Abstract
What we understand about diabetes from decades of genetics research is now being supplemented with exciting new evidence based on a better understanding of how one of the biggest “environmental” factors the body is exposed to is influencing the pathogenesis of disease. The recent discovery that certain dietary nutrients possessing a trimethylamine (TMA) moiety (namely choline/phosphatidylcholine and L-carnitine) participate in the development of atherosclerotic heart disease has renewed attention towards the contributions of gut microbiota in the development of cardiovascular diseases. Collectively, animal and human studies reveal that conversion of these nutrient precursors to trimethylamine N-oxide (TMAO) depends on both microbial composition and host factors, and can be induced by dietary exposures. In addition, circulating TMAO levels are strongly linked to cardiovascular disease risks and various adverse cardio-renal consequences. Our group and others have further demonstrated that circulating TMAO levels are elevated in patients with type 2 diabetes mellitus compared to healthy controls and gut microbiota-dependent phosphatidylcholine metabolism has been implicated in metabolic dysregulation and insulin resistance in animal models. Therefore, preventive strategies to minimize adverse consequences associated with TMAO generation in the diabetic population are warranted.
Keywords: Microbiome, Diabetes mellitus, Branched chain amino acids, Trimethylamine N-oxide
Introduction
The composition of our microbiome is diverse and complex. Although it is suggested that a core set of microbiota is shared among adults, the few bacterial phyla (Firmicutes and Bacteroides) that are generally represented in the gut can be expanded into thousands of species [1, 2]. The Human Microbiome Project has estimated the number of microbes at over 100 trillion representing at least 5000 different species, and this number will continue to evolve as we learn more about this vast and multifaceted system [3]. One important question to answer is whether certain compositions of micro-organisms in our gut are a reflection of disease pathogenesis or a marker of disease response?
Our understanding of diabetes development from decades of genetics research is now being augmented with novel perspectives on how one of the chief “environmental” factors the body is exposed to—what we eat—is influencing the pathogenesis of disease. The obesity epidemic in the world is a major driver of increased insulin resistance and type 2 diabetes mellitus (T2DM). Over 80 % of patients with T2DM are overweight. However, there is evidence that this relationship depends on more than just the caloric content of ingested food. Backhed and colleagues first observed that germ-free mice had less body weight compared to their conventional counterparts even with increased caloric intake and, furthermore, these germ-free mice were protected from the development of “western diet”-induced glucose intolerance and insulin resistance [4, 5]. We have also learned that obesity is more than just heritable but also transmissible through microbiome transplantation into germ-free mice [6]. The mechanism appears to involve increased efficiency of energy harvesting in the obesity-related microbiome that may potentially occur via the production of short chain fatty acids [5, 6]. Another factor contributing to insulin resistance may be chronic inflammation as a result of bacteria-promoted endotoxemia [7]. Notably, studies have associated obesity with increased levels of binding proteins against endotoxins as well as the development of insulin resistance [8, 9].
In order to approach the daunting task of assessing the microbiome, there are various approaches taken to better understand the microbiota composition, the dietary factors that can drive shifts in this composition, and the biomarkers that are reflective of this composition. In this review, we will describe established and novel gut-dependent metabolites and current understandings of the gut flora makeup in the context of T2DM.
Microbiota Produced Metabolites and the Evolving Relationship with Diabetes
Short Chain Fatty Acids (SCFA)
Short chain fatty acids are organic fatty acids with one to six carbons. These metabolites are key end products of colonic fermentation and are produced in the distal gut through bacterial fermentation of various macronutrients such as resistant starches, dietary fiber, various sugars, and proteins that escape the upper intestinal digestion. The large majority of SCFAs present in the colon are acetate, propionate, or butyrate [10], with an approximate intraluminal distribution of 60 % acetate and 20 % each of propionate and butyrate [6, 11]. SCFAs are rapidly and efficiently absorbed via apical solute transporters in a concentration-dependent manner and also help to maintain colonic acid-base balance [10, 12, 13]. Of the major SCFAs that are absorbed, only acetate appears in significant amounts in blood [14]. Acetate can be taken up by the liver or delivered to peripheral tissues and metabolized by muscle cells for energy. Propionate is primarily used in the liver for gluconeogenesis and butyrate is a major source of energy for the ceco-colonic epithelium [10]. Overall, SCFAs of colonic origins are believed to contribute 5–10 % of the energy source in healthy individuals. In addition, fiber-rich diets have been demonstrated to improve insulin sensitivity in subjects with T2DM [15–17].
