Abstract
Heavy light chain (HLC) assays allow precise measurement of the monoclonal and of the noninvolved polyclonal immunoglobulins of the same isotype as the M‐protein (e.g., monoclonal IgAκ and polyclonal IgAλ in case of an IgAκ myeloma), which was not possible before. The noninvolved polyclonal immunoglobulin is termed ‘HLC‐matched pair’. We investigated the impact of the suppression of the HLC‐matched pair on outcome in 203 patients with multiple myeloma, a phenomenon that likely reflects the host's attempt to control the myeloma clone. Severe (>50%) HLC‐matched pair suppression was identified in 54.5% of the 156 newly diagnosed patients and was associated with significantly shorter survival (45.4 vs. 71.9 months, P = 0.019). This correlation was statistically significant in IgG patients (46.4 vs. 105.1 months, P = 0.017), but not in patients with IgA myelomas (32.9 vs. 54.1 months, P = 0.498). At best response, HLC‐matched pair suppression improved only in patients with ≥VGPR, indicating partial or complete humoral immune reconstitution during remission in those with excellent response. Severe HLC‐matched pair suppression retained its prognostic impact also during follow‐up after first response. In the 47 pretreated patients with relapsed/refractory disease, a similar correlation between severe HLC suppression and survival was noted (22.8 vs. not reached, P = 0.028). Suppression of the polyclonal immunoglobulins of the other isotypes than the myeloma protein correlated neither with HLC‐matched pair suppression, nor with outcome. Multivariate analysis identified severe HLC‐matched pair suppression as independent risk factor for shorter survival, highlighting the impact of isotype specific immune dysregulation on outcome in multiple myeloma. Am. J. Hematol. 91:295–301, 2016. © 2015 The Authors. American Journal of Hematology Published by Wiley Periodicals, Inc.
Introduction
Survival in patients with multiple myeloma depends mainly on the biology of the malignant clone and on the patient's age‐dependent biological fitness, including organ, bone marrow, and particularly immune system function. Signs of severe immunosuppression, such as impairment of polyclonal B‐cell progenitors and plasma cell precursors 1 and suppression of uninvolved polyclonal immunoglobulins of a different isotype to the tumor monoclonal protein are frequently observed in patients with multiple myeloma and have been associated with adverse prognosis 2 while preserved polyclonal B cell proliferation and immunoglobulin synthesis usually is associated with favorable prognosis 3. The recently developed HLC assay allows the measurement of both pairs of a specific isotype, e.g., quantification of IgAλ and of IgAκ in a patient with IgAλ monoclonal gammopathy 4, 5. Thereby a ratio between involved and noninvolved HLC‐matched pairs similar to the FLC ratio can be calculated. Moreover, the test allows quantification of the suppressive effect of the malignant clone, or more likely, of activated suppressor T cells on the isotype matched polyclonal pair. This phenomenon termed HLC‐matched pair suppression could not be assessed before the introduction of the HLC assay and has been reported as being an independent risk factor in predicting malignant MGUS transformation in a large study 6 and as characteristic feature of an evolving type of MGUS in patients with an IgG M‐protein in a smaller series of MGUS patients 7. The purpose of this study was to analyze whether the suppression of the isotype‐matched pair is a hallmark of the aggressiveness of the disease and correlates with outcome in both newly diagnosed and in previously treated patients with multiple myeloma.
Patients and Methods
203 patients with MM and measurable disease were enrolled. 156 patients were newly diagnosed [median age: 66 (32–94) years, male/female: 82/74, ISS stage I: 59, II: 63, and III: 34; 63 IgGκ, 37 IgGλ and 33 IgAκ, 23 IgAλ]. 47 were relapsed/refractory [median age: 63 (40–86) years, male/female: 24/23, ISS stage I: 20, II: 17, III: 10; 19 IgGκ, 14 IgGλ, 8 IgAκ, 6 IgAλ]
Patients in the first cohort received different induction regimens (Thalidomide–Dexamethasone (27), Melphalan–Prednisolone (15), Vincristine–Adriamycin–Dexamethasone followed by autologous stem cell transplantation (23), Vincristine–Melphalan–Cyclophosphamide–Prednisolone (19), Bortezomib–Thalidomide–Dexamethasone with or without Cyclophosphamide (42) and Lenalidomide–Dexamethasone (30), and those in the second cohort with relapsed/refractory disease were uniformly treated with Bedamustine–Bortezomib–Dexamethasone as previously reported 8.
