Abstract
Legionella pneumophila, the causative agent of Legionnaires’ disease, replicates within alveolar macrophages and free-living amoebae. However, the lifestyle of L. pneumophila in the environment remains largely unknown. Here we established a novel natural host model of L. pneumophila endosymbiosis using the ciliate Paramecium caudatum. We also identified Legionella endosymbiosis-modulating factor A (LefA), which contributes to the change in life stage from endosymbiosis to host lysis, enabling escape to the environment. We isolated L. pneumophila strains from the environment, and they exhibited cytotoxicity toward P. caudatum and induced host lysis. Acidification of the Legionella-containing vacuole (LCV) was inhibited, and enlarged LCVs including numerous bacteria were observed in P. caudatum infected with L. pneumophila. An isogenic L. pneumophila lefA mutant exhibited decreased cytotoxicity toward P. caudatum and impaired the modification of LCVs, resulting in the establishment of endosymbiosis between them. Our results suggest that L. pneumophila may have a mechanism to switch their endosymbiosis in protistan hosts in the environment.
Symbiotic relationships between different species are thought to have a huge impact on the dynamics of organic evolution and biotic diversity. Bacteria in the natural environment are able to benefit by establishing these symbiotic relationships with protistan hosts1. It has been reported that some obligate intracellular bacteria, such as Neochlamydia, Parachlamydia, and Rickettsia, adapt to the intracellular conditions in free-living amoebae and establish an endosymbiotic relationship2,3,4. It could be speculated that these bacteria selected the lifestyle of a symbiont during the course of evolution because it provides advantages. Protistan hosts can protect the symbionts from various environmental stresses and provide an ideal environment for bacterial replication5,6. These benefits are thought to be advantageous for the survival of bacterial symbionts. Furthermore, previous studies have demonstrated that the evolution of bacterial pathogenesis and the acquisition of virulence toward humans and/or animals correlate with the virulence toward the protistan host7,8,9,10. It has also been indicated that newly isolated amoebae-resistant bacteria are pathogenic for humans and/or animals11,12.
Legionella pneumophila, the major causative agent of Legionnaires’ disease and Pontiac fever13,14, is a facultative intracellular Gram-negative bacterium that can establish an endoplasmic reticulum-derived vacuole, in which it replicates, by utilizing the dot/icm genes, which encode a type IVB secretion system (T4SS)15,16,17,18. This T4SS makes it possible for L. pneumophila to replicate in human alveolar macrophages. However, human infection is a dead end for L. pneumophila, from an evolutionary point of view, because human-to-human spread of L. pneumophila has not been observed19,20.
L. pneumophila is often found in natural and man-made aquatic environments where it can survive for long periods21,22. From a public health perspective, it can be a high-impact risk factor for human infection. Furthermore, it is well known that L. pneumophila can also survive in the environment in close association with free-living protists. Amoebae and Tetrahymena have been reported to be protistan hosts of Legionella in environmental fresh water23,24. This association leads to the replication and spread of L. pneumophila in the environment and the development of antibiotic resistance25. In addition, protistan hosts are thought to be the primary evolutionary factors for the acquisition and maintenance of virulence toward humans20,26,27. Although previous reports have suggested that the Dot/Icm system also contributes to intracellular survival and replication in amoeba20,28, the mechanisms of infection and endosymbiosis in protistan hosts are not fully understood.
So far, 14 species of amoebae and 2 species of ciliates are known as potential hosts of L. pneumophila23,29. Paramecium spp., found widely in environmental water, are free-living, single-celled, freshwater ciliates that feed on bacteria30. Paramecium spp. are appreciated as model organisms not only for the analysis of cellular and molecular biology, including phagocytosis and exocytosis31,32, but also for endosymbiosis33,34,35. Holospora spp. are endonuclear symbionts of the Paramecium spp36. P. caudatum, for example, can acquire salinity resistance37 and heat-shock resistance38,39,40 if infected with Holospora spp. Despite the fact that many endosymbiotic bacteria have been identified in Paramecium spp.41, interactions between Paramecium spp. and Legionella spp. are unknown.
In this study, we generated a novel protistan host for L. pneumophila and investigated the mechanisms by which L. pneumophila establishes endosymbiosis in the model. We observed not only the process of endosymbiosis but also a cytotoxicity that rejects endosymbiosis with L. pneumophila strains from Paramecium hosts. Our results suggest that L. pneumophila has functional systems to control endosymbiosis with Paramecium spp., including the selection of suitable host strains in the environment.
Results
Paramecium spp. as a novel host model of Legionella endosymbiosis
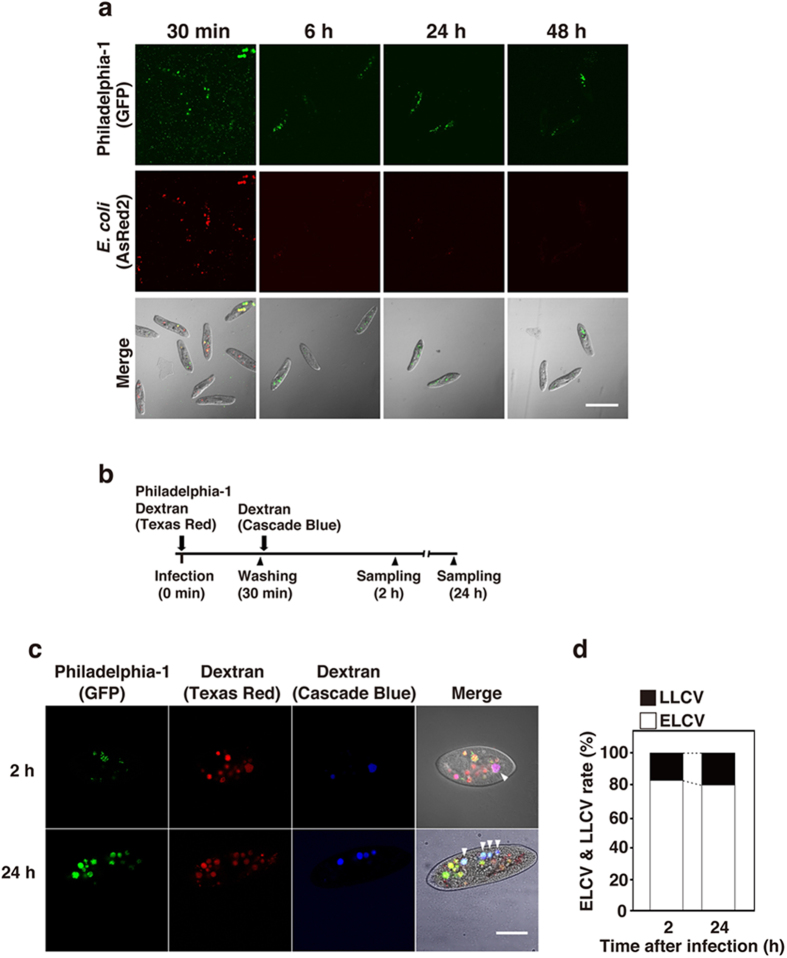
We established a novel host model of Legionella symbiosis using Paramecium spp. P. caudatum strain RB-1 was infected with L. pneumophila strain Philadelphia-1 (Phi-1) by mixing them; then the intracellular localization of the bacteria was determined. Escherichia coli used as a control were observed inside the phagosomes of P. caudatum RB-1 30 min after infection. L. pneumophila Phi-1 remained in P. caudatum RB-1 without digestion for at least 48 h after infection (Fig. 1a). In contrast, E. coli were reduced in number 6 h after infection, and disappeared completely within 48 h after infection (Fig. 1a). It is well known that P. caudatum have high phagocytic and exocytic activities42. Hence, it is possible that re-phagocytosis of free Legionella released from other P. caudatum RB-1 by exocytosis occurs in this assay. To confirm this possibility, a fluorescent dye-conjugated dextran uptake assay was performed according to the schedule shown in Fig. 1b. The percentage of early-formed Legionella-containing vacuoles (ELCV), which are only Texas Red-conjugated dextran (TRDx) positive, and that of late-formed Legionella-containing vacuoles (LLCV), which are positive for both TRDx and Cascade Blue-conjugated dextran (CBDx), were measured 2 and 24 h after infection. As a result, the rate of ELCV (82.7%) was higher than that of LLCV (17.3%) 2 h after infection (Fig. 1c,d). The ELCV were so stable in RB-1, such that the distribution between ELCV and LLCV was hardly changed 24 h after infection (ELCV, 80%; LLCV, 20%) (Fig. 1c,d). These results suggest that P. caudatum RB-1 is likely to serve as a protistan host of Legionella. Legionella may establish endosymbiosis and survive in Paramecium by controlling the turnover of LCV.
Figure 1. L. pneumophila Philadelphia-1 establishes endosymbiosis in P. caudatum RB-1.
