Abstract
Until recently, the agamid species, Japalura flaviceps, was recognized to have the widest geographic distribution among members of the genus occurring in China, from eastern Tibet to Shaanxi Province. However, recent studies restricted the distribution of J. flaviceps to the Dadu River valley only in northwestern Sichuan Province, suggesting that records of J. flaviceps outside the Dadu River valley likely represent undescribed diversity. During two herpetofaunal surveys in 2013 and 2015, eight and 12 specimens of lizards of the genus Japalura were collected from the upper Nujiang (=Salween) Valley in eastern Tibet, China, and upper Lancang (=Mekong) Valley in northwestern Yunnan, China, respectively. These specimens display a unique suite of diagnostic morphological characters. Our robust comparisons of phenotype reveal that these populations can be distinguished readily from J. flaviceps and all other recognized congeners. Herein, we describe the two Japalura lineages as new species, Japalura laeviventris sp. nov. and Japalura iadina sp. nov.. In addition, we provide updated conservation assessments for the new species as well as imperiled congeners according to the IUCN criteria for classification, discuss the importance of color patterns in the diagnosis and description of species in the genus Japalura, and discuss directions for future taxonomic studies of the group.
Keywords: Coloration, Conservation, Hengduan Mountains, Hydropower development, Japalura flaviceps
INTRODUCTION
The family Agamidae, a radiation of more than 300 species of iguanian lizards, is one of the most taxonomically diverse lizard groups in Eurasia, with members of the family possessinga wide range of morphologies and life history traits (Manthey, 2010; Moody, 1980; Townsend et al., 2011). Due to this large variation in phenotype, agamid lizards represent a great system for comparative studies in multiple fields of biology, including phylogeography(Macey et al., 1998; Melville et al., 2009; Moody, 1980), ethology(Kastle & Schleich, 1998; Murphy et al., 1978; Qi et al., 2011; Wei & Lin, 1981), and evolutionary biology (Grismer, 2010; Schulte et al., 2002; Stuart-Fox & Ord, 2004). Across mainl and Asia, China harbors the greatest diversity of agamid lizards, currently possessing 52 currently recognized species in the country representing four subfamilies (Ananjeva et al., 2011). Of this diversity, nearly half(25 species)are believed to be endemic to China, including majority of the enigmatic Mountain Dragons of the genus Japalura Gray, 1853 (Ananjeva et al., 2011; Wang et al., 2015).
To date, the genus Japalura is composed of 30 species, distributed across much of Asia(Wang et al., 2015). The vast majority of this diversity occurs in the territory of Greater China(16 species found in Mainl and China, five species on the isl and s of Taiwan; Ota et al., 1998; Wang et al., 2015). Until recently, Japalura flaviceps Barbour & Dunn, 1919 was recognized as having the widest geographic range in China, with populations distributed from eastern Tibet to Gansu Province in central China(Pope, 1935; Zhao et al., 1999). However, subsequent examinations of morphological variation among populations of J. flaviceps not only revealed the widespread species to be a complex of distinct evolutionary lineages, but also restricted the range of true J. flaviceps to the valleys of the Dadu River in northwestern Sichuan only(Gao & Hou, 2002; Li et al., 2001; Manthey et al., 2012; Wang et al., 2015). Furthermore, Manthey et al.(2012) and Wang et al.(2015) suggested that unexamined populations of J. cf. flaviceps outside of the Dadu River drainage may represent additional, unique evolutionary lineages within the species complex, worthy of additional systematic study.
During herpetofaunal surveys of eastern Tibet in 2013 and of northwestern Yunnan in 2015, we captured eight and 12 specimens of lizards in the upper Nujiang(=Salween)Valley, eastern Tibet, China, and the upper Lancang(=Mekong)Valley, northwestern Yunnan, China, respectively. All individuals were identified to the genus Japalura. Although these populations are considered to be part of the J. flaviceps species complex, they can be distinguished readily from true J. flaviceps sensu Manthey et al.(2012) and other members of the genus by suites of distinct morphological characters. In this study, we describe these populations as two distinct, new species within the genus. We review the taxonomic history of the J. flaviceps species complex, comment on the importance of coloration for species delimitation in the genus and discuss directions for future taxonomic studies of the group. Additionally, we assess the new species and other imperiled congeners against the IUCN criteria for classification(IUCN, 2013).
MATERIALS AND METHODS
Eight specimens of one new species were collected from the upper Nujiang Valley near the Nujiang Bridge at Baxoi, Qamdo Prefecture of eastern Tibet, China, including two adult males, four adult females, and two juveniles. Twelve specimens of a second new species were collected from the upper Lancang Valley at Ninong, Deqin, northwestern Yunnan, China, including 11 adult males and one adult female.
Following euthanasia, tissue samples were taken from livers and preserved in 95% ethanol, and voucher specimens were fixed in 10% buffered formalin and later transferred to 70% ethanol for long-term preservation. With the exception of a single female specimen collected from Tibet that possesses an incomplete tail(KIZ 014040), all adult specimens were chosen as the type series. All specimens(including KIZ 014040)are deposited in the Museum of Kunming Institute of Zoology, Chinese Academy of Sciences(KIZ).
Measurements were made with digital calipers to the nearest 0.1 mm, except for snout-vent length(SVL) and tail length(TAL), which was made with a ruler to the nearest 1 mm. With the exception of several new traits measured in this study, focal characters and character definitions follow Wang et al.(2015): snout-vent length(SVL), tail length(TAL), head width(HW), snout-eye length(SEL), fore-limb length(FLL), hind limb length(HLL), supralabial count(SL), infralabial count(IL), middorsal scale(MD), Toe IV subdigital lamellae(T4S), Toe IV length(T4L), trunk length(TRL), interorbital distance(IOD), number of scales between nasal and first supralabials(NSL), supraciliary count(SCL), and number of scale rows between sixth supralabial and orbit circle(SOR). Additionally, in this study we examined the following morphometric characters(definitions provided after colon): enlarged, conical, post-tympanic scale count(PTY): large conical scales posterior to tympanum; and enlarged, conical, post-rictal scale count(PRS): large conical scales posterior to the rictus. Values for paired characters(SL, IL, NSL, SOR)were recorded from both sides of the body, with counts provided in left/right order.
Summaries of specimens examined are listed in Appendix I. For comparisons, morphological data of the following phenotypically similar species were collected from vouchered specimens(from type or topotype specimens when available): J. batangensis, J. dymondi, J. micangshanensis, J. flaviceps, J. splendida, J. varcoae, J. vela, J. yunnanensis, and J. zhaoermii. Currently, with recognized populations of J. splendida distributed across multiple distinct zoogeographic regions of China (Xie et al., 2004; Zhao et al., 1999), and known to possess considerable variability in morphological characters (Manthey et al., 2012; Yang & Rao, 2008; Zhao et al., 1999), it is likely that the widespread species represents a complex of unique evolutionary lineages. Therefore, to avoid unnecessary confusion in drawing comparisons with potentially unique but undescribed diversity, we include morphological data from the type specimen of J. splendida as well as specimens from localities geographically proximate to the species’ type locality along the Yangtze River(e.g. Chongqing). Morphological data of the following species were obtained from the literature: J. brevicauda (Manthey et al., 2012), J. kumaonensis (Schleich & Kästle, 2002), J. luei(Ota et al., 1998), J. makii(Ota, 1989), and J. yulongensis (Manthey et al., 2012).
Comparisons of coloration in life are based on type descriptions and available color photographs (Manthey, 2010; Yang & Rao, 2008; Zhao et al., 1999). Museum abbreviations for specimens examined follow Sabaj Perez(2015), and include: Chengdu Institute of Biology, Chinese Academy of Sciences(CIB); Kunming Institute of Zoology, Chinese Academy of Sciences(KIZ); Museum of Comparative Zoology at Harvard University(MCZ), Boston, MA, USA; and National Museum of Natural History(USNM), Washington D.C., USA.
The topographic map shown in Figure 1 was created by N. A. Huron in ArcMap v.10.3.1 using the digital elevation model(DEM)layers based on NASA’s Shuttle Radar Topographic Mission(SRTM). The SRTM data are available for free at approximately 90 meters resolution(3 arc-second projections; Reuter et al., 2007; CIAT-CSI SRTM, 2015).
