Abstract
The lack of breast cancer screening in low and middle‐income countries results in later stage diagnosis and worsened outcomes for women. A cluster randomized trial was performed in Bogotá, Colombia between 2008 and 2012 to evaluate effects of opportunistic breast cancer screening. Thirteen clinics were randomized to an intervention arm and 13 to a control arm. Physicians in intervention clinics were instructed to perform clinical breast examination on all women aged 50–69 years attending clinics for non‐breast health issues, and then refer them for mammographic screening. Physicians in control clinics were not explicitly instructed to perform breast screening or mammography referrals, but could do so if they thought it indicated (“usual care”). Women were followed for 2‐years postrandomization. 7,436 women were enrolled and 7,419 (99.8%) screened in intervention clinics, versus 8,419 enrolled and 1,108 (13.1%) screened in control clinics. Incidence ratios (IR) of early, advanced and all breast cancers were 2.9 (95% CI 1.1–9.2), 1.0 (0.3–3.5) and 1.9 (0.9–4.1) in the first (screening) year of the trial, and the cumulative IR for all breast cancers converged to 1.4 (0.7–2.8) by the end of follow‐up (Year 2). Eighteen (69.2%) of 26 women with early stage disease had breast conservation surgery (BCS) versus 6 (42.5%) of 14 women with late‐stage disease (p = 0.02). Fifteen (68.2%) of 22 women with breast cancer in the intervention group had BCS versus nine (50.0%) of 18 women in the control group (p = 0.34). Well‐designed opportunistic clinic‐based breast cancer screening programs may be useful for early breast cancer detection in LMICs.
Keywords: low and middle income countries, breast cancer, opportunistic screening, cluster randomized controlled trial
Short abstract
What's new?
Breast cancer is a common malignancy in Colombia, and its mortality rates are rising. To catch the disease earlier, recently developed guidelines from the National Cancer Institute of Colombia center on opportunistic (hospital‐based) screening with biennial mammography and clinical breast examination. In this randomized trial involving patients at primary health‐care clinics in Bogotá, opportunistic breast screening was associated with increased rates of disease detection and use of breast‐conservation therapy. Cancers were diagnosed at earlier stages in women who underwent screening versus usual care. The data suggest that opportunistic breast screening can advance early detection in low‐resource settings.
Abbreviations
- BCS
breast conservation surgery
- BIRADS
breast imaging reporting and data system
- CBE
clinical breast examination
- CI
confidence interval
- CRT
cluster randomized trial
- GP
general practitioners
- HR
hazard ratio
- ICD
International Classification of Disease
- IR
incidence ratio
- LMIC
low and middle income countries
- NCIC
National Cancer Institute of Colombia
In the United States, breast‐cancer death rates dropped by 34% between 1990 and 2013,1 an impressive improvement attributed to the combination of earlier detection and effective adjuvant therapies.2 In Europe, comparable estimates of reductions in breast cancer mortality range from 25 to 31%.3, 4, 5 However, many low‐ and middle‐income countries (LMICs) are experiencing an increased incidence of breast cancer,6 and most have limited resources for early breast‐cancer detection programs, resulting in late diagnosis, which is more difficult to treat effectively, and is associated with increased morbidity and mortality.7
In Colombia, breast cancer was the most frequent cause of cancer‐related death among women between 1984 and 2008,8 and breast‐cancer mortality trends have increased steadily in recent years.9 In response, the National Cancer Institute of Colombia (NCIC) developed evidence‐based guidelines for breast‐cancer early detection commensurate with available resources, using stratified guidelines developed by the Breast Health Global Initiative.10 These guidelines are aimed at implementing opportunistic (hospital‐based) biennial mammographic screening of asymptomatic women aged 50–69 years, performed in conjunction with annual clinical breast examination (CBE),11 while ensuring appropriate diagnostic and treatment procedures for positive screened women and for symptomatic women irrespective of age.
In this study we implemented an opportunistic breast screening program in the context of a cluster randomized controlled trial (CRT) in 26 clinics in Bogotá, Colombia. The primary outcome was a relative reduction of advanced breast cancer (stage IIB or higher) in the intervention arm compared with the control arm. The intervention included training general practitioners (GPs) on breast‐cancer screening (CBE, mammography) and offering breast‐screening on all women 50–69 years attending clinics for non‐breast health related issues. The control arm received no explicit intervention and women received “usual care.”11
We hypothesized that intervention clinics would show (i) an increase in number of detected breast cancers, (ii) down‐staging in diagnosed breast cancer and (iii) greater rates of breast conserving surgery (BCS) compared to women receiving usual care in control clinics.
