Summary
Background
Bimatoprost ophthalmic solution 0·03% is approved in several countries for the treatment of eyelash hypotrichosis. Previous trials were limited to 4 months of treatment and primarily idiopathic hypotrichosis.
Objectives
To evaluate the long‐term safety and efficacy of bimatoprost in patients with idiopathic or chemotherapy‐induced hypotrichosis.
Methods
This multicentre, double‐masked, randomized, parallel‐group study included two 6‐month treatment periods [treatment period 1 (TP1) and treatment period 2 (TP2)]. Patients with idiopathic hypotrichosis were randomized to three treatment groups: (i) bimatoprost (TP1 and TP2); (ii) bimatoprost (TP1) and vehicle (TP2); and (iii) vehicle (TP1) and bimatoprost (TP2). Patients with chemotherapy‐induced hypotrichosis were randomized to two treatment groups: (i) bimatoprost or vehicle (TP1) and (ii) bimatoprost (TP2). Primary end point was a composite of at least a one‐grade improvement in investigator‐assessed Global Eyelash Assessment and at least a three‐point improvement in patient‐reported Eyelash Satisfaction Questionnaire Domain 2 at month 4. Secondary measures included digitally assessed eyelash characteristics.
Results
The primary efficacy end point was met in both populations (idiopathic responder rate was 40·2% for bimatoprost vs. 6·8% for vehicle; postchemotherapy responder rate was 37·5% for bimatoprost vs. 18·2% for vehicle). Efficacy by month 6 was maintained (idiopathic) or enhanced (postchemotherapy) at 12 months. Treatment effects were maintained for approximately 2 months but markedly diminished 4–6 months following treatment cessation in patients with idiopathic hypotrichosis. No drug‐related serious adverse events were reported.
Conclusions
Daily treatment with bimatoprost ophthalmic solution 0·03% for 1 year was effective and well tolerated in patients with idiopathic and chemotherapy‐induced hypotrichosis.
Short abstract
What's already known about this topic?
The safety and efficacy of bimatoprost ophthalmic solution 0·03% have been previously established for idiopathic hypotrichosis in studies lasting up to 4 months.
What does this study add?
Very little has been reported on eyelash loss and regrowth after chemotherapy, and there is currently no standard prevention or treatment for chemotherapy‐induced hypotrichosis of the eyelashes.
We provide long‐term (up to 12 months) safety and efficacy data for treatment with bimatoprost ophthalmic solution 0·03% in chemotherapy‐induced and idiopathic hypotrichosis.
Eyelash hypotrichosis is described as having inadequate, short or not enough eyelashes, whether due to the ageing process, genetic predisposition or other causes.1 Another aetiology for eyelash hypotrichosis is through chemotherapy‐induced alopecia. Chemotherapy‐induced hair loss is considered one of the most emotionally upsetting aspects of cancer and has a strong negative impact on the psychological well‐being of the patient.2 Although hair loss is typically partially or fully reversible after chemotherapy, there is a substantial delay in regrowth (generally 3–6 months for the scalp).3
Bimatoprost is a synthetic prostamide F2α analogue.4, 5 Bimatoprost ophthalmic solution 0·03% (Latisse®; Allergan, Inc., Irvine, CA, U.S.A.) was approved, based on studies that included adults with idiopathic hypotrichosis for up to 4 months of treatment, in December 2008 by the U.S. Food and Drug Administration for the treatment of hypotrichosis of the eyelashes.6, 7 A randomized clinical study demonstrated that bimatoprost solution 0·03% applied to the eyelid margins was safe and associated with longer, thicker and darker lashes.8 However, very little has been reported on the incidence of eyelash loss and regrowth after chemotherapy, and there is currently no treatment for eyelash hypotrichosis in this population. This represents the first study of bimatoprost 0·03% that has evaluated the long‐term safety and efficacy of this treatment in increasing overall eyelash prominence in patients with idiopathic hypotrichosis and with hypotrichosis due to recent chemotherapy. It also represents the first study to document the natural history of untreated hypotrichosis after chemotherapy (in the placebo group) and the patient‐reported impact of this medically induced cause of eyelash loss. The clinical trial is registered with ClinicalTrials.gov (www.clinicaltrials.gov/show/NCT00907426).
Materials and methods
This was a 1‐year, multicentre, double‐masked, randomized, parallel‐group study of 1‐year treatment duration with two 6‐month treatment periods. Two subpopulations evaluated in the study included patients with idiopathic hypotrichosis and those with hypotrichosis caused by recent chemotherapy. Additionally, the effect of drug discontinuation was studied in patients with idiopathic hypotrichosis.
Conducted over 1 year, the study consisted of nine face‐to‐face visits and two telephone conversations. Treatment period 1 (TP1) extended from screening to month 6, and included six visits: screening/baseline (day −14 to day 1); telephone visit (week 1); and visits at months 1, 2, 4 and 6. Treatment period 2 (TP2) began at the 6‐month visit and included visits at months 7, 8, 10 and 12/early termination), and one telephone conversation (1 week after month 6).
Patients with idiopathic hypotrichosis were randomized to three treatment arms in a 2 : 1 : 1 ratio: bimatoprost during both treatment periods; vehicle in TP1 and bimatoprost in TP2; and bimatoprost in TP1 and vehicle in TP2. Patients with chemotherapy‐induced hypotrichosis were randomized in a 3 : 1 ratio to receive bimatoprost during both treatment periods or vehicle in TP1 followed by bimatoprost in TP2. Treatment assignments for both TP1 and TP2 were determined by randomization at baseline. All study medications were identical in appearance, supplied in identical bottles and labelled with medication kit numbers. Patients, investigators and investigational staff were masked to treatment assignment throughout the entire study period. An automated, interactive web/voice response system was used to manage randomization and treatment assignments. Study sites dispensed study medication according to the instructions of the automated system.
Men or women at least 18 years of age who met the following criteria were eligible for inclusion in the study: a score of 1 or 2 on the clinician‐graded Global Eyelash Assessment (GEA); a score of 1 (very much disagree) or 2 (disagree) on each of three items [16 (confidence), 19 (attractiveness) and 18 (professionalism)] on the patient‐assessed Eyelash Satisfaction Questionnaire (ESQ); best‐corrected visual acuity (BCVA) score equivalent to a Snellen acuity of 20/100 or better in each eye; and an intraocular pressure (IOP) of ≤ 20 mmHg in each eye. Patients in the chemotherapy‐induced hypotrichosis population also met the following inclusion criteria: inadequate eyelashes after completing chemotherapy; treatment for solid tumour type stage 1, 2 or 3a cancer; had received their last chemotherapy treatment between 4 and 16 weeks prior to baseline; considered free of cancer. Key exclusion criteria included any eye disease or abnormality, or a history of eye surgery.