As described in more detail later, two recent metagenomic studies both demonstrated decreased SCFA butyrate-producing bacteria in patients with T2DM [18••, 19••]. This lends support to the idea that SCFA-producing bacteria regulate metabolism and possibly alter the intestinal permeability to drive chronic inflammatory processes. Acting as more than a nutrient source, SCFAs have also been shown to trigger cell-specific signaling cascades. SCFA receptors have been identified and are called G-protein coupled receptor 41 (Gpr41) (also known as free fatty acid receptor 3, FFAR3) and G-protein coupled receptor 43 (Gpr43) (free fatty acid receptor 2, FFAR2). Both receptors are expressed in various cell types including gut epithelial cells, adipocytes, neutrophils, and monocytes [20, 21]. The signaling mechanism of SCFA demonstrated the influence of microbiota when it was shown that Gpr43-deficient mice are obese when fed a normal diet and yet Gpr43-overexpressing mice remained lean independent of calories consumed [22]. Interestingly, when these mice were treated with antibiotics, both mice types displayed a normal phenotype regardless if they were raised in germ-free environments or not. The authors also demonstrated that SCFA activation of Gpr43 suppressed insulin signaling and acted as a sensor for excessive dietary energy. Conversely, both Gpr41-deficient mice with a conventional microbiota and gnoto biotic Gpr41−/− mice colonized with the human distal gut microbes Bacteroides thetaiotaomicron and Methanobrevibacter smithii are observed to have reduced adiposity compared to their wild-type counterparts. This difference is lost when Gpr41-deficient and wild-type mice are both raised germ-free [23].
Many other potential mechanisms of SCFAs and their functions in metabolism have linked the interactions of diet and microbiota content. For example, SCFAs such as butyrate may protect against diet-induced insulin resistance. This can occur through engagement of Gpr43 and 41 and the release of glucagon-like peptide 1 (GLP-1), an incretin hormone that can improve insulin secretion and resistance as well as preserve beta-cell function [24, 25]. The main location of GLP-1-secreting cells is the distal ileum and colon and, interestingly, rectal but not intravenous infusions of SCFAs in humans stimulates an abrupt increase in GLP-1 secretion [26]. Furthermore, increased insulin sensitivity was found after subjects with metabolic syndrome were infused with butyrate-producing intestinal microbiota from lean donors [27]. Intake of dietary fibers and the subsequent SCFA profile are also associated with an anti-inflammatory response in the body [28]. This is in contrast to a high fat diet that is associated with a reduced amount of SCFA and increased lipopolysaccharide levels suggesting the colonization and secretion of endotoxins by gram-negative bacteria in order to stimulate an inflammatory response [9, 29]. SCFAs appear to promote the integrity of gut epithelial tight junctions as well as prevent the secretion of proinflammatory mediators such as TNF-α and IL-12 while also increasing the release of anti-inflammatory cytokine IL-10 [30–32]. These observations could perhaps explain some of the beneficial effects of SCFAs in countering the endotoxemia and chronic inflammation that underlie the development of obesity and insulin resistance.
Trimethylamine N-oxide (TMAO)
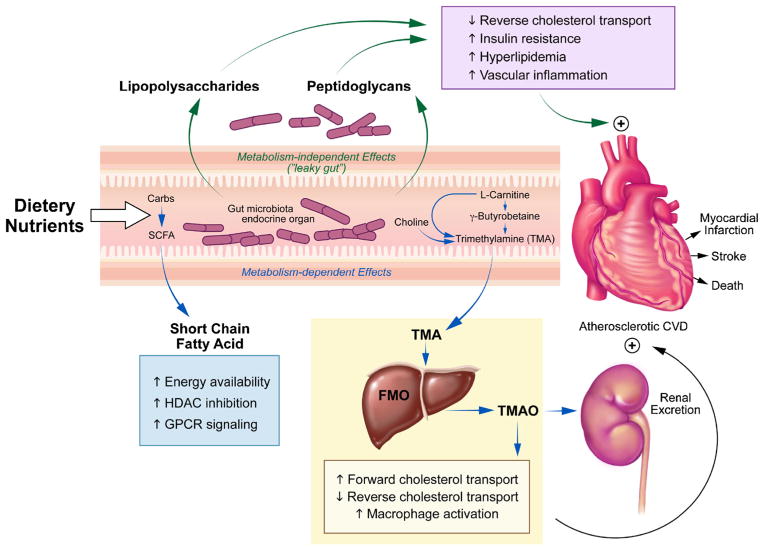
Recently, dietary nutrients contributing to the gut-dependent formation of trimethylamine N-oxide have become a novel research and therapeutic target as participants in cardiovascular disease. TMAO is a gut flora-dependent metabolite that has been linked to adverse cardiac and renal outcomes, illustrating the connection between diet and disease. Moreover, beyond the dietary relationship between TMAO precursors and disease, the discovery of TMAO has allowed us to utilize a potential new model to study the effects of gut metabolism that does not rely on comparing the complexities of the gut ecosystem as an endpoint. A meta-organismal pathway has been elucidated and refined to show that micronutrients such as phosphatidylcholine, choline, betaine, γ-butyrobetaine, and L-carnitine can participate in the development of atherosclerosis through the intestinal microbiota-dependent generation of TMA and subsequent absorption followed by host hepatic conversion of TMA to TMAO through flavin monooxygenase 3 (FMO3) [33, 34]. Furthermore, the susceptibility to disease phenotype transmitted by TMAO-generating microbiota has been demonstrated in mice cecal transplantation models by Gregory et al. [35]. Wang et al. utilized metabolomic profiles to find that TMAO, betaine, and phosphatidylcholine had significant relationships among one another and predicted an increased risk for cardiovascular disease (CVD) [36•]. These risk associations have been repeatedly shown in large observational trials [37, 38•, 39, 40]. Interestingly, a separate study that analyzed plasma metabolites in diabetes patients yielded similar plasma correlations among TMAO, carnitine, and choline although TMAO was not significant for predicting diabetes (p=0.11) in this group’s analysis [41]. However, due to the close relationship between diabetes and cardiovascular disease, it is not surprising that more recent TMAO studies found a higher presence of diabetes mellitus in various disease cohorts exhibiting elevated TMAO [37, 38•, 39, 40]. In addition, we have observed that the presence of diabetes in patients from a heart failure cohort was associated with statistically significant elevations in plasma TMAO [39]. More