Methods
Study setting and design
The cluster randomized trial (CRT) was conducted from 2008 to 2012 in Bogotá, Colombia, with the approval of NCIC and ethical committees of participating institutions. Written informed consent was obtained from all participants.
The Colombian population receives medical care through two major insurance plans, one for workers and their families, and one for low‐income families. Each plan is managed by insurance companies which define their own health services via a network of clinics. Twenty‐six primary care health clinics were randomized to either the intervention (N = 13) or control arm (N = 13). Randomization was stratified by insurance company to ensure that women in both arms had comparable access to diagnostic and treatment facilities. The Unit for Data Analysis at NCIC used computer‐generated random numbers for allocating clinics to the intervention or control arm, which were generated when at least two clinics (clusters) were identified by the corresponding insurance company to be part of the study. Radiologists and outcome assessors were blinded to randomization arm.
Study preparation in intervention and control clinics
GPs based in the intervention clinics received a 2‐day training course on breast‐cancer epidemiology, clinical signs and symptoms of breast cancer, and principles of mammography and BIRADS (Breast Imaging Reporting and Data System) grading. They received practical training on CBE screening based on the Barton technique.12 They were instructed to perform CBE on all eligible women, record results, and refer women with suspicious findings for further diagnostic procedures. GPs from control clinics were not specifically instructed to perform CBE or to refer women for further diagnostic procedures or screening mammograms, but could do so if they thought it appropriate (“usual care”).
An audit was carried out at mammography centers to confirm that screening quality recommendations were implemented in preparation for the trial. Radiologists and technicians based in mammography centers were trained at NCIC on mammography procedures and QC standards. Radiologists received a session on reading and reporting mammography results using BIRADS. Radiologists and mammography centers were common to both control and intervention clinics but radiologists were blinded to clinic intervention status. Mammograms were read by one radiologist (single‐read). Nurses were educated about breast‐screening and patient recruitment procedures. Nurses in the intervention clinics were additionally instructed to offer breast‐cancer screening to all eligible women.
Quality assurance
NCIC breast surgeons accompanied a 10% randomly selected sample of GPs during their consultations with patients and they monitored the GPs CBE technique, and performed CBE on the same patient. Two independent expert radiologists reviewed all mammograms categorized as BIRADS 4‐5 and 10% of the remaining mammograms, from both intervention and control clinics. If a disagreement was found with the initial report that would change clinical recommendations, a report was sent to the clinic recommending a re‐evaluation.
Follow‐up participant recruitment, inclusion and exclusion criteria
Women could participate if they were aged 50–69 years; resident in Bogotá or surrounding cities; had not had a mammogram in the previous 2 years; no personal history of breast cancer; and attending health‐centers for reasons unrelated to breast health. In all clinics, nurses were trained to identify potentially eligible women using hospital appointment registries, explain study objectives and procedures, obtain written informed consent, and administer a structured interview to obtain information on sociodemographic characteristics and breast‐cancer risk factors.
Women in control clinics were contacted and interviewed upon exiting physician offices, but were not explicitly offered screening. They received information on early breast‐cancer detection, and on how to seek screening in the healthcare system. Trial design and recruitment are depicted in Figure 1.
Figure 1.
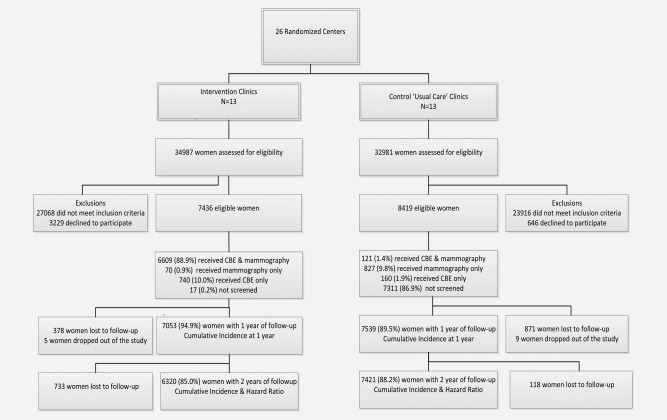
Flowchart of the study.