This study was conducted in accordance with the International Conference on Harmonisation guideline for Good Clinical Practice and with the Declaration of Helsinki. Local ethical committee approval was obtained prior to study initiation. Informed consent was obtained from all patients prior to any study‐related procedure.
Patients placed one drop of study treatment (i.e. bimatoprost 0·03% or vehicle) onto a sterile single‐use‐per‐eye applicator and applied it to the upper eyelid margin of one eye; a second applicator was used for the contralateral eye. Throughout the study, treatment was applied once daily in the evening.
The primary efficacy measure was the proportion of treatment responders based on a composite end point at month 4, defined a priori as at least a one‐grade improvement from baseline in the GEA score and at least a three‐point improvement from baseline in the total score for Domain 2 of the ESQ. The GEA is a validated four‐point photonumeric scale used by investigators at each site to assess overall eyelash prominence, as indicated by the physical attributes of eyelash length, darkness and thickness (1 = minimal, 2 = moderate, 3 = marked, 4 = very marked). The ESQ is a validated 23‐item patient‐reported outcome questionnaire that measures satisfaction in three domains: physical attributes of length, fullness and overall satisfaction (Domain 1); subjective attributes of confidence, attractiveness and professionalism (Domain 2); and impact on daily routine (Domain 3).7, 9 Reponses to questions in ESQ Domain 2 were rated on a five‐point Likert‐type scale (1 = very much disagree; 2 = disagree; 3 = neutral; 4 = agree; 5 = very much agree), with higher scores indicating a higher degree of patient‐reported satisfaction with the subjective attributes of the eyelashes.
Secondary efficacy measures were based on digital image analysis (DIA) of eyelash characteristics: upper eyelash length (mm), thickness (mm2) and darkness (intensity units). For darkness, a negative value in terms of change from baseline indicated darker eyelashes. At each study site, images were taken by trained staff photographers and DIA was performed by study technicians, all of whom were blinded to study treatment.
Safety measures included adverse events (AEs), ophthalmic examination [ophthalmoscopy (dilated), biomicroscopy, IOP, iris colour assessment, and BCVA]. AEs were monitored throughout the study by the investigators.
Statistical analysis
The 6‐month primary analysis was based on data from the first 6 months of the study, that is TP1, which followed a parallel‐group design, allowing for hypothesis testing and between‐group comparisons (bimatoprost 0·03% vs. vehicle). Statistical testing was not performed beyond the 6‐month analysis because all patients treated with vehicle during TP2 had been treated with bimatoprost in TP1; hence, there was no vehicle‐only control group for TP2.
The intent‐to‐treat (ITT) population consisted of all randomized patients. The ITT population was used for all efficacy analyses. The safety population consisted of all patients who received one or more doses of study medication.
The study was sized to have adequate power to evaluate the primary efficacy variable at month 4 for the overall study population (idiopathic and chemotherapy‐induced hypotrichosis subpopulations combined).
Global Eyelash Assessment and ESQ data were collected at each scheduled visit. The primary efficacy end point was defined as the proportion of treatment responders at month 4. The analysis of responders was performed using frequency distributions (counts and percentages). For the 12‐month study analysis, the baseline value was the value collected on day 1 or the most recent evaluation prior to day 1. For the TP2 analysis, the baseline value was the value collected at month 6; if month 6 data were missing, data from the most recent previous evaluation were used. For the ITT population analysis, the last observation carried forward method was used to impute missing values in each of the two components of the primary composite efficacy variable. Between‐group comparisons for TP1 were performed using the Cochran–Mantel–Haenszel test stratified by hypotrichosis aetiology. Individual components of the composite efficacy measure, as well as at least a two‐grade improvement from baseline GEA score, were also assessed at these time points.
Upper eyelash characteristics of length, thickness and darkness were determined by a validated DIA method. The reliability and reproducibility of DIAs have been verified within the acceptance criteria of 0·05% or less of mean coefficient of variance (data on file, Allergan, Inc.).8 The principal variables for the secondary efficacy assessments are change from baseline in upper eyelash length (mm); average progressive eyelash thickness (mm2); eyelash darkness (intensity units) at months 4, 6, 10 and 12; or early termination. Descriptive statistics of the raw value at baseline and percentage change from baseline at follow‐up visits were summarized by treatment group for each principal measure of the three secondary variables of eyelash characteristics. Between‐group comparisons were performed using a van Elteren test stratified by hypotrichosis aetiology.
Safety analysis
The 12‐month data were the primary focus of the safety evaluation. Safety data were summarized with descriptive statistics [n, or frequency distributions (counts or percentages)]. Biomicroscopical examinations and assessments of iris colour, IOP and BCVA were performed for each patient at screening/baseline and at months 1, 2, 4, 6, 8 and 12 (or early termination prior to month 12); ophthalmoscopic examinations (dilated) were performed at screening/baseline and months 6 and 12 (or early termination prior to month 12). Two IOP measurements were taken for each eye at each visit; a third measurement was taken if the difference between the first two measurements was > 2 mmHg. The average or median IOP was determined in the event that two or three measurements were made, respectively. The IOP value analysed at each visit was the average IOP of both the patient's eyes. Change and percentage change from baseline in IOP were summarized by descriptive statistics. Between‐group comparison was performed using one‐way anova.
Iris colour assessments were performed using a 10‐category subjective classification: blue, blue–grey, blue/grey–brown, grey, green, green–brown, hazel, brown, dark brown and other.
Results
A total of 368 patients were randomized at 39 sites (32 U.S. centres and seven European Union centres), of whom 238 had idiopathic hypotrichosis and 130 had chemotherapy‐induced hypotrichosis. Patient disposition and baseline characteristics are provided (Fig. 1 and Table 1, respectively). As per the inclusion criteria, all enrolled patients had baseline GEA scores of 1 (minimal; 39·8%) or 2 (moderate; 60·2%), with a similar distribution of GEA scores in both treatment groups at baseline, although a greater proportion of patients in the chemotherapy‐induced hypotrichosis subpopulation had baseline GEA scores of 1 compared with patients in the idiopathic hypotrichosis subpopulation (71·3% vs. 22·7%). The majority of patients completed the clinical trial, with only 14·9% of patients discontinuing the study. The most common reason for discontinuation was AEs for idiopathic hypotrichosis and loss to follow‐up for the chemotherapy‐induced hypotrichosis subpopulation.