(a) Intracellular localization of Philadelphia-1 (GFP) and E. coli (AsRed2) in RB-1 at 30 min, 6 h, 24 h, and 48 h after infection. Bacteria were simultaneously added to RB-1 at an MOI of 10000. Scale bar represents 100 μm. (b) Bacteria and each dextran were added to RB-1 according to this schedule. LCVs containing each dextran were observed by confocal laser scanning microscopy 2 h and 24 h after infection (c), and percentages of ELCV and LLCV are shown with the total of all LCVs being 100% (d). Arrowheads point to LLCV, which are LCVs positive for both Texas Red- and Cascade Blue-conjugated dextrans. Scale bar represents 30 μm.
Cytotoxicity toward Paramecium spp. infected with Legionella
To assess whether endosymbiosis is an inducible phenomenon only between L. pneumophila Phi-1 and P. caudatum RB-1, 62 strains of Paramecium spp. and 8 strains of L. pneumophila were examined, in all combinations, using the same infection assay. Although most L. pneumophila strains, particularly the 3 strains isolated from human lung, had no effect on host viability, only 2 strains, Ofk308 and Bnt314, isolated from environmental water exhibited cytotoxicity toward 8 and 15 strains of Paramecium within 48 h after infection, respectively (Table 1). The P. caudatum RB-1 strain used in Fig. 1 was also sensitive to their cytotoxicity. Thus, P. caudatum RB-1 was employed as a model host strain in the following experiments.
Table 1. Cytotoxicity toward Paramecium spp. by infection with Legionella.
| No |
Paramecium spp. |
L. pneumophila |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NBRP ID | Species | Strain Name | Philadelphia | Knoxville | Togus | Ymg289 | Twr292 | Ymt294 | Ofk308 | Bnt314 | |
| 1 | PA040015A | tetraurelia | d4-2 (7.2b) | − | − | − | − | − | − | − | − |
| 2 | PT040011A | tetraurelia | 51 | − | − | − | − | − | − | − | ++ |
| 3 | PT040015A | tetraurelia | d4-2 | − | − | − | − | − | − | − | − |
| 4 | PT041001A | tetraurelia | st110-1a | − | − | − | − | − | − | − | − |
| 5 | PT042002A | tetraurelia | st110-1b | − | − | − | − | − | − | − | − |
| 6 | PA060001A | sexaurelia | GSZ-3 | − | − | − | − | − | − | − | − |
| 7 | PA080002A | octaurelia | 131 | − | − | − | − | − | − | − | + |
| 8 | PA090001A | novaurelia | Ep-17 | − | − | − | − | − | − | − | − |
| 9 | PA120001A | dodecaurelia | 251 | − | − | − | − | − | − | + | + |
| 10 | PA130001A | tredecaurelia | 321 | − | − | − | − | − | − | − | + |
| 11 | PB000006A | bursaria | KNZ0902g | − | − | − | − | − | − | − | − |
| 12 | PB000014A | bursaria | HA1g | − | − | − | − | − | − | − | − |
| 13 | PB031001A | bursaria | HK4g | − | − | − | − | − | − | − | − |
| 14 | PB031002A | bursaria | KM2g | − | − | − | − | − | − | ++ | ++ |
| 15 | PB033003A | bursaria | HK1g | − | − | − | − | − | − | − | − |
| 16 | PB031010B | bursaria | Yad1g1N | − | − | − | − | − | − | − | − |
| 17 | PB032001A | bursaria | Dd1g | − | − | − | − | − | − | − | − |
| 18 | PB033001A | bursaria | CT39g | − | − | − | − | − | − | − | − |
| 19 | PB032002A | bursaria | YF1g | − | − | − | − | − | − | − | − |
| 20 | PB034003A | bursaria | STL3g | − | − | − | − | − | − | + | + |
| 21 | PB034004A | bursaria | HA1g | − | − | − | − | − | − | − | + |
| 22 | PC000001A | caudatum | BW1 | − | − | − | − | − | − | − | − |
| 23 | PC000021A | caudatum | Fura1 | − | − | − | − | − | − | − | − |
| 24 | PC000028A | caudatum | NDI1-12 | − | − | − | − | − | − | − | − |
| 25 | PC000030A | caudatum | NSA1 | − | − | − | − | − | − | + | ++ |
| 26 | PC000033A | caudatum | RC307 | − | − | − | − | − | − | + | ++ |
| 27 | PC000035A | caudatum | SDIA1-3 | − | − | − | − | − | − | − | + |
| 28 | PC000044A | caudatum | KOM1231 | − | − | − | − | − | − | − | − |
| 29 | PC000050A | caudatum | Kr302 | − | − | − | − | − | − | − | − |
| 30 | PC000052A | caudatum | YD-10 | − | − | − | − | − | − | − | − |
| 31 | PC000053A | caudatum | YD-11 | − | − | − | − | − | − | − | − |
| 32 | PC011011A | caudatum | Mmn64 | − | − | − | − | − | − | − | − |
| 33 | PC121023A | caudatum | KNZ1209 | − | − | − | − | − | − | − | − |
| 34 | PC012002A | caudatum | Myn92 | − | − | − | − | − | − | − | − |
| 35 | PC012005A | caudatum | KNZ1207 | − | − | − | − | − | − | − | − |
| 36 | PC032013A | caudatum | TAZ0460 | − | − | − | − | − | − | + | + |
| 37 | PC032024A | caudatum | HC3 | − | − | − | − | − | − | − | ++ |
| 38 | PC032004A | caudatum | TAZ0462 | − | − | − | − | − | − | − | - |
| 39 | PC041002A | caudatum | N93-027 | − | − | − | − | − | − | − | − |
| 40 | PC042001A | caudatum | RB-1 | − | − | − | − | − | − | ++ | ++ |
| 41 | PC042003A | caudatum | Hot1 | − | − | − | − | − | − | ++ | + |
| 42 | PC051001A | caudatum | YD-1 | − | − | − | − | − | − | − | − |
| 43 | PC061001A | caudatum | Yhb2-2 | − | − | − | − | − | − | − | − |
| 44 | PC122029A | caudatum | dKNZ1207x1209-1 | − | − | − | − | − | − | − | − |
| 45 | PC121031A | caudatum | dKNZ1207x1209-3 | − | − | − | − | − | − | − | − |
| 46 | PC122033A | caudatum | dKNZ1207x1209-5 | − | − | − | − | − | − | − | − |
| 47 | PC121001A | caudatum | My43C3d | − | − | − | − | − | − | − | − |
| 48 | PC131001A | caudatum | Atk | − | − | − | − | − | − | − | − |
| 49 | PD000001A | duboscqui | PD1 | − | − | − | − | − | − | − | − |
| 50 | PK000001A | calkinsi | GN5-3 | − | − | − | − | − | − | − | − |
| 51 | PM000003A | multimicro* | M03c4 | − | − | − | − | − | − | − | − |
| 52 | PM000006A | multimicro* | M09 | − | − | − | − | − | − | − | − |
| 53 | PM000009A | multimicro* | TH103 | − | − | − | − | − | − | − | − |
| 54 | PM000011A | multimicro* | YM8 | − | − | − | − | − | − | − | − |
| 55 | PM000014A | multimicro* | YM-26 | − | − | − | − | − | − | − | − |
| 56 | PM034001A | multimicro* | YM-25 | − | − | − | − | − | − | − | − |
| 57 | PN000001A | nephridiatum | Rw-1 | − | − | − | − | − | − | − | + |
| 58 | PO000001A | polycaryum | YnA (+) | − | − | − | − | − | − | − | − |
| 59 | PP000001A | putrinum | WS1 | − | − | − | − | − | − | − | − |
| 60 | PP001001A | putrinum | OM1 | − | − | − | − | − | − | − | − |
| 61 | PP002002A | putrinum | Sw2 | − | − | − | − | − | − | − | − |
| 62 | PP004001A | putrinum | OM4 | − | − | − | − | − | − | − | − |
−; no cytotoxicity (80–100% of survival rate), +; low cytotoxicity (less than 50% of survival rate), ++; high cytotoxicity (less than 10% of survival rate). *multimicro; multimicronucleatum.
In order to investigate the mechanisms of cytotoxicity, P. caudatum RB-1 was infected with L. pneumophila Ofk308 and Bnt314 for 48 h at various multiplicities of infection (MOIs). In this experiment, Ofk308 and Bnt314 infection clearly caused cytotoxicity in an MOI-dependent matter (Fig. 2a). Next, P. caudatum were treated with culture supernatants of these two strains of bacteria for 48 h. This treatment did not affect the number of live P. caudatum (Fig. 2b). When P. caudatum was mixed with killed bacteria, no difference was observed from the non-fed control (Fig. 2c). These results indicate that active live bacterial uptake to the host phagosomes is required to bring out their cytotoxicity. Most Legionella strains have the ability to establish endosymbiosis with Paramecium. However, environmental isolates of L. pneumophila, such as Ofk308 and Bnt314, do not have this ability, due to unknown factors that are cytotoxic for host cells at environmental temperatures.
Figure 2. Infection of L. pneumophila Ofk308 is required to exhibit the cytotoxicity toward RB-1.