evidence from Lever et al. has identified plasma TMAO and betaine (synthesized from choline) to be elevated in patients with T2DM as compared to those without diabetes. They also reported altered levels of these metabolites to be predictive of various adverse outcomes such as death, heart failure, and secondary myocardial infarction [42]. Similarly, our group has recently observed that the presence of diabetes in patients from a heart failure cohort was associated with statistically significant elevations in plasma TMAO [39]. In addition, Gao and colleagues showed that dietary TMAO supplementation in mice exacerbates impaired glucose tolerance, insulin signaling, and promotes adipose tissue inflammation [43]. Moving beyond studies of association, one exciting recent study identified FMO3 and TMAO through a metabolic and transcriptional screen to be highly elevated in Liver Insulin Receptor Knockout (LIRKO) mice [44••]. It was demonstrated that insulin downregulates the expression of FMO3 while glucagon has the opposite effect. In this study, Miao et al. observed that LIRKO mice (as well as several other mouse models of diabetes) are unable to respond to insulin and subsequently have robustly elevated FMO3 expression along with serum TMAO levels. They then showed that knockdown of elevated FMO3 completely normalized glucose tolerance and improved insulin tolerance in these LIRKO mice through the suppression of FoxO1. This recent report suggests that the FMO3/TMAO pathway may play an important role in the pathophysiology of diabetes. Interestingly, with known mechanisms between TMAO and cholesterol metabolism, FMO3 knockdown also prevents hypercholesterolemia and atherosclerosis allowing us to come full circle and link TMAO to both diabetes and cardiovascular disease [44••, 45]. A Sumary of TMAO metabolism can be reviewed in Fig. 1.
Fig. 1.
Overall scheme showing microbiota-dependent pathways for conversion of various precursors to the TMAO metabolite. Choline is metabolized into TMA through microbial mechanisms in the gut. Choline can also be directly oxidized to betaine, which plays a role in homocysteine detoxification as a methyl-group donor. Similarly, dietary L-carnitine is preferentially metabolized in the gut into gamma-butyrobetaine (γBB) at a 1000-fold higher rate than its direct conversion to trimethylamine (TMA). L-carnitine can also be endogenously synthesized from γBB but this latter metabolite is largely shunted towards TMA production. The gut microbiota can oxidize some TMA into trimethylamine N-oxide (TMAO) while the remaining TMA is absorbed and delivered to the liver and processed by flavin monooxygenases (FMO) to form TMAO
Previous studies on TMAO have focused on the deleterious influences on cardiovascular and kidney pathophysiology, and the results have consistently shown the close microbiota-dependence of TMAO generation [33, 46, 47•]. This conditional mechanism allows us to systematically assess the relationship between various metabolites and how they can influence physiology directly through the gut flora; it is an alternative method compared to trying to classify and identify specific species of bacteria which may vary greatly depending on numerous environmental factors. A common contributing factor towards cardiometabolic dysregulation, such as gut-mediated low grade chronic inflammation as a result of endotoxemia or the recently implicated FMO3/TMAO alterations, makes plasma TMAO a provocative marker to monitor how dietary efforts can modify phenotype. Moreover, the direct involvement of TMAO in disease processes such as atherosclerosis and insulin resistance suggests that TMAO and its related metabolites can be used as a guide towards the development of novel markers suitable for studying the diabetes disease process. Here, we briefly review relevant aspects of TMAO metabolism and the pathogenesis of cardiometabolic disorders.
Choline and Betaine
Choline is an essential nutrient found in animal and plant products and is required for phospholipid membrane formation as a component of phosphatidylcholine [48–50]. It is a precursor to the neurotransmitter acetylcholine and choline deficiency has been linked to neurological impairment [51]. Choline’s direct oxidation product is betaine, which plays important roles in cell volume regulation, cell stability, and the regulation and detoxification of homocysteine [52]. Although increased betaine excretion is believed to be a shared feature among those with cardiometabolic diseases, studies have also shown that both elevated and deficient levels of betaine are associated with these disease settings [53, 54]. It is possible that increased betaine-homocysteine methyltransferase activity or betaine efflux dysregulation, among many potential hypotheses, can result in increased betaine depletion in diabetes [42, 55]. Associations of choline and betaine with diabetes have also been made by several groups [42, 53, 56, 57]. Moreover, several studies have observed increased levels of choline and betaine associated with the increased incidence of coronary syndrome as well as increased blood lipid levels [39, 40, 42, 58–62]. This provides evidence for the relationship between choline and betaine with diabetes and cardiovascular health. However, the evidence is muddied by the fact that some choline and betaine intake studies show only limited associations with cardiovascular morbidity [63, 64]. Therefore, the mixed evidence compels us to further elucidate mechanisms of choline metabolism that may provide additional clues about the relationship between this dietary nutrient and cardiometabolic disease.
