Abstract
Tristetraprolin (TTP) is a 34-kDa, zinc finger-containing factor that in mammalian cells acts as a tumor suppressor protein through two different mechanisms. In the cytoplasm TTP promotes the decay of hundreds of mRNAs encoding cell factors involved in inflammation, tissue invasion, and metastasis. In the cell nucleus TTP has been identified as a transcriptional corepressor of the estrogen receptor alpha (ERα), which has been associated to the development and progression of the majority of breast cancer tumors. In this work we report that nuclear TTP modulates the transactivation activity of progesterone receptor (PR), glucocorticoid receptor (GR) and androgen receptor (AR). In recent years these steroid nuclear receptors have been shown to be of clinical and therapeutical relevance in breast cancer. The functional association between TTP and steroid nuclear receptors is supported by the finding that TTP physically interacts with ERα, PR, GR and AR in vivo. We also show that TTP overexpression attenuates the transactivation of all the steroid nuclear receptors tested. In contrast, siRNA-mediated reduction of endogenous TTP expression in MCF-7 cells produced an increase in the transcriptional activities of ERα, PR, GR and AR. Taken together, these results suggest that the function of nuclear TTP in breast cancer cells is to act as a corepressor of ERα, PR, GR and AR. We propose that the reduction of TTP expression observed in different types of breast cancer tumors may contribute to the development of this disease by producing a dysregulation of the transactivation activity of multiple steroid nuclear receptors.
Abbreviations: TTP, tristetraprolin; ERα, estrogen receptor; PR, progesterone receptor; AR, androgen receptor; GR, glucocorticoid receptor
Keywords: Tristetraprolin, Nuclear receptor, Corepressor, Transactivation activity, Estrogen, Progesterone, Glucocorticoid, Androgen, Breast cancer
1. Introduction
Tristetraprolin (TTP) is a 34-kDa protein characterized by the presence of two tandemly located CCCH-type zinc finger domains and three proline rich motifs [1], [2], [3], [4]. In mammalian cells TTP is localized both in the nucleus and cytoplasm and its total protein expression and organelle-specific distribution are regulated by signal transduction pathways activated by interleukins, growth factors and glucocorticoids [5], [6], [7], [8], [9]. Functional analysis performed in animal models and cells in culture revealed that the cytoplasmic form of TTP uses its zinc finger domains to interact with AU-rich elements (AREs) present in the 3′-untranslated region of mRNAs [10], [11], [12], [13]. The binding of TTP accelerates mRNA degradation by contributing to the recruitment of the Ccr4/Caf1/Not deadenylation complex [14].
Although AREs are present in 8% to 10% of all mammalian transcripts, TTP has been shown to selectively target around 250 mRNAs encoding factors involved in regulation of cell growth, inflammation, metastasis and apoptosis [15], [16], [17]. These results have led different investigators to suggest that TTP is involved in the control of neoplasic development in diverse tissues [18]. The function of TTP as a tumor suppressor protein has been supported by the finding that its protein expression levels negatively correlate with tumor progression in different types of cancer tumors [15], [19], [20].
Recently, our laboratory demonstrated that the nuclear form of TTP acts as a corepressor of estrogen receptor a (ERα), a ligand activated transcription factor associated to the development of 70%–80% of all breast cancer tumors [4], [21]. Mechanistically, TTP was shown to repress ERα transactivation by disrupting its interaction with the steroid receptor coactivator 1 (SRC-1) and by facilitating the recruitment of histone deacetylase 1 (HDAC1) to the promoter region of estradiol-responsive genes [4]. This study demonstrated that TTP overexpression reduces the proliferation rate and tumorigenicity potential of human breast cancer cells in mice [4]. These results allowed us to suggest that nuclear TTP also acts as a tumor suppressor protein through a mechanism that involves its ability to regulate ERα transactivation [4].
In this work, we sought to gain better insight into the function of nuclear TTP as a transcriptional corepressor by exploring its effect on the activity of other hormone nuclear receptors that have been recently shown to be of clinical or therapeutical relevance in breast cancer [22], [23], [24], [25]. Our results show that TTP is physically associated to progesterone receptor (PR), glucocorticoid receptor (GC) and androgen receptor (AR) in the nucleus of breast cancer MCF-7 cells in vivo. We show further that TTP overexpression reduces ERα, PR, GR and AR transactivation activity. In contrast, siRNA-mediated reduction of endogenous TTP protein levels produced a significant increase in the transcriptional activity of these steroid nuclear receptors without affecting their protein levels in MCF-7 cells. These results suggest that the corepressor activity of nuclear TTP may participate in the regulation of multiple hormone-dependent signaling pathways that play a key role in the development of human normal mammary gland epithelium and breast cancer tumors.
2. Materials and methods
2.1. Reagents and Antibodies
Estradiol (E8875, 17β-estradiol), progesterone (P-0130, 4-Pregnene-3,20 dione) and dexamethasone (D1756), were obtained from Sigma-Aldrich. Androgen (10300, 5α-Androstan-17β-ol-3-one) was obtained from Fluka Chemika. Human ERα antibody (Sc D-12, Sc HC-20) and Human TTP antibody (Sc-12565) were purchased from Santa Cruz Biotechnology, Inc. and TTP polyclonal (T5327) antibody was from Sigma-Aldrich. Human PR (ab-68195), GR (ab-2768) and AR (ab-74272) antibodies were from Abcam. TTP knockdown assays were performed using TTP siRNA mixture and control siRNA from Santa Cruz Biotechnology (Sc-36760, Sc-37007). Lipofectamine 2000 was purchased from Invitrogen by life Technologies (11668-019).