Breast‐screening (intervention arm)
Results of analogue two‐view mammography screening (cranio‐caudal and mediolateral‐oblique) were interpreted and scored according to BIRADS.13 Women with BIRADS 4‐5, women with BIRADS 1‐2 but with positive CBE, and women with CBE‐positive findings without a mammogram, underwent a diagnostic work‐up including diagnostic breast ultrasound, and either fine needle aspiration or surgical biopsy. Colombian guidelines require women with a BI‐RADS 3 diagnosis enter a surveillance protocol and have a 6‐month follow‐up mammogram, which is compliant with standard BI‐RADS recommendations.13 Patients from both intervention and controls clinics with histologic confirmation of breast cancer were referred to a breast surgeon for further management.
An active follow‐up protocol was implemented in the intervention arm for all abnormal screening findings to ensure access to confirmatory diagnosis and treatment. For ethical reasons, women attending control clinics with mammograms with a score of BIRADS 4‐5 were followed up via telephone. In total 4% of BIRADS 4‐5 mammograms in the control group underwent biopsy after 3 months. The delay is the result of not having an organized program as part of the regular practice (control arm); however, the research team intervened for ethical reasons by cautioning insurance companies about the situation in every specific case. The elapsed time between the abnormal result of the mammogram and the corresponding clinical follow‐up is likely to be longer without the intervention of the research team and in such case more disease in advanced stages would be expected for this group.
Outcome measures: Data collection
All participants in intervention and control clinics were followed for up to 2 years. Data from health insurance companies were utilized in clinics for workers and their relatives. International Classification of Disease (ICD)−10 codes were used to identify patients diagnosed with benign breast lesions and breast cancer; and diagnoses of lung, liver, and bone cancers were reviewed to identify possible cases of metastatic breast cancer. In addition, Specific Codes for Health Procedures were utilized to identify any procedure on the breast irrespective of diagnosis. For women in the low‐income insurance plan, follow‐up consisted of regular telephone or home surveys to determine if they had been diagnosed with benign lesions or breast cancer, or had undergone any procedure on the breast. The same survey was administered to women who were no longer covered by health insurance companies during the follow‐up period. Full medical record reviews were carried out for women who were diagnosed with any breast‐related disease or procedure on the breast. A panel of breast surgeons blinded to trial arm allocation reviewed medical records of all women with breast cancer and assigned clinical stage according to the TNM system. Demographic and breast cancer risk factors were collected by in‐person interview with participants in both arms, and the following covariates dichotomized as yes/no: previous mammogram, family history of breast cancer, menarche before 11 years, ever use of hormone replacement therapy, first pregnancy after 30 years of age, nulliparity, university education or higher, married, and occupation (housewife). In situ/Stage I/IIA cancers were categorized as early stage disease; and Stages IIB/IIIA/IIIB were categorized as advanced.
Power
The study was designed to have 80% power to detect a 50% reduction of advanced breast cancer (Stage IIB or higher) in the intervention arm in comparison with the control arm (with 5% Type‐I error). Expected incidence of breast cancer at stages IIA or less in the absence of the intervention was estimated to be 56/100,000, based on the overall estimated breast‐cancer incidence of 229/100,000 among 50–69 year‐old women from Bogotá, and the percentage of early cancers at the NCIC of about 24.3%.14, 15 We assumed that clusters consisting of an average of 2,000 women would provide about 3,800 person‐years of observation (assuming a yearly dropout rate of 10%). We assumed a coefficient of variation of 0.16 between clusters, requiring randomization of at least 13 clusters in each study arm.16
Statistical analysis
The demographic characteristics of women in the two arms of the study, and their breast cancer risk factors, were compared, as were the TNM classifications of all cases in the two groups. Differences in distributions for categorical variables were estimated using the Pearson χ 2 test. We calculated incidence rates up to 2 years of follow‐up in both intervention and control arms for early, advanced, and total breast cancer, and we calculated the incidence ratio and 95% confidence intervals (CI) for Year 1 data and for Years 1 and 2 data, respectively (Table 3). Person‐years of follow‐up were calculated from date of participant recruitment to date of diagnosis, death, or date of last follow‐up. Hazard ratios (HR) were used for comparing cumulative incidence up to 2 years postrecruitment, and 95% CI were based on the partial likelihood for Cox's proportional hazards model with adjustment for covariates (Fig. 2). Data were censored at 2 years postrecruitment. Variables considered for inclusion in the Cox models as potential confounders are shown in Table 1. Cluster effects due to clinics were adjusted using a frailty model. Age at menarche and age at first pregnancy were included in the final models. All tests of statistical significance are two‐sided. Statistical analyses were performed using Stata statistical software, version 12.0 (StataCorp, College Station, Texas).