It has been recently observed that TMAO is formed from orally ingested choline and betaine, and that the generation of TMAO from its precursors is dependent on intact gut flora [36•, 40, 47•]. Furthermore, TMAO has been demonstrated to play a mechanistic role in the development of accelerated atherosclerosis and chronic kidney disease [40, 47•]. Previous studies have also shown that the administration of antibiotics in humans had only limited effects on the blood circulating levels of choline and betaine, despite abolition of TMAO levels. Furthermore, the presence of elevated plasma levels of choline and betaine, which individually are associated with poorer prognosis, only confers this added risk when associated with similar increases in TMAO [40]. These observations suggested that TMAO, rather than the presence of exposure to its substrates such as choline or betaine, may be the major driver for the development of future cardiovascular events. Recent unpublished data from our research group has found associations between elevated TMAO, choline, and betaine levels and increased mortality across glycemic status, as well. In addition, TMAO also appeared to be the strongest predictor for adverse prognosis compared to choline and betaine in the setting of T2DM (unpublished work). Interestingly, a previous study from Dumas et al. detailed a close relationship between decreased choline bioavailability alongside increased TMA excretion in association with dietary-induced insulin resistance and nonalcoholic fatty liver disease. Mouse models in their study also exhibited strain-specific gut microbial metabolism with a high fat diet, which suggested genetic contributions to the determination of the gut microbiome [65]. Therefore, the dependence of TMAO on gut microbiota composition and its dietary relationship with choline and betaine lead us to believe that these phosphatidylcholine (PC) metabolites’ functional metabolic consequence may contribute to increased mortality as a potential pathogenic link between the gut microbiota pathways and diabetes progression.
Carnitine and γ-Butyrobetaine
L-carnitine is a vital molecule for lipid metabolism and plays a key role in the transport of activated fatty acids between the cytosol and mitochondria. Within the mitochondria, it acts as a cofactor in the beta-oxidation of long chain fatty acids through the facilitation of fatty acid entry via the carnitine-palmityl transferase system and exit via the carnitine acyl transferase system [66]. This non-protein amino acid is a conditionally essential nutrient that is mainly derived from dietary sources such as meat, fish, and dairy products but can also be endogenously synthesized in the liver and kidney from the amino acids lysine and methionine. Although the cells within our body can synthesize L-carnitine, only prokaryotic organisms such as those found within the enteric system can catabolize it. L-carnitine has been a popular supplement with data suggesting it would be associated with an improvement in glycemia and plasma lipids [67–70]. Alterations of fatty acid transport can result in accumulations of acyl-CoA, diacylglycerol, and fatty acids within the cytosol or mitochondrial and lead to dysregulation of insulin signaling. Therefore, carnitine’s role in the bidirectional transport of lipids is believed to help with problems of lipid overload to improve insulin sensitivity. The research on beneficial effects of L-carnitine in diabetes poses an interesting question when paralleled with recent research suggesting gut bacterial metabolism of carnitine to TMAO can accelerate the progression of atherosclerosis and increase CVD risk. Reviewing evidence from clinical studies in carnitine supplementation for T2DM and CVD has mixed results. A meta-analysis of placebo-controlled carnitine supplementation trials in T2DM suggested that carnitine can lower total cholesterol as well as fasting glucose [70]. However, this meta-analysis also noted a lack of studies with adequate methodological quality with only four studies and a total of 284 study subjects that were analyzed. Furthermore, this analysis also noted a decrease of HDL in carnitine-supplemented patients with T2DM. This supports experimental evidence of TMAO-mediated inhibition of reverse cholesterol transport; in this case, carnitine supplementation can result in increased production of TMAO [47•]. Similarly, meta-analyses of placebo-controlled trials to evaluate the role of L-carnitine in secondary prevention of CVD suggested reductions in lethal ventricular arrhythmias and development of angina. However, 11 of 13 studies in these meta-analyses, including two large studies with over 1000 subjects, failed to demonstrate any clinical benefit with short-term carnitine supplementation [71]. In addition, another recent meta-analysis on the effects of oral carnitine for the prevention of secondary acute MI concluded that there appeared to be no significant benefit [72].
γ-Butyrobetaine was previously known to be the immediate biosynthetic precursor for carnitine in the endogenous car-nitine synthesis pathway. A recent study to determine intermediates in the metabolism of TMAO has shown that γ-butyrobetaine is also produced from the gut conversion of L-carnitine as an alternate pathway for TMAO generation [33]. Koeth et al. discovered that the gut conversion of L-carnitine to γ-buytrobetaine to TMA is 1000× more efficient than the direct conversion of L-carnitine to TMA. Furthermore, their study revealed that the gut microbiota composition of mice supplemented with carnitine and those supplemented with γ-butyrobetaine differ significantly, despite the structural similarities of these metabolites. These findings are also supported by the fact that conversion of the upstream metabolites L-carnitine to γBB starts in the jejunum while conversion to TMA occurs mainly within the cecum and to a lesser extent along the rest of the intestinal tract. This suggests different sections of the gut are occupied by different microbial ecosystems that promote a collaborative metabolism of L-carnitine; as active carnitine transporters at the duodenum and jejunum are saturated, a two-step bacterial-mediated process converts remaining L-carnitine to γBB and finally to TMA. The authors further identified a potential enzyme complex, yeaW/X, that directly transforms L-carnitine to TMA. This shows the variety by which bacterial mechanisms can generate TMAO. In addition, it has been previously reported that expression of the cutC gene by bacteria such as Desulfovibrio can increase the conversion of choline to TMA [73]. Furthermore, a recently discovered unusual Rieske-type oxygenase from the human microbiota was shown to be able to convert L-carnitine in culture to TMA [74]. These insights compel us to consider how specific bacteria that provide metabolic benefits can be altered through analogous approaches of mechanistic alterations. Therefore, further investigation of how energy homeostasis can be affected by various routes of metabolism for important dietary nutrients is critical for the progression of this field.