2.2. Plasmids
ERα transactivation activity was determined using a luciferase reporter gene driven by a promoter containing three estrogen responsive elements (ERE-Tk-LUC) that has been previously described [4], [26]. To test the transactivation activity of PR, AR. and GR, human cells were transfected with the PGL3-MMTV in which the luciferase reporter gene is regulated by a promoter containing a palindromic sequence recognized by PR, GR and AR. pcDNA3.1-ERα and ERE-Tk-LUC vectors were kindly provided by Dr. W. Lee Kraus (Cornell University). Vectors PRE-dbCAT (PR), pSVhARo-BHEXE (AR), pcDNA3.1-GR and PGL3-MMTV were a gift from Dra. Rocío Ángeles García Becerra (Instituto Nacional de Ciencias Médicas y Nutrición-México). Human full-length TTP cDNA (GenBank™ accession no. NM_003407.3) was cloned into the mammalian expression vector pCMV-3Tag-1 A (Agilent Technologies, Santa Clara, CA) as previously described [4]. The sequences of all constructs were verified by DNA sequencing at LARAGEN Inc. (Culver City, CA).
2.3. Cell culture and transfection assays
MCF-7 cells (ATCC HTB-22, American Type Culture Collection, Manassas, VA) were maintained in Minimum Essential medium Alpha Medium (11900-024, Gibco) supplemented with 5% FBS, 100 units/ml penicillin, and 100 mg/ml streptomycin in a humidified atmosphere containing 5% CO2 at 37 °C. Cells were seeded into tissue culture dishes containing phenol red-free Minimum Essential Medium Eagle (M3024, Sigma-Aldrich) supplemented with 5% charcoal/dextran-treated FBS and cultured for 36–40 h before all experimental treatments with hormone. Cells were transfected using Lipofectamine 2000 with 2 mg of ERE-Tk-LUC reporter, 1 mg of pcDNA3. 1-ERα, PR, GR, AR and 0.5 mg pCMV-3Tag-1 A-TTP or 0.006 mg of Renilla vector (transfection control). After 4 h, the cells were washed twice with phosphate-buffered saline and treated with 100 nM of estradiol, progesterone, dexamethasone or androgens for 48 h in phenol red-free MEM supplemented with 5% stripped FBS. Cells were then washed and harvested in Passive Lysis Buffer (Promega). Control cells were incubated under the same experimental conditions in the absence of hormones. Luciferase and Renilla activities were measured using a Dual-Luciferase Reporter Assay System (E1960, Promega) and Glomax Multi JR Detection System (Promega). TTP knockdown assays were performed using TTP siRNA mixture and control siRNA from Santa Cruz Biotechnology and transfected using Lipofectamine 2000 (Invitrogen). The specificity of the TTP knockdown assay was determined by analyzing the expression of TTP, ERα, PR, GR and AR in MCF-7 cells transfected with a control siRNA or with a specific siRNA-TTP.
2.4. Immunoprecipitation and Western blot
MCF-7 cells were lysed with TNTE buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 0.5% Triton X-100 plus a mixture of protease and phosphatase inhibitors). Proteins were immunoprecipitated with mouse anti-ERα, anti-GR or rabbit anti-PR and anti-AR antibodies. Immunoprecipitated proteins were separated by SDS-PAGE, and Western blot analysis was performed using a specific antibodies anti-TTP and anti-mouse or anti-rabbit secondary HRP-conjugated antibody (Pierce). Proteins were visualized using an enhanced chemiluminescence assay (SuperSignal West Pico Chemiluminescent Substrate, Thermo Scientific).
2.5. Statistical analysis
Each transfection assay was performed by triplicate in three different experiments using different cell cultures. Data are presented as mean ± S.E. Statistical significance was analyzed at 0.05 levels of significance using Student's t-test.