Figure 1.
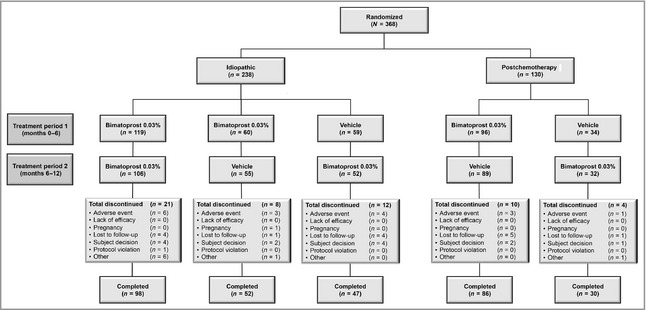
Study flow diagram. No patient was randomized to receive vehicle in both treatment periods.
Table 1.
Overall baseline demographic characteristics (intent‐to‐treat population)
| Characteristic | Overall (n = 368) | Idiopathic hypotrichosis (n = 238) | Postchemotherapy‐induced hypotrichosis (n = 130) |
|---|---|---|---|
| Age, years (mean ± SD) | 49·8 ± 10·5 | 49·3 ± 11·1 | 50·7 ± 9·2 |
| Sex | |||
| Male | 4 (1·1) | 3 (1·3) | 1 (0·8) |
| Female | 364 (98·9) | 235 (98·7) | 129 (99·2) |
| Ethnicity | |||
| White | 305 (82·9) | 202 (84·9) | 103 (79·2) |
| Black | 34 (9·2) | 19 (8·0) | 15 (11·5) |
| Asian | 13 (3·5) | 9 (3·8) | 4 (3·1) |
| Hispanic | 15 (4·1) | 7 (2·9) | 8 (6·2) |
| Othera | 1 (0·3) | 1 (0·4) | 0 (0·0) |
| GEA score | |||
| Minimal | 147 ± 39·3 | 54 ± 22·7 | 93 ± 71·5 |
| Moderate | 221 ± 60·1 | 184 ± 77·3 | 37 ± 28·5 |
| Marked | 0 | 0 | 0 |
| Very marked | 0 | 0 | 0 |
| Total ESQ Domain 2 (mean ± SD)b | 4·10 ± 1·35 | 4·20 ± 1·40 | 3·90 ± 1·23 |
Values are given as n (%) unless otherwise stated. GEA, Global Eyelash Assessment; ESQ, Eyelash Satisfaction Questionnaire. aBlack and white; bmissing data on total ESQ Domain 2 for one patient postchemotherapy.
Composite and individual component efficacy measures of bimatoprost treatment
During TP1, the primary composite efficacy end point at month 4 was met for the overall study population and both subpopulations. For both subpopulations, the composite efficacy end point responder rates at months 4 and 6 were significantly greater for active drug compared with vehicle (Table 2). The responder rates for patients with idiopathic hypotrichosis treated with bimatoprost compared with vehicle were 40·2% vs. 6·8% (P < 0·01) and 47·5% vs. 3·4% (P < 0·01) at months 4 and 6, respectively. Corresponding responder rates for patients with chemotherapy‐induced hypotrichosis were 37·5% vs. 18·2% (P = 0·04) at month 4 and 46·9% vs. 18·2% (P < 0·01) at month 6. Although a greater vehicle treatment effect was observed in patients postchemotherapy who experienced natural eyelash regrowth compared with those with idiopathic hypotrichosis, the results for vehicle‐treated groups were consistently lower than the corresponding results from bimatoprost‐treated groups across all end points. More than four of five patients postchemotherapy were still nonresponders in the placebo group at month 6.
Table 2.
Summary of improvements from baseline to months 4 and 6 for idiopathic hypotrichosis and chemotherapy‐induced hypotrichosis subpopulations: efficacy end points based on Global Eyelash Assessment (GEA) and/or Eyelash Satisfaction Questionnaire (ESQ) scores (intent‐to‐treat population)
| Efficacy end point | Time point | Idiopathic hypotrichosis bimatoprost (n = 179) treatment responders | Idiopathic hypotrichosis vehicle (n = 59) treatment responders | P‐value |
|---|---|---|---|---|
| Composite end point: ≥ 1‐grade increase in GEA score and ≥ 3‐point improvement in ESQ Domain 2 | Month 4a | 72 (40·2) | 4 (6·8) | < 0·01 |
| Month 6 | 85 (47·5) | 2 (3·4) | < 0·01 | |
| ≥ 1‐grade increase in GEA | Month 4 | 133 (74·3) | 8 (13·6) | < 0·01 |
| Month 6 | 139 (77·7) | 8 (13·6) | < 0·01 | |
| ≥ 3‐point increase in ESQ Domain 2 | Month 4 | 85 (47·5) | 9 (15·3) | < 0·01 |
| Month 6 | 100 (55·9) | 9 (15·3) | < 0·01 | |
| ≥ 2‐grade increase in GEA Score | Month 4 | 46 (25·7) | 0 (0·0) | < 0·01 |
| Month 6 | 55 (30·7) | 0 (0·0) | < 0·01 |
| Efficacy end pointa | Time point | Chemotherapy‐induced hypotrichosis patients receiving bimatoprost (n = 96) treatment responders | Chemotherapy‐induced hypotrichosis patients receiving vehicle (n = 34) treatment responders | P‐valueb |
|---|---|---|---|---|
| Composite end point: ≥ 1‐grade increase in GEA score and ≥ 3‐point improvement in ESQ Domain 2 | Month 4a | 36 (37·5) | 6 (18·2)c | 0·04 |
| Month 6 | 45 (46·9) | 6 (18·2)c | < 0·01 | |
| ≥ 1‐grade increase in GEA | Month 4 | 70 (72·9) | 18 (54·5)c | 0·05 |
| Month 6 | 77 (80·2) | 17 (51·5)c | < 0·01 | |
| ≥ 3‐point increase in ESQ Domain 2 | Month 4 | 39 (40·6) | 8 (24·2)c | 0·09 |
| Month 6 | 46 (47·9) | 12 (36·4)c | 0·25 | |
| ≥ 2‐grade increase in GEA score | Month 4 | 35 (36·5) | 2 (6·1)c | < 0·01 |
| Month 6 | 44 (45·8) | 3 (9·1)c | < 0·01 |
Values are given as n (%). Bimatoprost, bimatoprost ophthalmic solution 0·03%. aMonth 4 is the primary analysis time point. bA Pearson's χ2 test was performed; if ≥ 25% of the cells had expected counts < 5, then Fisher's exact test was used instead. cOverall population of patients with chemotherapy‐induced hypotrichosis receiving vehicle was 33.