Immunoglobulin heavy light chain pairs (IgGκ/IgGλ and IgAκ/IgAλ) were assessed using polyclonal antibodies targeted at unique junctional epitopes between heavy chain and light chain constant regions of intact immunoglobulin (Hevylite™ Binding Site, Birmingham, UK) on a Binding Site SPA plus™ Analyzer. Measurements of these parameters were used to derive IgGκ/IgGλ and IgAκ/IgAλ ratios, which were compared with reference ranges 9, 10. HLC ratios outside of the reference ranges were considered to be indicative of a clonal process. Tests were run independently in Birmingham and Vienna and results were read by experienced qualified clinical chemists. Immunoelectropheresis was run on Sebia Hydrasys™ in both laboratories and results were compared. Concentrations of conventional parameters, such as IgA, IgG, β2‐microglobulin (β2‐M), FLC, LDH, and creatinine were assessed by standard techniques.
Using a receiver operating characteristics (ROC) analysis, the optimum cutoff value for severe HLC matched pair suppression in the newly diagnosed patients with multiple myeloma was 48%. Therefore, a cutoff value of 50% was selected for further analysis. Moderate HLC‐matched pair suppression was defined as reduction below the lower level of normal (LLN) to 50% suppression and severe HLC‐matched pair suppression as >50% reduction below the LLN of the respective isotypes (IgGκ <1.92 g/L, IgGλ <0.95 g/l, IgAκ <0.28 g/l, IgAλ <0.22 g/l).
Systemic immunoparesis was defined as either one or two noninvolved polyclonal isotypes being below the LLN and severe immunoparesis as being 50% below the LLN of the respective isotypes (IgG <7 g/l, IgA <0.7 g/l, IgM <0.4 g/l). The following parameters were included in the univariate analysis: ISS stage, albumin, β2‐microglobulin, hemoglobin, creatinine, calcium, LDH, response to therapy, free light chain (FLC) ratio, heavy light chain (HLC) ratio, HLC‐matched pair suppression, severe HLC‐matched pair suppression, and systemic immunoparesis. The high‐risk FISH cytogenetic patient group was defined according to the recommendations of the IMWG for definition of high‐risk disease 11 and/or presence of t(14; 20) and/or amp1q21. Kaplan–Meier survival curves were compared using log‐rank test 12, univariate and multivariate analysis were performed using Cox proportional regression analysis and Pearson's correlations using SPSS, version 18 13, categorical values were compared using Fisher's exact test 14.
Results
HLC‐matched pair suppression in newly diagnosed patients and survival
Patient characteristics are shown in Table 1. Median follow‐up was 45 months in the newly diagnosed and 21 months in the relapsed refractory patient cohort. Severe HLC‐matched pair suppression (>50%) was identified in 85 (54.5%) of the 156 newly diagnosed patients. Fifty‐one (32.7%) patients presented with moderate (LLN, 50%) and 20 (12.8%) without any HLC‐matched pair suppression. Overall survival was significantly shorter in the entire group of newly diagnosed patients with severe HLC‐matched pair suppression compared to both other groups combined (median: 45.4 vs. 71.9 months, HR: 1.616, CI: 1.073–2.434, P = 0.019) (Fig. 1A). Severe HLC‐matched pair suppression was slightly, but not statistical significant more prevalent in patients with IgG (57/100, 57.0%) compared to those with IgA myeloma (27/56, 48.2%, P = 0.0764), and overall survival was significantly shorter in patients with IgG myeloma and severe HLC‐matched pair suppression (median, 46.4 vs. 105.1 months, HR 1.839, CI: 1.099–3.076, P = 0.017) (Fig. 1B), while in patients with IgA myeloma, no significant difference in survival between those with and without severe HLC‐matched pair suppression was found (median: 32.9 vs. 54.1 months, HR: 1.265, CI: 0.64–2.501, P = 0.498) (Fig. 1C). Within the group of 100 patients with IgG myeloma, 39 had moderate and only four no HLC‐matched pair suppression. In patients with IgA myeloma, 27 had severe, 13 had moderate (suppression <50% −LLN), and 16 had no HLC‐matched pair suppression; survival did not differ significantly between these three groups (32.9 vs. 59.4 vs. 54.1, P = 0.694).
Table 1.
Patient Characteristics
| Parameter | Newly diagnosed patients, numbers, or median (range), or percentages | Relapsed/refractory patients, numbers, or median (range) | |
|---|---|---|---|
| N | 156 | 47 | |
| Age (years) | 66 (32–94) | 63 (40–86) | |
| Male/female | 82/74 | 24/23 | |
| Albumin (g/l) | 38.0 (16.0–51.0) | 38.2 (24.8–48.0) | |
| β2‐microglobulin (mg/l) | 3.7 (1.1–85.9) | 3.5 (1.2–36.7) | |
| ISS stage | I | 59 | 20 |
| II | 63 | 17 | |
| III | 34 | 10 | |
| Isotype | IgG (IgGκ/IgGλ) | 100 (63/37) | 33 (14/19) |
| IgA (IgAκ/IgAλ) | 56 (33/23) | 14 (6/8) | |
| FISH (standard/high)a | 40/50 | 20/20 | |
| BMPC (%) | 40% (2–95%) | 25% (1–90%) | |
| Hemoglobin (g/dl) | 11.4 (4.6–16.7) | 11.3 (6.7–16.0) | |
| LDH (U/l) | 150.5 (36–1072) | 176.0 (68–746) | |
| Creatinine (mg/dl) | 1.1 (0.7–3.21) | 1.1 (0.6–4.8) | |
| Calcium (mg/dl) | 9.36 (7.28–12.84) | 9.08 (7.02–11.08) | |
| Follow‐up in months, median (range) | 45 (1–158) | 21 (3–33) | |
Available in 90 and 40 patients only.