Figure 1.
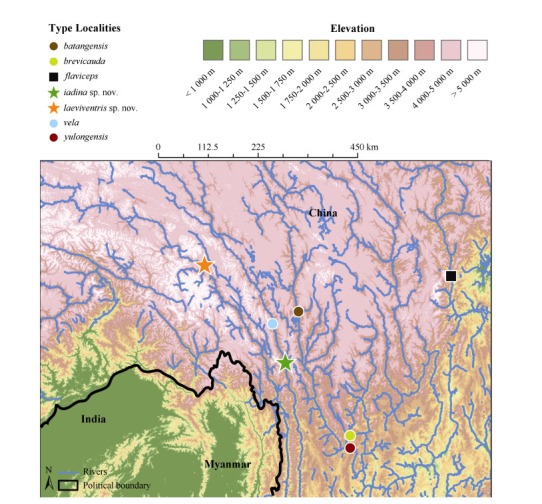
Distribution map of Japalura in the Hengduan Mountain Range, southwest China(Map created by Nicholas A. HURON and Cameron D. SILER). Color-coded shapes show the distribution of type localities for the new species(stars), true J. flaviceps sensu Wang et al.(2015) (square), and other referenced members of the J. flaviceps Species Complex(circles).
RESULTS
Japalura laeviventris sp. nov. Wang, Jiang, Siler, and Che (Figures 1-4)
Figure 4.
Preferred microhabitat of Japalura laeviventris sp. nov. near the Nujiang Bridge, Baxoi County, Qamdo Prefecture, eastern Tibet, China(Photo by Ya-Qiang SUN)
Synonyms
Japalura flaviceps Pope, 1935: 467; Zhao & Jiang, 1977: 293-298; Hu et al., 1987: 112; Zhao et al., 1999: 111-115; Li et al., 2010: 115.
Holotype: KIZ 014038, adult male, collected near the Nujiang Bridge in the upper Nujiang Valley at Baxoi(=Basu), Qamdo(=Changdu), eastern Tibet(=Xizang), PR China(N30.10034°, E97.22787°, 2 739 m elevation); collected by Ke JIANG on 3 July 2013.
Paratopotypes: One adult male(KIZ 014037) and three adult females(KIZ 014041-43); collected by Ke JIANG, Kai WANG, and Ya-Qiang SUN.
Diagnosis: Following Inger’s definition of the genus (Inger, 1960), the new species is assigned to the genus Japalura based on a number of diagnostic characters, including:(1)dorsal scales unequal in size;(2)enlarged crest scales present;(3)gular pouch present;(4)lateral fold of skin in axilla-groin region present;(5)supraciliary scales greatly imbricate;(6)head relatively long, flat;(7)tail long, slender;(8)tail cylindrical in shape; and (9)precloacal and femoral pores absent.
Japalura laeviventris sp. nov. can be distinguished from all congeners by the combination of the following suite of morphological characteristics:(1)small adult body size(SVL 67-72 mm in males, 64-70 mm in females);(2)moderate TAL(TAL/SVL 168%-200%);(3)moderate HLL(HLL/SVL 64.3%-78.4%);(4)NSL 1;(5)T4S 22-26;(6)SOR 3;(7)strongly-protuberant, conical, post-tympanic scale absent;(8)strongly-protuberant, conical, post-rictal scale absent;(9)tympanum concealed;(10)nuchal crests relatively raised on weak skin folds;(11)dorsal crests weakly developed without distinct skin folds in males;(12)transverse gular fold present;(13)gular pouch distinct, present;(14)scales of ventral surface of body smooth or weakly keeled;(15)MD 57-59, (16)ground dorsal coloration off-white in males, brownish-gray in females;(17)dorsal, lateral, and ventral surface of head, dorsal forelimbs, and lateral surface of body speckled with black;(18)distinct radial streaks around eyes;(19)dorsolateral stripes present, smooth-edged, pale-yellow in males;(20)dark-brown, “M”-shaped pigmentation patterns along dorsal midline in males; and (21)small, triangular, orange gular spots in adults of both sexes.
Description of holotype: Adult male, SVL 67 mm, TAL 133 mm, FLL 30.6 mm, HLL 49.8 mm, HW 15.5 mm, HL 21.3 mm. Rostral rectangular, three times broader than high, in contact with six small scales excluding supralabial. Nasal sub-circular, bordered by 10/9 small scales. Single scale between rostral and first supralabial. Supralabials 7/8, smooth, posteriormost longest. Loreal scales irregularly arranged and weakly keeled. Ciliaries circular, much smaller than other dorsal scales, forming orbit circle; supraciliaries 9/11, prominent, elongated, above orbit; first 5/7 supraciliaries overlapping one-half to two-third of its length with subsequent ones, last four slightly overlapping; three rows of scales between orbit circle and sixth supralabials on both sides of head, all weakly keeled, with scales of middle row largest. Scales posterior to eyes strongly keeled; five enlarged scales between orbit and tympanum on both sides; tympanum covered with much smaller scales. Dorsal head scales heterogeneous in size, approximately circular shaped, mildly keeled, convex, somewhat granular in appearance; single row of five scales forming a weak ridge along snout midline from one scale posterior rostral to mid-point between anterior corner of eyes; parietal smooth, enlarged, with a distinct pineal eyespot; post-occipital and posterior lateral head scales strongly keeled; conical scale 1/0 on occipital region, weakly developed; no large conical scales posterior to tympanum or rictus.
Ventral head scales homogeneous in size, mostly smooth, weakly keeled posterolaterally; transverse gular fold distinct, well developed; gular pouch present; shoulder fold posterior to gular fold on each side, from ventral surface of neck to just about 5 mm above pectoral joint; axillary fold present on each side of body. Middorsal crest scales 59, roughly equal in size to neighboring scales, imbricate along body midline; nuchal crest relatively raised on skin fold, while dorsal crest weakly developed without skin folds. A row of enlarged and distinctively keeled scales running parallel to dorsal crest from pectoral region of body to pelvis on each side of crest above dorsolateral stripes. Ground dorsal scales heterogeneous in size; scales of axilla much smaller than remaining dorsals; large, distinct, flat scales distributed irregularly across dorsal surface of body, circular in shape, roughly four times larger than ground scales, at times arranged in proximally transverse rows. Dorsal limb scales distinctively keeled, roughly equal in size to ventral scales, homogeneous in size on forelimbs, heterogeneous on hind limbs; Toe IV subdigital lamellae 25/24. Tail scales keeled in lateral rows; cloaca scales small. Ventral scales of body and limbs mostly homogeneous in size, smooth or weakly keeled close to lateral sides.
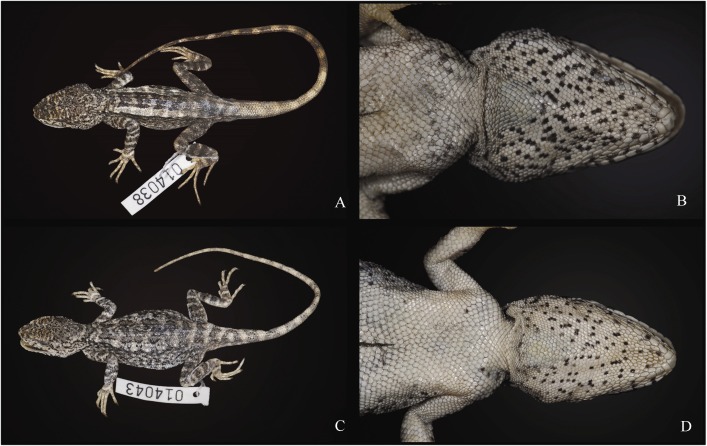
Coloration of holotype in life: In life, the dorsal surface of the head is light yellowish, speckled with small black scales, of which some connect to form dark X-shaped patterns. Two of these X-shaped patterns are observed on the snout, and another can be observed on the dorsal surface of the head between the orbits. The lateral surface of the head is off-white and is also speckled with black scales. Some black scales form radial stripes around the eyes, with the stripes directed posteriorly being the broadest. The ground coloration of the dorsal and lateral surfaces of the body is off-white. Smoothedged, pale-yellow dorsolateral stripes run along the entire length of the body on each side of the vertebral crest. Black vermiculate pigmentation patterns are present on the lateral surfaces of the body, beneath and proximate to the dorsolateral stripes. A series of three V-shaped brownish-black patterns of dark pigmentations are observed on the dorsal surface of the body, distributed from the occipital region of the head to the pectoral joint of the body along the dorsal midline. These shapes are followed posteriorly by a series of four brownish-black, M-shaped pigmentations, of which the last one is gradually faded and somewhat unclear. Numerous brownish-gray, transverse b and s are present on the posteriormost two-thirds of the tail. The b and s do not form complete rings around the tail ventrally. The dorsal surfaces of the forelimbs are off-white and speckled with black scales. The black scales in this region are connected in places by gray-blotched scales, and together, these colors create irregular transverse b and s of dark coloration. The dorsal surfaces of the hind limbs are light gray, with dark-gray transverse patterns similar to those observed on the fore-limbs. The ground coloration of the ventral surface of the head is uniform white, but heavily speckled with black scales. Sporadically, small series of these black scales from short, longitudinal, vermiculate stripes. A triangular, bright orange gular spot is present in the center of the gular pouch. The ventral surfaces of the body, limbs, and tail are uniform white, except for the ventral surfaces of the h and s and feet, which are more yellowish(Figure 2).