Statistically significant differences were observed in the responder rates for patients with idiopathic hypotrichosis achieving at least a one‐grade improvement in GEA score when the bimatoprost‐treated and vehicle‐treated groups were compared [74·3% vs. 13·6% (P < 0·01) at month 4 and 77·7% vs. 13·6% (P < 0·01) at month 6, respectively] (Table 2). The differences in the corresponding responder rates for patients with chemotherapy‐induced hypotrichosis were not as pronounced owing to natural eyelash regrowth in this subpopulation. However, this difference was statistically significant at month 6 [80·2% (bimatoprost 0·03%) vs. 51·5% (vehicle) (P < 0·01)].
A marked increase was observed in the percentage of patients in the idiopathic hypotrichosis subpopulation with at least a three‐point increase in ESQ Domain 2 at the 4‐ and 6‐month time points when the groups receiving bimatoprost and vehicle were compared (Table 2). Although the corresponding responder rates for the chemotherapy‐induced hypotrichosis subpopulation were higher in the bimatoprost‐treated compared with vehicle‐treated groups, the differences between these groups were not statistically significant at either months 4 or 6.
No patients with idiopathic hypotrichosis receiving vehicle met the more stringent criteria of at least a two‐grade improvement in GEA score, although this rate was 25·7% (month 4) and 30·7% (month 6) in the bimatoprost‐treated patients (Table 2). Although the corresponding responder rates for patients with chemotherapy‐induced hypotrichosis receiving vehicle were higher (6·1% at month 4 and 9·1% at month 6), the higher eyelash regrowth rates in the bimatoprost‐treated group were evident compared with the vehicle‐treated group [36·5% and 6·1% (P < 0·01), respectively, at month 4].
Analysis of eyelash characteristics by digital imaging analysis
Representative photographs of eyelash growth in patients with idiopathic hypotrichosis and chemotherapy‐induced hypotrichosis after up to 6 months of treatment are shown in Figure 2. Statistically significant changes in the percentage improvement from baseline of eyelash length, thickness and darkness were observed between bimatoprost‐treated and vehicle‐treated groups in both subpopulations at months 4 and 6 (Table 3). However, month 6 measurements of eyelash characteristics of patients postchemotherapy treated with vehicle were similar to the eyelash characteristics (Table 4) of patients with idiopathic hypotrichosis at baseline, indicating that natural eyelash regrowth in this subpopulation was insufficient at month 6.
Figure 2.
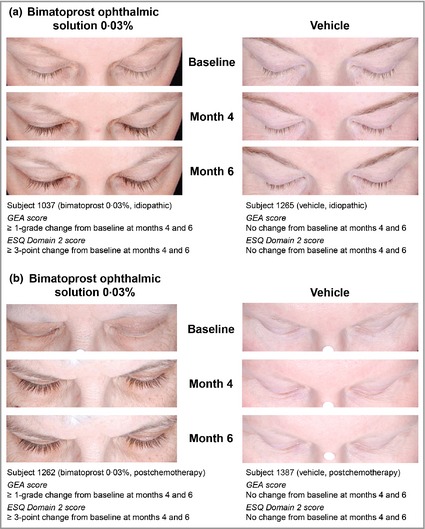
Representative photographs of patients with hypotrichosis undergoing 6 months of treatment with bimatoprost 0·03% or vehicle. (a) Patient with idiopathic hypotrichosis; (b) patient with chemotherapy‐induced hypotrichosis. GEA, Global Eyelash Assessment; ESQ, Eyelash Satisfaction Questionnaire.
Table 3.
Percentage improvement from baseline at months 4 and 6 for idiopathic hypotrichosis and chemotherapy‐induced hypotrichosis subpopulations: efficacy end points based on eyelash characteristics (intent‐to‐treat population)
| Efficacy end pointb | Time point | Idiopathic hypotrichosisa | Chemotherapy‐induced hypotrichosisa | ||||
|---|---|---|---|---|---|---|---|
| Bimatoprost (n = 179) | Vehicle (n = 59) | P‐valuec | Bimatoprost (n = 96) | Vehicle (n = 34) | P‐valuec | ||
| Upper eyelash length (mm) | Month 4 | 22·9 (177) | −4·9 (58) | < 0·01 | 28·5 (94) | 11·3 (31) | 0·02 |
| Month 6 | 26·0 (177) | −1·0 (58) | < 0·01 | 37·8 (94) | 16·3 (31) | < 0·01 | |
| Upper eyelash thickness (mm2) | Month 4 | 95·9 (150) | −7·2 (52) | < 0·01 | 180·1 (64) | 25·0 (19) | < 0·01 |
| Month 6 | 91·4 (150) | −7·7 (52) | < 0·01 | 245·9 (64) | 33·3 (19) | < 0·01 | |
| Upper eyelash darkness (intensity units)d | Month 4 | −15·7 (150) | 1·4 (52) | < 0·01 | −14·4 (65) | −5·7 (18) | 0·01 |
| Month 6 | −14·1 (150) | 1·6 (52) | < 0·01 | −16·3 (65) | −6·5 (18) | < 0·01 | |
Values are given as % (n). Bimatoprost, bimatoprost ophthalmic solution 0·03%. aMean values are presented for the idiopathic hypotrichosis subpopulation. The median is reported for the chemotherapy‐induced hypotrichosis subpopulation where the distribution of the values is skewed with a small number of very high values in percentage change from baseline from those patients who had very few or no eyelashes at baseline. bMonth 4 is the primary analysis time point. c P‐value for between‐group comparison is based on Wilcoxon rank‐sum test. dA negative change from baseline indicates darker eyelashes compared with baseline.
