Abstract
Injury to the ulnar collateral ligament (UCL) most commonly occurs in the overhead throwing athlete. Knowledge surrounding UCL injury pathomechanics continues to improve, leading to better preventative treatment strategies and rehabilitation programs. Conservative treatment strategies for partial injuries, improved operative techniques for reconstruction in complete tears, adjunctive treatments, as well as structured sport specific rehabilitation programs including resistive exercises for the entire upper extremity kinetic chain are all important factors in allowing for a return to throwing in competitive environments. In this review, we explore each of these factors and provide recommendations based on the available literature to improve outcomes in UCL injuries in athletes.
Keywords: Elbow, Ulnar collateral ligament, Valgus instability, Tommy John surgery, Rehabilitation, Overhead athlete, Improved outcomes
Core tip: While surgical techniques undoubtedly affect the outcome following ulnar collateral ligament (UCL) reconstruction, they do not independently do so. Rather, it is a complex milieu of pre-operative, intra-operative, and post-operative factors that combine to affect the overall outcome following UCL injury. Due to the variability in success rates for treatment of these injuries, careful review of each of these factors is required to ensure outcomes are optimized following treatment. This study serves as a review of these factors, providing recommendations based on available literature to improve outcomes following UCL injuries in athletes in future years.
INTRODUCTION
Ulnar collateral ligament (UCL) injuries have occurred with an increasing incidence among throwing athletes in recent years[1]. Once considered a career-ending injury, Dr. Jobe’s reconstructive technique revolutionized the treatment of these injuries, improving outcomes and return-to-sport following surgical reconstruction[2]. Since that time, attention has focused on optimizing the surgical technique, with several subsequent modifications aimed at improving outcomes and minimizing associated complication rates. While seemingly successful based on summative analyses in recent systematic reviews[3,4], the results are inconsistent, with return to play rates varying from 53%-90%[5-9] and complication rates varying from 3%-25%[10,11]. This disparity in outcomes following surgical reconstruction has prompted further study into the management of UCL injuries beyond advancements in surgical techniques. Consequently, knowledge surrounding UCL injury pathomechanics continues to improve, leading to better preventative treatment strategies and rehabilitation programs[1,12,13]. Additionally, the role of rigorous post-operative rehabilitation programs is a significant contributing factor to successful return-to-sport following surgical reconstruction[14,15].
While surgical techniques undoubtedly affect the outcome following UCL reconstruction, they do not independently do so. Rather, it is a complex milieu of pre-operative, intra-operative, and post-operative factors that combine to affect the overall outcome following UCL injury. Due to the variability in success rates for treatment of these injuries, careful review of each of these factors is required to ensure outcomes are optimized following treatment. This study serves as a review of these factors, providing recommendations based on available literature to improve outcomes following UCL reconstruction in future years (Table 1).
Table 1.
Key factors for improving outcomes in ulnar collateral ligament injuries at various time points
| Time point | Target points for improved outcomes |
| Pre-op | Patient selection |
| Intra-op | Do not transpose nerve unless symptoms present preoperatively |
| Docking > Jobe (complications) | |
| Post-op | Sport specific rehabilitation |
| Isokinetic testing | |
| Return to throw program | |
| Daily stretching exercises |
NON-RECONSTRUCTIVE OPTIONS
Throwing athletes who have sustained UCL injuries often require surgical reconstruction in order to return to their preinjury level of activity. There are, however, non-operative and non-reconstructive modalities that may be utilized in certain clinical scenarios permitting earlier return to sport without the morbidity associated with reconstruction.
Non-operative treatment can include rest, non-steroidal anti-inflammatory drugs (NSAIDs), and bracing along with physical therapy. Rettig et al[16] described a 2-phase non-operative rehabilitation program. Phase 1, typically 2 to 3 mo in duration, consisted of rest, bracing, NSAIDs and progressive range of motion (ROM) exercises. If pain-free at the end of this phase continued, strengthening and throwing progression programs were started. In some scenarios involving lifting or throwing, bracing was used to prevent elbow hyperextension. With this protocol, they had success in returning 42% of throwing athletes to their pre-injury level of activity at an average of 24.5 wk[16]. Additional non-operative measures include activity modifications, which may include sport cessation or a position change, allowing continued participation without surgical treatment. Beyond these non-operative measures, additional non-reconstructive treatment options include platelet-rich plasma (PRP) treatment or primary repair of the UCL.
PRP use has increased substantially for treatment of various tendon and ligament pathology, however little literature exists on its use specifically in UCL injuries. A recent retrospective review by Dines et al[17] examined 44 baseball players treated for partial thickness UCL injuries with PRP. Levels of participation varied from professional (6), collegiate (14) and high-school (24). Though a small cohort, outcomes following treatment with PRP were best in the professional group with 67% returning to play[17]. Only 36% of the collegiate athletes and 17% of the high school athletes had excellent outcome scores based on a modified Conway Scale[17]. Overall these outcomes are worse than UCL reconstruction and should be reserved for specific patient groups. One such group may include athletes who are late in their professional careers and unable to undergo necessary post-operative rehabilitation. Return to pitching following reconstruction requires 12+ mo of post-operative rehabilitation, while return from non-operative treatment with PRP is significantly shorter, based on the ability to progress through an interval-throwing program. Therefore, these older overhead athletes may receive the most benefit from a PRP injection following partial UCL injury[18].
While PRP may have a positive effect on UCL healing, corticosteroids have a negative effect on ligament healing and are not recommended for use following acute ligamentous injuries. Using a rabbit model, Walsh et al[19] injected betamethasone into a surgically created UCL defects, reporting negative effects on both biomechanical and histologic properties of the healing ligament. As a result of the deleterious effects on ligamentous healing, corticosteroids are not recommended in the treatment of acute UCL injuries[19].
Primary repair of the UCL, rather than reconstruction, may permit more rapid return to play and improved outcomes in specific patient groups[20,21]. Younger athletes who sustain UCL injuries have a distinct injury pattern from professional athletes that is more amenable to repair, typically confined to either the proximal and/or distal aspect of the UCL rather than a degenerative mid-substance injury attributable to repetitive micro-trauma[20,21]. In 2008, Savoie et al[21] reported the outcomes of primary repair of UCL injuries in patients averaging 17.2 years of age, with nearly 5 years of follow-up. Through their work, they identified that the best candidate for this treatment type is one with an acute avulsion injury without signs of previous degenerative injury and no noted concomitant injuries. Their described technique includes a diagnostic arthroscopy for confirmation of pathology, followed by a muscle-splitting approach[22] with capsular reflection along the anterior edge of the ligament permitting evaluation of intra-articular damage. Anatomic repair is performed using bone tunnels or a double loaded anchor, securing the injured ligament proximally at the base of the medial epicondyle or distally at the center of the sublime tubercle[21].