Figure 2.
Lateral and ventral views of adult male holotype KIZ 014038(A and B) and adult female paratopotype KIZ 014043(C and D)of Japalura laeviventris sp. nov. in life(Photos by Kai WANG). Note the dark reddish orange color posterior to the shoulder fold in the lateral view is the color of ectoparasites and not a coloration pattern of the new species.
Coloration of holotype in preservative: The coloration of the holotype in preservative closely matches its coloration in life; however, the following differences are observed:(1)the ground coloration of the dorsal surface of the head as well as the coloration of the dorsolateral stripes is light gray, and (2)the triangular orange gular spot turns white(Figure 3).
Figure 3.
Dorsolateral views and ventral close-ups views of adult male holotype KIZ 014038(A and B) and adult female paratopotype KIZ 014043(C and D)of Japalura laeviventris sp. nov. in preservative(Photos by Kai WANG)
Variation and sexual dimorphism: Variations in pholidosis and measurements are summarized in Table 1. The male paratopotype(KIZ 014037)is slightly darker in dorsal coloration than the holotype in preservative. Females are sexually dimorphic from males by having shorter snouts(SEL/HL), less speckled ventral surfaces of the heads(v.s. heavily speckled), more consistently arranged transverse rows of enlarged scales on the dorsal surfaces of the body(v.s. irregularly distributed), smaller orange gular spots(v.s. larger), much more distinct transverse patterns of pigmentations at bases of the tails(v.s. less distinct), as well as by the absences of M-shaped dark pigmentation patterns along the dorsal midline of the body(v.s. presence), and the presence of dark transverse b and s on the dorsal surface of the body that extend to the lateral surfaces of the body(v.s. absence). Yellow dorsolateral stripes are absent in most females, except for a single individual(KIZ 014043), which has wavy dorsolateral stripes. Juveniles(KIZ 014039, 014044)closely resemble adult females, except for the absence of orange gular spots. A single juvenile(KIZ 014039)was observed to possess a light-gray, dorsal, vertebral stripe from the pectoral region of the body to pelvis. This trait was not observed in any adult females.
Table 1.
Meristic and mensural data of the type series of japalura laeviventris sp. nov
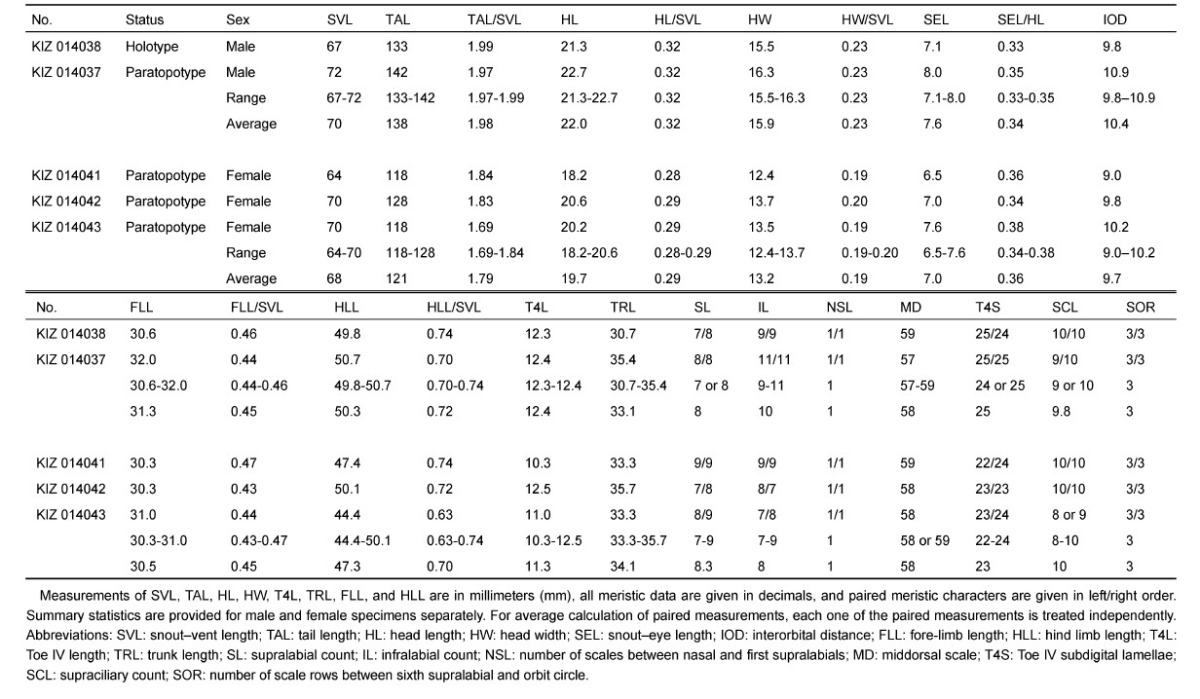 |
Comparisons: Populations of the new species were identified previously as J. flaviceps. However, the new species can bedistinguished readily from the latter by having smooth or weakly keeled scales on the ventral surface of the head and the body(v.s. distinctively keeled), a greater number of MD(57-59 v.s. 43-48), heavily speckled ventral surfaces of the head, with speckles rarely forming short lines(v.s. speckles absent, but broad, dark stripes present and interconnected into a mosaic pattern), as well as by the absence of strongly-protuberant, conical, post-rictal scale(v.s. presence), presence of X-shaped patterns of dark pigmentation on the dorsal surface of the head(v.s. absence, or presence of few transverse streaks), presence of distinct radial patterns around the eyes(v.s. absence), presence of M-shaped patterns of dark pigmentation along the dorsal midline between the two dorsolateral stripes in males(v.s. rhombus-shaped patterns with distinct yellow centers), and presence of distinct orange gular spots in both sexes(v.s. absence in both sexes).
Japalura laeviventris sp. nov. is most similar to J. kumaonensis(Annandale, 1907) and J. yunnanensis Anderson, 1879 in coloration patterns. All three species possess radial patterns of dark pigmentation around the eyes and light dorsolateral stripes in males. However, the new species can be distinguished readily from the latter two by having smooth or weakly keeled scales on the ventral surfaces of the head and the body(v.s. distinctively keeled), a greater number of MD(≥57 v.s. ≤52), as we as by the absence of strongly protuberant, conical, post-tympanic scales(presence and in high numbers) and absence of strongly-protuberant, conical, post-rictal scale(v.s. presence). Additionally, Japalura laeviventris sp. nov. differs from from J. kumaonensis by having a greater number of SL(7- 9 v.s. 5 or 6), a concealed tympanum(v.s. exposed), a relatively well developed gular pouch(v.s. weakly developed), M-shaped patterns along the dorsal-midline in males(v.s. chevron-shaped), the posteriorly directed radial-stripes of the eyes less prominent and short, ending before reaching the tympanums(v.s. distinct, broad, enclosing the tympanums), as well as by the presence of a transverse gular fold(v.s. absence) and presence of orange gular spots in both sexes(v.s. absence in both sexes); and from J. yunnanensis by having a shorter tail(TAL/SVL ≤200% v.s. ≥235%), fewer T4S(22-26 v.s. 27-31), greater number of NSL(1 v.s. 0), broad dorsolateral stripes with smooth edges in males(v.s. narrow and jagged), an off-white ground coloration on the dorsal surface of the body(v.s. green or brown), the terrestrial lifestyle(v.s. arboreal), as well as by the presence of a transverse gular fold(v.s. absence) and presence of orange gular spots in both sexes(v.s. light yellow gular spots in males, sometimes absence in females).