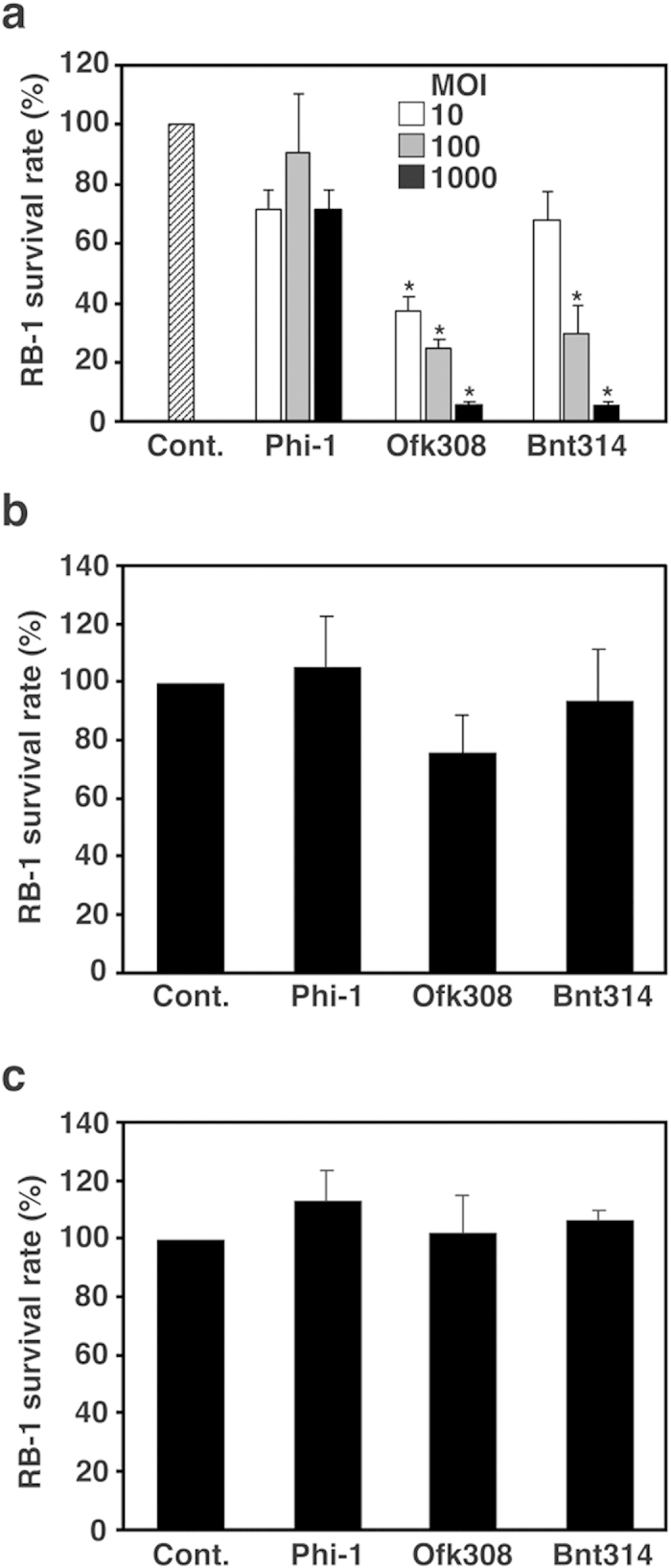
(a) RB-1 was infected with L. pneumophila Phi-1, Ofk308, and Bnt314 at MOIs of 10, 100, and 1000. RB-1 was treated with culture supernatants of Phi-1, Ofk308, and Bnt314 (b), or with killed bacteria (c). Cont., no infection. Relative RB-1 survival rates are indicated, with Cont. being defined as 100%. Data are averages of triplicate samples from three identical experiments, and error bars represent standard deviations. Statistically significant differences, compared with Cont., are indicated by asterisks (*P < 0.01).
Isolation of a cytotoxicity-defective mutant of L. pneumophila Ofk308
To identify the factors contributing its cytotoxicity toward P. caudatum RB-1, we mutagenized L. pneumophila Ofk308 randomly with the mini-Tn5Km2 transposon. Mutants carrying the transposon inserted into the chromosome were resistant to kanamycin, and were selected on BCYE agar plates containing kanamycin. Using this method, 240 transconjugants were isolated and then screened in a P. caudatum infection assay. Finally, 4 cytotoxicity-defective mutants were isolated. We tried to identify the transposon insertion site in these 4 mutants, but 3 of them were not identified. We identified the mutated gene in only one mutant. The gene was lpofk01540, which is a homolog of the sodium/hydrogen antiporter of L. pneumophila Phi-1 (lpg1507). It is likely that the gene is involved in establishing endosymbiosis in Paramecium. Therefore, we named the gene “Legionella endosymbiosis-modulating factor A (lefA).” This lefA mutant has lost its cytotoxicity toward P. caudatum to the same level as L. pneumophila Phi-1, and its complemental strain showed recovery of cytotoxicity comparable with that of the parental strain Ofk308 (Fig. 3a). We also evaluated the intracellular growth of bacterial strains in P. caudatum LCVs. L. pneumophila Phi-1 and lefA mutant showed slight growth in P. caudatum and no damage was observed in infected P. caudatum (Fig. 3b,c). The number of Ofk308 increased significantly in P. caudatum and the shapes of infected P. caudatum were changed unnaturally (Fig. 3b,c), which is likely to lead to cell death due to eventual rupture. The lefA complemental strain also showed high intracellular growth efficiency and damaged infected P. caudatum. E. coli was digested and its numbers reduced rapidly, as shown in Figs 1a and 3c.
Figure 3. lefA mutant has no cytotoxicity toward RB-1.
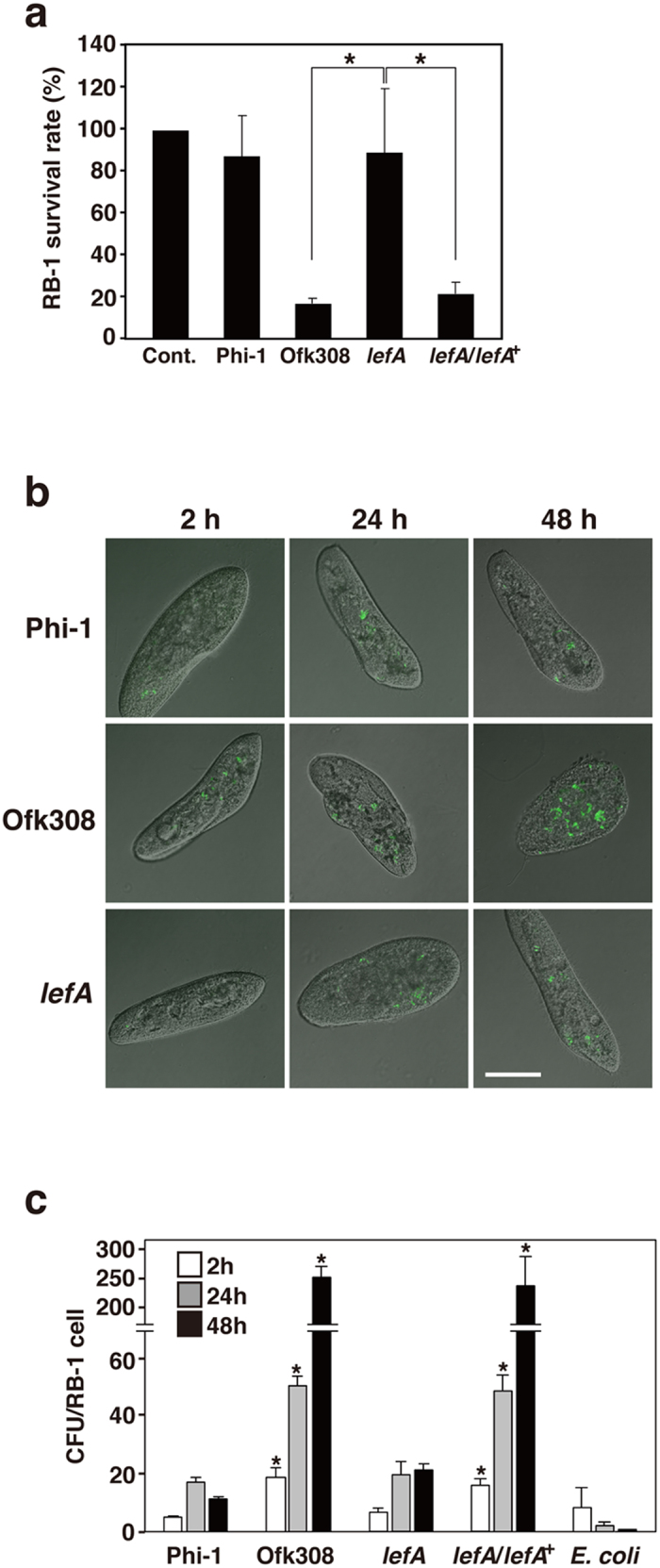
(a) RB-1 was infected with Phi-1, Ofk308, the lefA mutant (lefA), and the lefA complemental strain (lefA/lefA+) at MOIs of 1000. Cont., no infection. Relative RB-1 survival rates are indicated, with Cont. being defined as 100%. (b) Cell shapes of RB-1 infected with each strain of L. pneumophila 2 h, 24 h, and 48 h after infection. Scale bar represents 30 μm. (c) Number of bacteria per RB-1 cell. Data are averages of triplicate samples from three identical experiments, and error bars represent standard deviations. Statistically significant differences compared with lefA (a) or Phi-1 (c) are indicated by asterisks (*P < 0.01).