Post-operative rehabilitation includes splinting followed by full time hinged ROM brace wear and an expedited standard rehabilitation protocol[15]. Progressive return to play was permitted in an ROM brace at 6-8 wk post-operatively with progression out of the brace at 12 wk with return to full activities upon graduation from the return to play program at 16 to 24 wk post-operatively[21]. Results for primary repair in this specific patient cohort were excellent with 93% (56 of 60) patients returning to sporting activities within 6 mo. This included 40 patients with proximal UCL repairs, 11 with distal UCL repairs and 9 with combined proximal and distal UCL repairs[21].
The results of Savoie et al[21] are similar to results of primary repair reported by Richard et al[20] in a collegiate patient population with an average age of 27 who sustained combined acute UCL and flexor-pronator avulsion from the humeral origin. Of the 11 patients who underwent primary repair through bone tunnel fixation, 9 returned to collegiate athletics between 4 and 6 mo post-operatively[20]. Though this study is limited by short, 16-mo follow-up and a wider variety of sporting activities with fewer overhead athletes, it illustrates a patient population that may benefit from primary UCL repair.
Both of these studies’ outcomes differ from earlier results from Conway et al[10] who reported outcomes for patients treated with either UCL repair or reconstruction. In their study of throwing athletes only 50% of patients undergoing a direct repair returned to their previous level of sport compared to 68% of those undergoing a reconstruction[10]. Even worse outcomes were obtained in major-league baseball players undergoing primary repair with only 2 of the 7 being able to return to sport[10].
In short, UCL repair may be a viable surgical option in young athletes with acute injuries, resulting in excellent outcomes and permitting earlier return to play than following UCL reconstruction. This procedure, however, should be limited to young athletes without degenerative UCL injuries as is often encountered in collegiate and professional baseball players.
RECONSTRUCTIVE OPTIONS
Ulnar collateral ligament reconstruction has been effective in returning athletes to sport in approximately 80% of cases[3]. However, the results differ widely depending on the surgical series, with highly variable return-to-play rates (as low as 53%) and complication rates (as high as 25%), often related to the specific reconstructive techniques. In addition, studies have identified significantly inferior functional outcomes among those who re-tear their UCL and require revision UCL reconstruction, with a return to play rate ranging from 33%-78%[23-25]. Due to the variability in achieving a successful outcome following UCL reconstruction, and the ramifications of re-injury and revision surgery, careful review of surgical techniques is necessary to ensure that appropriate surgical steps are taken to optimize outcomes and limit complication and re-rupture rates.
Surgical techniques
Surgical reconstruction of the UCL was first described by Dr. Frank Jobe in 1986. His primary reconstructive method involved detachment of the flexor-pronator musculature, submuscular transposition of the ulnar nerve and reconstruction of the UCL with a palmaris longus or plantaris tendon graft in a figure-8 configuration with repair of the flexor-pronator tenotomy[2]. The first successful procedure was performed on pitcher Tommy John in 1974. In Jobe’s initial series of 16 patients he reported a return-to-play rate of 63%, with a complication rate of 32%, most commonly related to ulnar neuropathy[2]. In a follow-up series of 71 patients using the same reconstruction method, he noted 68% return to sport with a 21% complication rate[10].
While offering an improved outcome compared with conservative treatment or acute UCL repair, concern remained over the relatively high complication rate associated with Jobe’s reconstructive method[10]. As a result, the modified Jobe technique was subsequently described by Smith et al[22], who introduced a flexor-pronator muscle-splitting approach that obviated the need for an obligatory ulnar nerve transposition and avoided tenotomizing the flexor-pronator origin. This modification resulted in an excellent outcome in 93% of a series of 83 athletes, with a 100% return to play rate[26]. Complications were reported in 5% and were limited to transient ulnar neuropathy, which resolved in all patients. It should be noted that while this approach no longer required a submuscular ulnar nerve transposition, many surgeons continue to perform subcutaneous ulnar nerve transpositions with the modified Jobe technique in select cases with preceding ulnar nerve symptoms, or routinely in all cases, depending on individual preference. Cain et al[27] reported on the outcomes of 743 patients that underwent UCL reconstruction utilizing the modified Jobe reconstruction with concomitant subcutaneous ulnar nerve transpositions. They identified an 83% return-to-play rate, and 20% complication rate. Notably, the authors of this study reported re-operation rates of 19% for residual posteromedial impingement.
As experience continued to grow in treating these injuries, concern was raised over the method of graft tensioning and fixation using the figure-of-8 configuration. Subsequently, Rohrbough et al[9] introduced a reconstruction method known as the docking technique to reduce the size of the humeral tunnels and improve fixation strength of the reconstruction. This procedure is performed through a muscle-splitting approach and does not require ulnar nerve transposition. It also involves looping the graft through a similar bone tunnel in the proximal ulna, however it differs in that both free limbs of the graft are then passed into a single tunnel on the humerus, with sutures exiting posteriorly through smaller drill holes, allowing the sutures to be tied over a posterior bony bridge. In addition, authors recommended routine elbow arthroscopy to treat concomitant pathology in the elbow joint, which was noted in up to 45% of patients[9]. In their index cohort of 36 patients, they reported a 92% return-to-play rate at the same level of competition, with only a 5.5% complication rate, including one transient ulnar neuropathy and one wound hematoma[9]. A larger follow-up study of 100 patients over 3 years revealed a 90% return to play rate, with only a 3% complication rate. Similarly, Paletta et al[28] described a modified docking technique utilizing a quadrupled, rather than doubled, palmaris graft, with slight differences in humeral bone tunnel preparation. Their procedure offered similar outcomes with 92% return to pre-injury level of competition, with slightly higher complication rates of 8%[28]. Additionally, Bowers et al[29] treated 21 overhead athletes with a modified docking technique using a triple-strand Palmaris graft. They had 19 (90%) excellent results, 2 good results, and no complications[29].