In addition to the four species compared above, the new species can be diagnosed from all remaining congeners by having smooth or weakly keeled scales on the ventral surfaces of the head and the body(v.s. distinctively keeled), an off-white ground coloration of the body(brown, black, or green), heavily speckled surfaces of the head and lateral surfaces of the body(v.s. absence or weakly speckled lateral body only), M-shaped dark patterns of pigmentations along the dorsal midline of the body between the two dorsolateral stripes in males(v.s. rectangular blotches of dark pigmentations), and the orange gular spots in both sexes in life(v.s. other colorations, in males only), as well as by the absence of large, conical post-rictal and posttympanic scales(v.s. presence and in high numbers).
Distribution and Natural History: Japalura laeviventris sp. nov. is known only from its type locality in the upper Nujiang Valley(Figure 1); however, the species may also occur along geographically proximate stretches of Nujiang. Little vegetation exists in the Hot- and -Dry Valley(Figure 4). The new species is terrestrial, with individuals have been observed on the s and y hills alongside the river, utilizing rock crevices and rodent burrows as shelters. No larger reptiles have been recorded from the area. Males of the new species are more conspicuous in behavior than females, and can be observed basking for greater lengths of time in open habitat. In contrast, females appear to stay in closer proximity to shelters.
Etymology: We derive the new species name from the Latin word “laeviventris, ” meaning “smooth venter, ” in reference to one of the major diagnostic characteristics of the new species: smooth or weakly keeled ventral body scales. Suggested common name: Smooth-venter Mountain Dragon(English); Hua Fu Pan Xi(Chinese; 滑腹攀蜥).
Japalura iadina sp. nov. Wang, Jiang, Siler, and Che (Figures 5-Figure 7)
Figure 5.
Dorsolateral(A), ventral(B), and ventral head close-up views(C)of the adult male holotype(KIZ 019321)of Japalura iadina sp. nov. in life(Photos by Kai WANG)
Figure 7.
Habitat of Japalura iadina sp. nov. in the Dry-Hot Valley of Lancang River, Deqin, Northwest Yunnan, China(Photo by Kai WANG)
Synonyms
J. flaviceps Zhao et al., 1999: 293-298; Yang and Rao, 200: 200-201; 8; Xu and Zhang 2011: 202-203; J. splendida Xu and Zhang 2011: 202-203; J. cf. flaviceps Manthey et al., 2012
Holotype: KIZ 019321, adult male, collected by Kai WANG on 27 May 2015, from the Lancang Valley at Ninong, Deqin, northwest Yunnan, China(N28.370255°, E98.865287°, 2 062 m elevation).
Allotopotype: KIZ 09398, adult female. Collected by Da-Hu ZOU. Specimen shares the same locality and collection information as the holotype.
Paratopotypes: KIZ 09401-03, 019322, 019323, 019325-28, all adult males. Collected by Kai WANG, Ke JIANG, and Da-Hu ZOU.
Diagnosis: Following Inger’s(1960) definition of the genus, the new species is assigned to Japalura based on a number of diagnostic characters, including:(1)dorsal scales unequal in size;(2)enlarged crest scales present;(3)gular pouch present;(4)lateral fold of skin in axilla-groin region present;(5)supraciliary scales greatly imbricate;(6)head relatively long, flat;(7)tail long, slender;(8)tail cylindrical in shape; and (9)precloacal and femoral pores absent.
The new species differs from all congeners by a combination of the following morphological characters:(1)gular fold present;(2)distinct gular pouch present;(3)relative hind-limb length moderate HLL/SVL 69.6%-80.1%;(4)relative tail length moderate TAL/SVL 173%-198%;(5)F4S 15-17;(6)T4S 19-25;(7)MD 35-46;(8)three lateral rows of enlarged scales present on the dorsal surface of the body parallel to the dorsal crest;(9)nuchal and dorsal crests moderately raised on skin folds;(10)nuchal and dorsal crests relatively low and discontinuous in males;(11)dorsal and lateral surfaces of body emerald green in males, yellowish brown speckled with large, light yellow scales in females;(12)tail yellowish green in males, brownish in females;(13)ventral surface of body bluish or whitish gray, sometimes with black speckles, in males, uniform yellow in females;(14)two smooth-edged, light-green, dorsolateral stripes present in males;(15)vermiculate stripes on ventral surface of head present, distinct, blackish blue in males, black in females;(16)gular region dark blue in males, yellow in females.
Description of holotype: Adult male, SVL 60 mm, TAL 116 mm, TrL 28.3 mm, HL 18.5 mm, HW 12.4 mm, IOD 9.1 mm, SEL 6.9 mm, FLL 28.8 mm, HLL 44.3 mm, T4L 10.3 mm. Rostral rectangular, three times broader than high, in contact with six small scales excluding supralabial; nasal sub-circular; single scale betweennasal and first supralabial; supralabials eight on both sides of head, weakly keeled; loreal scales irregularly arranged and moderatly keeled; ciliaries circular, much smaller than other scales of lateral head, forming orbit circle; supraciliaries six on both sides of head, prominent, elongated; three rows of scales between orbit circle and sixth supralabial on both sides of head, all weakly keeled; scales posterior to eyes strongly keeled; orbit and tympanum separated by five enlarged scales on both sides of head; tympanum covered with small scales; dorsal head scales heterogeneous in size, distinctively keeled, convex; single row of five scales forming weak ridge along snout midline from just posterior to rostral to point in line with anterior corner of eyes; parietal keeled, enlarged; pineal eyespot present; post-occipital and posterior lateral head scales strongly keeled; conical scales on occipital region numerous, strongly protuberant; conical scales posterior to tympanum two, large; conical scale posterior to tympanum or rictus single, large, on each side of head.
Ventral head scales homogeneous in size, distinctively keeled; transverse gular fold present, distinct; gular pouch present; shoulder fold posterior to gular fold on each side of body present, distributed from ventral surface of throat to dorsolateral stripes; axillary fold present on each side of body. Middorsal scales 40, larger than neighboring scales, imbricate along dorsal midline; nuchal and dorsal crests slightly raised on skin folds, with distinct break separating regional folds; three dorsolateral rows of enlarged, distinctively keeled scales running parallel to dorsal crest from pectoral region of body to pelvis on each side of body, with one dorsal to dorsolateral stripe and two along upper and lower edges of dorsolateral stripe; ground dorsal scales heterogeneous in size; scales of axilla smaller than dorsal scales; large conical scales present, on dorsal and lateral surface of body, distinctly keeled, r and omly scattered; dorsal limb scales distinctively keeled, slightly larger than ventral scales, homogeneous in size on fore-limbs, heterogeneous on hind limbs; F4S 16/16; T4S 20/21. Tail scales keeled, in lateral rows; cloaca scales small. Ventral scales of body and limbs near homogeneous in size, distinctively keeled.
Coloration of holotype in life: The ground coloration of the dorsal, lateral, and ventral surfaces of the head is emerald green. Four broad, black transverse b and s are observed on the dorsal surface of the head, and are equally spaced across the region between the nares and a point in line with the posterior edge of the orbits. Black reticulated patterns are observed posterior to the last transverse b and s on the dorsal surface of the head. Nine black streaks are observed radiating around the eye on each side of the head. The streaks extend ventrally to the supralabial scales, with the posteriormost streaks broadest. The infralabial scales and the margin of the lower jaw are emerald green with black bars present on several of the infralabial scales. These infralabial bars match up with streaks present on the supralabial scales, and extend extend further posteroventrally, gradually transitioning into blackish blue, vermiculate stripes on the ventral surface of the head. The vermiculate stripes connect posteriorly with the large, triangular shaped, blue gular spot located in the center of the gular pouch.