What Our Gut Microbiota Composition May Tell Us About Our Health
One of the goals of sequencing the gut flora is to identify species of bacteria that provide specific functional advantages for the human metabolism. Attempts to identify core species that colonize our intestines have revealed certain players such as Faecalibacterium prausnitizii, Roseburia intestinalis, and Bacteroides uniformes [75]. However, in some individuals, these species can still represent less than 0.5 % of the microbes present [76]. Similarly, specific species in the setting of diabetes have been studied with the goal of quantifying specific gut signatures that may aid in the diagnosis and treatment of disease. F. prausnitizii is a prototypical anti-inflammatory component of the gut flora while R. intestinalis is a butyrate producer. Many studies have associated lower concentrations of these bacteria in patients with metabolic syndrome, diabetes, and obesity [18••, 19••, 77–79]. However, functional evidence is still lacking for these associations.
Metagenome-wide Associations
Recent metagenome-wide association studies (MGWAS) by two independent groups from China and Europe demonstrated the potential relationship between the gut microbiota and T2DM pathophysiology [18••, 19••]. Both groups reported the discovery of metagenomic differences between patients with T2DM and a group of healthy control subjects. The first evidence from high-throughput sequencing on patient stool samples was published by Qin et al. In their study, patients with T2DM were described to exhibit a “moderate” intestinal dysbiosis with more than a 3 % difference in gut microbial genes [19••]. This group utilized a concept that they termed metagenomic linkage group analysis, which draws comparisons based on groups of linked genetic material as one unit as opposed to independently distributed like in traditional taxonomic-based approaches. Within their clinical cohort, the patients with T2DM were characterized by a lower proportion of the butyrate-producing Clostridiales members R. intestinalis and F. prausnitizii and subsequently reduced butyrate biosynthesis as well as increased abundance of opportunistic pathogens and oxidative stress response. Interestingly, the Chinese group also noted increases in Desulfovibrio species in their T2DM cohort, which have been shown to be capable of converting choline to TMA. A second large MGWAS study from Karlsson et al. studied a cohort of 70-year-old European women consisting of participants with normal glucose tolerance, impaired glucose tolerance, or T2DM [18••]. They also utilized a technique that went beyond the traditional species-based approach and assembled metagenomic clusters, which consisted of gene sets that had high correlation based on their presence in subject analysis profiles. Their investigation also discovered a depleted abundance of prototypical butyrate producers Roseburia and F. prausnitizii that was highly discriminant for T2DM and increases in opportunistic pathogens such as Clostridium clostridioforme, which is associated with bacteremia.
Despite these similarities, the overall results of the two studies did not align as closely as one would imagine. Different discriminatory metagenomic clusters were found when the European group used their classification model on the Chinese T2DM cohort. Given the smaller number of patients analyzed in the European study (n=53), different sequencing techniques, and the heterogeneity (age, gender, use of diabetes medication) of the Chinese cohort, there are many potentially confounding factors that may have contributed to the classification differences. Nonetheless, these groups independently identified greater proportions of non-butyrate-producing bacteria in their respective cohorts compared to non-diabetic patients and raised an interest in the association between gut dysbiosis and the diabetic disease state. These studies are a foundation for future research in the refinement and expansion of the metagenomics toolbox to aid in the development of novel diagnostic biomarkers.
Other Bacterial Species
Other bacteria such as Akkermansia muciniphila, part of the Verrucomicrobia phylum, while not a dominant phyla within the gut is found in many individuals as a minor constituent [1]. Akkermansia is a mucin-degrading bacterium and its gut concentration has been shown to be inversely correlated with higher body weight and diabetes [80, 81]. A recent study by Everard et al. in mice models showed that a prebiotic diet containing oligofructose, a constituent of the luminal mucin layer that activates goblet cells, can increase levels of Akkermansia and improve metabolic functions [82]. Furthermore, they showed that similar treatment by direct administration of Akkermansia reduced body weight and reversed hyperglycemia. Evidence has also shown increased Akkermanisa concentrations after metformin treatment [83]. These studies suggest that Akkermansia might restore the gut mucus layer and thereby decrease gut permeability, and may exert an anti-inflammatory effect as well. However, controversial data also show increased Akkermansia in rats fed a high fat diet, and Akkermansia was observed to be more abundant in patients with T2DM from the Qin et al. metagenomics study [19••]. In the investigations of TMAO metabolism, as previously mentioned, different bacterial compositions resulted from γBB and carnitine supplementation. It was notable that Akkermansia was significantly associated with TMAO production in γBB-supplemented mice but not L-carnitine-supplemented mice [33]. Although great uncertainty still lies within this field, these examples show us the potential of what can be achieved by increasing our understanding of the mechanisms through which microorganisms influence our energy balance.