Figure 1.
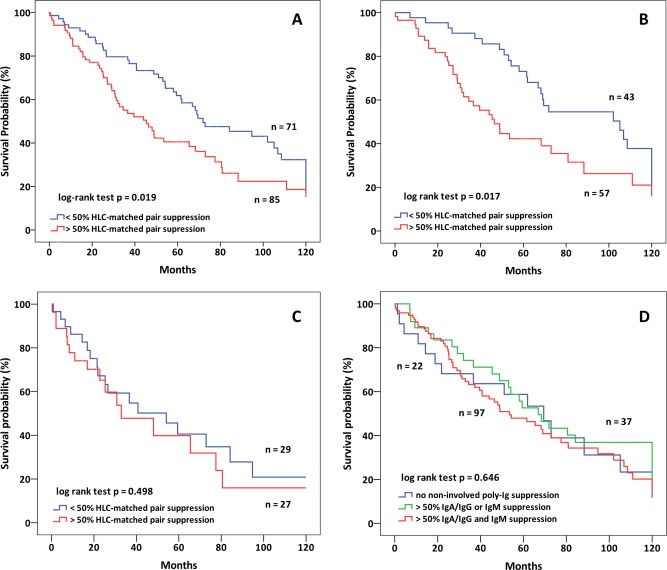
(A) Overall survival in 156 newly diagnosed patients (100 IgG and 56 IgA) with and without severe HLC‐matched suppression (45.4 vs. 71.9 months, P = 0.019). (B) Overall survival in 100 IgG patients with and without severe HLC‐matched suppression (46.4 vs. 105.1 months, P = 0.017). (C) Overall survival in 56 IgA patients with and without severe HLC‐matched suppression (32.9 vs. 54.1 months, P = 0.498). (D) Overall survival in patients without polyclonal immunoglobulin suppression (n = 22), with IgA/IgG or IgM suppression only (n = 37) or with IgG/IgA and IgM suppression (n = 97) (median 69.5 vs. 66.9, vs. 53.5 months, P = 0.646) using values just below the normal range provided no additional values (P = 0.25).
In patients with IgG myeloma, HLC‐matched pair suppression showed a weak correlation with the concentration of the M‐protein (IgGκ: r = 0.465, P < 001; IgGλ: r = 0.5, P = 0.003), while in those with IgA myeloma no correlation was noted (IgAκ: r = −0.059, P = 0.779, IgAλ: r = 0.228, P = 0.433).
Suppression of the noninvolved polyclonal immunoglobulins
Severe suppression of the noninvolved polyclonal immunoglobulin isotypes did not correlate with survival (Fig. 1D). Survival was similar between the 37 patients with suppression of noninvolved polyclonal immunoglobulins of either IgG, IgA, or IgM isotypes (median 66.9 months), and the 97 patients with suppression of both, IgG or IgA and IgM noninvolved polyclonal immunoglobulins (median: 53.5 months), and in the 22 patients without severe suppression of any of the noninvolved polyclonal isotypes (median: 69.5 months, HR 1.065, CI: 0.806–1.406, P = 0.646). Similar results were obtained when these groups were compared defining suppression as all values below the lower level of normal (data not shown). Also, there was no difference in survival when suppression of the noninvolved polyclonal isotypes was analyzed separately in patients with IgG or IgA M‐protein (data not shown). According with these findings, no correlation between HLC matched pair isotype suppression and suppression of noninvolved polyclonal immunoglobulins was found, both for patients with IgG or with IgA myeloma, independently whether severe suppression or moderate suppression was compared (Pearson's correlation coefficients, detailed data not shown).
Cytogenetic findings
FISH cytogenetic data were available in 90 of the newly diagnosed patients. High‐risk cytogenetics were associated with shorter survival (66.9 vs. 108.5 months, HR: 2.131, CI: 1.189–3.819, P = 0.009) but showed only a weak, statistical not significant tendency for an association with severe HLC‐matched pair suppression (r = 0.250). When overall survival was compared in patients with and without severe HLC‐matched pair suppression and with or without high‐risk cytogenetics (Fig. 2A), a stepwise decrease in survival was observed from patients with none of both risk factors (median: 120.0 months), no severe HLC‐matched pair suppression and high risk cytogenetics (median: 84.2 months), severe HLC‐matched pair suppression without high risk cytogenetics (median: 80.9 months) and with both, severe HLC‐pair suppression and high risk cytogenetics (median: 32.9 months, HR: 1.540, CI: 1.177‐2.016, P = 0.002). Multivariate analysis (including FISH high‐risk cytogenetics, severe HLC‐matched pair suppression, response, β2‐microglobulin, and stage) revealed severe matched‐pair suppression (HR: 1.851, CI: 1.029–3.332, P < 0.04), high‐risk FISH cytogenetics (HR: 2.308, CI: 1.258–4.235, P < 0.007), and response (VGPR or better) (HR: 2.544, CI: 1.401–4.623, P < 0.002) as being significantly associated with survival.