A black vertebral stripe runs from the occipital region of the head to the pelvis along the dorsal midline of the body. Several crest scales and a few small, ground scales along the vertebral stripe are green. The lateral and dorsolateral surfaces of the body are emerald green, with reticulated patterns of black pigmentation on the lateral surfaces of the body. These reticulated patterns of pigmentation form a thin, black, dorsolateral line along the ventral edge of the dorsolateral stripe on each side of the body. The dorsolateral stripes are green and smooth-edged, running from the posterior occipital region of the head to the pelvis. A series of enlarged, distinctively keeled, green scales are observed in a dorsolateral series on each side of the dorsal crest, running from the neck to the pelvis and distributed along the black vertebral stripe. The ventral surface of the body is uniform whitish blue and is slightly lighter anteriorly.
The ground coloration of the dorsal surfaces of the limbs is emerald green, with numerous transverse b and s running from the proximal to distal regions of the limbs. The ventral surfaces of the fore-limbs and hind limbs are whitish blue and greenish yellow, respectively. The ventral surfaces of the h and s and feet are gray in coloration.
The dorsal surface of the tail is greenish yellow, with numerous dark gray, transverse b and s running along its length. The transverse b and s do not form complete rings around the tail ventrally. The ventral side of the tail is significantly duller gray in coloration (Figure 5).
Coloration of holotype in preservative: The coloration of the holotype in preservative closely resembles its coloration in life; however, the following differences are observed: 1)the emerald green coloration on some parts of the dorsal surfaces of the head, body, and limbs changed to light blue, and 2)the blue coloration of the gular spot on the ventral surface of the head and body faded significantly.
Variation and sexual dimorphism: Variation in morphometric characters and pholidosis patterns is summarized in Table 2. The new species is sexually dimorphic, with females possessing distinct coloration and pigmentation patterns from males. The female allotype of the new species differs from males by having light, yellowish brown ground coloration on the dorsal surfaces of the head, body, and limbs(v.s. emerald green), distinct, light brown transverse b and s on the dorsal surface of the body(v.s. single, black vertebral stripe), a greenish yellow gular spot(v.s. blue), and a white ground coloration on the ventral surfaces of the body and limbs (v.s. blue; Figure 6).
Table 2.
Meristic and mensural data of the type series of Japalura iadina sp. nov
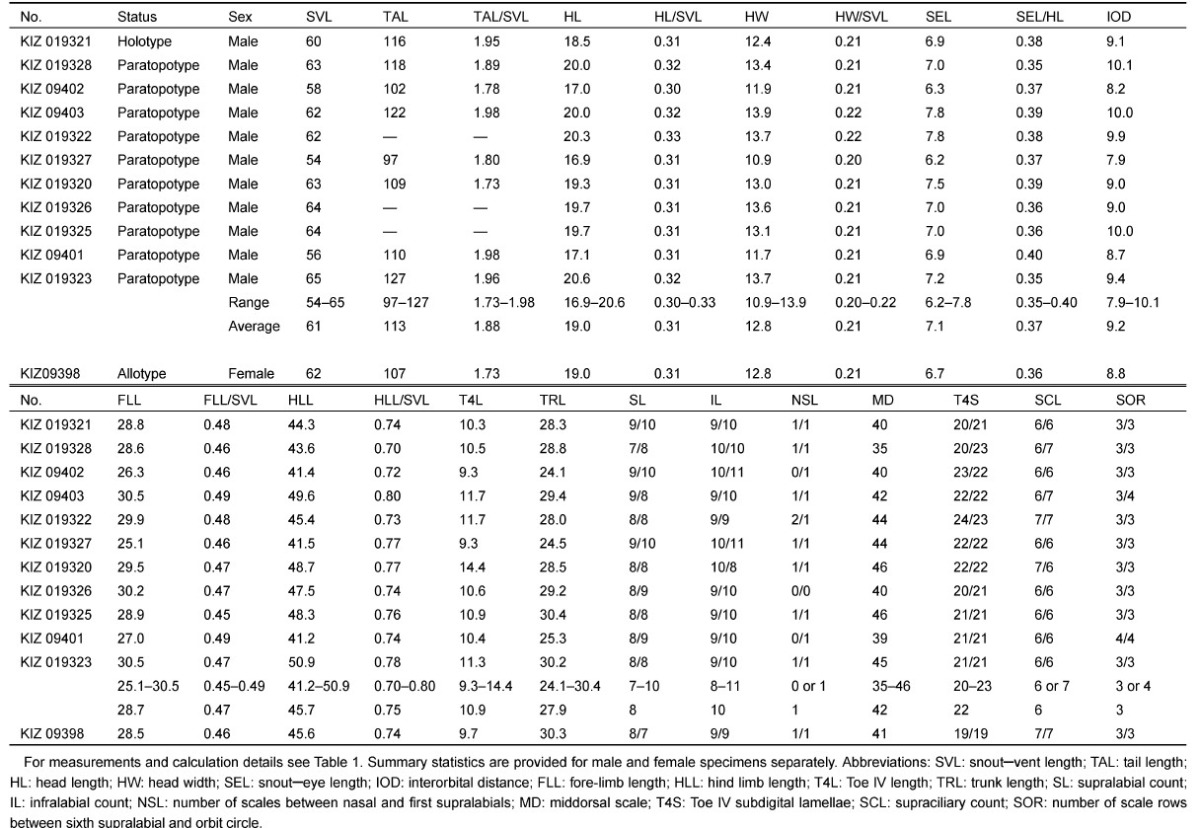 |
Figure 6.
Dorsolateral(A), ventral(B), and ventral head close-up views(C)of the adult female allotype of Japalura iadina sp. nov. (KIZ 09398)in life(Photos by Kai WANG)
Table 3.
Morphological comparisons of Japalura laeviventtris sp.nov., j.iadina sp.nov., and phenotypically similar congeners
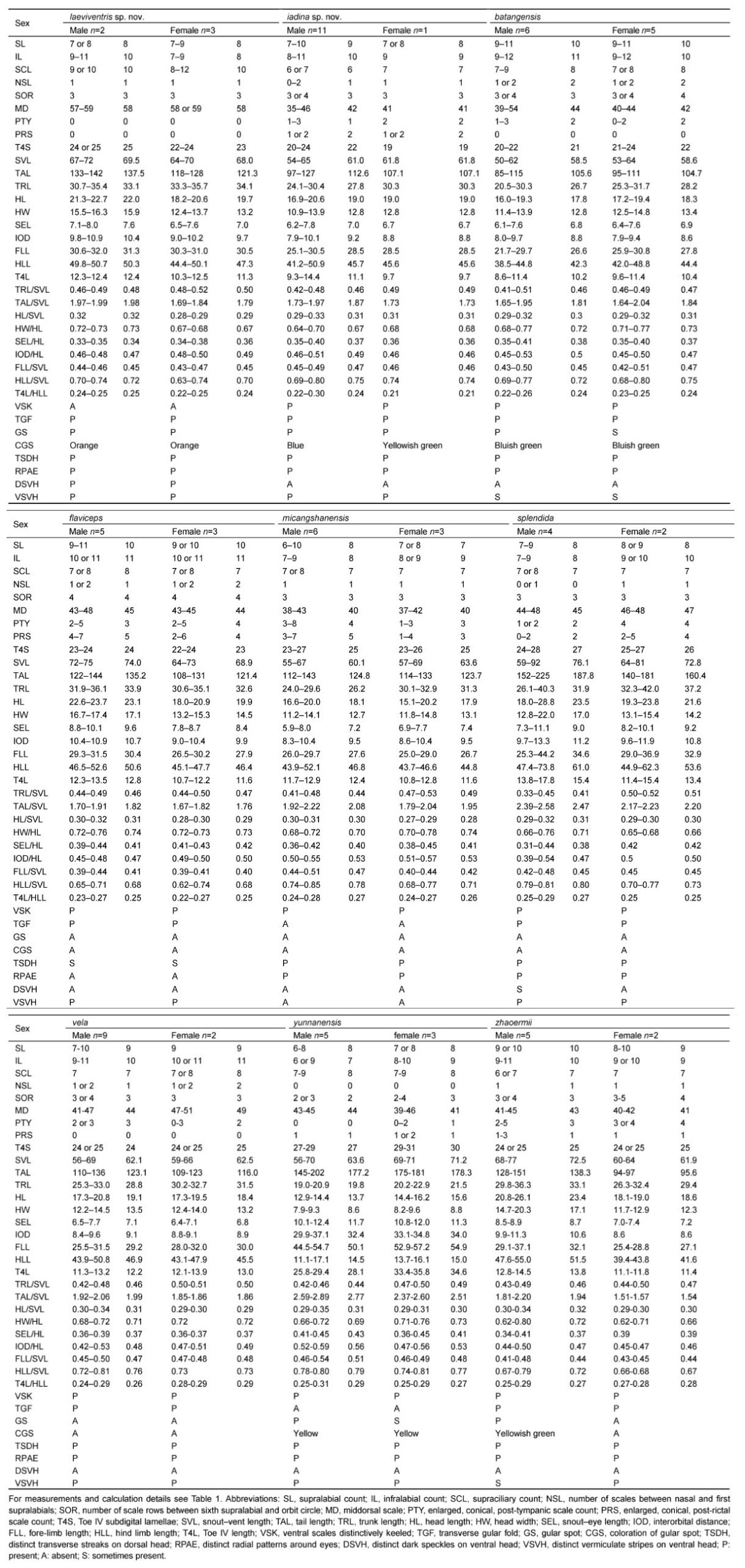 |
Comparisons: The new species Japalura iadina sp. nov. has been confused previously with J. flaviceps; however, it can be distinguished readily from the latter by having a smaller adult body size(SVL 54-65 mm v.s. 64-75 mm), a tendency towards a shorter relative snout length(SEL/HL 34.9%-40.2% v.s. 40.0%-44.2%), smaller, less protuberant nuchal and dorsal skin folds in males(v.s. strongly protuberant), distinct ground coloration on the dorsal surfaces of the head, limbs, and body in males(emerald green v.s. brown), and distinct patterns of pigmentation along the dorsal midline of the body in males(vertebral black stripes v.s. a series of dark rhomboid- shaped patterns of pigmentation), as well as by the presence of distinct gular spots in both sexes(v.s. absence), and by the presence of distinct black radial stripes around the eyes(v.s. absence).
