OH). Urine organic acid profile may demonstrate elevated lactic, 3-OH isovaleric, 3-OH propionic, 3-MCC, methylcitric, and tiglylglycine consistent with loss of function of the above carboxylases. HCLS is caused by pathogenic variants in HLCS (21q22.1) which result in loss of function, through either reduced activity or absent activity of the holocarboxylase synthase enzyme [3].
The natural history of HCLS in the medical literature describes this condition as clinically severe often with significant intellectual disability and frequent hospitalizations with profound metabolic acidosis. Here we describe a cohort of patients, 2 diagnosed pre-NBS and 3 post-NBS with broad differences in initial presentation and phenotype. In addition, prior to the advent of universal NBS, there are isolated reports of late-onset holocarboxylase synthetase deficiency in the medical literature, which describe patients diagnosed between 1 and 8 years of life [2], [4], [5], [6], however to our knowledge there are no reports of late-onset HCLS being missed by NBS. Also we report two cases, each with novel pathogenic variants HCLS, diagnosed at age 3 years and 21 months respectively. The first patient had a normal newborn screen whilst the second had an abnormal newborn screen but was misdiagnosed as 3-methylcrotonylcarboxylase (3-MCC) deficiency and subsequently lost to follow-up.
2. Materials and Methods
2.1. Case 1
Case 1 is now a 5 year old female who was born to a 24-year-old G1P1 via normal vaginal delivery. Pregnancy had no complications except minor gestational diabetes that did not require treatment and no adverse exposures were reported. The patient's NBS was performed in Illinois and was normal. She had been healthy until age 2 years 6 months when she presented to the ER with an episode of emesis and lethargy. She received IV fluids, recovered rapidly and was subsequently discharged, with no biochemical testing being performed at this time.
She represented at age 3 years with 2 days of emesis, tachypnea, and anion gap metabolic acidosis with pH 7.1 (Normal 7.35–7.45), bicarbonate 11.1 mmol/l (normal 20–28), elevated lactate 5.6 mmol/l (Normal < 3), ketosis (beta-OH butyrate 5 mmol/l, normal < 0.3) and elevated ammonia 90 mmol/l (normal < 48).
Biochemical labs on the day of admission (see Table 1) demonstrated normal plasma amino acids, acylcarnitine profile with multiple elevations including C3 of 10,257 nmol/l (normal 0–870) and C5-OH 540 nmol/l (normal 0–540), urine organic acids with elevations in 3-hydroxybutyric, acetoacetic, lactic, 3-OH propionic, 3-OH isovaleric acids, 3-MCC and tiglylglycine consistent with a diagnosis of HCLS and urine acylglycines showed elevated 3-methylcrotonylglycine.
Table 1.
Biochemical & molecular results.
| Age at presentation | Initial presentation ACP C3 (normal 0–870 mmol/l) C5-0H (normal 0–110 mmol/l) |
Initial presentation organic acids elevations | HCLS sequencing & Del/Dup | Metabolically stable clinic follow-up ACP C3 (normal 0–870 mmol/l) C5-0H (normal 0–110 mmol/l) |
|
|---|---|---|---|---|---|
| Case 1 | 3 years 6 months | C0 – 30 C3 – 10,257 C5-OH – 540 |
|
|
C0 – 42 C3 – 1017 C5-OH – 18 |
| Case 2 | 24 months | CO – 42 C3 – 17,298 C5-OH – 2659 |
|
|
C0 – 44, 29, 13, 21, NR C3 – 26,560, 7719, 7690, 5366, 2570 C5-OH – 2267, 2446, 1922, 982, 700 |
| Case 3 | 18 days | C3 – elevated C5-OH – elevated |
|
|
C0 – 38, NR, NR, NR C3 – 3705, 960, 1520, 730 C5-OH – 411, 770, 360, 260 |
| Case 4 | 4 months | C3 – elevated C5-OH – elevated |
NR | NR - HCS enzyme in lymphoblasts showed no activity | C0 – 22 C3 – 602 C5-OH – 36 |
| Case 5 | 5 months | NR |
|
|
C0 – 24 C3 – 558 C5-OH – 68 |
Key: ACP–acylcarnitine profile, NR–no result available.