Figure 2.
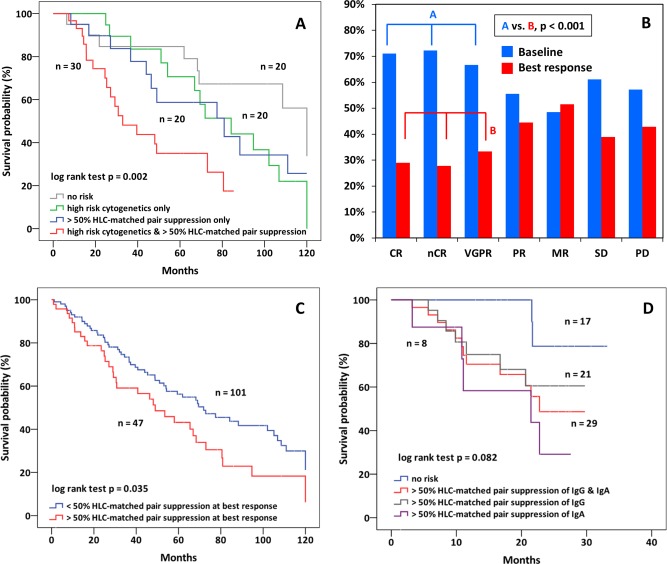
(A) Overall survival in 90 newly diagnosed patients with FISH data available. No risk factor (median: 120.0 months), high risk cytogenetics only (median: 84.2 months), severe HLC‐matched pair suppression only (median: 80.9 months), and both, high risk cytogenetics and severe HLC‐matched pair suppression (median: 32.9 months, P = 0.002). (B) HLC‐matched pair suppression in newly diagnosed patients at baseline and at best response according to response category. HLC‐matched pair suppression improved significantly only in patients with VGPR or better (P < 0.001). (C) Overall survival after achievement of best response in newly diagnosed patients with and without severe HLC‐matched pair suppression (49.0 vs. 71.9 months, P = 0.035). (D) Overall survival in relapsed/refractory patients with and without HLC‐matched pair suppression (median. 22.8 vs. not reached months, HR: 4.632, CI: 1.034–20.751, P = 0.028) and separately in patients with severe HLC‐matched pair suppression and IgG (median: not reached) or IgA (median: 21.5 months) myeloma.
HLC‐matched pair suppression and response
Severe HLC‐matched pair suppression at baseline before start of therapy did not correlate with subsequent response, which was observed in 112/156 (71.8%) newly diagnosed patients. However, baseline HLC‐matched pair suppression improved significantly in patients with excellent response, namely those with VGPR or better (41.7%, P < 0.001), but not in those with less than VGPR (30.1%) at time of best response (Fig. 2B). HLC‐matched pair suppression at best response correlated significantly with subsequent survival (49.0 vs. 71.9 months, HR: 1.581, CI: 1.026–2.434, P = 0.035), thus highlighting the prognostic relevance of severe HLC‐matched pair suppression, both at start of first line therapy, and at best response (Fig. 2C).
HLC‐matched pair suppression in patients with relapsed/refractory disease
Of the 47 patients with relapsed/refractory disease, severe HLC‐matched pair suppression was identified in 29 (61.7%) patients, namely in 21 of 32 (65.6%) of patients with IgG, and in 7 of 14 (50.0%) in patients with IgA myeloma, and showed a significant correlation with shortened survival (median OS: 22.8 months vs. not reached, HR: 4.632, CI: 1.034–20.751, P = 0.028) (Fig. 2D). The small numbers limit comparisons between patients with IgG and with IgA M‐protein, but in IgG myeloma 7/21 (33%) patients with and 2/11 (18%) without severe HLC‐matched pair suppression died during follow‐up. The respective figures in patients with IgA myeloma were 5/14 (36%) and 0/7 (0%). Twenty‐nine patients with relapsed/refractory disease presented with severe suppression of one or two noninvolved isotypes, 12 had moderate, and six no suppression. Similar to newly diagnosed patients, suppression of either one, or of both noninvolved isotypes was not associated with prognosis (data not shown).