The new species is morphologically most similar to J. splendida, J. makii, and J. luei, of which all four species have the green background coloration. However, Japalura iadina sp. nov. can be diagnosed from J. splendida by having a terrestrial life style(v.s. arboreal), a smaller adult body size(SVL 54-65 mm v.s. 59-92 mm), fewer T4S(19-24 v.s. 24-28), a shorter relative tail length(TAL/SVL≤198% v.s. ≥217%), distinct tail coloration in males(greenish yellow v.s. brownish gray), as well as by the absence of white lip stripes(v.s. presence), presence of gular spots in both sexes(v.s. absence), and absence of green coloration on the dorsal surfaces of the body in females(v.s. presence); from J. makii by having a smaller adult body size(SVL 54-65 mm v.s. 61-79 mm), a relatively shorter tail(TAL/SVL 1.73-1.98 v.s. 2.09-2.61), fewer T4S(19-24 v.s. 24-30), a pink coloration of tongue and oral cavity in life(v.s. yellow), a terrestrial lifestyle(vs. arboreal), as well as by the absence of a gular fold(v.s. presence), absence of green, transverse b and s across the dorsal surface of body(v.s. presence), and absence of lip strips below eyes(v.s. presence); and from J. luei by having a smaller adult body size(SVL 54-65 mm v.s. 65-75 mm), a relatively shorter tail(TAL/SVL 1.73-1.98 v.s. 2.31-2.48), fewer T4S(19-24 v.s. 24-28), a terrestrial lifestyle(v.s. arboreal), continuous dorsolateral stripes in males(v.s. a dorsolateral series of disconnected, irregular, large markings), as well as by the absence of a gular fold(v.s. presence), and absence of lip stripes below eyes(v.s. presence).
Japalura iadina sp. nov. differs from J. laeviventris by having a smaller adult body size(SVL 54-65 mm v.s. 64-72 mm), distinctively keeled ventral scales of head and body(v.s. smooth or weakly keeled), fewer MD(35-46 v.s. 57-59), distinct ground coloration of the dorsal surfaces of head and body in males(emerald green v.s. off-white), distinct coloration of gular spots(blue in males, greenish yellow in females v.s. orange in both sexes), and distinct patterns of pigmentations on the dorsal surfaces of the body along the dorsal midline(black vertebral stripes speckled with green v.s. M-shaped patterns of dark brown pigmentation).
Additionally, when compared with species distributed in close geographic proximity along the Lancang River, J. iadina sp. nov. can be distinguished from J. vela by having less protuberant, discontinuous vertebral crests in males(v.s. sail-like, strongly protuberant, continuous), distinct ground coloration on the dorsal surfaces of the body(emerald green v.s. black) and tail(yellow v.s. gray)in males, uniquely shaped dorsolateral stripes in males(smooth edged v.s. strongly jagged), as well as by the presence of gular spots in both sexes(v.s. absence in both sexes), and by the absence of weakly defined, reddish dorsolateral lines in females(v.s. presence); and from J. yunnanensis by having a terrestrial lifestyle(v.s. arboreal), shorter relative tail length(TAL/SVL ≤198% v.s.≥237%), fewer T4S(19-24 v.s. 27-31), distinct coloration of gular spots(blue in males, greenish yellow in females vs. yellow in both sexes when present or absence in females), as well as by the presence of a transverse gular fold(v.s. absence).
Distribution and natural history: Although locally abundent, Japalura iadina sp. nov. is known only from the type locality(Figure 1). The new species is terrestrial, inhabiting dry, rocky habitats along the Lancang River(Figure 7). The emerald green coloration of the males makes them st and out from the rocky, environmental background. Tail autotomy has been observed for this population.
Etymology: The Latin name “iadina” means “emerald like, ” which describes the diagnostic emerald green body coloration of males of the new species. Suggested common name: Emerald Mountain Dragon(English), Fei Cui Pan Xi(Chinese; 翡翠攀蜥).
DISCUSSION
Body coloration, particularly the coloration of the gular region, has been suggested to play an important role in the recognition of conspecifics, sexual selection, and the general diversification of agamid lizards in general(Bastiaans et al., 2014; LeBas & Marshall, 2000; Stuart-Fox & Ord, 2004), particularly members of the genus Japalura(Kästle & Schleich, 1998; Wei & Lin, 1981). Within the genus Japalura, color patterns vary among species from mainl and Asia, but appear to be conservative within species, and hence serve as good diagnostic characters for systematic studies of species diversity (Wang et al., 2015). However, to date, detailed descriptions of coloration in life, have not been published for many species in the genus, including J. brevicauda, J. chapaensis, J. grahami, J. hamptoni, J. otai, J. yulongensis;(Mahony, 2010; Manthey, 2010; Manthey et al., 2012; Ota & Weidenhöfer, 1992; Zhao et al., 1999). Since specimen coloration is recognized to fade significantly after preservation for many specimens in natural history collections, color descriptions of preserved specimens may not be as applicable to diagnostic comparisons. Therefore, we recommend future research focusing on describing coloration patterns based on new observations of species of Japalura in life.