An additional modification attempted to address the inability of reconstruction techniques to restore the biomechanical strength comparable to the native ligament. Ahmad et al[30] identified improved fixation strength with cadaveric testing of a reconstructive technique using interference screw fixation, resulting in the development of a hybrid technique with ulnar interference screw fixation and humeral docking, known as the DANE TJ technique[31]. Otherwise, the procedure was unchanged, performed through a flexor-pronator muscle-splitting approach without ulnar nerve transposition. Results from the initial technique description, reported 86% return-to-play rates, with 18% complication rate of either transient ulnar neuropathy (9%) or post-operative adhesions requiring re-operation (9%)[31].
With many of these surgical techniques, there is concern over the size of the bone tunnels and the effect on graft tensioning and the potential for bone bridge compromise. A recent biomechanics study on 10 cadaveric elbows investigated the relationship graft size had on resistance to valgus load[32]. They found no significant difference in angular valgus deformation between palmaris longus, triceps brachii, extensor carpi radialis longus, and semitendinosus.
Further review of all available surgical technique descriptions and clinical series on UCL reconstructions was performed in two recent systematic reviews, which allowed for pooling of data to provide further comparative analysis between the different surgical techniques[3,4]. In the first review, Vitale et al[3] reported outcomes associated with different aspects of each surgical approach. They report that transitioning from a flexor-pronator detachment to a muscle-splitting surgical approach improved the success rates from 70% to 87%, while also reducing the rate of post-operative ulnar neuropathy from 20% to 6%. Additionally, adoption of the muscle-splitting approach reduced the need for an obligatory ulnar nerve transposition. Outcomes were noted to improve from a success rate of 75% in those who had an obligatory transfer to 89% in those who did not. Also, those undergoing an obligatory nerve transfer had a 9% rate of post-operative ulnar neuropathy, while only 4% of those who did not undergo a transposition reported the same. Finally, adoption of the docking and modified docking techniques also significantly improved outcomes with 90% and 95% of patients reporting excellent outcomes with these respective techniques, compared with only 76% of those undergoing reconstruction with the figure-of-8 technique. Similarly, a decrease in post-operative ulnar neuropathy rates was also noted among those undergoing docking and modified docking reconstructions compared with the figure-of-8 technique, with only 3% and 5% experiencing these complications in the docking groups while 8% of those with the figure-of-8 technique were observed to experience this complication.
A second systematic review by Watson et al[4] provided a comparison of the overall complication rates associated with each reconstructive technique. Cumulatively, when considering all reported outcomes from UCL reconstruction clinical series, they identified a complication rate of 16.6%, with the majority of these complications being ulnar neuropathy (12.9%). Further stratification of these results revealed different rates dependent on procedure, with the original Jobe reconstruction carrying a complication rate of 29.2%, while the modified Jobe technique carried a complication rate of 19.1%. The docking technique and modified docking technique had lower rates or 6% and 4.3% respectively.
Based on the results of these reported series and systematic analyses, it appears that newer reconstructive methods, including the docking and modified docking procedures, are associated with higher return-to-play rates and lower complication rates than earlier techniques, including the Jobe and modified Jobe techniques, as well as in comparison to the DANE TJ technique. Additionally, it appears that use of a muscle splitting surgical approach, without obligate ulnar nerve transposition, is also associated with improved outcome rates and lower complication rates. Finally, consideration should be given to both open or arthroscopic assessment and treatment of concomitant pathology, specifically posteromedial impingement, which was treated in 34%-45% of cases in larger volume series[3,11,27]. While no randomized trial exists to corroborate these conclusions, they are based on the best-available literature, including clinical data from over 1300 patients.
Adjunctive treatments
In addition to modifications in surgical techniques, basic science research is ongoing to determine if there are any adjunctive therapies that may expedite or improve the quality of tendon-to-bone healing following UCL reconstruction. As identified in both ACL reconstruction and rotator cuff repair surgery, the structure and composition of the insertion site is complex, with a gradual transition from tendon to bone with interposed unmineralized and mineralized fibrocartilage[33,34]. This architecture is typically not reconstituted in the normal healing process following ligament reconstruction or rotator cuff repair, although several attempts have been made at adding biologic agents to stimulate regenerative, rather than reparative, healing in both ACL and rotator cuff injuries.
For ACL injuries, addition of a collagen-platelet rich plasma scaffold following direct ligament repair was found to improve biomechanical and histologic properties of the healing ligament in both animals and humans[35,36]. Application of PRP following ACL reconstruction in animal models has also shown positive results in stimulating revascularization and re-innervation of the ACL graft[37,38]. Clinical results of PRP addition following ACL reconstruction have been less impressive, with only mild or no clinical improvement noted[39,40]. Similarly, stem cell use has also been studied in conjunction with ACL reconstruction, where addition of tendon-derived stem cell sheets and bioengineered periosteal progenitor cell sheets have demonstrated encouraging results in small animal models with improved fibrocartilage and bone formation at the tendon-bone junction, although clinical results are limited due to restrictions regarding stem cell utilization[41,42].
While there is a paucity of literature on the effect of these various orthobiologic agents in UCL reconstruction, results of the literature for both ACL reconstruction and rotator cuff repair can potentially be extrapolated to this group. Further study is necessary to see if these biologic agents can potentially improve or expedite healing to allow for improved clinical outcomes and lower re-injury rates.
POST-OPERATIVE
Every athlete who is evaluated for an ulnar collateral ligament injury should have a thorough evaluation of all intrinsic and extrinsic factors that can contribute to valgus instability. It is important to address poor mechanics related to underlying factors, including capsular stiffness in glenohumeral internal rotation deficit (GIRD), scapular dyskinesis, and deficiencies of core and single leg strength. Post-operative and nonsurgical treatment are related to the restoration of normal scapulohumeral rhythm, which begins with establishing trunk and core stability, elbow range of motion and strength, as well as using triplanar exercises, including lunges and balance exercises[43].
GIRD should be evaluated by stabilizing the scapula, placing the arm in 90° of abduction in the scapular plane, and internally and externally rotating the arm. Bilateral measurements should be obtained, and treatment initiated if the side-to-side difference in the total arc of rotational motion is greater than 5°[44]. Modified sleeper stretches and modified side-lying cross body are excellent for improving GIRD[45,46]. The modified sleeper stretch is performed in the lateral position with the patient lying on the affected extremity using their unaffected arm to stretch the posterior capsule (Figure 1). The modification of rotating slightly posteriorly stabilizes the scapula without causing subacromial impingement. The modified side-lying cross body stretch is performed in the lateral position with the athlete lying on the affected extremity, using the opposite hand to horizontally adduct the targeted shoulder (Figure 2). The opposite forearm is aligned on top and restricts external rotation of the humerus. The side-lying position stabilizes the scapula and resists scapular protraction allowing optimal stretch of the posterior shoulder. Each of these stretches are held for 30 s and repeated 3 times. There is evidence that the side-lying cross body stretch is more effective than the sleeper stretch[45,46].