FISH cytogenetics were available in 40 patients. High‐risk cytogenetics were associated with shorter survival (median 23 months vs. not reached, HR = 3.180, CI: 0.860–11.761, P = 0.067), and showed a weak, not significant correlation with severe HLC‐matched pair suppression (r = 0.126, P = 0.446). When survival was compared in patients with different risk factors, similar findings as in newly diagnosed patients were obtained. The combination of severe HLC‐matched pair suppression with high‐risk cytogenetics was associated with shortest survival, compared to patients with only one or none risk factor (median 21 months vs. not reached vs. not reached, HR: 2.946, CI: 1.280–6.785, P = 0.004).
Abnormal HLC ratio and survival in patients with relapsed/refractory disease
Previously, we reported a significantly shorter overall survival in newly diagnosed patients with highly abnormal HLC ratio (<0.022; >45) compared to those with less abnormal HLC ratios (median 35 vs. 60 months, HR: 2.07, CI: 1.15–3.75, P = 0.001) 15. In this study, a similar significant correlation between a highly abnormal HLC ratio and shorter survival was found in previously treated patients with relapsed/refractory disease (22.8 months vs. not reached, HR: 3.051, CI: 0.955–9.747, P = 0.048).
Univariate and multivariate analysis of the role of HLC‐matched pair suppression and other risk factor for survival
Univariate analysis in the 156 patients revealed severe HLC‐matched pair suppression to be closely associated with short survival (HR: 1.616, CI: 1.073–2.434, P = 0.019) in addition to previously established risk factors such as highly abnormal HLC ratio, β2‐microglobulin, ISS stage, hemoglobin, calcium, and response to therapy (statistical details are shown in Table 2). Results of multivariate Cox analysis proved severe HLC‐matched pair suppression to be independently associated with survival (HR: 2.553, CI: 1.214–5.367, P < 0.013) in addition to response (VGPR or better) to therapy (HR: 1.856, CI: 1.140–3.012, P = 0.013) and calcium (HR: 2.46, CI: 0.997–6.068, P = 0.051).
Table 2.
Results of Univariate and of Multivariate Analysis of Prognostic Factors with Survival
| Parameter | Hazard ratio | Confidence interval (95%) | Significance P |
|---|---|---|---|
| Univariate analysis | |||
| Albumin < 35 g/l | 0.849 | 0.527–1.367 | 0.500 |
| β2‐Microglobulin >5.5 g/l | 2.146 | 1.340–3.436 | 0.001 |
| ISS Stage (I vs. II vs. III) | 1.554 | 1.177–2.051 | 0.002 |
| Creatinine >2 mg/dl | 1.115 | 0.485–2.560 | 0.798 |
| LDH > 250 U/l | 1.538 | 0.794–2.981 | 0.202 |
| Response ≥ VGPR | 1.757 | 1.147–2.692 | 0.010 |
| Hemoglobin ≤ 10 g/dl | 1.864 | 1.059–3.282 | 0.031 |
| Calcium > 10.5 mg/dl (>2.6 mmol/l) | 3.239 | 1.599–6.559 | 0.001 |
| FLC ratio <0.03 or >32 | 1.018 | 0.677–1.531 | 0.932 |
| HLC ratio <0.022 or > 45 | 1.712 | 1.135–2.583 | 0.010 |
| HLC matched pair suppression <LLN | 0.826 | 0.468–1.460 | 0.508 |
| HLC matched pair suppression >50% | 1.581 | 1.026–2.434 | 0.038 |
| Noninvolved isotype suppression <LLN | 0.994 | 0.754–1.312 | 0.981 |
| Noninvolved isotype suppression >50% | 0.932 | 0.729–1.193 | 0.811 |
| Risk of FISH cytogenetics (90 patients) | 2.131 | 1.189–3.819 | 0.009 |
| Multivariate analysis | |||
| β2‐Microglobulin >5.5 g/l | 1.389 | 0.669–2.884 | 0.378 |
| ISS stage (I vs. II vs. III) | 1.526 | 0.890–2.614 | 0.124 |
| Response > VGPR | 1.853 | 1.140–3.012 | 0.013 |
| Hemoglobin > 10 g/dl | 1.502 | 0.760–2.967 | 0.242 |
| Calcium > 10.5 mg/dl (>2.6 mmol/l) | 2.460 | 0.997–6.068 | 0.051 |
| HLC ratio <0.022 or > 45 | 1.721 | 0.885–3.345 | 0.110 |
| HLC matched pair suppression >50% | 2.553 | 1.214–5.367 | 0.013 |
Discussion
The significant correlation between severe HLC‐matched pair suppression and short survival is the most important new finding of this study. In addition, our study confirms the predictive value of highly abnormal HLC ratios for shortened survival also in patients with relapsed/refractory disease, previously shown for newly diagnosed patients 15. HLC‐matched pair suppression correlated with survival, both in newly diagnosed and in previously treated patients with relapsed/refractory disease, but in the previously untreated patients, this interrelationship was mainly due to the significant correlation within the cohort of patients with IgG myeloma, while in IgA patients, only a statistical nonsignificant tendency for poorer outcome was found. When the same analysis was conducted in pretreated patients with relapse/refractory disease, a tendency for increased mortality was found both in patients with IgG and IgA myeloma, but the impact of these results is limited by the small number of patients with relapsed/refractory disease.