Studies of gut microbiota response to dietary modification have demonstrated the ability to rapidly alter gut microbiome composition [84]. Diets rich in animal protein and saturated fats are seen to favor a Bacteroides enterotype while carbohydrate-enriched diets favor a Prevotella enterotype [85]. However, among several studies, it has also been observed that a Prevotella enterotype correlates with higher TMAO levels, but the individuals in this group were predominantly omnivores rather than vegetarians/vegans suggesting more complexity than anticipated [47•]. Another recent study from David et al. showed that short-term dietary interventions can significantly alter the gut’s microbiota. In this study, the group observed changes from baseline microbial communities after as little as 1 day [86]. They noted interesting findings such as decreased SCFAs as well as an increase of bile-tolerant organisms when following animal-based diets compared to plant-based diets. A rapid response to differing dietary functional profiles in humans raises confidence that our gut flora is easily modifiable in ways that can have a major impact on both the cause and treatment of the dysbiosis that contributes to diseases like diabetes.
Conclusions
The investigation into how the microbiome can drive therapeutic interventions is a stimulating and blossoming field. Recent metagenomic studies have provided new insights into the composition of normal and diseased microbiomes. These studies oblige us to consider the usefulness of metagenomic diagnostics in the clinical setting. However, it is important to note that current logistical complications make quantifying proportions of bacteria in feces for clinical assessment more difficult, while the process of transport, handling, and sequencing may influence results [87]. Furthermore, without solid mechanistic insight into how pre and probiotics associate with disease phenotypes, the hastened clinical utilization of novel therapies like fecal transplant to alter the gut flora may not yield the expected results [18••]. In contrast to the quantification of various microbial species through either genetics, functional classes, or more advanced algorithms, the ability to measure blood levels of a microbial-dependent product with biologically active effects may be a more appealing alternative therapeutic indicator. One such example is TMAO in the setting of cardiovascular disease. Although relatively new, TMAO is a marker that has been shown to be predictive of future CVD risk in multiple large clinical studies [37, 38•, 39, 40]. While definitive results are not yet available for TMAO as a gut microbial-dependent marker for diabetes, efforts should be made to search for similar types of metabolites which can be used to monitor the efficacy of dietary or other means of therapeutic interventions. Numerous research efforts are currently underway to investigate mechanisms by which intestinal microbiota can alter various human metabolic processes. Because a large majority of currently available evidence has been based on in vitro work, long-term prospective studies in humans are necessary in order to determine if the observations from the lab can be applied to individuals from various regional backgrounds. Exciting times lie ahead as future studies discover the mechanistic links between the associations observed today. These future discoveries will propel novel therapeutics towards the manipulation of unique micro-biota compositions and functions with the ultimate goal of preventing disease.
Acknowledgments
This work is supported in part by grants from the National Institutes of Health and the Office of Dietary Supplements (R01HL103931, P20HL113452).
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest Daniel Li, Jennifer Kirsop, and W. H. Wilson Tang declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
This article is part of the Topical Collection on Pathogenesis of Type 2 Diabetes and Insulin Resistance
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
• Of major importance
- 1.Eckburg PB, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–8. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claesson MJ, et al. Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLoS One. 2009;4(8):e6669. doi: 10.1371/journal.pone.0006669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turnbaugh PJ, et al. The human microbiome project. Nature. 2007;449(7164):804–10. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Backhed F, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backhed F, et al. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104(3):979–84. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 7.Amar J, et al. Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept. Diabetologia. 2011;54(12):3055–61. doi: 10.1007/s00125-011-2329-8. [DOI] [PubMed] [Google Scholar]
- 8.Moreno-Navarrete JM, et al. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int J Obes (Lond) 2012;36(11):1442–9. doi: 10.1038/ijo.2011.256. [DOI] [PubMed] [Google Scholar]
- 9.Cani PD, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 10.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81(3):1031–64. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 11.Cummings JH, et al. The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am J Clin Nutr. 1979;32(10):2094–101. doi: 10.1093/ajcn/32.10.2094. [DOI] [PubMed] [Google Scholar]
- 12.Goncalves P, Martel F. Butyrate and colorectal cancer: the role of butyrate transport. Curr Drug Metab. 2013;14(9):994–1008. doi: 10.2174/1389200211314090006. [DOI] [PubMed] [Google Scholar]
- 13.Cook SI, Sellin JH. Review article: short chain fatty acids in health and disease. Aliment Pharmacol Ther. 1998;12(6):499–507. doi: 10.1046/j.1365-2036.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- 14.Pouteau E, et al. Acetate, propionate and butyrate in plasma: determination of the concentration and isotopic enrichment by gas chromatography/mass spectrometry with positive chemical ioniza-tion. J Mass Spectrom. 2001;36(7):798–805. doi: 10.1002/jms.181. [DOI] [PubMed] [Google Scholar]
- 15.Ray TK, et al. Long-term effects of dietary fiber on glucose tolerance and gastric emptying in noninsulin-dependent diabetic patients. Am J Clin Nutr. 1983;37(3):376–81. doi: 10.1093/ajcn/37.3.376. [DOI] [PubMed] [Google Scholar]