Figure 1.
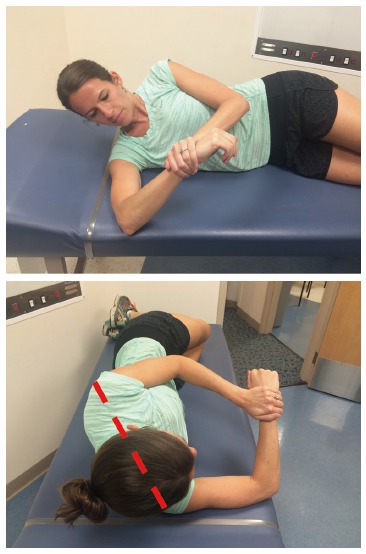
Modified sleeper stretch. The athlete lies on her side with her scapula retracted, rotates slightly posteriorly to place the shoulder in the scapular plane (dashed line), and passively internally rotates the shoulder until a mild stretch is felt. Hold for 30 s, relax, and repeat 3 times.
Figure 2.
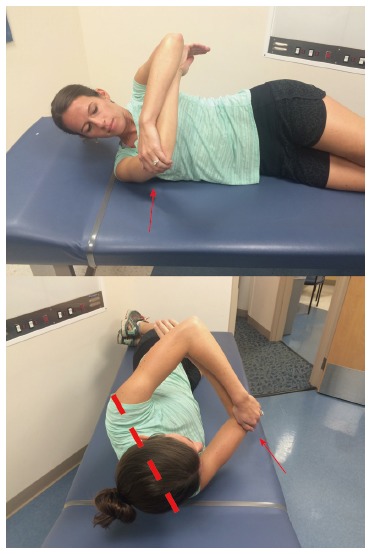
Modified side-lying cross body stretch. The athlete lies on the affected shoulder and stabilizes the scapula by rotating posteriorly (dashed line) against the table as the shoulder is horizontally adducted (arrow). External rotation is restricted via counter pressure of the opposite forearm. Hold for 30 s, relax, and repeat 3 times.
The open book and corner stretches can improve pectoralis minor and biceps short head flexibility. The open book stretch is performed in the lateral position lying on the unaffected extremity with the patient’s knees bent and arms stretched out in front. Opening the chest and laying the affected extremity on the opposite side and looking in the same direction stretches the pectoralis major and biceps short head (Figure 3). The corner stretch is performed facing a corner with the shoulders abducted and elbows flexed to 90° and the athlete slowly leans into the corner (Figure 4). Each of these stretches are held for 90 s and repeated 3 times.
Figure 3.
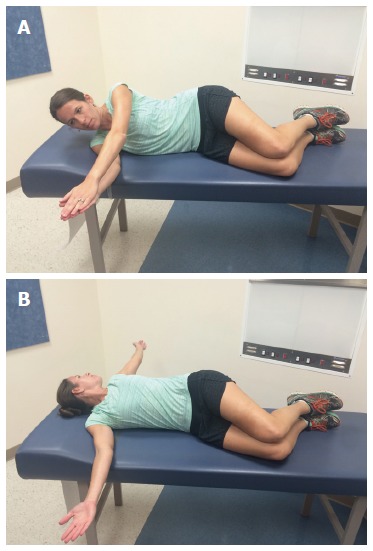
Open book stretch. A: The athlete lies on the unaffected shoulder with hips and knees bent 90° and arms straight out in front of him; B: Opening the chest and laying the affected extremity on the opposite side and looking in the same direction stretches the pectoralis major and biceps short head. Hold for 90 s, relax, and repeat 3 times.
Figure 4.
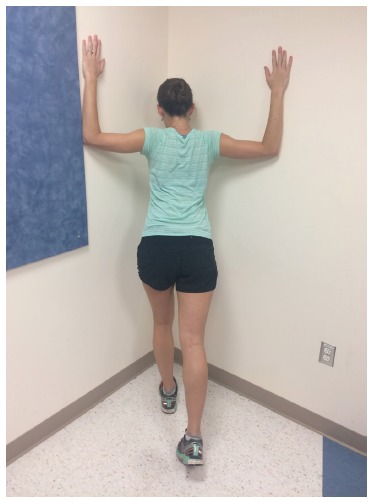
Corner stretch. The athlete faces a corner with the shoulders abducted, elbows flexed to 90° and slowly leans into the corner. Hold for 90 s, relax, and repeat 3 times.
Scapular dyskinesis is characterized by loss of upward acromial rotation, excessive scapular internal rotation, and excessive scapular anterior tilt[47]. These positions create scapular protraction, which decreases demonstrated rotator cuff strength[48]. Evaluation of scapular dyskinesis is accomplished by observation of static position and dynamic motions. The emphasis for rehabilitation for ulnar collateral ligament injuries should start proximally and end distally. Proximal control of core stability leads to control of three-dimensional scapular motion, with a goal to achieve the position of optimal scapular function - posterior tilt, external rotation, and upward elevation[43]. The serratus anterior functions most importantly as an external rotator of the scapula, and the lower trapezius acts as a stabilizer. Maximal rotator cuff strength is achieved from a stabilized, retracted scapula[49]. Periscapular strengthening should be accomplished by taking advantage of the synergistic activity of proximal trunk and hip muscle activation[49]. Exercise sets should include lawn mower pulls and low row exercises[49].
Kinetic chain factors may be evaluated by screening methods. Hip and trunk stability can be assessed using the single leg stance and single leg squat maneuvers[43]. In the single leg stance test, a positive Trendelenburg sign indicates gluteus medius weakness. Forward or lateral trunk tilt or rotation of the trunk around the leg in a single leg squat maneuver indicates a loss of dynamic control. Lunge and balance exercises should be incorporated into a rehabilitation program to improve trunk and core stability.
Rehabilitation programs vary institutionally and by treating physician. There is currently no validated comprehensive program. Rehabilitation following elbow injury or elbow surgery should follow a sequential and progressive multiphased approach that involves a gradual and protected return of ROM and an extensive resistance exercise program for the entire upper extremity kinetic chain. The rehab program should include proprioceptive exercises to stimulate mechanoreceptors as well as total arm strengthening, emphasizing proximal scapular stabilization. Low-resistance, high-repetition programs promote an optimal return to uncompensated throwing.