The expression of lefA in L. pneumophila Ofk308
Although the results described above suggest that lefA contributes to the cytotoxicity toward P. caudatum, all Legionella strains used in this study have a homolog of lefA, regardless of their cytotoxicity. Because the LefA protein of Ofk308 shares 99.2% amino acid sequence homology with that of Phi-1 and these sequence analyses did not clearly explain the relationship between amino acid profile and cytotoxicity (Supplementary Fig. 1), we determined the expression levels of these genes using quantitative real time PCR. The expression of Ofk308 lefA was significantly increased within P. caudatum from 20 to 30 min after infection (Fig. 4a); although, it was stable under in vitro culture conditions and no significant difference was observed when compared with the Phi-1 lefA (Fig. 4b). Expression of the lefA in Bnt314, which has the same cytotoxicity as Ofk308 (Fig. 2a), was also upregulated by infection. On the other hand, such inducible upregulation of lefA was not observed in Phi-1.
Figure 4. Expression of lefA in Ofk308 and Bnt314 is upregulated within RB-1.
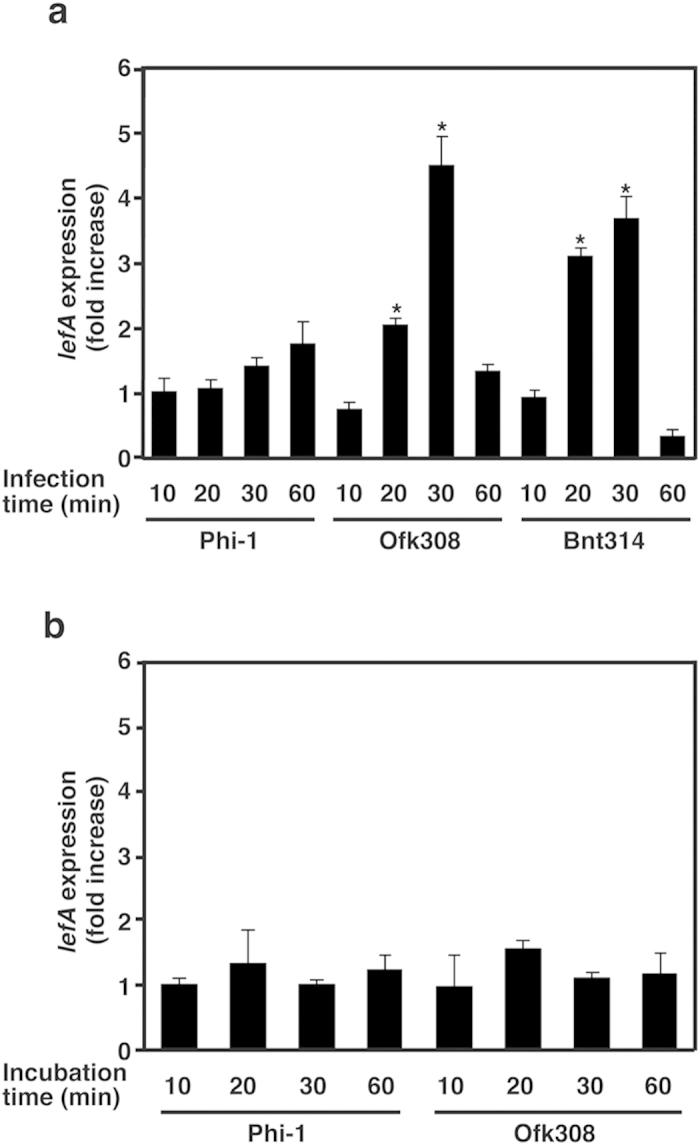
(a) Each strain of L. pneumophila was used to infect RB-1 for 10 to 60 min, and RNA samples were collected from the bacteria. Expression of lefA was determined by real-time PCR. The fold increase of lefA was normalized to 16S rRNA; the expression levels are represented relative to a sample obtained 10 min after infection with Phi-1. (b) Each strain was incubated at 25 °C for 10 to 60 min without RB-1, and then RNA samples were collected. The expression was determined as described above. Statistically significant differences compared to Phi-1 are indicated by asterisks (*P < 0.01).
L. pneumophila Ofk308 modulates LCV acidification in RB-1
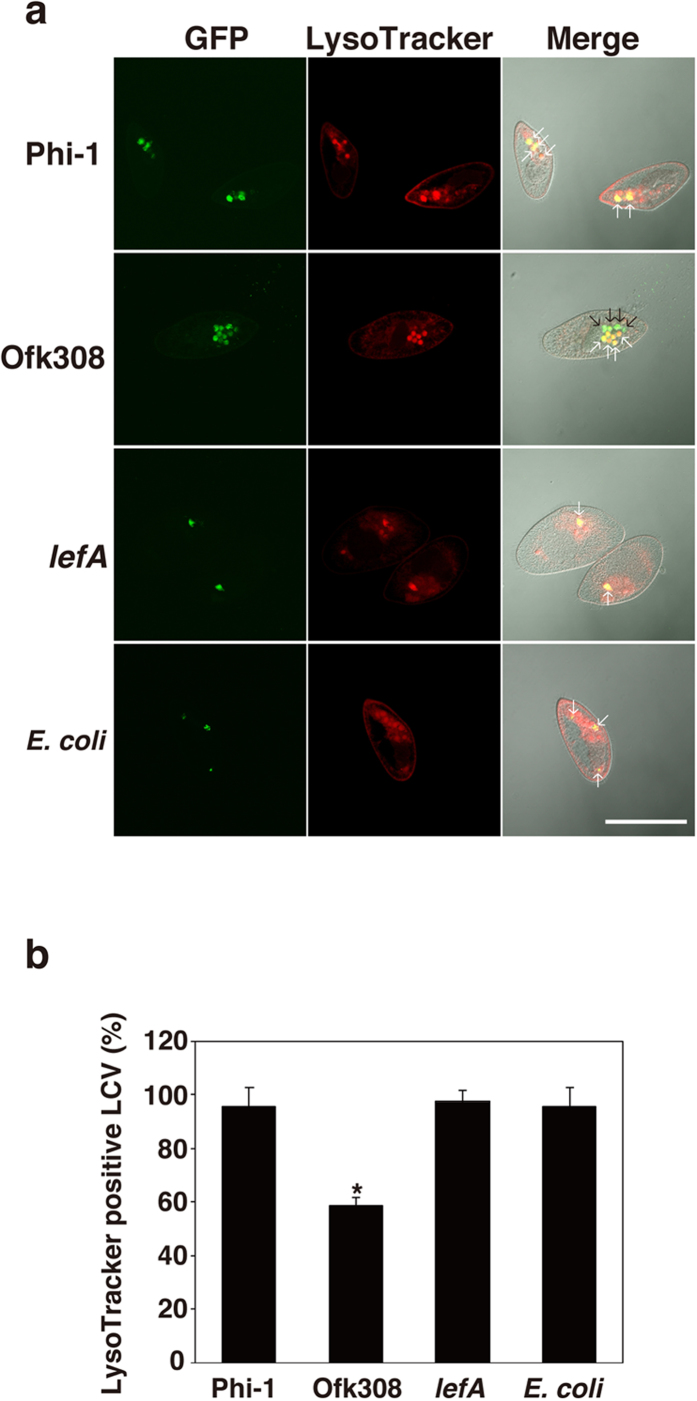
To investigate how lefA modulates the endosymbiosis of Ofk308 in Paramecium, the maturation process of P. caudatum phagosomes that contain bacteria was evaluated using a LysoTracker. As a result, almost all of the phagosomes containing L. pneumophila Phi-1 (95.8%), the lefA mutant (97.4%), or E. coli (95.8%) were LysoTracker-positive 30 min after infection (Fig. 5a,b), indicating that majority of these phagosomes are acidified. In contrast, the percentage of the LysoTracker-positive phagosomes containing Ofk308 was lower (58.9%) (Fig. 5a,b).
Figure 5. The maturation of host LCVs containing Ofk308 is inhibited.
(a) LCV maturation 30 min after infection was evaluated with LysoTracker. Bacteria were added to RB-1 at an MOI of 10000. White arrows point to LysoTracker-positive LCVs (or phagosomes containing E. coli). Black arrows point LysoTracker-negative LCVs. Scale bar represents 100 μm. (b) Relative LysoTracker-positive LCVs percentages are shown, with the total of all LCVs being 100%. Data are the averages of triplicate samples from three identical experiments, and error bars represent standard deviations. Statistically significant differences compared to Phi-1 are indicated by asterisks (*P < 0.01).