3. Results
3.1. Correlation between TTP mRNA expression levels and breast carcinoma using the Oncomine cancer microarray database
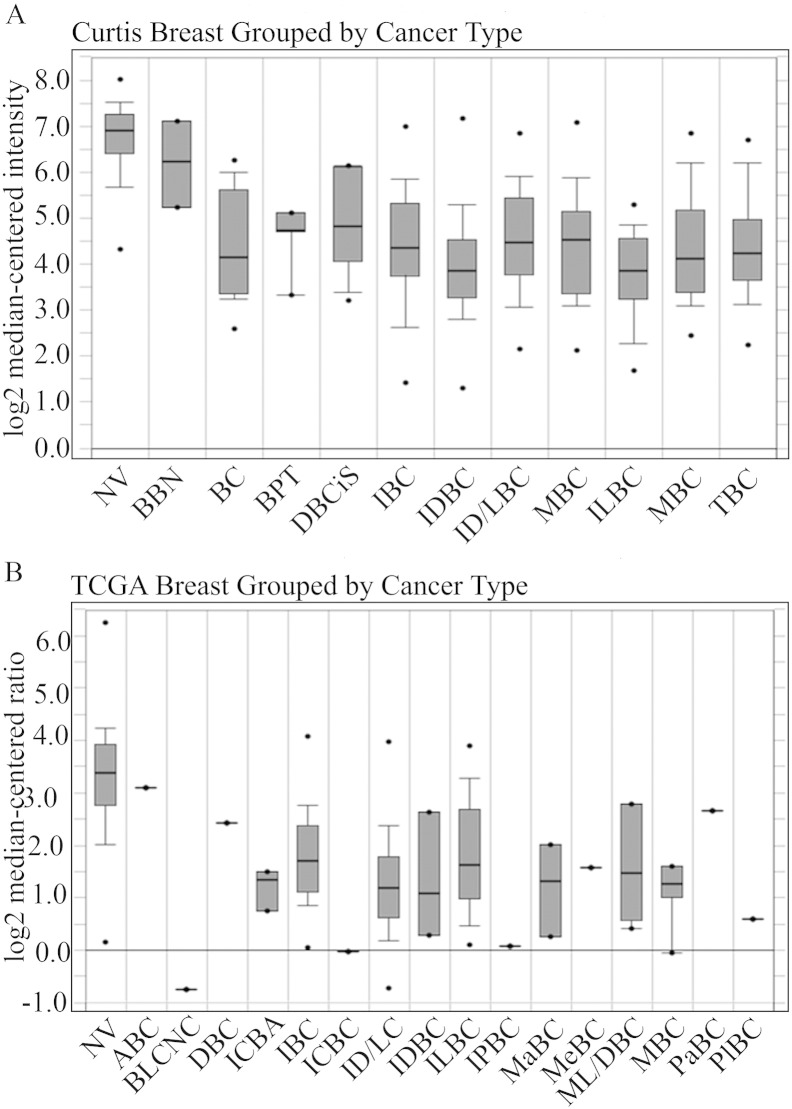
In this study, we set out to determine the effect of TTP on the transactivation activity of the steroid nuclear receptors PR, GR and AR that along with ERα have been associated to cell proliferation, tumor development, apoptosis control and response to hormonal therapy in breast cancer. To explore the relationship between TTP expression and breast cancer tumorigenesis, we compared TTP mRNA levels in normal tissues and different types of breast carcinomas using the Oncomine database (http://www.oncomine.org, Compendia Bioscience, Ann Arbor, MI). The analysis of data sets containing gene chip profiles classified as normal or breast carcinoma tissues, showed a significant reduction in TTP mRNA levels in breast carcinomas compared to normal tissue. Two representative results of two independent data sets [27] are shown in Fig. 1.
Fig. 1.
TTP mRNA expression in a breast cancer microarray database. TTP mRNA expression in normal and breast carcinoma tissues was analyzed using the Oncomine database. Two representative results obtained from independent data sets are shown. (A) The tissues analyzed in Curtis data set are normal breast (N), benign breast neoplasm (BBN), breast carcinoma (BC), breast phyllodes tumor (BPT), ductal breast carcinoma in situ (DBCis), invasive breast carcinoma (IBC), invasive ductal breast carcinoma (IDBC), invasive ductal and invasive lobular breast carcinoma (ID/LBC), invasive lobular breast carcinoma (ILBC), medullary breast carcinoma (MeBC), mucinous breast carcinoma (MuBC) and tubular breast carcinoma (TBC). (B) The tissues analyzed in TCGA data set are normal breast (N), apocrine breast carcinoma (ABC), breast large cell neuroendocrine carcinoma (BLCNC), ductal breast carcinoma (DBC), intraductal cribriform breast adenocarcinoma (ICBA), invasive breast carcinoma (IBC), invasive cribriform breast carcinoma (ICBC), invasive ductal and lobular carcinoma (ID/LC), invasive ductal breast carcinoma (IDBC), invasive lobular breast carcinoma (ILBC), invasive papillary breast carcinoma (IPBC), male breast carcinoma (MaBC), metaplastic breast carcinoma (MeBC), mixed lobular and ductal breast carcinoma (ML/DBC), mucinous breast carcinoma (MBC), papillary breast carcinoma (PaBC), pleomorfic breast carcinoma (PlBC).
3.2. TTP interacts with different steroid nuclear receptors in MCF-7 cells in vivo
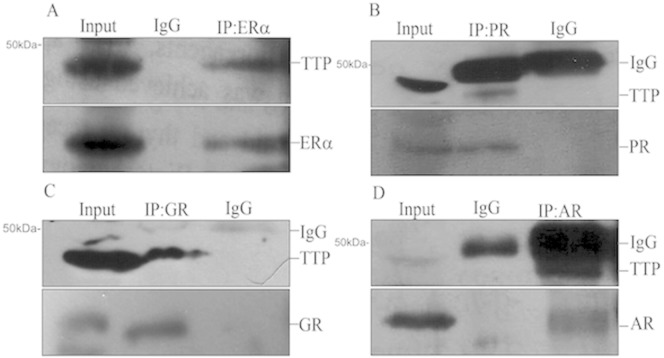
To study the functional interaction between TTP and steroid nuclear receptors we used breast cancer MCF-7 cells that had previously shown to express multiple steroid nuclear receptors [25], [28], [29], [30], [31], [32], [33], [34], [35], [36]. To explore the physical interaction between TTP and the steroid nuclear receptors ERα, PR, GR and AR, we performed coimmunoprecipitation assays in MCF-7 cells. Endogenous nuclear hormone receptors present in MCF-7 protein extracts were immunoprecipitated with anti-ERα, anti-PR, anti-GR, anti-AR antibodies or IgG (negative control). The interaction of nuclear receptors with TTP was visualized by immunoblotting using anti-TTP antibody. As previously shown TTP is associated with ERα in MCF-7 cells [4] (Fig. 3, upper panel). Our results showed that TTP also interacts with PR, GR and AR in MCF-7 cells in vivo (Fig. 2B, C and D, upper panels). The absence of a TTP reactive band when the protein extracts were immunoprecipitated in the presence of IgG suggest that interaction between TTP and ERα, PR, GR and AR is specific. As a control, 10% of the protein extracts used in each immunoprecipitation assay were analyzed by Western blot using anti-ERα, anti-PR, anti-GR and anti-AR to confirm the presence of the nuclear receptors in the protein extracts (Fig. 2A–D, input lanes). The immunoprecipitation efficiency in every assay was tested by subjecting the membranes to a second immunoblotting with the specific antibody used for protein immunoprecipitation (Fig.2A–D, lower panels).