HLC‐matched pair suppression tended to be more common in IgG compared to IgA myelomas, both in previously untreated (56 vs. 48%, P = 0.0764) and in those with relapsed/refractory disease (63 vs. 43%, P = 0.109) and was weakly correlated with the M‐protein concentration in IgG myelomas, but not in IgA patients where no correlation between the respective parameters was observed. These findings resemble observations in MGUS patients where HLC‐matched pair suppression was more frequent in patients with higher IgG M‐protein concentration, while in IgA and IgM MGUS a more heterogeneous pattern without clear correlation with M‐protein concentration was observed. As HLC‐matched pair suppression is a function of both, a high concentration of the myeloma M‐protein and low levels of the HLC‐matched polyclonal pair, an early observation by Wang et al. 16 is of interest. These authors determined the polyclonal immunoglobulin concentration by subtracting the M‐component concentration from the gamma fraction based on densitometer scans of serum protein–electrophoresis gels and found significantly lower polyclonal immunoglobulin levels in patients with IgG as compared to those with IgA myeloma. Speculatively, one could argue that the concentration dependent increase in the catabolic rate of IgG immunoglobulins 17 may have a relatively greater effect on the much less abundantly produced HLC‐matched pair polyclonal isotype, shifting the HLC ratio towards the clonal protein. Another, and more likely explanation, is an isotype specific suppressive effect on immunoglobulin production, which obviously cannot, or not sufficiently, suppress the involved isotype, but may have a pronounced effect on the HLC‐matched polyclonal isotype pair. This process most likely is affected by T regulatory cells (Treg), which were reported to be significantly increased in multiple myeloma 18, 19. Tregs have been attributed to suppress immunoglobulin production and secretion without affecting proliferation of the myeloma cells 20, a phenomenon which has been found to be isotype specific 21, 22. Tregs suppress antitumor cytotoxicity 23, and increased Tregs levels have been linked with shorter survival in various cancers 24 and in multiple myeloma 19, 25. More specifically, increased Treg levels in myeloma were found to correlate with low polyclonal plasma cell numbers, adverse clinical features and shorter survival 25. Accordingly, in multiple myeloma patients with long standing disease control and survival, significantly lower numbers of Tregs were found compared to patients with active disease 26, 27. These facts support the notion that T cell mediated isotype specific regulatory factors play a major role as inducer of HLC‐matched pair suppression and contribute to shortened survival of affected patients. In MGUS HLC‐matched pair suppression below the lower level of normal is associated with an increased risk for transformation into active myeloma 6. Thus, this phenomenon is both a hallmark of poor prognosis in myeloma, and a predictor of transformation of MGUS to active myeloma.
Another interesting finding is the normalization of HLC‐matched pair suppression in patients with CR, nCR and VGPR, but not in those with lesser quality of response, indicating immune reconstitution of the HLC‐matched pair in patients with significant tumor response. Similar findings have recently been reported by Fouquet et al. 28 who observed a ≥20% improvement in HLC‐matched pair concentration in 55 and 75% of patients achieving ≥PR or ≥VGPR, respectively, compared to 18.5% in patients with less than PR at time of best response. Notably, in our patients, no correlation between baseline HLC‐matched pair isotype suppression and response was observed, but persistence of HLC‐matched pair suppression at best response was associated with shorter survival, confirming its prognostic role in different phases of the disease.
In our study, suppression of one or two of the noninvolved polyclonal isotypes, in myeloma commonly termed immunoparesis, was not associated with adverse survival in newly diagnosed patients (Fig. 1D). This was true for all isotypes independently whether one or two noninvolved isotypes were suppressed and whether they were severely suppressed or only below the lower level of normal. The role of immunoparesis of the noninvolved isotypes had been addressed before. In MGUS, suppression of the noninvolved polyclonal isotypes was associated with a higher risk for progression into active disease 6, 29, while in smoldering myeloma discrepant findings have been reported. Pérez‐Persona et al. 30 identified immunoparesis as a significant risk factor for progression and included this parameter in a risk model for patients with smoldering myeloma 31. On the contrary, immunopareresis could not be confirmed as risk factor for progression by the Greek myeloma group 32. Similar discrepancies apply to patients with active myeloma. Kastritis et al. 2 reported shorter survival in myeloma patients with immunoparesis as opposed to the 13% of patients without suppression of one or two of the noninvolved isotypes, while in our patients no difference in outcome, neither in those with moderate, nor in those with severe immunosuppression was noted.