- 16.Mendeloff AI. Dietary fiber and human health. N Engl J Med. 1977;297(15):811–4. doi: 10.1056/NEJM197710132971506. [DOI] [PubMed] [Google Scholar]
- 17.Robertson MD, et al. Prior short-term consumption of resistant starch enhances postprandial insulin sensitivity in healthy subjects. Diabetologia. 2003;46(5):659–65. doi: 10.1007/s00125-003-1081-0. [DOI] [PubMed] [Google Scholar]
- 18••.Karlsson FH, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. doi: 10.1038/nature12198. These two studies (18,19) provide the first metagenomic evidence of differing profiles between diabetes and normal individuals and suggest the potential to predict development of disease from metagenomic sequences. [DOI] [PubMed] [Google Scholar]
- 19••.Qin J, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. These two studies (18,19) provide the first metagenomic evidence of differing profiles between diabetes and normal individuals and suggest the potential to predict development of disease from metagenomic sequences. [DOI] [PubMed] [Google Scholar]
- 20.Le Poul E, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278(28):25481–9. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 21.Brown AJ, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312–9. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 22.Kimura I, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuel BS, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105(43):16767–72. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tolhurst G, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–71. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 26.Freeland KR, Wolever TM. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-alpha. Br J Nutr. 2010;103(3):460–6. doi: 10.1017/S0007114509991863. [DOI] [PubMed] [Google Scholar]
- 27.Vrieze A, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–6. e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 28.Amar J, et al. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87(5):1219–23. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 29.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saemann MD, et al. Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000;14(15):2380–2. doi: 10.1096/fj.00-0359fje. [DOI] [PubMed] [Google Scholar]
- 31.Roelofsen H, Priebe MG, Vonk RJ. The interaction of short-chain fatty acids with adipose tissue: relevance for prevention of type 2 diabetes. Benefic Microbes. 2010;1(4):433–7. doi: 10.3920/BM2010.0028. [DOI] [PubMed] [Google Scholar]
- 32.Peng L, et al. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139(9):1619–25. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Koeth RA, et al. gamma-Butyrobetaine is a proatherogenic diate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20(5):799–812. doi: 10.1016/j.cmet.2014.10.006. This study and reference [47] provide novel insights into the metabolism of TMAO and its associations with various gut microbiome profiles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124(10):4204–11. doi: 10.1172/JCI72331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gregory JC, et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2014;290:5647–60. doi: 10.1074/jbc.M114.618249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. In this study, Wang et al. uses a metabolomic screen to identify TMAO as a novel gut microbiota-dependent metabolite that is a potential modifiable risk factor for cardiovascular disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang WH, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64(18):1908–14. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Tang WH, et al. Intestinal microbial metabolism of phosphatidyl-choline and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84. doi: 10.1056/NEJMoa1109400. In this clinical study with over 4000 participants, the authors reconfirmed the gut microbiota dependence of TMAO in human subjects and found that serum TMAO levels predicted 3-year incident cardiovascular risk in a dose-dependent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang WH, et al. Intestinal microbiota-dependent phosphatidylcho-line metabolites, diastolic dysfunction and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2014;21:91–6. doi: 10.1016/j.cardfail.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014;35(14):904–10. doi: 10.1093/eurheartj/ehu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang TJ, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–53. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lever M, et al. Betaine and trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: an observational study. PLoS ONE. 2014;9(12):e114969. doi: 10.1371/journal.pone.0114969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao X, et al. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118(4):476–81. doi: 10.1016/j.jbiosc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 44••.Miao J, et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6:6498. doi: 10.1038/ncomms7498. This study shows that knockdown of FMO3 and subsequently TMAO levels can prevent the development of hy-perglycemia, hyperlipidemia, and atherosclerosis in a diabetes mouse model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shih DM, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56(1):22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang WH, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–55. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.Koeth RA, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. doi: 10.1038/nm.3145. See reference 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeisel SH, da Costa KA. Choline: an essential nutrient for public health. Nutr Rev. 2009;67(11):615–23. doi: 10.1111/j.1753-4887.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chu DM, et al. Choline and betaine food sources and intakes in Taiwanese. Asia Pac J Clin Nutr. 2012;21(4):547–57. [PubMed] [Google Scholar]
- 50.Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. J Lipid Res. 2008;49(6):1187–94. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Paoletti L, et al. Role of phosphatidylcholine during neuronal differentiation. IUBMB Life. 2011;63(9):714–20. doi: 10.1002/iub.521. [DOI] [PubMed] [Google Scholar]