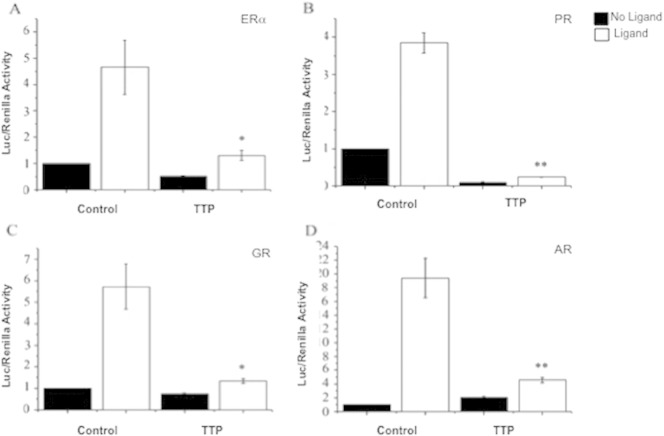
Fig. 3.
TTP overexpression represses ERα, PR, GR and AR transactivation in MCF-7 cells. Human breast cancer MCF-7 cells were transiently transfected with empty pCMV-3TAG vector (control) or with 0.25 μg of pCMV-3TAG-TTP (FLAG-TTP) along with the corresponding luciferase reporter vector. The effect of TTP on nuclear hormone receptors transactivation was determined by assay of luciferase activity, as described under “Experimental Procedures.” Assays were performed in triplicate in three independent experiments in the presence (white bars) or absence (black bars) of the corresponding nuclear receptor ligands (17β-estradiol for ERα, progesterone for PR, 5α-Androstan-17β-ol-3-on for AR and dexamethasone for GR. The results are represented as mean ± S.E. Differences in ERα activity between control MCF-7 cells and TTP-expressing MCF-7 cells are statistically significant (p < 0.05).
Fig. 2.
TTP interacts with ERα, PR, GR and AR in vivo. Endogenous ERα (A), PR (B), AR (C) and GR (C) were immunoprecipitated from protein extracts prepared from MCF-7 cells using specific antibodies. Immunoprecipitated proteins were resolved by PAGE, and the binding of TTP was visualized by Western blot. The input lane represents 10% of the protein extract used in the coimmunoprecipitation assays. IP: immunoprecipitation, WB: Western blot.
3.3. TTP represses ERα, PR, GR and AR transcriptional activity
To test whether TTP expression affects PR, GR and AR transactivation we performed transient transfection assays in MCF-7 cells. In these experiments, pcDNA3.1-TTP was the source of TTP, and the luciferase reporter vectors ERE-Tk-LUC and MMTV-LUC were used as indicators of ERα, PR, GR and AR transcriptional activity as described in materials and methods. Endogenously expressed ERα, PR, GR and AR showed baseline luciferase activity in MCF-7 cells that increased upon stimulation with their respective hormone ligands (Fig. 3A–D). As previously reported [4] transfection of MCF-7 cells with 250 ng of pCMV-3Tag-1 A-TTP in the presence of 17β-estradiol reduced the transcriptional activity of ERα by 73% (Fig. 3A). The same treatment reduced the ligand-dependent transactivation of PR, GR and AR by 93%, 77% and 76%, respectively (Fig. 3B–D). These results suggest that TTP acts as a common transcriptional corepressor for ERα, PR, GR and AR in MCF-7 cells.