In the 90 newly diagnosed patients with FISH data available, only a statistical not significant tendency for an association between high‐risk FISH cytogenetics and severe HLC‐matched pair suppression was found. Expectedly, high risk FISH cytogenetics were associated with shorter survival, but survival was shortest in patients with both severe HLC‐matched pair suppression and unfavorable cytogenetic features (Fig. 2A). Similar findings were obtained in the pretreated patient cohort with relapsed/refractory disease.
Previously, we 15 and others 33 have shown a close correlation of a highly abnormal HLC ratio with short survival. In this study, we showed that this applies to patients with relapsed/refractory disease as well. In Cox multivariate analysis, however, severe HLC‐matched pair suppression was identified as most relevant predictor of poor outcome, followed by response to therapy and increased calcium levels. HLC‐matched pair suppression was a more powerful predictor than the HLC ratio, which no longer retained significance as independent predictor.
The use of stored serum samples and the long follow‐up of up to 10 years of previously untreated patients is one of the important features of our study as well as the inclusion of a uniformly treated group of patients with relapsed/refractory disease. Limitations of our study are the retrospective nature of the analysis and the incomplete set of FISH data. In addition, more recently introduced technologies for MRD evaluation were not available when most of the newly diagnosed patients were started on therapy.
In conclusion, our study revealed severe HLC‐matched pair suppression as an interesting phenomenon associated with poor prognosis in previously untreated patients with IgG myeloma and in those with relapsed/refractory disease. HLC‐matched pair suppression was not associated with suppression of noninvolved isotypes and their suppression was not associated with poor outcome. HLC‐matched pair suppression improved in patients with marked response to myeloma therapy (VGPR or better), but not in those with less tumor reduction. Persistence of HLC‐matched pair suppression correlated with shorter survival after best response. Suppression of the HLC‐matched pair likely reflects the immune system's attempt to confine the myeloma clones, which affects both the involved clonal and the noninvolved polyclonal plasma cells of the same isotype. Elucidating further the nature and role of immune effector cells responsible for the herein described isotype‐specific immune suppression should provide important insights into the complex interplay between myeloma cells and their environment.
Conflict of interest: HL received honoraria for speaker's bureau from Binding Site. St. H and O.B are employees of binding site and hold shares. D.M., N.Z., and V.F declare no conflict of interest.
Author Contribution: H.L. and St.H. designed the study, analyzed data, and drafted the manuscript. D.M and O.B. tested the sera, St. H., H.L., D.M., and V.F., and O.B analyzed the data. All authors commented on the manuscript.
References
- 1. Rawstron AC, Davies FE, Owen RG, et al. B‐lymphocyte suppression in multiple myeloma is a reversible phenomenon specific to normal B‐cell progenitors and plasma cell precursors. Br J Haematol 1998;100:176–183. [DOI] [PubMed] [Google Scholar]
- 2. Kastritis E, Zagouri F, Symeonidis A, et al. Preserved levels of uninvolved immunoglobulins are independently associated with favorable outcome in patients with symptomatic multiple myeloma. Leukemia 2014;28:2075–2079. [DOI] [PubMed] [Google Scholar]
- 3. Girnius S, Munshi NC. Individualized therapy in multiple myeloma: Are we there? Semin Oncol 2013;40:567–576. [DOI] [PubMed] [Google Scholar]
- 4. Bradwell AR, Harding SJ, Fourrier NJ, et al. Assessment of monoclonal gammopathies by nephelometric measurement of individual immunoglobulin kappa/lambda ratios. Clin Chem 2009;55:1646–1655. [DOI] [PubMed] [Google Scholar]
- 5. Boyle EM, Fouquet G, Guidez S, et al. IgA kappa/IgA lambda heavy/light chain assessment in the management of patients with IgA myeloma. Cancer 2014;120:3952–3957. [DOI] [PubMed] [Google Scholar]
- 6. Katzmann JA, Clark R, Kyle RA, et al. Suppression of uninvolved immunoglobulins defined by heavy/light chain pair suppression is a risk factor for progression of MGUS. Leukemia 2013;27:208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Espino M, Medina S, Blanchard MJ, Villar L. Involved/univolved immunoglobulin ratio identifies monoclonal gammopathy of undetermined significance patients at high risk of progression to multiple myeloma. Br J Haematol 2014;164:752–755. [DOI] [PubMed] [Google Scholar]
- 8. Ludwig H, Milosavljevic D, Zojer N, et al. Bendamustine–bortezomib–dexamethasone is an active and well‐tolerated regimen in patients with relapsed or refractory multiple myeloma. Blood 2014;123:985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaur A, Snelus T, Mitchell F, et al. Turbidimetric immunoassays for IgAκ and IgAλ quantification for the assessment of patients with multiple myeloma. Clin Chem 2010;56:B170a. [Google Scholar]