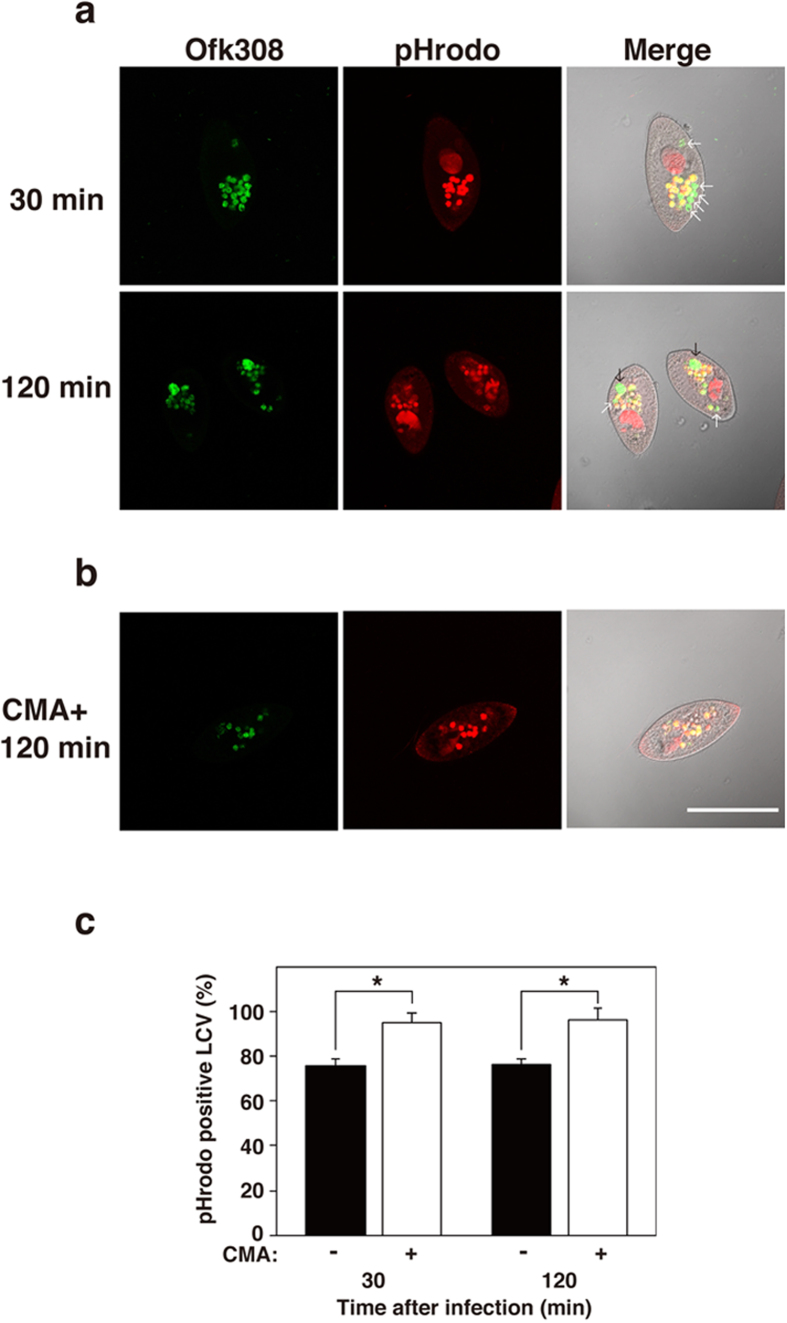
To address the acidification of phagosomes containing Ofk308 in detail, pH indicator (pHrodo)-conjugated dextran (pHDx) and Ofk308 were added to a P. caudatum culture at the same time. Thirty min after infection, 76% of the LCVs exhibited low pH and 24% of the LCVs were negative for pHDx, as in the experiment with LysoTracker shown in Figs 5 and 6a,c. Furthermore, one or two enlarged LCVs per host cell were also observed 2 h after infection. The enlarged LCVs are a quite unique structure observed in P. caudatum infected with Ofk308 at high MOI (Fig. 6a). These enlarged LCVs were also completely negative for pHDx, which means that their acidification was inhibited. These results suggest that Ofk308 modifies both the size and pH of LCVs.
Figure 6. Concanamycin A treatment affects the acidification and enlargement of host LCVs formed by infection of Ofk308.
RB-1 was infected with Ofk308 at an MOI of 10000 and pHrodo-conjugated dextran was added simultaneously (a) without or (b) with concanamycin A (CMA). White arrows point to pHrodo-negative LCVs. Black arrows point to enlarged LCVs. Scale bar represents 100 μm. (c) Relative pHrodo-positive LCVs percentages are shown, with the total of all LCVs being 100%. Data are the averages of triplicate samples from three identical experiments, and error bars represent standard deviations. Statistically significant differences are indicated by asterisks (*P < 0.01).
Concanamycin A (CMA) is a specific inhibitor of vacuole-type ATPase (V-ATPase). It has been reported that the CMA inhibits the acidification of P. caudatum phagosomes43. We investigated the effects of CMA treatment on LCV modification by Ofk308 infection in P. caudatum. When P. caudatum were pre-treated with CMA before Ofk308 infection, these treatments reduced the phagocytosis of P. caudatum, which made it difficult to observe enough LCVs (Supplementary Fig. 2). Thus, we treated P. caudatum with CMA 5 min after infection with Ofk308. CMA treatment kept 95% of the LCVs in a low pH condition from 30 min to 2 h (Fig. 6b,c). Moreover, the enlarged LCVs did not appear at all. We have confirmed that CMA treatment had no direct effect on Ofk308 viability itself at the concentration used in this assay (Supplementary Fig. 3). These results suggest that the modulation of LCVs by Ofk308 depends on P. caudatum V-ATPase.
Intracellular growth of lefA mutant in THP-1 cells
Finally, the contribution of lefA to intracellular growth in mammalian cells was examined in a human macrophage cell line, THP-1 cells. As shown in our previous study, L. pneumophila Phi-1 and Ofk308 displayed potent growth 24 h after infection. However, the lefA mutant failed to grow in THP-1. In addition, the lefA complemental strain showed restitution of potent growth at the same level as parental strain Ofk308 (Fig. 7). These results indicate that, although lefA was identified as an endosymbiosis modulation factor in protistan hosts in this study, it is also an essential factor for Legionella to grow intracellulary in THP-1 cells.
Figure 7. lefA mutant fails to grow in human monocytic THP-1 cells.
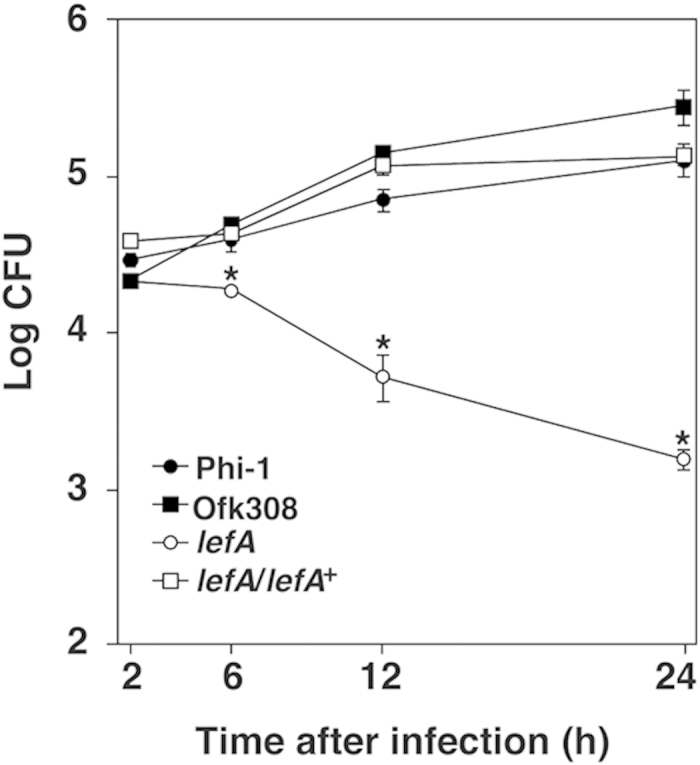
Infected THP-1 cells were cultured for 2, 6, 12, and 24 h. Data are the averages of triplicate samples from three identical experiments, and error bars represent standard deviations. Statistically significant differences compared to Phi-1 are indicated by asterisks (*P < 0.01).
Discussion
Protists are deeply involved in the life cycle of L. pneumophila44. Therefore, the development of protistan host models is greatly useful for investigating the mechanisms of Legionella infection and endosymbiosis, which occur in the natural environment. In addition, prior adaptation to intracellular growth within primitive eukaryotic hosts such as protists is thought to be required for L. pneumophila to gain the ability to infect humans and survive in macrophages20,45. In this study, we established a novel, endosymbiotic host model of Legionella symbiosis using Paramecium spp. The high mobility and high cell division rate of Paramecium in nature may be the major advantages for the spread of bacteria. In addition, all infection assays in our present work were performed at 25 °C, which is typical for Paramecium spp. culture conditions. Although this temperature is lower than that used in infection procedures reported in other protistan hosts46,47 or in macrophages, the cytotoxicity or endosymbiosis of Legionella strains in Paramecium hosts were clearly observed under these conditions. They reflect the natural environmental conditions under which Legionella survive, and reveal the true aspects of Legionella in the environment.