Phase 1 involves immediate motion. Reestablishing full elbow extension, typically defined as preinjury motion, is the primary goal of early ROM activities. Another goal of this phase is to decrease pain and inflammation. Modalities including cryotherapy, high voltage stimulation, and laser therapy can be helpful. Once the acute inflammatory response has subsided, moist heat, warm whirlpool, and ultrasound may be used at the beginning of treatment to prepare the tissue for stretching[50]. If the patient continues to have difficulty achieving full extension using ROM and mobilization techniques, a low load, long duration stretch may be performed to aid tissue elongation[50]. Submaximal isometrics are performed initially for the elbow flexor and extensor, as well as the wrist flexor, extensor, pronator, and supinator muscle groups. Scapular strengthening and activation exercises are also initiated immediately following surgery.
Phase 2, the intermediate phase, starts when the patient exhibits full ROM with minimal pain and involves improving muscular strength and endurance and reestablishing neuromuscular control of the elbow. Particular emphasis is placed on shoulder external and internal rotation at 90° abduction. External rotation helps avoid increased strain on the medial elbow structures during the overhead throwing motion[51] while internal rotation may create a protective varus force at the elbow[50]. A complete upper extremity strengthening program, such as the Thrower’s Ten Program, which focuses on the muscles needed for dynamic stability, should be included[52] (Figure 5).
Figure 5.
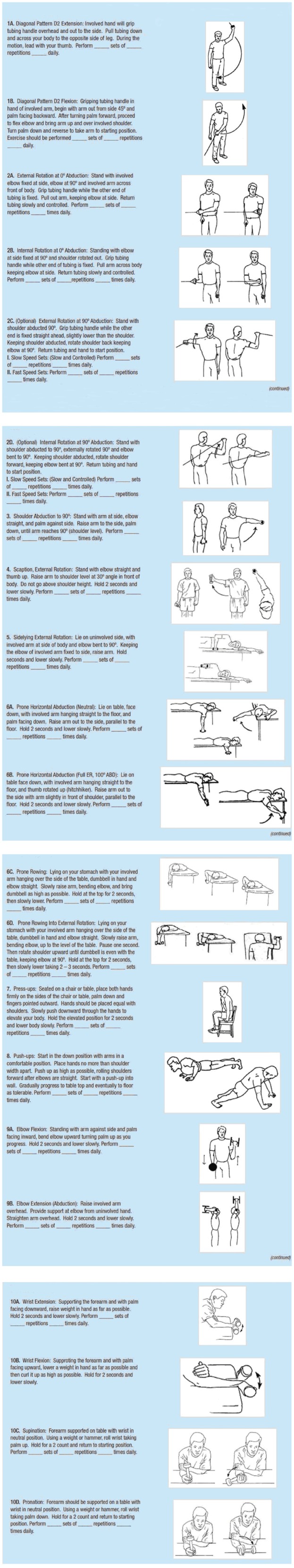
Thrower’s ten program. The Thrower’s Ten Program is designed to exercise the major muscles necessary for throwing. The Program’s goal is to be an organized and concise exercise program. In addition, all exercises included are specific to the thrower and are designed to improve strength, power, and endurance of the shoulder complex musculature.
Phase 3 encompasses advanced strengthening in preparation for a gradual return to sport. To enter this phase, the athlete must demonstrate strength that is 70% of the contralateral extremity. The advanced Thrower’s Ten Program[53] is used at this stage and involves exercises based on the principles of coactivation, dynamic stabilization, muscular facilitation, endurance, and coordination[53].
Phase 4, the final phase, involves an interval throwing program allowing the athlete to return to full competition. These throwing programs are sport specific and differ for golf and tennis athletes[54]. Isokinetic testing is commonly performed at this stage to determine the readiness of the athlete for an interval throwing program[54]. The interval throwing program has two phases, beginning with progressive long tosses and ending with throwing off the mound. The validity of this order has been questioned as some believe that long toss creates more stress at the medial elbow when compared with off the mound throwing.
Specific postoperative rehabilitation guidelines are based on the operative technique used for UCL reconstruction. The rehabilitation program used at the Andrews Sports Medicine Institute is outlined in Table 2[50] and the rehabilitation program used at Hospital for Special Surgery is outlined in Table 3[50]. Dynamic stabilization of the medial elbow is accomplished by concentric and eccentric strengthening the flexor carpi ulnaris and flexor digitorum superficialis. Given their anatomic location overlying the UCL, these muscles assist the UCL in stabilizing valgus stress at the medial elbow[50].
Table 2.
Airfield surface movement indicator postoperative rehabilitation protocol following ulnar collateral ligament reconstruction with palmaris longus autograft
| Time period | Phase | Goal |
| Day 0-7 | Splinted at 90° flexion | Early healing of graft and fascial sling for nerve transposition |
| Weeks 1-5 | Hinged elbow ROM brace | Protect healing tissues from valgus stress |
| Weeks 3-4 | Light resistance isotonic exercises | Develop dynamic stabilization of the medial elbow |
| Week 6 | Thrower’s Ten Program | |
| Weeks 8-9 | Progressive resistance exercises incorporated | |
| Week 12 | Advanced Thrower’s Ten Program | |
| Two-hand plyometric drills | ||
| Week 14 | One-hand plyometric drills | |
| Week 16 | Interval throwing program | |
| Week 22/24 | Throwing from the mound | |
| Months 9-12 | Return to competitive throwing |
ROM: Range of motion.
Table 3.