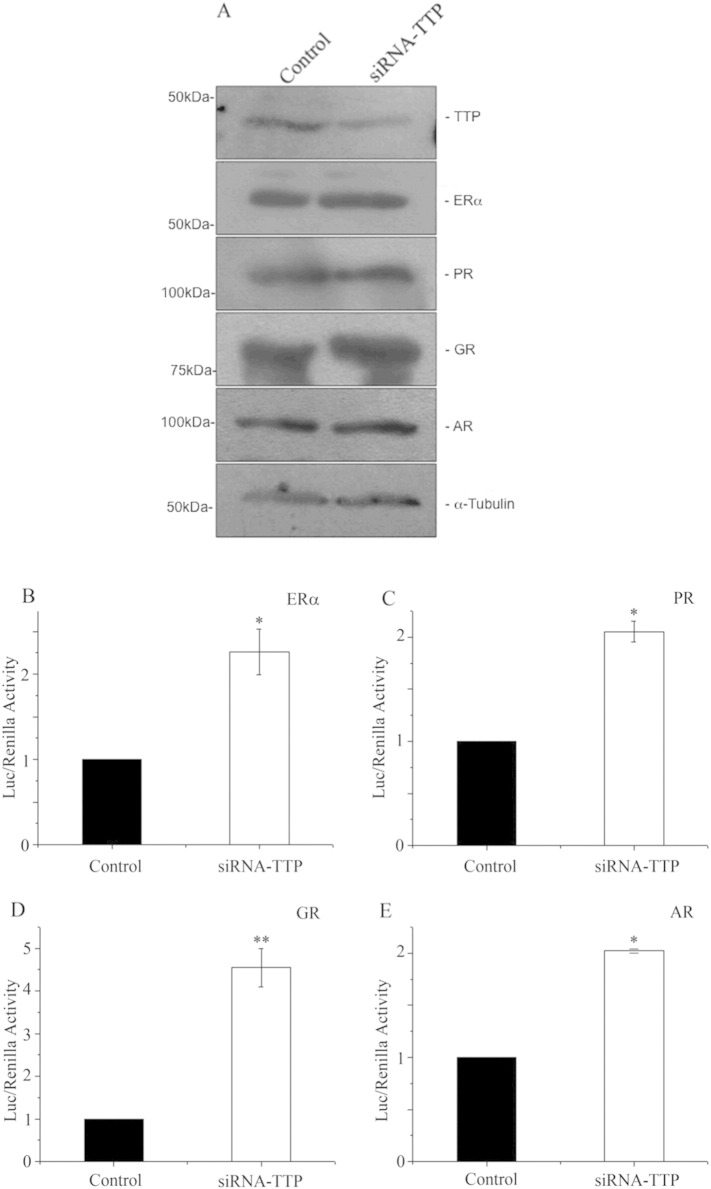
Next, we used siRNA-mediated knockdown assays to mimic the TTP loss of expression observed in different carcinomas and to determine the functional relevance of this factor in the transactivation activity of endogenously expressed ERα, PR, GR and AR in MCF-7 cells. The siRNA-mediated reduction of TTP expression was confirmed by WB analysis of protein extracts prepared from MCF-7 cells transfected with a specific TTP-siRNA or with an unrelated control siRNA. The results showed a significant reduction in TTP expression in cells transfected with TTP-siRNA compared to MCF-7 cells transfected with the control siRNA (Fig. 4(A), TTP panel). Transfection of MCF-7 cells with TTP-siRNA did not affect the expression of ERα, PR, GR and AR, or the protein levels of tubulin that was used as an unrelated loading control protein (Fig. 4(A)). These results confirm the specificity of the siRNA on TTP expression and suggest that TTP does not affect the expression of ERα, PR, GR and AR. Next, we determined the hormone-dependent activity of ERα, PR, GR and AR in MCF-7 cells transfected with TTP-siRNA or the control siRNA. Our results showed that siRNA-mediated knockdown of TTP expression increased the hormone-dependent transactivation activity of all steroid nuclear receptors tested, compared to MCF-7 cells transfected with siRNA control (Fig. 4(B)). These results indicate that TTP is an important participant in the regulation of ERα, PR, GR and AR transactivation and suggest the possibility that loss of TTP expression observed in breast cancer tumors may cause a dysregulation of multiple steroid nuclear receptor-dependent signaling pathways.
Fig. 4.
siRNA-mediated knockdown of TTP protein levels in MCF-7 cells. MCF-7 cells were transfected with specific siRNA-TTP (1.25 μg) or with an unrelated control siRNA (2.5 μg). (A) Protein extracts from MCF-7 cultures were resolved by PAGE, and expression levels of TTP, ERα, PR, GR, AR and tubulin, as a loading control protein, were evaluated by Western blot using specific antibodies as described under “Experimental Procedures.” TTP knockdown increases ERα, PR, GR and AR transactivation. MCF-7 cells were transfected with an unrelated siRNA (control) or a specific TTP-siRNA (1.5 μg), along with TK-LUC, in the presence of the corresponding nuclear receptor ligands. The effect of TTP-siRNA on ERα (B), PR (C), GR (D) and AR (E) transactivation was determined by a luciferase assay and compared with luciferase activity in MCF-7 cells transfected with empty pCMV-3TAG vector and the corresponding Tk-LUC reporter vector. Results, in triplicate in three independent experiments, are represented as mean ± S.E. (error bars). Differences in ERα activity in MCF-7 cells transfected with TTP or with TTP-siRNA were shown to be statistically significant (p < 0.05).
4. Discussion
Functional studies performed in mammalian cells had previously shown that TTP function as a tumor suppressor is mediated by two different mechanisms that depend on its cellular localization [4], [37]. In the cytoplasm, TTP promotes the decay of mRNAs associated to inflammation, angiogenesis, tissue invasion, and metastasis (53–58). The nuclear form of TTP acts as a transcriptional corepressor via its interaction with HDACs. However, while cytoplasmic TTP regulates the expression levels of hundreds of mRNAs, its nuclear regulatory function had only been found to modulate the transcriptional activity of p65/NF-kB and ERα [4], [37].