Two L. pneumophila strains isolated from the environment, Ofk308 and Bnt314, refused to establish endosymbiosis in 8 and 15 strains of Paramecium, including RB-1, respectively (Table 1). These L. pneumophila strains may have strict protistan host tropism or switching systems to select suitable hosts in the environment. Ofk308, in particular, may have employed systems correlating with lefA to survive and, as a result, to achieve some sort of advantage in the environment. It was reported that endosymbiont Neochlamydia in amoeba negatively affects subsequent L. pneumophila infection2. Thus, lefA-mediated switching systems may play a role in the elimination or avoidance of these inconvenient host cells.
Sodium/hydrogen antiporters are ubiquitous membrane proteins considered to be the major Na+ excretion system in bacteria48,49. Mutants lacking sodium/hydrogen antiporters are unable to grow in the presence of high concentrations of NaCl at pH 750. However, their participation in virulence or intracellular growth is not well known in Legionella. The lefA mutant of L. pneumophila did not show growth depression in a medium containing a high concentration of NaCl, compared with parental strain Ofk308 (Supplementary Fig. 4). L. pneumophila Ofk308 is likely to have a number of genes encoding sodium/hydrogen antiporters or alternative transport systems that control Na+ excretion. Thus, it is possible that these genes substitute for lefA. On the other hand, the lefA mutant exhibited a complete deficiency of cytotoxicity toward P. caudatum RB-1 because of this single gene mutation. These results indicate that LefA has other unique functions contributing to cytotoxicity toward P. caudatum RB-1, aside from its role as a sodium/hydrogen antiporter. It was reported that another type of antiporter gene (kefB) from Mycobacterium tuberculosis arrests phagosomal maturation and acidification51. Ofk308 also inhibited acidification of LCVs in P. caudatum RB-1 and induced enlarged LCVs (Fig. 6). Ofk308 could modify the properties of LCVs depending on LefA, and make it possible for Ofk308 to grow, notably in RB-1. Thus, LefA may lead to the cytotoxicity toward RB-1. However, it was confirmed that other Legionella strains used in this study also have a homolog of lefA. We have investigated the expression levels of lefA in these Legionella strains, and observed up-regulation of lefA in Ofk308 due to uptake by RB-1 (Fig. 4b). These unidentified expression-regulating systems of lefA, or other factors associated with lefA, may be critical to exhibit the cytotoxicity.
Furthermore, although the Ofk308 strain showed potent growth, at the same level as that of L. pneumophila Phi-1, its lefA mutant failed to grow in THP-1 cells (Fig. 7). Our findings possibly provide another function of lefA; it is involved in intracellular growth in macrophage cells. It has been reported that several Legionella virulence factors identified in macrophages are also required for successful intracellular growth within protistan hosts such as amoebae52. It is well known that the Dot/Icm T4SS is critical for the intracellular growth of L. pneumophila53,54. The Dot/Icm secretion system injects effectors into the host cell, and enables L. pneumophila to adapt to intracellular life within both protistan and mammalian hosts20. Thus, we investigated the cytotoxicity of a dotH-deletion mutant of Ofk308 in P. caudatum RB-1. As a result, reduction of the cytotoxicity was not observed (Supplementary Fig. 5). These results suggest that the lefA may play a central role in the modulation of Ofk308 endosymbiosis in P. caudatum RB-1, independent of the Dot/Icm system.
In this study, we used 62 strains of 14 species in Paramecium as protistan hosts. There were differences in sensitivity among these strains to the cytotoxicity exerted by infection with Ofk308 and Bnt314 (Table 1). No definitive tendency of this sensitivity to cytotoxicity was found among these species. Two major types of taxonomy based on similarity of morphology have been reported in Paramecium spp. One method divides them into two groups: the aurelia group (P. aurelia species, P. caudatum, P. multimicronucleatum) and the bursaria group (P. bursaria, P. putrinum, P. trichium, P. calkinsi)55; and the other method divides them into three groups: the putrinum group (P. putrinum, P. bursaria), the woodruffi group (P. woodruffi, P. calkinsi, P. polycarium, P. arcticum, P. preudotrichium), and the aurelia group (P. aurelia species, P. caudatum, P. jenningsi, P. africanum, P. multimicronucleatum, P. wichtermani)56. We also evaluated whether any tendencies in the sensitivity to cytotoxicity exist according to these taxonomies. However, we could not find any clear correlations here, either. Studies of host-related factors are absolutely imperative in order to analyze the mechanisms of endosymbiosis by L. pneumophila in protistan hosts. Further examinations of the genetic backgrounds of these Paramecium strains may explain the differences in their sensitivity to cytotoxicity.
In conclusion, the results from our study suggest that L. pneumophila has a potential mechanism to control endosymbiosis in Paramecium in which the lefA gene is prominently involved. When the pathogenicity or infectious risks of pathogens that exist in environment, including Legionella, are investigated, it is important to know as much about their environmental phase before they infect humans. In that regard, the findings about the mechanism of endosymbiosis modulation shown in the L. pneumophila-Paramecium model contribute to the development of further research concerning environmental pathogens.
Methods
Bacterial strains
All bacterial strains and plasmids used in this work are listed in Table 2. Legionella pneumophila Philadelphia-1 (GTC_00296), Knoxville-1 (GTC_00745), and Togus-1 (GTC_00746) were obtained from National BioResouce Project (NBRP) of the Ministry of Education, Culture, Sports, Science and Technology, Japan ( http://www.nbrp.jp/). Five isolated environmental strains were reported in our previous study21,57. These Legionella strains were maintained as frozen glycerol stocks and cultured on either N-(2-acetamido)-2-aminoethanesulphonic acid -buffered charcoal yeast extract agar (BCYE) or in the same medium without agar and charcoal (AYE), at 37 °C. E. coli strains were cultured either in LB broth or on the LB containing 1.5% agar. If necessary, ampicillin (100 μg/mL), chloramphenicol (10 μg/mL), and kanamycin (30 μg/mL) were used. GFP expression in Legionella was induced by adding isopropyl-β-D-thiogalactopyranoside (IPTG) (1 μM) to AYE.
Table 2. Bacterial strains and plasmids used in this study.
| Strain | Characteristics | Reference or Sourse |
|---|---|---|
| L. pneumophila | ||
| Philadelphia-1 | Isolated from human lung | GTC 00296 (ATCC 33216) |
| Knoxville-1 | Isolated from human lung | GTC 00745 (ATCC 33153) |
| Togus-1 | Isolated from human lung | GTC 00746 (ATCC 33154) |
| Ymg289 | Isolated from environmental water | Tachibana et al.21 |
| Twr292 | Isolated from environmental water | Tachibana et al.21 |
| Ymt294 | Isolated from environmental water | Tachibana et al.21 |
| Ofk308 | Isolated from environmental water | Tachibana et al.21 |
| Bnt314 | Isolated from environmental water | Tachibana et al.21 |
| lefA | mini-Tn5Km inserted into lefA gene of Ofk308 | This work |
| lefA/lefA+ | lefA carrying pMS8-lefA | This work |
| E. coli | ||
| DH5α | Φ80lacZΔM15, Δ(lacZYA-argF)U169, recA1, endA1, hsdR17, supE44,thi-1, gyrA96, relA1 | Takara |
| DH5α λpir | DH5α (λpir) tet::Mu recA | Takara |
| JM109 | recA1, endA1, gyrA96, thi-1, hsdR17, e14- (mcrA-), supE44, relA1, Δ (lac-proAB) | Takara |
| Plasmids | ||
| pAM239-GFP | pMMB-derived vector encording GFP | Watarai et al.60 |
| pAcGFP1 | pUC-derived vector encoding GFP | Clontech |
| pAsRed2 | pUC19-derived vector encording AsRed2 | Clontech |
| pUTmini-Tn5Km | pUT vector containing mini-Tn5 carring Km resistance gene | BioMedal |
| pMS8-lefA | pMS8 vector expressing lefA | This work |
Paramecium spp. strains
All Paramecium spp. used in this study were provided by Symbiosis Laboratory, Yamaguchi University with support, in part, by the NBRP. Culture and maintenance of Paramecium spp. were described previously58. In brief, the culture medium used for Paramecium was 2.5% (w/v) fresh lettuce juice in Dryl’s solution59, inoculated with a non-pathogenic strain of Klebsiella pneumoniae 1 day before use. The cultivation of Paramecium spp. was performed at 25 °C.
Cytotoxicity measurement
Bacteria were added to each Paramecium spp. in 1.5 mL tubes at a multiplicity of infection (MOI) of 10, 100, 1000, and 10000, respectively. Killed bacteria were obtained by treatment with 4% paraformaldehyde for 60 min. After washing 3 times with PBS, these killed bacteria were added to RB-1 in a 1.5 mL tube at a substantial MOI of 1000. Overnight cultures of bacteria were centrifuged at 15 000 rpm for 5 min, and then the supernatants were filtered through a 0.45-μm filter. These culture supernatants were also added to RB-1 in a 1.5 mL tube. These tubes were incubated at 25 °C for 48 h. After incubation, the number of live Paramecium was counted by microscopy.