High speed steels postoperative rehabilitation protocol following ulnar collateral ligament reconstruction with palmaris longus autograft
| Time period | Treatment strategies | Goal |
| Day 0-10 | Splinted or hinged elbow ROM brace at 60 degrees flexion | Promote graft healing, reduce pain, and swelling |
| Weeks 1-4 | Hinged elbow ROM brace at all times No PROM Elbow AROM in brace | Restore ROM 30°-90° Promote graft healing Independent home exercise program |
| Weeks 4-6 | Continue brace wear at all times Avoid PROM Avoid valgus stress Continue AROM in brace Isometric exercises of deltoid, wrist, elbow | Restore ROM 15°-115° Minimal pain and swelling |
| Weeks 6-12 | Minimize valgus stress Avoid PROM by the clinician Avoid pain with exercises Continue AROM Low intensity, long duration stretch for extension Isotonic exercises of the scapula, shoulder, elbow, forearm and wrist Eccentric training when strength is adequate | Restore full ROM All upper extremity strength 5/5 Begin to restore muscular endurance |
| Week 8 | Begin internal/external rotation strengthening Begin forearm pronation/supination strengthening | |
| Weeks 12-16 | Pain free plyometric exercises Advance internal/external rotation to 90/90 position Neuromuscular drills Plyometric program Endurance training | Restore full strength and flexibility Prepare for return to activity |
| Week 16 | Begin interval throwing program | |
| Weeks 16-36 | Avoid pain with throwing or hitting Avoid loss of strength or flexibility Continue flexibility training Continue strengthening program | Return to activity Prevent reinjury |
| Week 20 | Begin hitting program |
ROM: Range of motion; AROM: Active range of motion; PROM: Passive range of motion.
Injury to the UCL most commonly occurs in baseball pitchers, but is also seen in other subsets of athletes, including javelin throwers, football quarterbacks and softball pitchers. Each sport requires different throwing mechanics and imparts different stresses to the elbow due to the varied angular velocities produced at the elbow (Table 4). Rehabilitation protocols should be sport specific and take into account the unique movements associated with these activities.
Table 4.
Angular velocity by sport
| Sport | Baseball | Softball | Football | Javelin | Tennis |
| Angular velocity | 2400°/s | 570°/s | 1760°/s | 1900°/s | 982°/s |
The javelin event involves throwing a 2.6-m spear weighing at least 800 g. Throwers lengthen the path of acceleration by maintaining an extended elbow for as long as possible until foot strike[55]. The throwing motion is broken down into four phases: Approach run, cross steps, delivery stride, and thrust phase. During the thrust phase, the elbow flexes from 40°-60°[55]. As contrasted with baseball pitchers who undergo rapid extension, javelin throwers undergo rapid flexion. Although throwing a javelin and pitching a baseball both produce large valgus forces on the medial side of the elbow, leading to UCL injuries, the mechanics of throwing are vastly different. Perhaps there should be changes to post-operative protocols that specifically address these specialized movement differences. No consensus postoperative protocol and throwing program exists for javelin throwers in the literature. As a javelin is much heavier than a baseball (1.76 pounds vs 0.32 pounds), we prefer to wait 8 mo from surgery (as compared to 4 in baseball pitchers) to begin an interval throwing program. We also recommend focusing more on lower extremity core strengthening to account for the increased weight of the javelin. Javelin throwers should be counseled that due to their unique motion and weight of the javelin, their return to play will be longer than in baseball players, and should be expected around 15 mo.
The motion of throwing a football is similar to throwing a baseball pitch. The lower incidence of elbow injuries in football quarterbacks is multifactorial. With a larger size ball, arm velocities are much slower, therefore producing less stress. The motion is also more over-the-top which produces less valgus force at the elbow. It is also hypothesized that the follow-through phase is abbreviated as the quarterback needs to be prepared for the impact from an opposing player, possibly lowering forces and torques produced at the elbow. Finally, quarterbacks perform the throwing motion significantly few times per game and per season compared to major league pitchers, and therefore are cumulatively placing less stress on their elbows. While some quarterback UCL injuries are chronic, the vast majority in the literature are from acute contact injuries[56,57]. Results from Dodson et al[56] and Kenter et al[57] suggest that these players can be successfully treated nonoperatively and return to competitive play.
Softball pitchers are a unique subset of throwers due to the underhand nature of their motion. While the overhead thrower is extending the elbow at ball release, the underhand softball pitcher is flexing the elbow. Although reasons are unclear, the female athlete, especially the underhand softball pitcher, imparts less stress to the elbow, making the injury more amenable to repair[58]. There is some evidence to suggest positive outcomes in ligament reconstruction for these athletes. However, the data on these athletes lacks the data that we have for their male counterparts. Further research into female throwing injuries is necessary. Currently, repair is a viable option.
UCL injuries have also been reported tennis, gymnastics, wrestling, volleyball and in baseball position players[27]. The demands of their sports and positions result in a much lower frequency of injury and usually do not necessitate UCL reconstruction for return to play. Further research is needed to investigate sport-specific protocols and treatment outcomes for athletes who play sports that place the UCL at risk.
CONCLUSION
We still need to answer the unknown. For example, currently we throw long-toss before mound throwing. There is some evidence to support that this actually puts more stress on the UCL reconstruction. Return to sport at the same or higher level may be easier for a high school athlete compared to a professional pitcher, but currently these are not differentiated in the literature. In order to have functional screening and quantitative return to play after UCL reconstruction like we currently have for ACL reconstruction, we need to know what is normal at every level of participation and position, including professional, college, high school athletes as well as distinctions between pitchers vs position players.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest. No financial support.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 7, 2015