Table 4.
Eyelash characteristics: mean raw values ± SD at baseline and months 4 and 6 (intent‐to‐treat population)
| Efficacy end point | Time point | Idiopathic hypotrichosis bimatoprost 0·03% (n = 179) | Idiopathic hypotrichosis vehicle (n = 59) |
|---|---|---|---|
| Upper eyelash length (mm) | Baseline | 5·69 ± 0·90 (177) | 5·83 ± 0·67 (58) |
| Month 4 | 6·94 ± 1·28 (177) | 5·53 ± 0·80 (59) | |
| Month 6 | 7·12 ± 1·32 (177) | 5·75 ± 0·80 (59) | |
| Upper eyelash thickness (mm2) | Baseline | 0·79 ± 0·34 (150) | 0·87 ± 0·36 (52) |
| Month 4 | 1·33 ± 0·55 (162) | 0·78 ± 0·37 (53) | |
| Month 6 | 1·30 ± 0·50 (165) | 0·77 ± 0·38 (55) | |
| Upper eyelash darknessa (intensity units) | Baseline | 149·29 ± 23·90 (150) | 143·49 ± 24·78 (52) |
| Month 4 | 125·39 ± 25·88 (162) | 143·73 ± 24·31 (53) | |
| Month 6 | 127·95 ± 26·39 (165) | 146·08 ± 24·24 (55) |
| Efficacy end point | Time point | Chemotherapy‐induced hypotrichosis subjects receiving bimatoprost 0·03% (n = 96) | Chemotherapy‐induced hypotrichosis subjects receiving vehicle (n = 34) |
|---|---|---|---|
| Upper eyelash length (mm) | Baseline | 4·87 ± 1·20 (94) | 4·65 ± 1·41 (31) |
| Month 4 | 6·32 ± 1·46 (96) | 5·34 ± 1·20 (33) | |
| Month 6 | 6·84 ± 1·49 (96) | 5·61 ± 1·12 (33) | |
| Upper eyelash thickness (mm2) | Baseline | 0·39 ± 0·30 (66) | 0·67 ± 0·10 (19) |
| Month 4 | 1·01 ± 0·50 (80) | 0·63 ± 0·37 (28) | |
| Month 6 | 1·18 ± 0·59 (84) | 0·72 ± 0·41 (28) | |
| Upper eyelash darkness (intensity units)a | Baseline | 156·16 ± 25·91 (65) | 164·22 ± 25·65 (18) |
| Month 4 | 138·41 ± 25·53 (82) | 149·07 ± 22·70 (28) | |
| Month 6 | 132·65 ± 25·71 (85) | 148·50 ± 20·86 (28) |
Values are given as mean ± SD (n). Bimatoprost 0·03%, bimatoprost ophthalmic solution 0·03%. aA negative change from baseline indicates darker eyelashes compared with baseline.
The improvement in eyelash prominence observed by months 4 or 6 of treatment were either maintained (idiopathic) or further enhanced (postchemotherapy) through the 12‐month period with a once‐daily treatment regimen (Fig. 3).
Figure 3.
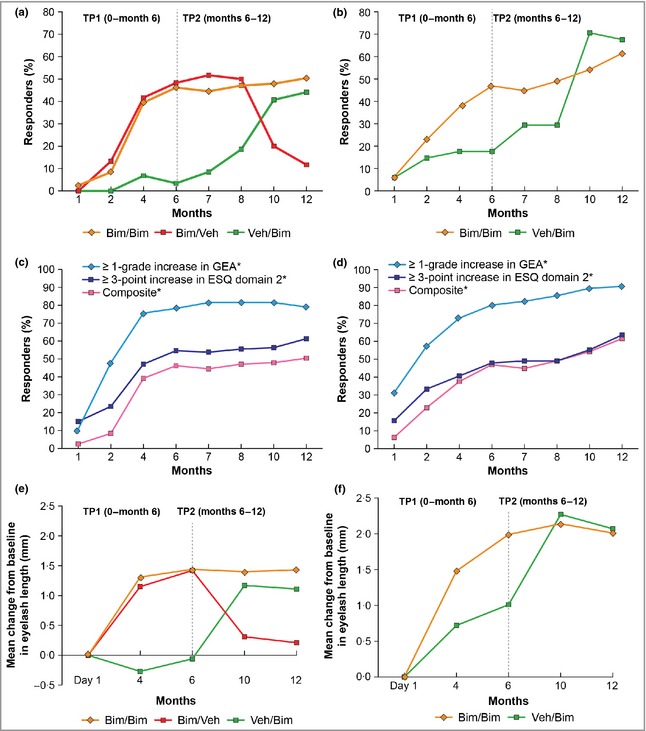
Responder rates for idiopathic hypotrichosis and chemotherapy‐induced hypotrichosis subpopulations according to study treatments and time points. (a) Responder rates in patients with idiopathic hypotrichosis treated with bimatoprost 0·03% (Bim) or vehicle (Veh) – primary composite efficacy measure; (b) responder rates in patients with chemotherapy‐induced hypotrichosis treated with bimatoprost 0·03% or vehicle – primary composite efficacy measure; (c) responder rates in patients with idiopathic hypotrichosis treated with bimatoprost 0·03% – individual components of the primary composite efficacy measure; (d) responder rates in patients with chemotherapy‐induced hypotrichosis treated with bimatoprost 0·03% – individual components of the primary composite efficacy measure; (e) mean changes in eyelash length for patients with idiopathic hypotrichosis; (f) mean changes in eyelash length for subjects with chemotherapy‐induced hypotrichosis. TP1, time period 1; TP2, time period 2; GEA, Global Eyelash Assessment; ESQ, Eyelash Satisfaction Questionnaire. *No patient was randomized to receive vehicle in both treatment periods.
The effect of treatment discontinuation was studied in the idiopathic hypotrichosis subpopulation only. For patients treated with bimatoprost during TP1 and switched to vehicle during TP2, the efficacy demonstrated at the end of TP1 was maintained through month 8 before diminishing at months 10 and 12, as demonstrated by the proportion of responders on the composite end point, and the efficacy end points based on components of the composite end point (Table 5). Figure 3(a) illustrates the effect of treatment discontinuation at 6 months with the composite end point responder rate. Similar trends were observed with eyelash characteristics following treatment discontinuation with bimatoprost at 6 months (Table 6; Fig. 3e).