Sequencing of HCLS revealed a heterozygous known pathogenic variant, c.1993C > T (p.R655X) in exon 8 and a heterozygous novel pathogenic variant, c.500A > C (p.Y167S) in exon 2. The c.500A > C variant results in an amino acid substitution of tyrosine with serine at amino acid position 167. The tyrosine residue at this position is evolutionary conserved from yeast to humans, and PolyPhen-2 and SIFT predict this variant to be deleterious. Deletion/duplication testing of HCLS did not reveal any copy number changes. Biotinidase assay was normal.
Initial management consisted of fluid rehydration, correction of acidosis with sodium bicarbonate 1 mEq/kg, biotin 10 mg daily, thiamine 100 mg daily and levocarnitine 75 mg/kg daily. Once the diagnosis was confirmed thiamine was stopped. Her acidosis improved and she was discharged home with continuing biotin and levocarnitine.
Follow-up at age 4 showed she was doing well and had no further hospitalizations. Her C3 on ACP was down from 10,000 to 1000 nmol/l. Follow-up at age 5 showed that the patient continued to do well. She had one 24 h hospitalization due to emesis which resolved quickly with IV fluids. During this admission her biotin dose was doubled to 40 mg.
Physical exam consistently demonstrates a non-dysmorphic female child with no evidence of hypotonia. Her growth parameters at age 5 are: head circumference 52.2 cm (83rd percentile), height 110.4 cm (68th percentile) and weight 17.5 kg (41st percentile). Her development has been normal to date, parents are non-consanguineous and family history is non-contributory. She continues on 20 mg of biotin and ~ 25 mg/kg of levocarnitine daily.
2.2. Case 2
Case 2 is now a 5-year-old Hispanic male who was born to a 24-year-old mother with uneventful pregnancy and delivery. On day of life (DOL) 1, he had tachypnea, vomiting, feeding difficulties and was evaluated for possible sepsis. On DOL 5 his NBS reported abnormal values with elevated C5-OH and borderline elevated C3; plasma acylcarnitines, plasma amino acids, and urine organic acids were suggestive of 3-MCC deficiency, and he was discharged on DOL 10 with this diagnosis. He was lost to follow-up and re-presented acutely to hospital at 21 months with an episode of fever and emesis. He was noted to be severely acidotic with bicarbonate < 5 mmol/l, pH of 7.02 and lactate of 5.2 mmol/l. Other results included slightly elevated ammonia at 51 mmol/l and elevated beta-OH butyrate at 2.3 mmol/l.
Biochemical labs on day of admission (see Table 1) demonstrated plasma amino acids with low arginine (16 μmol/l, normal 42–132) with no other significant abnormalities, acylcarnitine profile with elevations of C3 17,298 nmol/l (normal 0–870) and C5-OH 2659 nmol/l (normal 0–540), urine organic acids with elevations in 3-OH isovaleric, 3-OH propionic, 3-MCC, lactic and methyl citric consistent with a diagnosis of HCLS.
Initial management was largely similar to Case 1 with the mainstay of treatment being IV fluids, sodium bicarbonate and biotin. Once his acidosis improved, the patient was discharged home with biotin and levocarnitine treatment.
Sequencing of HCLS revealed compound heterozygous novel pathogenic variants, c.1532A > T (p.N511I) in exon 6 and c.2078G > C (p.G693A) in exon 9. The c.1532A > T variant is predicted to result in an amino acid substitution of asparagine with isoleucine at amino acid position 511, which is evolutionary conserved from yeast to humans. A c.1533T > A (p.N511K) variant affecting the same amino acid residue was previously reported in trans with a c.1744G > A (p.G582R) variant [7]. This child had an onset of symptoms at 6 months with skin lesions, alopecia, atopic dermatitis, and mild acidosis and was responsive to biotin supplementation. The c.2078G > C variant is predicted to result in an amino acid substitution of glycine with alanine at amino acid position 693, which is also a highly conserved residue across species. PolyPhen-2 and SIFT predicted both variants to be deleterious. Deletion/duplication testing of HCLS was not performed.