First decision: November 4, 2015
Article in press: January 7, 2016
P- Reviewer: Erickson BJ, Seijas R S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
References
- 1.Fleisig GS, Andrews JR. Prevention of elbow injuries in youth baseball pitchers. Sports Health. 2012;4:419–424. doi: 10.1177/1941738112454828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68:1158–1163. [PubMed] [Google Scholar]
- 3.Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36:1193–1205. doi: 10.1177/0363546508319053. [DOI] [PubMed] [Google Scholar]
- 4.Watson JN, McQueen P, Hutchinson MR. A systematic review of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2014;42:2510–2516. doi: 10.1177/0363546513509051. [DOI] [PubMed] [Google Scholar]
- 5.Erickson BJ, Gupta AK, Harris JD, Bush-Joseph C, Bach BR, Abrams GD, San Juan AM, Cole BJ, Romeo AA. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42:536–543. doi: 10.1177/0363546513510890. [DOI] [PubMed] [Google Scholar]
- 6.Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, Return to Competition, and Reinjury After Tommy John Surgery in Major League Baseball Pitchers: A Review of 147 Cases. Am J Sports Med. 2014;42:1323–1332. doi: 10.1177/0363546514528864. [DOI] [PubMed] [Google Scholar]
- 7.Osbahr DC, Cain EL, Raines BT, Fortenbaugh D, Dugas JR, Andrews JR. Long-term Outcomes After Ulnar Collateral Ligament Reconstruction in Competitive Baseball Players: Minimum 10-Year Follow-up. Am J Sports Med. 2014;42:1333–1342. doi: 10.1177/0363546514528870. [DOI] [PubMed] [Google Scholar]
- 8.Park JY, Oh KS, Bahng SC, Chung SW, Choi JH. Does well maintained graft provide consistent return to play after medial ulnar collateral ligament reconstruction of the elbow joint in elite baseball players. Clin Orthop Surg. 2014;6:190–195. doi: 10.4055/cios.2014.6.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohrbough JT, Altchek DW, Hyman J, Williams RJ, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30:541–548. doi: 10.1177/03635465020300041401. [DOI] [PubMed] [Google Scholar]
- 10.Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74:67–83. [PubMed] [Google Scholar]
- 11.Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34:1926–1932. doi: 10.1177/0363546506290988. [DOI] [PubMed] [Google Scholar]
- 12.Fleisig GS, Andrews JR, Cutter GR, Weber A, Loftice J, McMichael C, Hassell N, Lyman S. Risk of serious injury for young baseball pitchers: a 10-year prospective study. Am J Sports Med. 2011;39:253–257. doi: 10.1177/0363546510384224. [DOI] [PubMed] [Google Scholar]
- 13.Olsen SJ, Fleisig GS, Dun S, Loftice J, Andrews JR. Risk factors for shoulder and elbow injuries in adolescent baseball pitchers. Am J Sports Med. 2006;34:905–912. doi: 10.1177/0363546505284188. [DOI] [PubMed] [Google Scholar]
- 14.Bernas GA, Ruberte Thiele RA, Kinnaman KA, Hughes RE, Miller BS, Carpenter JE. Defining safe rehabilitation for ulnar collateral ligament reconstruction of the elbow: a biomechanical study. Am J Sports Med. 2009;37:2392–2400. doi: 10.1177/0363546509340658. [DOI] [PubMed] [Google Scholar]
- 15.Ellenbecker TS, Wilk KE, Altchek DW, Andrews JR. Current concepts in rehabilitation following ulnar collateral ligament reconstruction. Sports Health. 2009;1:301–313. doi: 10.1177/1941738109338553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rettig AC, Sherrill C, Snead DS, Mendler JC, Mieling P. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29:15–17. doi: 10.1177/03635465010290010601. [DOI] [PubMed] [Google Scholar]
- 17.Dines JS. Platelet rich plasma can successfully treat elbow ulnar collateral ligament insufficiency in high-level throwers. Amer J Orthp. 2016;5:2016. [PubMed] [Google Scholar]
- 18.Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41:1689–1694. doi: 10.1177/0363546513487979. [DOI] [PubMed] [Google Scholar]
- 19.Walsh WR, Wiggins ME, Fadale PD, Ehrlich MG. Effects of a delayed steroid injection on ligament healing using a rabbit medial collateral ligament model. Biomaterials. 1995;16:905–910. doi: 10.1016/0142-9612(95)93114-s. [DOI] [PubMed] [Google Scholar]
- 20.Richard MJ, Aldridge JM, Wiesler ER, Ruch DS. Traumatic valgus instability of the elbow: pathoanatomy and results of direct repair. J Bone Joint Surg Am. 2008;90:2416–2422. doi: 10.2106/JBJS.G.01448. [DOI] [PubMed] [Google Scholar]
- 21.Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36:1066–1072. doi: 10.1177/0363546508315201. [DOI] [PubMed] [Google Scholar]
- 22.Smith GR, Altchek DW, Pagnani MJ, Keeley JR. A muscle-splitting approach to the ulnar collateral ligament of the elbow. Neuroanatomy and operative technique. Am J Sports Med. 1996;24:575–580. doi: 10.1177/036354659602400503. [DOI] [PubMed] [Google Scholar]
- 23.Dines JS, Yocum LA, Frank JB, ElAttrache NS, Gambardella RA, Jobe FW. Revision surgery for failed elbow medial collateral ligament reconstruction. Am J Sports Med. 2008;36:1061–1065. doi: 10.1177/0363546508314796. [DOI] [PubMed] [Google Scholar]
- 24.Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22:642–646. doi: 10.1016/j.jse.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 25.Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43:1051–1056. doi: 10.1177/0363546515579636. [DOI] [PubMed] [Google Scholar]
- 26.Thompson WH, Jobe FW, Yocum LA, Pink MM. Ulnar collateral ligament reconstruction in athletes: muscle-splitting approach without transposition of the ulnar nerve. J Shoulder Elbow Surg. 2001;10:152–157. doi: 10.1067/mse.2001.112881. [DOI] [PubMed] [Google Scholar]
- 27.Cain EL, Andrews JR, Dugas JR, Wilk KE, McMichael CS, Walter JC, Riley RS, Arthur ST. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: Results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38:2426–2434. doi: 10.1177/0363546510378100. [DOI] [PubMed] [Google Scholar]
- 28.Paletta GA, Wright RW. The modified docking procedure for elbow ulnar collateral ligament reconstruction: 2-year follow-up in elite throwers. Am J Sports Med. 2006;34:1594–1598. doi: 10.1177/0363546506289884. [DOI] [PubMed] [Google Scholar]
- 29.Bowers AL, Dines JS, Dines DM, Altchek DW. Elbow medial ulnar collateral ligament reconstruction: clinical relevance and the docking technique. J Shoulder Elbow Surg. 2010;19:110–117. doi: 10.1016/j.jse.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31:332–337. doi: 10.1177/03635465030310030201. [DOI] [PubMed] [Google Scholar]