Table 5.
Summary of efficacy results for patients with idiopathic hypotrichosis treated with bimatoprost in treatment period 1 and switched to vehicle in treatment period 2 (intent‐to‐treat population)
| Efficacy variable)/visita | Treatment | Primary composite end point (n = 60) | ≥ 1‐grade improvement from baseline GEA score (n = 60) | ≥ 3‐point improvement from baseline ESQ Domain 2 score (n = 60) | ≥ 2‐grade improvement from baseline GEA score (n = 60) |
|---|---|---|---|---|---|
| Month 1 | Bimatoprost | 0 (0·0) | 6 (10·0) | 8 (13·3) | 0 (0·0) |
| Month 2 | 8 (13·3) | 24 (40·0) | 15 (25·0) | 2 (3·3) | |
| Month 4 | 25 (41·7) | 43 (71·7) | 29 (48·3) | 15 (25·0) | |
| Month 6 | 29 (48·3) | 46 (76·7) | 34 (56·7) | 18 (30·0) | |
| Month 7 | Vehicle | 31 (51·7) | 47 (78·3) | 34 (56·7) | 18 (30·0) |
| Month 8 | 30 (50·0) | 47 (78·3) | 33 (55·0) | 17 (28·3) | |
| Month 10 | 12 (20·0) | 28 (46·7) | 26 (43·3) | 5 (8·3) | |
| Month 12 | 7 (11·7) | 21 (35·0) | 23 (38·3) | 3 (5·0) |
Values given as n (%). Bimatoprost, bimatoprost ophthalmic solution 0·03%; GEA, Global Eyelash Assessment; ESQ, Eyelash Satisfaction Questionnaire. aVisit was based on visit window. Baseline was defined as the most recent evaluation prior to day 1. Last observation carried forward was performed.
Table 6.
Summary of upper eyelash characteristics in patients with idiopathic hypotrichosis treated with bimatoprost in treatment period 1 and switched to vehicle in treatment period 2 (intent‐to‐treat population)
| Upper eyelash characteristics/visita | Treatment | Mean ± SD at baseline (mean % change from baseline) | ||
|---|---|---|---|---|
| Length (mm) (n = 60) | Thickness (mm2) (n = 60) | Darkness (intensity units) (n = 60)b | ||
| Baseline | Bimatoprost | 5·7 (0·90) | 0·8 (0·32) | 152·5 (20·05) |
| Month 4 | 6·8 (21·01) | 1·2 (73·70) | 126·3 (−16·01) | |
| Month 6 | 7·1 (25·70) | 1·2 (79·90) | 128·0 (−14·89) | |
| Month 10 | Vehicle | 6·0 (6·46) | 0·8 (13·00) | 143·7 (−3·97) |
| Month 12 | 5·9 (4·62) | 0·9 (15·90) | 143·6 (−4·29) | |
Bimatoprost, bimatoprost ophthalmic solution 0·03%. aVisit was based on visit window. Baseline was defined as the most recent evaluation prior to day 1. Last observation carried forward was performed. bThe scale of intensity units is 0–255, where 0 is black and 255 is white. Therefore, lower values indicate darker eyelash colour and higher values indicate lighter eyelash colour; negative changes from baseline indicate darker eyelashes compared with baseline.
For patients with idiopathic hypotrichosis treated with vehicle during TP1 and switched to bimatoprost for TP2, the bimatoprost treatment effect was observed during the same time in TP2 that had been observed for patients treated with bimatoprost in TP1 (Fig. 3a). Patients with chemotherapy‐induced hypotrichosis assigned to vehicle for TP1 followed by treatment with bimatoprost for TP2 demonstrated eyelash characteristics equal, or similar, to those patients who received 12 months of treatment with bimatoprost (Fig. 3f). These results indicate that bimatoprost solution 0·03% is an effective treatment for hypotrichosis of the eyelashes across both subpopulations, regardless of whether it was started on day 1 or month 6, as well as the severity of the condition prior to treatment. It also indicates that early treatment (TP1) with bimatoprost 0·03% can achieve patient‐desired results for eyelash growth sooner than would be achieved through natural regrowth or by starting later. Thus, the inclusion criterion of being at least 4 weeks postchemotherapy before initiating therapy may be a reasonable time for physicians to start bimatoprost if such therapy is desired and warranted.
Safety observations
A summary of the AEs occurring among at least 2·0% of patients from either treatment group by treatment period and aetiology subpopulation is shown in Table 7. The majority of AEs reported in the study were described as mild. There were no serious AEs attributed to study treatment. Ten (2·8%) patients, all of whom were in the idiopathic hypotrichosis subpopulation, discontinued owing to a treatment‐related AE. AEs leading to discontinuation in this subpopulation were conjunctival hyperaemia(n = 3) and erythema (n = 3) of eyelid, eye irritation (n = 2), allergic conjunctivitis (n = 1), enophthalmos (i.e. deepened eyelid sulcus) (n = 1), eye pruritus (n = 1), dry eye (n = 1), eyelid margin crusting (n = 1), eyelid oedema (n = 1), eyelid pruritus (n = 1) and eyelid exfoliation (n = 1). One patient discontinued owing to decreased IOP, and another for contact dermatitis. The most frequently reported (≥ 2·0%) AEs for the overall population during bimatoprost treatment periods were conjunctival hyperaemia (12·1%), punctuate keratitis (5·6%), eyelid pruritus (4·7%) and erythema (4·2%). Most treatment‐related AEs resolved without sequelae at the end of the study. One case of mild punctuate keratitis resolved with sequelae for which treatment and follow‐up were deemed unnecessary; one case of madarosis was ongoing. A higher rate of conjunctival hyperaemia was noted in the chemotherapy‐induced subpopulation (16·7%) compared with the idiopathic population (8·5%).
Table 7.