In this work, we have substantially extended the known targets of nuclear TTP by demonstrating it corepresses the transactivation of the steroid nuclear receptors PR, GR and AR in breast cancer MCF-7 cells. The functional association of TTP with these steroid nuclear hormone receptors was confirmed by different experimental strategies. First, coimmunoprecipitation assays showed that endogenous TTP physically interacts with PR, GR and AR in MCF 7 cells in vivo. Second, transient transfection of TTP produced a significant reduction in the transactivation activity of PR, GR and AR. Third, siRNA-mediated reduction of endogenous TTP protein levels increased the transcriptional activity of all the steroid nuclear receptors tested. Further, reduction of endogenous TTP protein levels in MCF-7 cells did not affect the apparent abundance of ER, PR, GR and AR, suggesting that the mechanism responsible for TTP-mediated repression of steroid nuclear receptors transactivation does not involve its mRNA destabilizing activity.
The tumor suppressor activity of nuclear TTP in breast cancer cells has been attributed to its ability to repress the transcriptional activity of ERα, a ligand activated transcription factor that is associated to the growth and progression of 70% to 80% of all breast cancer tumors. However, the results obtained in this study suggest that TTP regulates the activity and functions of other steroid nuclear receptors that also play an important role in tumor development and may influence tumor response to treatment [38]. A recent report has shown that PR acts as an ERα associated protein that redirects the binding of ERα to different chromatin sites. The PR-ERα complex is responsible for a novel gene expression pattern that inhibits the estradiol-dependent proliferation of breast cancer cells. Interestingly, the PR-dependent reprogramming of ERα regulates the transcription of the ZFP36 gene which encodes TTP [39]. Other studies have shown that the nuclear receptor GR is present in 50% of invasive breast cancers and most of the breast cancer cell lines used in biomedical research. Ligand-dependent activation of GR has been associated to inhibition of the apoptotic response to chemotherapy treatment in breast cancer cells through its effect on the transcriptional activation of Bcl-xL, Bak, SGK-1 and MKP-1 [40], [41], [42], [43], [44], [45]. Finally, different studies have suggested that crosstalk between AR and ERα is involved in the regulation of normal mammary gland and breast cancer development [46]. In ERα-positive breast cancer tumors, higher AR expression levels are associated with lower grade, reduced lymph node involvement and longer disease-free survival [47], [48], [49], [50].
The function of TTP as a corepressor of multiple steroid nuclear receptors in MCF-7 cells suggest that this protein may participate in the down-regulation of the different functions of ERα, PR, GR and AR in mammary gland cells. The results from this work and previous studies that show that TTP expression is reduced in different types of cancer tumors supports the role of nuclear TTP as a tumor suppressor protein [19]. We propose that alterations in TTP expression may contribute to the development and progression of breast cancer tumors by the dysregulation of ERα and other steroid nuclear receptors signaling pathways. This hypothesis is supported by the observation that siRNA-mediated reduction of endogenous TTP expression levels produced a significant change in ERα, PR, GR and AR transactivation in MCF-7 cells. Based on the impact of the steroid nuclear receptors on the human genome, it is conceivable that TTP could participate in the transcriptional control of a numerous collection of genes. Further studies will be necessary to identify and characterize the genes regulated by nuclear TTP and to explore their potential as novel tumor markers or therapeutic targets for the diagnostic and treatment of breast cancer.
Funding
These studies were supported by grants of Consejo Nacional de Ciencia y Tecnologia (CB2014-236405-B) and Programa de Apoyo a Proyectos de Investigacion e Innovacion Tecnologica, Universidad Nacional Autonoma de Mexico (IN206215). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We thank Bibiana Ortega Dominguez and Rafael Cervantes Roldan, Universidad Nacional Autonoma de México for technical assistance. T.B.G. is a recipient of a posdoctoral fellowship from Programa de Investigación de Cáncer de Mama del Instituto de Investigaciones Biomédicas-UNAM. V.G.R is a student of Programa de Posgrado en Ciencias Biomédicas, Universidad Nacional Autónoma de México and is a recipient of a scholarship from Consejo Nacional de Ciencia y Tecnología. A.T.C. is a recipient of a posdoctoral fellowship from DGAPA-Universidad Nacional Autónoma de México.
Contributor Information
Tonatiuh Barrios-García, Email: tonabarrios@yahoo.com.
Vania Gómez-Romero, Email: vania.romero27@iibiomedicas.unam.mx.
Ángeles Tecalco-Cruz, Email: atecalco@iibiomedicas.unam.mx.
Viviana Valadéz-Graham, Email: vvaladez@ibt.unam.mx.
Alfonso León-Del-Río, Email: leon@biomedicas.unam.mx.