- 31.Dines JS, ElAttrache NS, Conway JE, Smith W, Ahmad CS. Clinical outcomes of the DANE TJ technique to treat ulnar collateral ligament insufficiency of the elbow. Am J Sports Med. 2007;35:2039–2044. doi: 10.1177/0363546507305802. [DOI] [PubMed] [Google Scholar]
- 32.Dargel J, Küpper F, Wegmann K, Oppermann J, Eysel P, Müller LP. Graft diameter does not influence primary stability of ulnar collateral ligament reconstruction of the elbow. J Orthop Sci. 2015;20:307–313. doi: 10.1007/s00776-014-0688-y. [DOI] [PubMed] [Google Scholar]
- 33.Muller B, Bowman KF, Bedi A. ACL graft healing and biologics. Clin Sports Med. 2013;32:93–109. doi: 10.1016/j.csm.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Nossov S, Dines JS, Murrell GA, Rodeo SA, Bedi A. Biologic augmentation of tendon-to-bone healing: scaffolds, mechanical load, vitamin D, and diabetes. Instr Course Lect. 2014;63:451–462. [PubMed] [Google Scholar]
- 35.Joshi SM, Mastrangelo AN, Magarian EM, Fleming BC, Murray MM. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med. 2009;37:2401–2410. doi: 10.1177/0363546509339915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray MM, Spindler KP, Devin C, Snyder BS, Muller J, Takahashi M, Ballard P, Nanney LB, Zurakowski D. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24:820–830. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 37.Bissell L, Tibrewal S, Sahni V, Khan WS. Growth factors and platelet rich plasma in anterior cruciate ligament reconstruction. Curr Stem Cell Res Ther. 2014;10:19–25. doi: 10.2174/1574888x09666140710102002. [DOI] [PubMed] [Google Scholar]
- 38.Xie X, Zhao S, Wu H, Xie G, Huangfu X, He Y, Zhao J. Platelet-rich plasma enhances autograft revascularization and reinnervation in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;183:214–222. doi: 10.1016/j.jss.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Del Torto M, Enea D, Panfoli N, Filardo G, Pace N, Chiusaroli M. Hamstrings anterior cruciate ligament reconstruction with and without platelet rich fibrin matrix. Knee Surg Sports Traumatol Arthrosc. 2015;23:3614–3622. doi: 10.1007/s00167-014-3260-6. [DOI] [PubMed] [Google Scholar]
- 40.Valentí Azcárate A, Lamo-Espinosa J, Aquerreta Beola JD, Hernandez Gonzalez M, Mora Gasque G, Valentí Nin JR. Comparison between two different platelet-rich plasma preparations and control applied during anterior cruciate ligament reconstruction. Is there any evidence to support their use. Injury. 2014;45 Suppl 4:S36–S41. doi: 10.1016/S0020-1383(14)70008-7. [DOI] [PubMed] [Google Scholar]
- 41.Chang CH, Chen CH, Liu HW, Whu SW, Chen SH, Tsai CL, Hsiue GH. Bioengineered periosteal progenitor cell sheets to enhance tendon-bone healing in a bone tunnel. Biomed J. 2012;35:473–480. doi: 10.4103/2319-4170.104412. [DOI] [PubMed] [Google Scholar]
- 42.Lui PP, Wong OT, Lee YW. Application of tendon-derived stem cell sheet for the promotion of graft healing in anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42:681–689. doi: 10.1177/0363546513517539. [DOI] [PubMed] [Google Scholar]
- 43.Kibler WB, Press J, Sciascia A. The role of core stability in athletic function. Sports Med. 2006;36:189–198. doi: 10.2165/00007256-200636030-00001. [DOI] [PubMed] [Google Scholar]
- 44.Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30:136–151. doi: 10.1177/03635465020300011201. [DOI] [PubMed] [Google Scholar]
- 45.Wilk KE, Hooks TR, Macrina LC. The modified sleeper stretch and modified cross-body stretch to increase shoulder internal rotation range of motion in the overhead throwing athlete. J Orthop Sports Phys Ther. 2013;43:891–894. doi: 10.2519/jospt.2013.4990. [DOI] [PubMed] [Google Scholar]
- 46.McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37:108–114. doi: 10.2519/jospt.2007.2337. [DOI] [PubMed] [Google Scholar]
- 47.Kibler WB, Uhl TL, Maddux JW, Brooks PV, Zeller B, McMullen J. Qualitative clinical evaluation of scapular dysfunction: a reliability study. J Shoulder Elbow Surg. 2002;11:550–556. doi: 10.1067/mse.2002.126766. [DOI] [PubMed] [Google Scholar]
- 48.Smith J, Dietrich CT, Kotajarvi BR, Kaufman KR. The effect of scapular protraction on isometric shoulder rotation strength in normal subjects. J Shoulder Elbow Surg. 2006;15:339–343. doi: 10.1016/j.jse.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 49.Ben Kibler BW, Sciascia A. What went wrong and what to do about it: pitfalls in the treatment of shoulder impingement. Instr Course Lect. 2008;57:103–112. [PubMed] [Google Scholar]
- 50.Wilk KE, Ellenbecker TS, Macrina LC. Rehabilitation of the Overhead Athlete’s Elbow, in Elbow Ulnar Collateral Ligament Injury. In: Dines JS, Altchek DW, editors. Springer Science Business Media: New York; 2015. pp. 227–259. [Google Scholar]
- 51.Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23:233–239. doi: 10.1177/036354659502300218. [DOI] [PubMed] [Google Scholar]
- 52.Wilk KE, Obma P, Simpson CD, Cain EL, Dugas JR, Andrews JR. Shoulder injuries in the overhead athlete. J Orthop Sports Phys Ther. 2009;39:38–54. doi: 10.2519/jospt.2009.2929. [DOI] [PubMed] [Google Scholar]
- 53.Wilk KE, Yenchak AJ, Arrigo CA, Andrews JR. The Advanced Throwers Ten Exercise Program: a new exercise series for enhanced dynamic shoulder control in the overhead throwing athlete. Phys Sportsmed. 2011;39:90–97. doi: 10.3810/psm.2011.11.1943. [DOI] [PubMed] [Google Scholar]
- 54.Reinold MM, Wilk KE, Reed J, Crenshaw K, Andrews JR. Interval sport programs: guidelines for baseball, tennis, and golf. J Orthop Sports Phys Ther. 2002;32:293–298. doi: 10.2519/jospt.2002.32.6.293. [DOI] [PubMed] [Google Scholar]
- 55.O’Hagan T, Stucken C, Dodson CC. Sports Specific Outcomes for Ulnar Collateral Ligament Reconstruction, in Elbow Ulnar Collateral Ligament Injury. In: Dines JS, Altchek DW, editors. Springer Science Business Media: New York; 2015. pp. 219–226. [Google Scholar]
- 56.Dodson CC, Slenker N, Cohen SB, Ciccotti MG, DeLuca P. Ulnar collateral ligament injuries of the elbow in professional football quarterbacks. J Shoulder Elbow Surg. 2010;19:1276–1280. doi: 10.1016/j.jse.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 57.Kenter K, Behr CT, Warren RF, O’Brien SJ, Barnes R. Acute elbow injuries in the National Football League. J Shoulder Elbow Surg. 2000;9:1–5. doi: 10.1016/s1058-2746(00)80023-3. [DOI] [PubMed] [Google Scholar]
- 58.Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34:431–437. doi: 10.1177/0363546505281240. [DOI] [PubMed] [Google Scholar]