- 52.Lever M, Slow S. The clinical significance of betaine, an osmolyte - with a key role in methyl group metabolism. Clin Biochem. 2010;43(9):732–44. doi: 10.1016/j.clinbiochem.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Lever M, et al. Variability of plasma and urine betaine in diabetes mellitus and its relationship to methionine load test responses: an observational study. Cardiovasc Diabetol. 2012;11:34. doi: 10.1186/1475-2840-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lever M, et al. Betaine and secondary events in an acute coronary syndrome cohort. PLoS One. 2012;7(5):e37883. doi: 10.1371/journal.pone.0037883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wijekoon EP, Brosnan ME, Brosnan JT. Homocysteine metabolism in diabetes. Biochem Soc Trans. 2007;35(Pt 5):1175–9. doi: 10.1042/BST0351175. [DOI] [PubMed] [Google Scholar]
- 56.Konstantinova SV, et al. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J Nutr. 2008;138(5):914–20. doi: 10.1093/jn/138.5.914. [DOI] [PubMed] [Google Scholar]
- 57.Schartum-Hansen H, et al. Assessment of urinary betaine as a marker of diabetes mellitus in cardiovascular patients. PLoS One. 2013;8(8):e69454. doi: 10.1371/journal.pone.0069454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lever M, et al. Plasma lipids and betaine are related in an acute coronary syndrome cohort. PLoS One. 2011;6(7):e21666. doi: 10.1371/journal.pone.0021666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rajaie S, Esmaillzadeh A. Dietary choline and betaine intakes and risk of cardiovascular diseases: review of epidemiological evidence. ARYA Atheroscler. 2011;7(2):78–86. [PMC free article] [PubMed] [Google Scholar]
- 60.Danne O, et al. Whole blood choline and plasma choline in acute coronary syndromes: prognostic and pathophysiological implications. Clin Chim Acta. 2007;383(1–2):103–9. doi: 10.1016/j.cca.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 61.Danne O, et al. Prognostic implications of elevated whole blood choline levels in acute coronary syndromes. Am J Cardiol. 2003;91(9):1060–7. doi: 10.1016/s0002-9149(03)00149-8. [DOI] [PubMed] [Google Scholar]
- 62.LeLeiko RM, et al. Usefulness of elevations in serum choline and free F2)-isoprostane to predict 30-day cardiovascular outcomes in patients with acute coronary syndrome. Am J Cardiol. 2009;104(5):638–43. doi: 10.1016/j.amjcard.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 63.Bidulescu A, et al. Usual choline and betaine dietary intake and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc Disord. 2007;7:20. doi: 10.1186/1471-2261-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bidulescu A, et al. Repeatability and measurement error in the assessment of choline and betaine dietary intake: the Atherosclerosis Risk in Communities (ARIC) study. Nutr J. 2009;8:14. doi: 10.1186/1475-2891-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dumas ME, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103(33):12511–6. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mingrone G. Carnitine in type 2 diabetes. Ann N Y Acad Sci. 2004;1033:99–107. doi: 10.1196/annals.1320.009. [DOI] [PubMed] [Google Scholar]
- 67.Capaldo B, et al. Carnitine improves peripheral glucose disposal in non-insulin-dependent diabetic patients. Diabetes Res Clin Pract. 1991;14(3):191–5. doi: 10.1016/0168-8227(91)90020-e. [DOI] [PubMed] [Google Scholar]
- 68.Ferrannini E, et al. Interaction of carnitine with insulin-stimulated glucose metabolism in humans. Am J Physiol. 1988;255(6 Pt 1):E946–52. doi: 10.1152/ajpendo.1988.255.6.E946. [DOI] [PubMed] [Google Scholar]
- 69.Mynatt RL. Carnitine and type 2 diabetes. Diabetes Metab Res Rev. 2009;25(Suppl 1):S45–9. doi: 10.1002/dmrr.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vidal-Casariego A, et al. Metabolic effects of L-carnitine on type 2 diabetes mellitus: systematic review and meta-analysis. Exp Clin Endocrinol Diabetes. 2013;121(4):234–8. doi: 10.1055/s-0033-1333688. [DOI] [PubMed] [Google Scholar]
- 71.DiNicolantonio JJ, et al. L-carnitine in the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Mayo Clin Proc. 2013;88(6):544–51. doi: 10.1016/j.mayocp.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 72.Shang R, Sun Z, Li H. Effective dosing of L-carnitine in the secondary prevention of cardiovascular disease: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2014;14:88. doi: 10.1186/1471-2261-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A. 2012;109(52):21307–12. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu Y, et al. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci U S A. 2014;111(11):4268–73. doi: 10.1073/pnas.1316569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qin J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turnbaugh PJ, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Furet JP, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–57. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang X, et al. Human gut microbiota changes reveal the progres-sion of glucose intolerance. PLoS One. 2013;8(8):e71108. doi: 10.1371/journal.pone.0071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Remely M, et al. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene. 2014;537(1):85–92. doi: 10.1016/j.gene.2013.11.081. [DOI] [PubMed] [Google Scholar]
- 80.Karlsson CL, et al. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring) 2012;20(11):2257–61. doi: 10.1038/oby.2012.110. [DOI] [PubMed] [Google Scholar]
- 81.Everard A, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60(11):2775–86. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Everard A, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–71. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shin NR, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63(5):727–35. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 84.Walker AW, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5(2):220–30. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu GD, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.David LA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lozupone CA, et al. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–30. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]