He was followed regularly in the metabolic clinic after discharge. Compliance with biotin supplementation was problematic to the degree that G-tube placement was considered. At age 4 years 3 months he presented to the ER with emesis and diarrhea. Labs demonstrated profound metabolic acidosis with a pH 6.9 and bicarbonate levels of 3 mmol/l. Other labs on admission included an ammonia level of 76 mmol/l and lactate level of 8.9 mmol/l. He was treated with IV fluid and double dose bicarbonate (40 mg) with a bicarbonate infusion rate of 0.2 mEq/kg/h to correct his acidosis. He has since represented acutely multiple times due to failure to comply with his biotin treatment.
Physical exam consistently demonstrates a non-dysmorphic male child with no evidence of hypotonia. At age 5 his growth parameters are: head circumference 51.3 cm (50th percentile), height 104.2 cm (50th percentile) and weight 18.3 kg (57th percentile). His development has been normal to date, parents are non-consanguineous and family history is non-contributory. He continues on a biotin dosage of 40 mg with ~ 50 mg/kg of levocarnitine daily.
2.3. Case 3
Case 3 is now a 3 year 10 month old female diagnosed by abnormal NBS with elevated C5-OH. She was born at term to a 33 year old G3P1 mother by vaginal delivery. There were no complications noted in the pregnancy. She was initially evaluated at DOL 18 and was asymptomatic. Subsequent testing revealed elevated C3 and C5-OH on the plasma acylcarnitine profile. Her urine organic acid analysis revealed elevated levels of 3-hydroxyisovaleric acid and 3-MCC and slight elevations of 3-OH propionic and methylcitric acid. HLCS sequence analysis revealed a known heterozygous nonsense pathogenic variant, c.1693C > T (p.R565X), and a novel heterozygous missense variant, c.977G > A (p.G326E). Glycine at amino acid position 326 of the HLCS protein is evolutionarily conserved across species and SIFT and PolyPhen-2 algorithms predict p.G326E to be deleterious. Parental segregation analysis confirmed the variants were present in trans.
Physical exam consistently demonstrates a non-dysmorphic female child with no evidence of hypotonia. At age 33 months her growth parameters are: head circumference 48.9 cm (51st percentile), height 96 cm (> 100th percentile, Z-score 8.22) and weight 14.6 kg (> 100th percentile, Z-score 8.22). At age 2, her parents noticed she was suffering from alopecia on the left side of her head, likely secondary to HCLS. Her development has been normal to date, parents are non-consanguineous and family history is non-contributory. She continues on biotin 30 mg twice daily.
2.4. Case 4
Case 4 is now a 23-year-old female born to a 21-year-old mother after an uncomplicated pregnancy who presented at 4 months of age with emesis, skin rash, recurrent infections and acidosis which required multiple hospitalizations. At age 14 months, during a hospitalization with acidosis, urine organic acids were sent and her results were consistent with HCLS. A biotinidase assay done at this time was normal. HCS enzyme activity was sent and showed no detectable activity in lymphocytes which confirmed the diagnosis. From birth to age 3 she had multiple admissions, she then had a long period without hospitalizations until age 12. At follow-up appointments she reported long periods, up to several months, where she did not take biotin. She reported feeling more weak and would develop a skin rash on her face, chest and extremities and developed alopecia. The symptoms resolved when she restarted biotin. Compliance continues to be a problem although she has not suffered any recent hospitalizations with acidosis. She is developmentally normal and physical exam shows a non-dysmorphic female of normal stature (168.9 cm) and weight (57.3 kg, BMI 20.09 kg). She does not demonstrate any tremor, ataxia, seizures, vision or hearing complaints. She has successfully had 2 children without complication. She continues on 200 mg daily of biotin.