References
- 1.Phillips R.S., Ramos S.B.V., Blackshear P.J. Members of the tristetraprolin family of tandem CCCH zinc finger proteins exhibit CRM1-dependent nucleocytoplasmic shuttling. J. Biol. Chem. 2002;277(13):11606–11613. doi: 10.1074/jbc.M111457200. [DOI] [PubMed] [Google Scholar]
- 2.Varnum B.C. The TIS11 primary response gene is a member of a gene family that encodes proteins with a highly conserved sequence containing an unusual Cys-His repeat. Mol. Cell. Biol. 1991;11(3):1754–1758. doi: 10.1128/mcb.11.3.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DuBois R.N. A growth factor-inducible nuclear protein with a novel cysteine/histidine repetitive sequence. J. Biol. Chem. 1990;265(31):19185–19191. [PubMed] [Google Scholar]
- 4.Barrios-García T. Tristetraprolin represses estrogen receptor α transactivation in breast cancer cells. J. Biol. Chem. 2014;289(22):15554–15565. doi: 10.1074/jbc.M114.548552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J.Y. Tumor suppressor p53 plays a key role in induction of both tristetraprolin and let-7 in human cancer cells. Nucleic Acids Res. 2013;41(11):5614–5625. doi: 10.1093/nar/gkt222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goddio M.V. Mammary differentiation induces expression of Tristetraprolin, a tumor suppressor AU-rich mRNA-binding protein. Breast Cancer Res. Treat. 2012;135(3):749–758. doi: 10.1007/s10549-012-2216-0. [DOI] [PubMed] [Google Scholar]
- 7.King E.M. Regulation of tristetraprolin expression by interleukin-1β and dexamethasone in human pulmonary epithelial cells: roles for nuclear factor-κB and p38 mitogen-activated protein kinase. J. Pharmacol. Exp. Ther. 2009;330(2):575–585. doi: 10.1124/jpet.109.151423. [DOI] [PubMed] [Google Scholar]
- 8.Smoak K., Cidlowski J.A. Glucocorticoids regulate tristetraprolin synthesis and posttranscriptionally regulate tumor necrosis factor alpha inflammatory signaling. Mol. Cell. Biol. 2006;26(23):9126–9135. doi: 10.1128/MCB.00679-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jalonen U. Inhibition of tristetraprolin expression by dexamethasone in activated macrophages. Biochem. Pharmacol. 2005;69(5):733–740. doi: 10.1016/j.bcp.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Ciais D., Cherradi N., Feige J.-J. Multiple functions of tristetraprolin/TIS11 RNA-binding proteins in the regulation of mRNA biogenesis and degradation. Cell. Mol. Life Sci. 2013;70(12):2031–2044. doi: 10.1007/s00018-012-1150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks S.A., Blackshear P.J. Tristetraprolin (TTP): interactions with mRNA and proteins, and current thoughts on mechanisms of action. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2013;1829(6–7):666–679. doi: 10.1016/j.bbagrm.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciais D. Destabilization of vascular endothelial growth factor mRNA by the zinc-finger protein TIS11b. Oncogene. 2004;23(53):8673–8680. doi: 10.1038/sj.onc.1207939. [DOI] [PubMed] [Google Scholar]
- 13.Stoecklin G. A novel mechanism of tumor suppression by destabilizing AU-rich growth factor mRNA. Oncogene. 2003;22(23):3554–3561. doi: 10.1038/sj.onc.1206418. [DOI] [PubMed] [Google Scholar]
- 14.Sandler H. Not1 mediates recruitment of the deadenylase Caf1 to mRNAs targeted for degradation by tristetraprolin. Nucleic Acids Res. 2011;39(10):4373–4386. doi: 10.1093/nar/gkr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross C.R., Brennan-Laun S.E., Wilson G.M. Tristetraprolin: roles in cancer and senescence. Ageing Res. Rev. 2012;11(4):473–484. doi: 10.1016/j.arr.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoecklin G. Genome-wide analysis identifies interleukin-10 mRNA as target of tristetraprolin. J. Biol. Chem. 2008;283(17):11689–11699. doi: 10.1074/jbc.M709657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai W.S. Novel mRNA targets for tristetraprolin (TTP) identified by global analysis of stabilized transcripts in TTP-deficient fibroblasts. Mol. Cell. Biol. 2006;26(24):9196–9208. doi: 10.1128/MCB.00945-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanduja S. The role of tristetraprolin in cancer and inflammation. Front. Biosci.J. virtual library. 2012;17:174–188. doi: 10.2741/3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennan S.E. The mRNA-destabilizing protein tristetraprolin is suppressed in many cancers, altering tumorigenic phenotypes and patient prognosis. Cancer Res. 2009;69(12):5168–5176. doi: 10.1158/0008-5472.CAN-08-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrick D.M., Blackshear P.J. Comparative expression of tristetraprolin (TTP) family member transcripts in normal human tissues and cancer cell lines. Arch. Biochem. Biophys. 2007;462(2):278–285. doi: 10.1016/j.abb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Jordan V.C. Third annual William L. McGuire Memorial Lecture. “Studies on the estrogen receptor in breast cancer”—20 years as a target for the treatment and prevention of cancer. Breast Cancer Res. Treat. 1995;36(3):267–285. doi: 10.1007/BF00713399. [DOI] [PubMed] [Google Scholar]