Despite the importance of the coloration patterns in the taxonomic studies of members of the genus Japalura, the diagnostic coloration of J. flaviceps has been historically confused, which has continued to the species’ recognized wide spread distribution. Originally described by Barbour & Dunn(1919) from the Tung River Valley(today Dadu River Valley)in northwest Sichuan Province, J. flaviceps was described as possessing a uniform, “dusky brown” dorsal head coloration and lacked “strongly marked stripe from the eye to the angle of the mouth.” Together with photographs of the holotype of J. flaviceps(MCZ R-12469) and topotypic males from the Dadu River Valley, Manthey et al.(2012) argued that J. flaviceps did not possess dorsal and lateral pigmentation patterns on the head, and proposed that the absence of such patterns should be used as a diagnostic feature of the species. Although most topotypic specimens of J. flaviceps that we examined possess no distinct, radial pigmentation patterns around the eyes, we did observe several individuals, particularly subadults, that possess several distinct transverse b and s of darker pigmentation across the dorsal region of the head(CIB 2333 and 2549). Therefore, we suggest that the absence of radial patterns of darker pigmentation around the eyes may be a more consistent feature of J. flaviceps for use in diagnostic comparison. Furthermore, we propose that the diagnostic feature of the true J. flaviceps should be restricted to the following combination of morphological char acters: SVL 70-83 mm in adult males, 58-78 mm in adult females; TAL/SVL 165%-192%; HLL/SVL 62%-78%; SEL/HL 39%-43%; SOR 4; SL 9-11; IL 9-11; MD 43-54; T4S 21-24; tympanum concealed; distinct gular fold present; distinct gular pouch present; enlarged, conical, post-rictal, post-occipital, and post-tympanic scales present, numerous, prominent; ventral scales distinctively keeled; dorsal ground coloration of head and body brownish gray; distinct radial stripes around eyes absent; dark, broad, interconnected, vermiculated stripes on the ventral surface of head present; gular spot absent; smooth-edged, yellow dorsolateral stripes in males present; a series of rhombshaped patterns of dark brown pigmentation with distinct yellow centers present along the dorsal midline of the body.
Due to the continued taxonomic confusion over comparisons of populations of the J. flaviceps species complex, J. flaviceps was thought to be a widespread species, and its conservation status was assessed as Least Concern (IUCN, 2013). However, because of the absence of data allowing for confirmation of species level diversity within the J. flaviceps Species Complex, and the paucity of available information about intraspecific ecological and genetic diversity, we recommend that J. flaviceps and all conspecifics in the species complex be considered Data Deficient, and recommended immediate research be focused on better underst and ing this unique complex of agamid lizards.
Unfortunately, habitats in the Hengduan Mountain Range are experiencing currently considerable human-mediated modifications as a result of the rapid development of hydropower plants (Pan et al., 2002; Chen & Rao, 2010; personal communication with Mr. Li CHENG). Although populations of Japalura can be abundant, continued alteration of these valley habitats could result in the extirpation of micro-endemic populations, or worse, unique evolutionary lineages not yet recognized formally as species. Additionally, many species of Japalura are experiencing over-exploitation through the illegal pet-trade, both domestically and internationally(personal communication with Mr. Jia- Wei WU and Mr. Mian HOU). Therefore, it is critical that researchers and conservation and government agencies work together to assess the conservation statuses and ecological requirements of species in the genus, particularly species and populations endemic to the river valleys in the range of the Hengduan Mountain Range.
APPENDIX
The following specimens were examined:
J. batangensis(n=16): CIB 2227, 2233, 2243, 1902-1908, KIZ 84011, 801081, Batang, Sichuan, P.R. China; KIZ 019314, KIZ 09404, 019311, 019312, Markam, Tibet, China.
J. dymondi(n=7): CIB 87234, 1869, Panzhihua, Sichuan, P.R. China; KIZ 95I1001, 1002, 1016, 1018, 1022, Dayao, Yunnan, China.
J. grahami(n=1): USMN 65500(holotype), Yibin, Sichuan, China. J. micangshanensis(n=9): CIB 86351, 86348, Xianyang, Shaanxi, P.R. China; CIB 86360, 86361, 86356, 86357, Luonan, Shaanxi, China; CIB 2572, 2578, 2582, Wenxian, Gansu, China.
J. flaviceps(n=13): CIB 2234, 2332, 2333, 2341, 2354, 2355, 2549, 2554, 2556, 2561, 2567; KIZ 05181, 05182; Luding, Sichuan, China.
J. splendida (n=6): USNM 35522(holotype), Yichang, Hubei, PR China; CIB 2588, 2591, 2596, 72468, 72469, Chongqing, China.
J. varcoae(n=3): CIB 2651, 2650, KIZ 85II0006, Kunming, Yunnan, China. J. vela(n=11): KIZ 013801(holotype), KIZ 013802, 013813, 013800, 013805-013811(paratopotypes), Jerkalo, Tibet, China.
J. yunnanensis(n=8): CIB 2684, 2686, 2687, 2689, KIZ 82081, Longling, Yunnan, PR China; KIZ 74II0240, 0248, 79I469, Tengchong, Yunnan, China.
J. zhaoermii(n=12): CIB 86432, 86435, 85721, 85722, 86433, 86434, 86436, Wenchuan, Sichuan, PR China; CIB 2232, 2244, 2240, KIZ 84032, 85030, Lixian, Sichuan, China.
ACKNOWLEDGEMENTS
We would like to thank Dr. Gernot Vogel(Society for Southeast Herpetology, Germany), Mr. Xin-Lei ZHAO(Chinese Academy of Medical Sciences), Mr. Scott Farnsworth(Washington State University), and Dr. Su LIU(Shanghai Chenshan Botanical Garden)for providing suggestions on the scientific names of the new species, Mr. Duan YOU and Mr. Ya-Qiang SUN for assisting our fieldwork in Tibet, Mr. Wynn Addison(NMNH), Professor Yue-Zhao WANG(CIB), Professor Yue-Ying CHEN(CIB), Mr. Ke LU(CIB), and Mr. Gui- Wu HE(KIZ)for kindly letting us examine specimens under their care, Mr. Nicholas A. Huron(University of Oklahoma)for assistance with ArcGIS and in the creation of the Figure 1 map, Ms. Jing-Ting LIU(Washington State University) and Ms. Dan-Lin LI(University of Idaho)for editing the photos, Mr. Mian HOU for providing valuable information, and members of the Siler Lab for their generous comments on the manuscript.
Funding Statement
This work was supported by the Ministry of Science and Technology of China (2014FY210200, 2011FY120200) and the Animal Branch of the Germplasm Bank of Wild Species of Chinese Academy of Sciences (the Large Research Infrastructure Funding).