2.5. Case 5
Case 5 is now a 17-year-old male born to a 27-year-old G1P1 after an uncomplicated pregnancy and delivery. He was hospitalized for 2 weeks after delivery for pneumonia. He was doing well up until 5 months of age when parents noted focal seizures. Urine organic acids were ordered as part of the workup and showed elevations in 3-OH isovaleric acid and 3-methylcrotonoylglycine and he was initially given a diagnosis of 3-MCC deficiency. He was treated with IV fluids, bicarbonate, biotin and levocarnitine and improved. He was hospitalized for tachypnea and dehydration at 12 months and admission labs showed a pH of 7.08, bicarbonate of 5 mmol/l and a lactate of 10.7 mmol/l. Mild developmental delays were apparent at this time. He continued to have multiple hospitalizations and biotinidase testing at this time was normal. HCS enzyme activity was measured and showed reduced activity of PCC, MCC and PC and a large decrease in Vmax, 3% of controls confirming the diagnosis of HCLS. Around age 12, HCLS gene sequencing was performed and revealed compound heterozygous novel variants, including c.1710C > G (p.N570K) and c.1519 + 5G > A. Asparagine at position 570 of the HLCS protein is highly conserved from yeast to human. SIFT and PolyPhen-2, predict p.N570K to be deleterious. NetGene2 and BDGP were used to predict the possible effects of the c.1519 + 5G > A variant on normal splicing. Both algorithms predicted that this variant will not affect normal splicing.
He continued to have intractable seizures with as many as 4 to 6 per day until the vagal nerve stimulator was placed which reduced the frequency of seizures. Multiple medications including phenobarbital, clonazepam, ethosuximide and lamotrigine had failed to control his seizures previously. At MRI was performed around 2 years of age showed microcephaly, mild-to-moderate ventricular enlargement, and delayed myelination. The patient continues to suffer from seizures, has severe intellectual disability, is nonverbal and wheelchair bound. Physical exam demonstrates microcephaly with head circumference 52 cm (< 1st percentile, Z-score − 8.22), height 158.5 cm (1st percentile, Z-score − 2.32) and weight 47 kg (1st percentile, Z-score − 2.44). He continues on 50 mg BID of biotin, glycopyrrolate, lamotrigine and baclofen.
3. Discussion
HCLS is an autosomal recessive disorder of biotin metabolism which typically presents in the neonatal period. With the advent of universal NBS in the United States affected patients can be identified prior to the onset of symptoms. This helps prevent the life threating complications of metabolic acidosis, seizure, and hyperammonemia that can result in long-term neurological sequelae and developmental disability.
Case 1 presented 6 months prior to her diagnosis with an episode of emesis, lethargy and metabolic acidosis. This case highlights the need for prompt analysis by specialists in inborn errors of metabolism and early consideration of biochemical testing such as an acylcarnitine profile or urine organic acid testing. We posit that any case of severe acidosis without clear cause in a child warrants a metabolic evaluation by a biochemical geneticist.
Case 2 demonstrates that HCLS may be misdiagnosed by NBS if analyte testing does not show pathognomonic analytes for the disorder and the clinical phenotype is not consistent with the classic presentation. Both Case 2 and Case 5 were misdiagnosed as 3-MCC until patients presented again with severe acidosis which prompted additional testing. Case 2 highlights the need for combined biochemical and molecular testing for disorders which may have similar biochemical abnormalities, particularly in cases with a milder phenotypic presentation that may be characteristic of more severe disorders which can be missed. Additionally, late presentation does not necessarily mean a milder disease course as this patient had multiple hospitalizations with severe acidosis, all of which are a result of lack of biotin supplementation, either through lack of compliance or medical illness.
Case 3 highlights the success and impact of expanded universal NBS. This patient was quickly diagnosed and promptly placed on biotin at DOL 18 and has not had any complications except mild alopecia. Conversely, Case 1 and 2 highlight that there are patients that are not identified by NBS and do go on to develop life-threatening complications of HCLS. Often when evaluating an infant we are reassured the NBS is normal and so the disorders on the NBS testing panel are thought less likely to be present. These cases serve as a reminder that patients require a full metabolic workup even if NBS testing has been normal.