- 10. Bradwell A, Harding S, Fourrier N, et al. Prognostic utility of intact immunoglobulin Ig'κ/Ig'λ ratios in multiple myeloma patients. Leukemia 2013;27:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chng WJ, Dispenzieri A, Chim CS, et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia 2014;28:269–277. [DOI] [PubMed] [Google Scholar]
- 12. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 13. Cox DR. Regression models and life tables. J R Stat Soc 1972;34:187–220. [Google Scholar]
- 14. Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. J R Stat Soc 1922;85:87–94. [Google Scholar]
- 15. Ludwig H, Milosavljevic D, Zojer N, et al. Immunoglobulin heavy/light chain ratios improve paraprotein detection and monitoring, identify residual disease and correlate with survival in multiple myeloma patients. Leukemia 2013;27:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang L, Young D. Suppression of polyclonal immunoglobulin production by M‐proteins shows isotype specificity. Ann Clin Lab Sci 2001;31:274–278. [PubMed] [Google Scholar]
- 17. Waldmann TA, Strober W. Metabolism of immunoglobulins. Prog Allergy 1969;13:1–110. [DOI] [PubMed] [Google Scholar]
- 18. Joshua DE, Brown RD, Ho PJ, Gibson J. Regulatory T cells and multiple myeloma. Clin Lymph Myeloma 2008;8:283–286. [DOI] [PubMed] [Google Scholar]
- 19. Giannopoulos K, Kaminska W, Hus I, Dmoszynska A. The frequency of T regulatory cells modulates the survival of multiple myeloma patients: Detailed characterisation of immune status in multiple myeloma. Br J Cancer 2012;106:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Milburn GL, Lynch RG. Immunoregulation of murine myeloma in vitro. II Suppression of MOPC‐315 immunoglobulin secretion and synthesis by idiotype‐specific suppressor cells. J Exp Med 1982;135:852–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Waldschmidt TJ, Williams KR, Lynch RG. Isotype‐specific recognition and regulation by T cells studied with tumor models. Int Rev Immunol 1987;2:201–220. [DOI] [PubMed] [Google Scholar]
- 22. Guglielmo P, Cunsolo F, Milone G, et al. Cytoplasmic heavy and light chain isotype suppression by peripheral Fcµ+ T‐cells from multiple myeloma. Haematologica 1987;72:465–468. [PubMed] [Google Scholar]
- 23. Chen ML, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor‐specific CD8 T cell cytotoxicity through TGF‐beta signals in vivo . Proc Natl Acad Sci USA 2005;102:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilke CM, Wu K, Zhao E, et al. Prognostic significance of regulatory T cells in tumor. Int J Cancer 2010;127:748–758. [DOI] [PubMed] [Google Scholar]
- 25. Raja M, Rihova L, Zhradova L, et al. Increased T regulatory cells are associated with adverse clinical features and predict progression in multiple myeloma. PLoS One 2012;7:e47077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bryant C, Suen H, Brown R, et al. Long‐term survival in multiple myeloma is associated with a distinct immunological profile, which includes proliferative cytotoxic T‐cell clones and a favourable Treg/Th17 balance. Blood Cancer 2013;3:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pessoa de Magalhães RJ, Vidriales MB, Paiva B, et al. Analysis of the immune system of multiple myeloma patients achieving long‐term disease control by multidimensional flow cytometry. Haematologica 2013;98:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fouquet G, Schraen S, Faucompré JL, et al. Hevylite to monitor hypogammaglobulinemia, a predictor of response to therapy in multiple myeloma. Am Soc Hematol Ann Meet San Francisco 2014;abstract 3383. [Google Scholar]
- 29. Cesana C, Klersy C, Barbarano L, et al. Prognostic factors for malignant transformation in monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. J Clin Oncol 2002;20:1625–1634. [DOI] [PubMed] [Google Scholar]
- 30. Pérez‐Persona E, Vidriales MB, Mateo G, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood 2007;110:2586–2592. [DOI] [PubMed] [Google Scholar]
- 31. Pérez‐Persona E, Mateo G, García‐Sanz R, et al. Risk of progression in smouldering myeloma and monoclonal gammopathies of unknown significance: Comparative analysis of the evolution of monoclonal component and multiparameter flow cytometry of bone marrow plasma cells. Br J Haematol 2010;148:110–114. [DOI] [PubMed] [Google Scholar]
- 32. Kastritis E, Terpos E, Moulopoulos L, et al. Extensive bone marrow infiltration and abnormal free light chain ratio identifies patients with asymptomatic myeloma at high risk for progression to symptomatic disease. Leukemia 2013;27:947–953. [DOI] [PubMed] [Google Scholar]
- 33. Koulieris E, Panayiotidis P, Harding SJ, et al. Ratio of involved/uninvolved immunoglobulin quantification by Hevylite™ assay: Clinical and prognostic impact in multiple myeloma. Exp Hematol Oncol 2012;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]