Number of patients treated with bimatoprost 0·03% for up to 12 months reporting adverse events (incidence of ≥ 2%) in the eye or skin system organ classes (safety population)
| System organ class preferred terma | 0–12 months | 0–6 months | 6–12 months | |||
|---|---|---|---|---|---|---|
| Idiopathic hypotrichosis (n = 118) | Chemotherapy‐induced hypotrichosis (n = 96) | Idiopathic hypotrichosis (n = 118) | Chemotherapy‐induced hypotrichosis (n = 96) | Idiopathic hypotrichosis (n = 106) | Chemotherapy‐induced hypotrichosis (n = 89) | |
| Eye disorders | ||||||
| Conjunctival hyperaemia | 10 (8·5) | 16 (16·7) | 7 (5·9) | 15 (15·6) | 4 (3·8) | 1 (1·1) |
| Punctate keratitis | 3 (2·5) | 9 (9·4) | 3 (2·5) | 8 (8·3) | 0 (0·0) | 1 (1·1) |
| Eyelid pruritus | 7 (5·9) | 3 (3·1) | 6 (5·1) | 3 (3·1) | 1 (0·9) | 1 (1·1) |
| Erythema of eyelid | 6 (5·1) | 3 (3·1) | 2 (1·7) | 1 (1·0) | 5 (4·7) | 2 (2·2) |
| Eye pruritus | 1 (0·8) | 6 (6·3) | 1 (0·8) | 5 (5·2) | 0 (0·0) | 1 (1·1) |
| Skin and subcutaneous disorders | ||||||
| Skin hyperpigmentation | 2 (1·7) | 5 (5·2) | 0 (0·0) | 3 (3·1) | 2 (1·9) | 2 (2·2) |
Values are given as n (%). aWithin each preferred term, a patient was counted once for either treatment period or once for the entire treatment period.
The percentage of patients with biomicroscopy and ophthalmoscopy findings of at least a two‐grade increase in severity from baseline for one or more visits during the bimatoprost treatment periods were 4·2% (9/214) for the group receiving bimatoprost during both treatment periods (12‐month treatment period), 5·0% (3/60) for the group receiving bimatoprost 0·03% followed by vehicle (6‐month treatment period) and 2·4% (2/84) for the group receiving vehicle followed by bimatoprost (6‐month treatment period). No new safety signals arose in the second 6‐month period of treatment, indicating that longer‐term treatment was not associated with an increased incidence of AEs. AEs of particular interest with prostamide F2α analogues include enophthalmos, IOP reduction and iris hyperpigmentation.
Enophthalmos leading to treatment discontinuation occurred in only one patient treated with bimatoprost, and this was the only enophthalmos‐related AE reported in the study. Reported as mild/moderate in severity, the onset of this AE was approximately 2 months after the initiation of bimatoprost. The AE was reported to be ongoing 6 months after the patient discontinued from the study.
The greatest magnitude of mean change from baseline IOP at any time point, for any group, was < 2 mmHg. The mean change from baseline in IOP was greater at all follow‐up visits for patients with idiopathic hypotrichosis treated with bimatoprost compared with those treated with vehicle. These differences, although statistically significant, were not considered to be clinically relevant. At month 4, the mean change from baseline was −1·17 in patients treated with bimatoprost and 0·17 in patients receiving vehicle. In the chemotherapy‐induced subpopulation, changes in IOP from baseline for patients treated with bimatoprost compared with patients treated with vehicle were statistically significant at month 2 only; at the 2‐month visit the mean change from baseline was −1·22 and −0·14 in the bimatoprost‐treated and vehicle‐treated groups, respectively (P = 0·02).
A one‐category change in iris hyperpigmentation was reported as an AE for only one patient, occurring approximately 2 months after discontinuation of bimatoprost and initiation of vehicle. The AE was reported as mild in severity, with a colour change of blue–grey to blue/grey–brown, which was a one‐grade change on the 10‐grade subjective classification. The patient did not discontinue from the study and the AE was reported to have resolved in a poststudy communication. Resolution of iris hyperpigmentation is not typical of true prostaglandin‐induced hyperpigmentation. For patients treated with bimatoprost, there were reports of five patients with a one‐category iris colour change from light to dark; six patients had iris colour changes corresponding to a one‐ to two‐category change in the direction of dark to light. In addition, iris colour changes were reported in two patients who had not received treatment with bimatoprost 0·03%. None of these colour changes were considered to be clinically relevant. Some changes of one or two points on a 10‐point scale are not unexpected from chance alone and may be consistent, in part, with the less‐than‐perfect intrarater reliability of the scale.
The AE profile demonstrated by this study was consistent with the known safety profile of bimatoprost solution 0·03%.
Discussion
The composite efficacy end point used in this study, supported by the individual efficacy component measurements, provided for clinician and patient assessments of treatment results. In addition, DIA of eyelash characteristics allowed for an objective independent measure of treatment efficacy. For all efficacy end points after 4 months of treatment, every group receiving bimatoprost in both subpopulations demonstrated statistically significant improvements from baseline compared with control patients treated with vehicle. These results were independent of whether treatment with bimatoprost was administered at baseline or delayed 6 months from baseline. This latter finding in the chemotherapy‐induced hypotrichosis subpopulation indicated that bimatoprost can accelerate eyelash regrowth, even when initiated months after chemotherapy cessation. In addition, for patients in the groups receiving bimatoprost during both treatment periods, a more pronounced treatment effect from bimatoprost was observed in the chemotherapy‐induced subpopulation compared with the idiopathic subpopulation at month 12. These results may be attributed to natural eyelash regrowth in patients with chemotherapy‐induced hypotrichosis in addition to the treatment effect of bimatoprost. However, month 6 measurements of mean eyelash characteristics of patients with chemotherapy‐induced hypotrichosis treated with vehicle were below mean eyelash characteristics of those with idiopathic hypotrichosis at baseline. Furthermore, about 80% of patients postchemotherapy were still nonresponders by the composite end point definition at month 6, suggesting that natural regrowth at 6 months is, in the vast majority of patients, well below their desired and objective measures of growth. Thus, the natural eyelash regrowth following completion of chemotherapy appears to be a slow recovery process without intervention with bimatoprost.
The impact of bimatoprost discontinuation, evaluated in the idiopathic hypotrichosis subpopulation only, demonstrated that the treatment effect diminished after 2 months. Based on the similarities between the subpopulations, the known mechanism by which cytotoxic agents cause hair loss (i.e. no permanent damage to the hair follicle) and the putative mechanism of action of bimatoprost in stimulating hair growth following hair loss due to chemotherapy, these study findings are expected to be applicable to the chemotherapy‐induced hypotrichosis subpopulation. However, whereas patients with idiopathic hypotrichosis revert back to their baseline levels of eyelash prominence upon treatment discontinuation, the eyelash characteristics of patients with chemotherapy‐induced hypotrichosis would be expected to revert back to a level of prominence that their eyelashes would naturally have achieved after chemotherapy cessation, and this result may be quite variable between individuals.