References
- 1.Ananjeva N, Guo XG, Wang YZ. Taxonomic diversity of Agamid lizards (Reptilia, Sauria, Acrodonta) from China: a comparative analysis. Asian Herpetological Research. 2011;2(3):117–128. [Google Scholar]
- 2.Anderson J. 1879 "1878". Comprising an Account of the Zoological Results of the Two Expeditions to Western Yunnan in 1868 and 1875. Zoological Results of the Two Expeditions to Western Yunnan in 1868 and 1875. :803–804. [Google Scholar]
- 3.Annandale N. Reptiles. In: Boulenger GA, Annandale N, Wall F, Regan CT. Report on a collection of Batrachia, Reptiles and Fish from Nepal and the Western Himalayas. Records of Indian Museum. 1907;1:149–158. [Google Scholar]
- 4.Barbour T, Dunn ER. Two new Chinese Japaluras. Proceeding of the New England Zoological Club. 1919;7:15–19. [Google Scholar]
- 5.Bastiaans E, Bastiaans MJ, Morinaga G, Gaytán JGC, Marshall JC, Bane B, Cruz FM, Sinervo B. Female preference for sympatric vs. allopatric male throat color morphs in the mesquite lizard (Sceloporus grammicus) species complex. PLOS ONE. 2014;9(4) doi: 10.1371/journal.pone.0093197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen XL, Rao DQ. Investigation of fish, amphibian, and reptile species in the Shangri-La County and areas around small Zhongdian Key Water Control Project. Journal of Yunnan Agricultural University. 2010;25(4):535–541. [Google Scholar]
- 7.CIAT-CSI SRTM. The CGIAR Consortium for Spatial Information. Available. 2015 [Google Scholar]
- 8.Gao ZF, Hou M. Description of a new Japalura species from western Sichuan Province, China. Sichuan Journal of Zoology. 2002;21(1):3–5. [Google Scholar]
- 9.Gray JE. Descriptions of some undescribed species of reptiles collected by Dr. Joseph Hooker in the Khassia Mountains, East Bengal, and Sikkim Himalaya. Annals and Magazine Natural History. 1853;12(2):386–392. [Google Scholar]
- 10.Grismer JL. Phylogeny, taxonomy, biogeography and evolution of asexuality within the southeast Asian lizard genus Leiolepis Cuvier, (1829) Master Thesis. Villanova University. 2010;1 [Google Scholar]
- 11.Hu S, Zhao E, Jiang Y, Fei L, Ye C, Hu Q, Huang Q, Huang Y, Tian W. Amphibia-Reptilia of Xizang. Beijing: Science Press. 1987;1 [Google Scholar]
- 12.Inger RF. A review of the Agamid lizards of the genus Phoxophrys Hubrecht. Copeia. 1960;1960(3):221–225. [Google Scholar]
- 13.IU CN. IUCN Red List of Threatened Species. Version 2013.2, 2013 [Google Scholar]
- 14.Kästle W, Schleich HH. 1998 Studies on the Systematics and Biology of the Genus Japalura (Sauria: Agamidae). Notes on Comparative Ethology and Taxonomy of the Genus Japalura. In: Kästle W, Schleich HH (Ed.). The Contributions to The Herpetology of South Asia (Nepal, India), Fuhlrott Museum. 1998:233–246. [Google Scholar]
- 15.LeBas NR, Marshall NJ. The role of colour in signalling and male choice in the agamid lizard Ctenophorus ornatus. Proceedings of the Royal Society of London B: Biological Sciences. 2000;267(1442):445–452. doi: 10.1098/rspb.2000.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Deng QX, Wu Y, Wang Y. A new species of Japalura from Sichuan (Agamidae Gray. Japalura). Journal of Sichuan Teachers College (Natural Sciences) 2001;22(4):329–331. [Google Scholar]
- 17.Li PP, Zhao EM, Dong BJ. Amphibians and Reptiles of Tibet. Beijing: Science Press. 2010;1 [Google Scholar]
- 18.Macey, J R, Schulte JA, Ananjeva NB, Larson A, Rastegar-Pouyani N, Shammakov SM, Papenfuss TJ. Phylogenetic relationships among Agamid lizards of the Laudakia caucasia species group: testing hypotheses of biogeographic fragmentation and an area cladogram for the Iranian Plateau. Molecular Phylogenetics and Evolution. 1998;10(1):118–131. doi: 10.1006/mpev.1997.0478. [DOI] [PubMed] [Google Scholar]
- 19.Mahony S. Systematic and taxonomic revaluation of four little known Asian agamid species, Calotes kingdonwardi Smith, 1935, Japalura kaulbacki Smith, 1937, Salea kakhienensis Anderson, 1879 and the monotypic genus Mictopholis Smith, 1935 (Reptilia: Agamidae) Zootaxa. 2010;7(2514):1–23. [Google Scholar]
- 20.Manthey U. Agamid Lizards of Southern Asia-Draconinae 2, Leiolepidinae. Chimaira, Frankfurt/M. 2010;1 [Google Scholar]
- 21.Manthey U, Wolfgang D, Hou M, Wang XH. Discovered in historical collections: two new Japalura species (Squamata: Sauria: Agamidae) from Yulong Snow Mountains, Lijiang Prefecture, Yunnan, PR China. Zootaxa. 2012;30(1664):27–48. [Google Scholar]
- 22.Melville J, Hale J, Mantziou G, Ananjeva NB, Milto K, Clemann N. Historical biogeography, phylogenetic relationships and intraspecific diversity of agamid lizards in the central Asian deserts of Kazakhstan and Uzbekistan. Molecular Phylogenetics and Evolution. 2009;53(1):99–112. doi: 10.1016/j.ympev.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Moody S. Phylogenetic and historical biogeographic relationships of the genera in the Agamidae (Reptilia, Lacertilia) PhD Dissertation, University of Michigan. 1980;1 [Google Scholar]
- 24.Murphy JB, Lamoreaux WE, Carpenter CC. Threatening behavior in the angle-headed dragon, Goniocephalus dilophus (Reptilia, Lacertilia, Agamidae) Journal of Herpetology. 1978;12(4):455–460. [Google Scholar]
- 25.Ota H, Weidenhöfer T. The first male specimen of the poorly known agamid lizard Japalura chapaensis Bourret, 1937 (Reptilia: Sauria), from northern Vietnam, with notes on its taxonomic status. Raffles Bulletin of Zoology. 1992;40(2):193–199. [Google Scholar]
- 26.Ota H. A new species of Japalura (Agamidae: Lacertilia: Reptilia) from Taiwan. Copeia. 1989;1989(3):569–576. [Google Scholar]
- 27.Ota H, Chen SL, Shang G. Japalura luei: A new agamid lizard from Taiwan (Reptilia: Squamata) Copeia. 1998;1998(3):649–656. [Google Scholar]
- 28.Pan XF, Zhou W, Zhou YW, Wu F, Zhang Q. Amphibian and reptile in Zhongdian area of northwest Yunnan. Sichuan Journal of Zoology. 2002;21(2):88–91. [Google Scholar]
- 29.Pope CH. The Reptiles of China: Turtles, Crocodilians, Snakes, Lizards. Natural History of Central Asia. Vol. X. New York: American Museum of Natural History. 1935;466 [Google Scholar]
- 30.Qi Y, Wan HF, Gu HJ, Wang YZ. Do displays and badges function in establishing the social structure of male toad-headed lizards, Phrynocephalus vlangalii. Journal of Ethology. 2011;29(2):381–387. [Google Scholar]
- 31.Reuter HI, Nelson A, Jarvis A. An evaluation of void filling interpolation methods for SRTM data. International Journal of Geographic Information Science. 2007;21(9):983–1008. [Google Scholar]
- 32.Sabaj Perez MH. Standard symbolic codes for institutional resource collections in herpetology and ichthyology: an online reference. Version 5.0 Washington, DC. Available from: http://www.asih.org/(8 September 2015). . 2015;1 [Google Scholar]
- 43.Schleich HH, Kästle W. Amphibians and Reptiles of Nepal. Ruggell: Koeltz Scientific Books. 2002 [Google Scholar]
- 33.Schulte JA, Macey JR, Pethiyagoda R, Larson A. Rostral horn evolution among agamid lizards of the genus Ceratophora endemic to Sri Lanka. Molecular Phylogenetics and Evolution. 2002;22(1):111–117. doi: 10.1006/mpev.2001.1041. [DOI] [PubMed] [Google Scholar]
- 34.Stuart-Fox DM, Ord TJ. Sexual selection, natural selection and the evolution of dimorphic coloration and ornamentation in agamid lizards. Proceedings of the Royal Society of London, Series B: Biological Sciences. 2004;271(1554):2249–2255. doi: 10.1098/rspb.2004.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Townsend T, Mulcahy DG, Noonan BP, Sites JW Jr Kuczynski CA, Wiens JJ, Reeder TW. Phylogeny of iguanian lizards inferred from 29 nuclear loci and a comparison of concatenated and species-tree approach for an ancient, rapid radiation. Molecular Phylogenetics and Evolution. 2011;61(2):363–380. doi: 10.1016/j.ympev.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Wang K, Jiang K, Pan G, Hou M, Siler CD, Che J. A new species of Japalura (Squamata: Sauria: Agamidae) from Eastern Tibet, PR China. Asian Hepetological Research. 2015;6(3):159–168. [Google Scholar]
- 37.Wei SY, Lin JY. Behavioral study of Japalura swinhonis formosensis (Sauria: Agamidae) Tunghai Journal of Biology. 1981;22:33–48. [Google Scholar]
- 38.Xie Y, Mackinnon J, Li D. Study on biogeographic divisions of China. Biodiversity and Conservation. 2004;13(7):1391–1417. [Google Scholar]
- 39.Xu J, Zhang WW. A field guide to the wildlife of Meili Snow Mountain National Park. Beijing: Encyclopedia of China Publishing House. 2011;1 [Google Scholar]
- 40.Yang DT, Rao DQ. Amphibia and Reptilia of Yunnan. Kunming: Yunnan Publishing Group Corporation. 2008;1 [Google Scholar]
- 41.Zhao EM, Jiang YM. A survey of reptiles in Xizang Autonomous Region, with faunal analysis and descriptions of new forms. Acta Zoological Sinica. 1977;23(1):64–71. [Google Scholar]
- 42.Zhao EM, Zhao KT, Zhou KY. Fauna Sinica, Reptilia, Vol. 2: Squamata, Lacertilia. Beijing: Science Press. 1999;1 [Google Scholar]