- 22.Xu L. 2015. Tristetraprolin induces Cell Cycle Arrest in Breast Tumor Cells Through Targeting AP-1/c-Jun and NF-κB Pathway. (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fallahi M. CREB targets define the gene expression signature of malignancies having reduced levels of the tumor suppressor tristetraprolin. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0115517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Souhibani N. The RNA-binding zinc-finger protein tristetraprolin regulates AU-rich mRNAs involved in breast cancer-related processes. Oncogene. 2010;29(29):4205–4215. doi: 10.1038/onc.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conzen S.D. Minireview: nuclear receptors and breast cancer. Mol. Endocrinol. 2008;22(10):2215–2228. doi: 10.1210/me.2007-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meneses-Morales I. SIP1/NHERF2 enhances estrogen receptor alpha transactivation in breast cancer cells. Nucleic Acids Res. 2014;42(11):6885–6900. doi: 10.1093/nar/gku311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curtis C. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu D. Identification of androgen receptor splice variant transcripts in breast cancer cell lines and human tissues. Horm. Cancer. 2014;5(2):61–71. doi: 10.1007/s12672-014-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fioretti F.M. Revising the role of the androgen receptor in breast cancer. J. Mol. Endocrinol. 2014;52(3):R257–R265. doi: 10.1530/JME-14-0030. [DOI] [PubMed] [Google Scholar]
- 30.Krishnan A.V., Swami S., Feldman D. Estradiol inhibits glucocorticoid receptor expression and induces glucocorticoid resistance in MCF-7 human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2001;77(1):29–37. doi: 10.1016/s0960-0760(01)00030-9. [DOI] [PubMed] [Google Scholar]
- 31.Fazzari A. The control of progesterone receptor expression in MCF-7 breast cancer cells: effects of estradiol and sex hormone-binding globulin (SHBG) Mol. Cell. Endocrinol. 2001;172(1–2):31–36. doi: 10.1016/s0303-7207(00)00397-x. [DOI] [PubMed] [Google Scholar]
- 32.Cassanelli S. Image cytometry of progesterone receptor expression during the cell cycle in the MCF-7 cell line. J. Histochem. Cytochem. 1991;39(12):1713–1718. doi: 10.1177/39.12.1940323. [DOI] [PubMed] [Google Scholar]
- 33.Hackenberg R. Down-regulation of androgen receptor by progestins and interference with estrogenic or androgenic stimulation of mammary carcinoma cell growth. J. Cancer Res. Clin. Oncol. 1990;116(5):492–498. doi: 10.1007/BF01613000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullick A., Katzenellenbogen B.S. Progesterone receptor synthesis and degradation in MCF-7 human breast cancer cells as studied by dense amino acid incorporation. Evidence for a non-hormone binding receptor precursor. J. Biol. Chem. 1986;261(28):13236–13246. [PubMed] [Google Scholar]
- 35.Horwitz K.B. Steroid receptor analyses of nine human breast cancer cell lines. Cancer Res. 1978;38(8):2434–2437. [PubMed] [Google Scholar]
- 36.Muscat G.E.O. Research resource: nuclear receptors as transcriptome: discriminant and prognostic value in breast cancer. Mol. Endocrinol. 2013;27(2):350–365. doi: 10.1210/me.2012-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang J. RNA-destabilizing factor tristetraprolin negatively regulates NF-κB signaling. J. Biol. Chem. 2009;284(43):29383–29390. doi: 10.1074/jbc.M109.024745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kittler R. A comprehensive nuclear receptor network for breast cancer cells. Cell Rep. 2013;3(2):538–551. doi: 10.1016/j.celrep.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Mohammed H. Progesterone receptor modulates ER[agr] action in breast cancer. Nature. 2015;523(7560):313–317. doi: 10.1038/nature14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu W. Glucocorticoid receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits paclitaxel-associated MAPK activation and contributes to breast cancer cell survival. J. Biol. Chem. 2005;280(6):4117–4124. doi: 10.1074/jbc.M411200200. [DOI] [PubMed] [Google Scholar]
- 41.Wu W. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res. 2004;64(5):1757–1764. doi: 10.1158/0008-5472.can-03-2546. [DOI] [PubMed] [Google Scholar]
- 42.Mikosz C.A. Glucocorticoid receptor-mediated protection from apoptosis is associated with induction of the serine/threonine survival kinase gene, sgk-1. J. Biol. Chem. 2001;276(20):16649–16654. doi: 10.1074/jbc.M010842200. [DOI] [PubMed] [Google Scholar]
- 43.Schorr K., Furth P.A. Induction of bcl-xL expression in mammary epithelial cells is glucocorticoid-dependent but not signal transducer and activator of transcription 5-dependent. Cancer Res. 2000;60(21):5950–5953. [PubMed] [Google Scholar]
- 44.Meßmer U.K. Suppression of apoptosis by glucocorticoids in glomerular endothelial cells: effects on proapoptotic pathways. Br. J. Pharmacol. 2000;129(8):1673–1683. doi: 10.1038/sj.bjp.0703255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vilasco M. Glucocorticoid receptor and breast cancer. Breast Cancer Res. Treat. 2011;130(1):1–10. doi: 10.1007/s10549-011-1689-6. [DOI] [PubMed] [Google Scholar]
- 46.Yeh S. Abnormal mammary gland development and growth retardation in female mice and MCF-7 breast cancer cells lacking androgen receptor. J. Exp. Med. 2003;198(12):1899–1908. doi: 10.1084/jem.20031233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park S. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann. Oncol. 2011;22(8):1755–1762. doi: 10.1093/annonc/mdq678. [DOI] [PubMed] [Google Scholar]
- 48.Castellano I. Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res. Treat. 2010;124(3):607–617. doi: 10.1007/s10549-010-0761-y. [DOI] [PubMed] [Google Scholar]
- 49.Søiland H. Prognostic relevance of androgen receptor detection in operable breast cancer. J. Surg. Oncol. 2008;98(7):551–558. doi: 10.1002/jso.21156. [DOI] [PubMed] [Google Scholar]
- 50.Schippinger W. Evaluation of the prognostic significance of androgen receptor expression in metastatic breast cancer. Virchows Arch. 2006;449(1):24–30. doi: 10.1007/s00428-006-0213-6. [DOI] [PubMed] [Google Scholar]