The majority of common AEs observed throughout the 12‐month period occurred during the first 6 months of treatment, indicating that continuous long‐term treatment does not lead to an increased incidence of AEs. In general, the AEs reported during this study were similar to those reported in previous studies of bimatoprost 0·03% for the treatment of hypotrichosis of the eyelashes; the AEs deemed by the investigator to be related to treatment were largely localized to the treatment area, nonserious, mild in severity, reversible with treatment cessation and predictable based on the known pharmacology of bimatoprost The incidence of treatment‐related conjunctival hyperaemia, punctate keratitis and eye pruritus were higher in the chemotherapy‐induced hypotrichosis subpopulation than in the idiopathic hypotrichosis subpopulation. This difference may be owing to the higher likelihood of eye conditions in a population with recent exposure to chemotherapeutic agents due to an effect of the chemotherapy.10 With respect to changes in IOP, it has been observed that IOP measurements in healthy patients can vary by 3–6 mmHg, even in a single day.11, 12 Thus, the mean changes in IOP were not considered clinically relevant in any treatment group. Similarly, the limited number of changes in iris colour observed during the study was not considered clinically relevant, as most recorded changes were one‐ or two‐category changes on a 10‐category scale, and were transient. The reported changes are most likely attributable to assessment variability.
One limitation of this study is that the patients were predominantly white women and hence may not be representative of the general population seeking treatment for eyelash hypotrichosis. However, similar findings were reported in two randomized, placebo‐controlled clinical trials of bimatoprost for Japanese patients with idiopathic or chemotherapy‐induced eyelash hypotrichosis.13
Bimatoprost 0·03% application once daily over a 12‐month period to the upper eyelids was found to be effective, safe and well tolerated in patients with idiopathic and chemotherapy‐induced hypotrichosis. The primary treatment goals of these subpopulations are not identical as the patients with chemotherapy‐induced hypotrichosis, a constant reminder of their disease, seek to rapidly restore diminished eyelash prominence to a prechemotherapy state. Nevertheless, treatment with bimatoprost provided statistically significant and clinically meaningful benefits to both populations, regardless of the severity of the condition prior to treatment. The AE profile demonstrated during this study was consistent with the previously reported safety profile of bimatoprost 0·03%. No new safety concerns were observed during the second 6‐month treatment period compared with the safety profile observed in the first 6 months of treatment.
Acknowledgments
Writing and editorial assistance was provided by Antoinette Campo of SCI Scientific Communications and Information (SCI), Parsippany, NJ, U.S.A., and by Susan Moench PhD, PA‐C, and Greg Tardie PhD, former employees of SCI.
Funding sources The authors received research grant support from Allergan, Inc., Irvine, CA, U.S.A., for this study and for manuscript preparation. Funding for editorial support was provided by Allergan, Inc., Irvine, CA, U.S.A.
Conflicts of interest D.A.G. is a consultant and investigator for Allergan, Inc. P.H. is a paid consultant for Allergan, Inc. W.P. and T.G. have served as consultants to Allergan, Inc., and have no stock in that company. G.A. and E.W. are employees of Allergan, Inc., and receive compensation in salary, as well as stock or stock options (or both). F.C.B. was an employee of Allergan, Inc., and received compensation in salary, as well as stock or stock options (or both), at the time the study was conducted.
References
- 1. Law SK. Bimatoprost in the treatment of eyelash hypotrichosis. Clin Ophthalmol 2010; 4:349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang J, Lu Z, Au J‐L. Protection against chemotherapy‐induced alopecia. Pharm Res 2006; 23:2505–14. [DOI] [PubMed] [Google Scholar]
- 3. Dorr VJ. A practitioner's guide to cancer‐related alopecia. Semin Oncol 1998; 25:562–70. [PubMed] [Google Scholar]
- 4. Smid SD. Role of prostaglandins and specific place in therapy of bimatoprost in the treatment of elevated intraocular pressure and ocular hypertension: a closer look at the agonist properties of bimatoprost and the prostamides. Clin Ophthalmol 2009; 3:663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Woodward DF, Liang Y, Krauss AH‐P. Prostamides (prostaglandin‐ethanolamides) and their pharmacology. Br J Pharmacol 2008; 153:410–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoelin S, Walt JG, Earl M. Safety, effectiveness, and subjective experience with topical bimatoprost 0.03% for eyelash growth. Dermatol Surg 2010; 36:638–49. [DOI] [PubMed] [Google Scholar]
- 7. Fagien S, Walt JG, Carruthers J et al Patient‐reported outcomes of bimatoprost for eyelash growth: results from a randomized, double‐masked, vehicle‐controlled, parallel‐group study. Aesthet Surg J 2013; 33:789–98. [DOI] [PubMed] [Google Scholar]
- 8. Smith S, Fagien S, Whitcup SM et al Eyelash growth in subjects treated with bimatoprost: a multicenter, randomized, double‐masked, vehicle‐controlled, parallel‐group study. J Am Acad Dermatol 2012; 66:801–6. [DOI] [PubMed] [Google Scholar]
- 9. Dang J, Hansen JE, Burgess SM. Validation and assessment of measurement invariance of the Eyelash Satisfaction Questionnaire (ESQ) in US cancer patients [abstract PSS32]. Value Health 2009; 12:A458. [Google Scholar]
- 10. Hazin R, Abuzetun JY, Daoud YJ et al Ocular complications of cancer therapy: a primer for the ophthalmologist treating cancer patients. Curr Opin Ophthalmol 2009; 20:308–17. [DOI] [PubMed] [Google Scholar]
- 11. Drance SM. The significance of the diurnal tension variations in normal and glaucomatous eyes. Arch Ophthalmol 1960; 64:494–501. [DOI] [PubMed] [Google Scholar]
- 12. Liu JH, Sit AJ, Weinreb RN. Variation of 24‐hour intraocular pressure in healthy individuals: right eye versus left eye. Ophthalmology 2005; 112:1670–5. [DOI] [PubMed] [Google Scholar]
- 13. Harii K, Arase S, Tsuboi R et al Bimatoprost for eyelash growth in Japanese subjects: two multicenter controlled studies. Aesthetic Plast Surg 2014; 38:451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