Case 4 had a severe classical presentation at 3 months yet has not had any major problems and is now 23-years-old. To our knowledge this is the longest follow-up of a case of HCLS and demonstrates a relatively benign phenotype after early childhood if biotin is given regularly. This phenotype is despite not taking biotin for months at a time, during which time she develops a skin rash and feels more lethargic. Taken together with Case 5, who also presented with a severe classical presentation at 4 months it is clear the phenotypic spectrum of HCLS is very broad, from severe intractable seizures and intellectual disability to normal growth and development. In addition we feel that prompt treatment of Case 3 has prevented possible adverse outcomes.
In addition we report 6 novel pathogenic variants in HCLS, c.500A > C (p.Y167S), c.1532A > T (p.N511I), c.2078G > C (p.G693A), c.977G > A (p.G326E), c.1710C > G (p.N570K) and c.1519 + 5G > A, and thus expand the pathogenic variant spectrum of HCLS.
4. Conclusion
In summary we report 5 cases of HCLS with phenotype ranging from severe neonatal presentation with a severe neurological presentation to initial presentation after age 3 with normal growth and development. We highlight the fact that two of these cases presented with severe life-threatening metabolic acidosis outside the neonatal period and now require life-long biotin supplementation. In addition once they are diagnosed their course can be complicated with multiple hospitalizations with acidosis as highlighted in Case 2.
Disclosure
None.
Contribution statement
All authors above made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work AND drafting the work or revising it critically for important intellectual content AND gave final approval of the version to be published AND agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgments
None.
References
- 1.Van Hove J.L., Josefsberg S., Freehauf C., Thomas J.A., le P. Thuy, Barshop B.A., Woontner M., Mock D.M., Chiang P.W., Spector E., Meneses-Morales I., Cervantes-Roldan R., Leon-Del-Rio A. Management of a patient with holocarboxylase synthetase deficiency. Mol. Genet. Metab. 2008;95:201–205. doi: 10.1016/j.ymgme.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suormala T., Fowler B., Jakobs C., Duran M., Lehnert W., Raab K., Wick H., Baumgartner E.R. Late-onset holocarboxylase synthetase-deficiency: pre- and post-natal diagnosis and evaluation of effectiveness of antenatal biotin therapy Eur. J. Pediatr. 1998;157:570–575. doi: 10.1007/s004310050881. [DOI] [PubMed] [Google Scholar]
- 3.B. W. Biotinidase deficiency. In: RA Pagon, MP Adam, HH Ardinger., editors. GeneReviews® [Internet] Seattle (WA): University of Washington; Seattle: 1993–2015. (2000 Mar 24 [Updated 2013 Dec 5]) [Google Scholar]
- 4.Gibson K.M., Bennett M.J., Nyhan W.L., Mize C.E. Late-onset holocarboxylase synthetase deficiency. J. Inherit. Metab. Dis. 1996;19:739–742. doi: 10.1007/BF01799165. [DOI] [PubMed] [Google Scholar]
- 5.Hwu W.L., Suzuki Y., Yang X., Li X., Chou S.P., Narisawa K., Tsai W.Y. Late-onset holocarboxylase synthetase deficiency with homologous R508W mutation. J. Formos. Med. Assoc. 2000;99:174–177. [PubMed] [Google Scholar]
- 6.Vitoria I., Rausell D., Gonzalez I., Perez-Cerda C., Dalmau J. Delayed onset holocarboxylase synthetase deficiency with normal pyruvate carboxylase activity. An. Pediatr. (Barc.) 2014;80:184–186. doi: 10.1016/j.anpedi.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Morrone A., Malvagia S., Donati M.A., Funghini S., Ciani F., Pela I., Boneh A., Peters H., Pasquini E., Zammarchi E. Clinical findings and biochemical and molecular analysis of four patients with holocarboxylase synthetase deficiency. Am J Med Gen. 2002;111:10–18. doi: 10.1002/ajmg.10532. [DOI] [PubMed] [Google Scholar]