Transposon mutagenesis
Random mini-Tn5 transposon mutagenesis was used to generate mutants of the L. pneumophila Ofk308 strain. The mini-Tn5-bearing plasmid pUTmini-Tn5 Km (BioMedal S. L.) was introduced into the Ofk308 strain by electroporation with a Gene Pulser electroporator (Bio-Rad Laboratories) in a 10% glycerol solution at 2.5 kV/25 μF. The mutants were purified on agar plates containing kanamycin (30 μg/mL) and were screened for cytotoxicity toward RB-1. Chromosomal DNA of the mutant was extracted from an overnight culture using the DNeasy Blood & Tissue kit (QIAGEN). The identification of mini-Tn5 inserted region was performed by whole-genome sequencing of the mutant using paired-end sequencing with an Illumina MiSeq kit v3.
Determination of bacterial load in RB-1
RB-1 was infected with GFP-expressing Legionella or E. coli at an MOI of 1000. After incubation at 25 °C, the RB-1 was washed using a Celltrics Fillter (mesh size, 10 μm) (Sysmex Partec GmbH) to remove the extracellular bacteria. Furthermore, to purge the K. pneumonia fed to the RB-1, samples were treated at 50 °C for 30 min. Colony forming units (CFU) were determined by serial dilution on BCYE containing chloramphenicol (10 μg/mL).
Fluorescence microscopy
GFP- or AsRed-expressing bacteria were added to RB-1 and were then incubated at 25 °C for 30 min to 48 h. Samples were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature. Subsequently, samples were washed twice with PBS. Fluorescent images were obtained using a FluoView FV100 confocal laser scanning microscope (Olympus). To observe the ELCV and LLCV, P. caudatum RB-1 was infected with L. pneumophila Phi-1 and TRDx was added to the medium simultaneously. And then extracellular bacteria and TRDx were washed out by filtration 30 min after infection. At this point, CBDx was added to the medium (Fig. 1b). TRDx, CBDx, and pHDx (Life Technologies) were added to RB-1 with bacterial infection or at the indicated times at concentrations of 50 μg/mL. LysoTracker (Life Technologies) was used after fixation for 30 min at a concentration of 50 nM. Concanamycin A (1 μM, Wako), a V-type proton pump inhibitor, was added to RB-1 5 min after infection. The number of these indicator-positive vacuoles was counted by microscopy, and shown as an average of 10 RB-1 cells.
Quantitative real-time PCR
The RB-1 infection assay were performed as described above. The bacterial RNA were extracted using the TRIzol regent (Life Technologies). Total bacterial RNA were subjected to reverse transcription using the ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo) according to the manufacturer’s instructions. Quantitative assays were performed on each cDNA with the THUNDERBIRD SYBR qPCR Mix (Toyobo) using an Applied Biosystems StepOne Real-Time PCR System. Dissociation curve analysis was performed in order to verify product homogeneity. The 16S rRNA amplicon was used as an internal control in order to normalize all data. The relative expression levels of the genes of interest were calculated using the relative quantification method (ΔΔCT). The specific primers for each target were designed as listed below: 5′-GTAGGCAAGTGCAGCAAACA and 5′-TGCATTTACAGCCCCAGAAT for lefA, and 5′-TAAGGAGACTGCCGGTGACA and 5′-GGCCATTGTAGCACGTGTGT for 16S rRNA.
THP-1 cells culture and infection assay
Cells from the human monocytic cell line THP-1 were grown in RPMI 1640 medium (Sigma–Aldrich), supplemented with 10% heat-inactivated FBS at 37 °C under an atmosphere containing 5% CO2. THP-1 cells were differentiated with 100 nM phorbol 12-myristate 13-acetate (Sigma–Aldrich) 48 h prior to use. Bacteria were added to a monolayer of THP-1 cells in 48-well tissue culture dishes at an MOI of 1. These plates were centrifuged for 10 min at 900 × g and incubated for 1 h at 37 °C. Extracellular bacteria were killed by gentamicin (30 μg/mL) treatment for 30 min. To measure the intracellular growth, the cells were incubated in fresh medium at 37 °C for the designated amount of time, washed three times with PBS, and then lysed with cold distilled water. CFU were determined by serial dilution on BCYE.
Statistical analyses
Statistical analyses were performed using Student’s t-test. Statistically significant differences compared with the control are indicated by asterisks (*P < 0.01). Data are the averages of triplicate samples from three identical experiments, and the error bars represent standard deviations.
Additional Information
How to cite this article: Watanabe, K. et al. Ciliate Paramecium is a natural reservoir of Legionella pneumophila. Sci. Rep. 6, 24322; doi: 10.1038/srep24322 (2016).
Supplementary Material
Acknowledgments
We thank H. Suzuki (Yamaguchi University) for useful suggestions. This work was supported, in part, by the Japan Society for the Promotion of Science Grant-in-Aid for Young Scientists (B), Grant Numbers 15K18782, to KW, and by the Japan Ministry of Education, Culture, Sports Science and Technology, TOKUBETSUKEIHI, to MF and MW.
Footnotes
Author Contributions K.W. and M.W. conceived and designed the experiments; K.W., R.N., M.T. and M.W. performed the experiments; K.W., M.F., T.S. and M.W. analyzed data; M.F. contributed reagents/materials/analysis tools; K.W. and M.W. wrote the paper.
References
- Taylor M., Mediannikov O., Raoult D. & Greub G. Endosymbiotic bacteria associated with nematodes, ticks and amoebae. FEMS Immunol Med Microbiol 64, 21–31, doi: 10.1111/j.1574-695X.2011.00916.x (2012). [DOI] [PubMed] [Google Scholar]
- Ishida K. et al. Amoebal endosymbiont Neochlamydia genome sequence illuminates the bacterial role in the defense of the host amoebae against Legionella pneumophila. PLoS One 9, e95166, doi: 10.1371/journal.pone.0095166 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann R. et al. Obligate intracellular bacterial parasites of acanthamoebae related to Chlamydia spp. Appl Environ Microbiol 63, 115–121 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz F. et al. A Rickettsiales Symbiont of Amoebae with Ancient Features. Environ Microbiol, doi: 10.1111/1462-2920.12881 (2015). [DOI] [PubMed] [Google Scholar]
- Barbaree J. M., Fields B. S., Feeley J. C., Gorman G. W. & Martin W. T. Isolation of protozoa from water associated with a legionellosis outbreak and demonstration of intracellular multiplication of Legionella pneumophila. Appl Environ Microbiol 51, 422–424 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borella P., Guerrieri E., Marchesi I., Bondi M. & Messi P. Water ecology of Legionella and protozoan: environmental and public health perspectives. Biotechnol Annu Rev 11, 355–380, doi: 10.1016/s1387-2656(05)11011-4 (2005). [DOI] [PubMed] [Google Scholar]
- Fields B. S. et al. Comparison of guinea pig and protozoan models for determining virulence of Legionella species. Infect Immun 53, 553–559 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner L. et al. Are clinical isolates of Pseudomonas aeruginosa more virulent than hospital environmental isolates in amebal co-culture test? Crit Care Med 34, 823–828, doi: 10.1097/01.ccm.0000201878.51343.f1 (2006). [DOI] [PubMed] [Google Scholar]
- Goy G. et al. The Neff strain of Acanthamoeba castellanii, a tool for testing the virulence of Mycobacterium kansasii. Res Microbiol 158, 393–397, doi: 10.1016/j.resmic.2007.01.003 (2007). [DOI] [PubMed] [Google Scholar]
- Steinberg K. M. & Levin B. R. Grazing protozoa and the evolution of the Escherichia coli O157:H7 Shiga toxin-encoding prophage. Proc Biol Sci 274, 1921–1929, doi: 10.1098/rspb.2007.0245 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greub G. & Raoult D. Microorganisms resistant to free-living amoebae. Clin Microbiol Rev 17, 413–433 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molmeret M., Horn M., Wagner M., Santic M. & Abu Kwaik Y. Amoebae as training grounds for intracellular bacterial pathogens. Appl Environ Microbiol 71, 20–28, doi: 10.1128/aem.71.1.20-28.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shands K. N. & Fraser D. W. Legionnaires’ disease. Dis Mon 27, 1–59 (1980). [DOI] [PubMed] [Google Scholar]
- McDade J. E. et al. Legionnaires’ disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med 297, 1197–1203, doi: 10.1056/nejm197712012972202 (1977). [DOI] [PubMed] [Google Scholar]
- Marra A., Blander S. J., Horwitz M. A. & Shuman H. A. Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc Natl Acad Sci USA 89, 9607–9611 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K. H. & Isberg R. R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol 7, 7–19 (1993). [DOI] [PubMed] [Google Scholar]
- Sadosky A. B., Wiater L. A. & Shuman H. A. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect Immun 61, 5361–5373 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C. R., Berger K. H. & Isberg R. R. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol 28, 663–674 (1998). [DOI] [PubMed] [Google Scholar]
- Moliner C., Fournier P. E. & Raoult D. Genome analysis of microorganisms living in amoebae reveals a melting pot of evolution. FEMS Microbiol Rev 34, 281–294, doi: 10.1111/j.1574-6976.2010.00209.x (2010). [DOI] [PubMed] [Google Scholar]
- Al-Quadan T., Price C. T. & Abu Kwaik Y. Exploitation of evolutionarily conserved amoeba and mammalian processes by Legionella. Trends Microbiol 20, 299–306, doi: 10.1016/j.tim.2012.03.005 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M., Nakamoto M., Kimura Y., Shimizu T. & Watarai M. Characterization of Legionella pneumophila isolated from environmental water and ashiyu foot spa. Biomed Res Int 2013, 514395, doi: 10.1155/2013/514395 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliermans C. B. et al. Ecological distribution of Legionella pneumophila. Appl Environ Microbiol 41, 9–16 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham T. J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol 33, 1179–1183 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. S., Shotts E. B. Jr., Feeley J. C., Gorman G. W. & Martin W. T. Proliferation of Legionella pneumophila as an intracellular parasite of the ciliated protozoan Tetrahymena pyriformis. Appl Environ Microbiol 47, 467–471 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winiecka-Krusnell J. & Linder E. Free-living amoebae protecting Legionella in water: the tip of an iceberg? Scand J Infect Dis 31, 383–385 (1999). [DOI] [PubMed] [Google Scholar]
- Luo Z. Q. Legionella secreted effectors and innate immune responses. Cell Microbiol 14, 19–27, doi: 10.1111/j.1462-5822.2011.01713.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton H. J., Ang D. K., van Driel I. R. & Hartland E. L. Molecular pathogenesis of infections caused by Legionella pneumophila. Clin Microbiol Rev 23, 274–298, doi: 10.1128/cmr.00052-09 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A. M., Von Dwingelo J. E., Price C. T. & Abu Kwaik Y. Cellular microbiology and molecular ecology of Legionella-amoeba interaction. Virulence 4, 307–314, doi: 10.4161/viru.24290 (2013). [DOI] [PMC free article] [PubMed]
- Fields B. S. The molecular ecology of legionellae. Trends Microbiol 4, 286–290 (1996). [DOI] [PubMed] [Google Scholar]
- Wichterman R. The Biology of Paramecium. Plenum Press (1986). [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A. & Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol 96, 1–27 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner H. Membrane trafficking in protozoa SNARE proteins, H+-ATPase, actin, and other key players in ciliates. Int Rev Cell Mol Biol 280, 79–184, doi: 10.1016/s1937-6448(10)80003-6 (2010). [DOI] [PubMed] [Google Scholar]
- Fujishima M. Infection and Maintenance of Holospora Species in Paramecium caudatum. Microbiology Monographs 12, 201–225 (2009). [Google Scholar]
- Fujishima M. & Kodama Y. Endosymbionts in paramecium. Eur J Protistol 48, 124–137, doi: 10.1016/j.ejop.2011.10.002 (2012). [DOI] [PubMed] [Google Scholar]
- Kodama Y. & Fujishima M. Secondary symbiosis between Paramecium and Chlorella cells. Int Rev Cell Mol Biol 279, 33–77, doi: 10.1016/s1937-6448(10)79002-x (2010). [DOI] [PubMed] [Google Scholar]
- Fokin S. I., Brigge T., Brenner J. & Görtz H. D. Holospora species infected the nuclei of Paramecium appear to belong into two groups of bacteria. Eur. J. Protistol 32, 19–24 (1996). [Google Scholar]
- Smurov A. O. & Fokin S. I. Resistance of Paramecium caudatum infected with endonuclear bacteria Holospora against salinity impact. Proc Zool Ins RAS 276, 175–178 (1998). [Google Scholar]
- Hori M. & Fujishima M. The endosymbiotic bacterium Holospora obtusa enhances heat-shock gene expression of the host Paramecium caudatum. J Eukaryot Microbiol 50, 293–298 (2003). [DOI] [PubMed] [Google Scholar]
- Fujishima M., Kawai M. & Yamamoto R. Paramecium caudatum acquires heat-shock resistance in ciliary movement by infection with the endonuclear symbiotic bacterium Holospora obtusa. FEMS Microbiol Lett 243, 101–105, doi: 10.1016/j.femsle.2004.11.053 (2005). [DOI] [PubMed] [Google Scholar]
- Hori M., Fujii K. & Fujishima M. Micronucleus-specific bacterium Holospora elegans irreversibly enhances stress gene expression of the host Paramecium caudatum. J Eukaryot Microbiol 55, 515–521, doi: 10.1111/j.1550-7408.2008.00352.x (2008). [DOI] [PubMed] [Google Scholar]
- Görtz H. D. & Fokin S. I. Diversity of Endosymbiotic Bacteria in Paramecium. Microbiology Monographs 12, 131–160 (2009). [Google Scholar]
- Allen R. D. & Fok A. K. Membrane trafficking and processing in Paramecium. Int Rev Cytol 198, 277–318 (2000). [DOI] [PubMed] [Google Scholar]
- Fujishima M. & Kawai M. Acidification in Digestive Vacuoles is an Early Event Required for Holospora Infection of Paramecium Nucleus. Eukaryotism and Symbiosis 367–370 (1997).
- Hoffmann C., Harrison C. F. & Hilbi H. The natural alternative: protozoa as cellular models for Legionella infection. Cell Microbiol 16, 15–26, doi: 10.1111/cmi.12235 (2014). [DOI] [PubMed] [Google Scholar]
- Franco I. S., Shuman H. A. & Charpentier X. The perplexing functions and surprising origins of Legionella pneumophila type IV secretion effectors. Cell Microbiol 11, 1435–1443, doi: 10.1111/j.1462-5822.2009.01351.x (2009). [DOI] [PubMed] [Google Scholar]
- Cianciotto N. P. & Fields B. S. Legionella pneumophila mip gene potentiates intracellular infection of protozoa and human macrophages. Proc Natl Acad Sci USA 89, 5188–5191 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk S. G. et al. Packaging of live Legionella pneumophila into pellets expelled by Tetrahymena spp. does not require bacterial replication and depends on a Dot/Icm-mediated survival mechanism. Appl Environ Microbiol 74, 2187–2199, doi: 10.1128/aem.01214-07 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Cheng J. & Guffanti A. A. The role of monovalent cation/proton antiporters in Na(+)-resistance and pH homeostasis in Bacillus: an alkaliphile versus a neutralophile. J Exp Biol 196, 457–470 (1994). [DOI] [PubMed] [Google Scholar]
- Padan E., Venturi M., Gerchman Y. & Dover N. Na(+)/H(+) antiporters. Biochim Biophys Acta 1505, 144–157 (2001). [DOI] [PubMed] [Google Scholar]
- Sakuma T., Yamada N., Saito H., Kakegawa T. & Kobayashi H. pH dependence of the function of sodium ion extrusion systems in Escherichia coli. Biochim Biophys Acta 1363, 231–237 (1998). [DOI] [PubMed] [Google Scholar]
- Khare G., Reddy P. V., Sidhwani P. & Tyagi A. K. KefB inhibits phagosomal acidification but its role is unrelated to M. tuberculosis survival in host. Sci Rep 3, 3527, doi: 10.1038/srep03527 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoll P., Rolando M., Gomez-Valero L. & Buchrieser C. From amoeba to macrophages: exploring the molecular mechanisms of Legionella pneumophila infection in both hosts. Curr Top Microbiol Immunol 376, 1–34, doi: 10.1007/82_2013_351 (2013). [DOI] [PubMed] [Google Scholar]
- Segal G. & Shuman H. A. Characterization of a new region required for macrophage killing by Legionella pneumophila. Infect Immun 65, 5057–5066 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J. P., Andrews H. L., Wong S. K. & Isberg R. R. Conjugative transfer by the virulence system of Legionella pneumophila. Science 279, 873–876 (1998). [DOI] [PubMed] [Google Scholar]
- Woodruff L. L. The Structure, Life History, and Intrageneric Relationships of Paramecium calkinsi, sp. nov. Biological Bulletin 41, 171–180, doi: 10.2307/1536748 (1921). [DOI] [Google Scholar]
- Jankowski A. W. Cytogenetic of Paramecium putrunum C. et L., (1958). Acta Protozool 10, 285–394 (1972). [Google Scholar]
- Watanabe K., Suzuki H., Nakao R., Shimizu T. & Watarai M. Draft Genome Sequences of Five Legionella pneumophila Strains Isolated from Environmental Water Samples. Genome Announc 3, doi: 10.1128/genomeA.00474-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishima M., Sawabe H. & Iwatsuki K. Scanning electron microscopic observation of differentiation from the reproductive short form to the infectious long form of Holospora obtusa. J. Protozool 37, 123–128 (1990). [Google Scholar]
- Dryl S. Antigenic transformation in Paramecium aurelia after homologous antiserum treatment during autogamy and conjugation. J. Protozool 6 (1959). [Google Scholar]
- Watarai M. et al. Legionella pneumophila is internalized by a macropinocytotic uptake pathway controlled by the Dot/Icm system and the mouse Lgn1 locus. J Exp Med 194, 1081–1096 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.