Table 3.
Breast cancer Year 1 and 2‐year incidence rates according to stage at diagnosis and study group
| Outcome | Intervention | Control | |||||
|---|---|---|---|---|---|---|---|
| Cases | Person‐years | Crude rate (95% CI per 100,000) | Cases | Person‐years | Crude rate (95% CI per 100,000) | Incidence ratio | |
| Year 1 follow‐up | |||||||
| Advanced breast cancer | 6 | 6,288 | 95.4 (44.8–207.7) | 7 | 7,388 | 94.7 (46.8–195.2) | 1.0 (0.3–3.5) |
| Early breast cancer | 15 | 6,288 | 238.5 (145.8–207.7) | 6 | 7,388 | 81.2 (38.1–176.8) | 2.9 (1.1–9.2) |
| All breast cancers | 21 | 6,288 | 334.0 (219.3–510.5) | 13 | 7,388 | 176.0 (103.6–300.9) | 1.9 (0.9–4.1) |
| Years 1 and 2 follow‐up | |||||||
| Advanced breast cancer | 6 | 11,510 | 52.1 (23.9,132.2) | 9 | 12,932 | 69.6 (36.6,132.2) | 0.7 (0.2,2.4) |
| Early breast cancer | 17 | 11,510 | 147.7 (92.2,236.4) | 9 | 12,932 | 69.6 (36.6,132.2) | 2.1 (0.9,5.4) |
| All breast cancers | 23 | 11,510 | 199.8 (133.2,299.7) | 18 | 12,932 | 139.2 (88.1,219.9) | 1.4 (0.7,2.8) |
Figure 2.
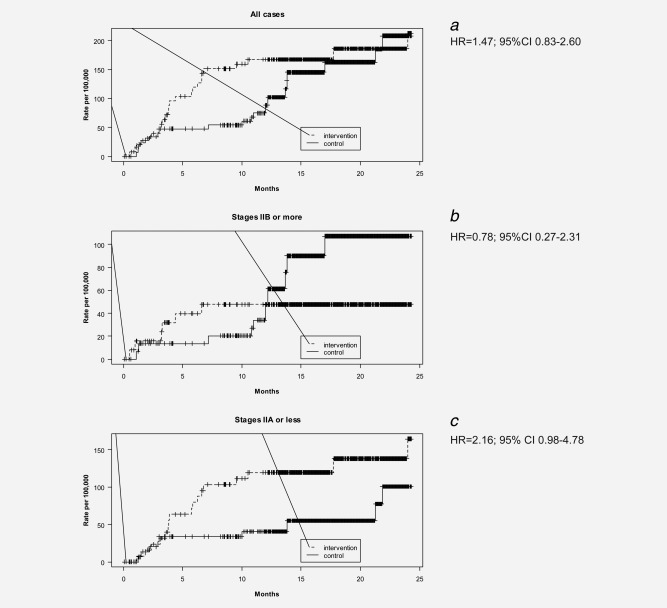
Cumulative incidence of breast cancer according to study group.
Table 1.
Baseline characteristics of eligible women
| Baseline characteristics of eligible women a | Intervention arm N = 7,436 (%) | Control arm N = 8,419 n (%) |
|---|---|---|
| Age (mean) | 58 | 58.1 |
| Previous mammogram | 4,358 (55.7) | 4,790 (58.7) |
| Family history of breast cancer | 310 (4.1) | 303 (3.8) |
| Menarche before 11 years of age | 432 (5.2) | 221 (2.7) |
| First pregnancy after 30 years of age | 660 (10.0) | 575 (7.6) |
| Hormone replacement therapy (ever use) | 842 (11.3) | 826 (9.8) |
| Nulliparous | 235 (3.0) | 237 (3.1) |
| Education (university or higher) | 1,193 (17.0) | 928 (12.0) |
| Marital status (married) | 3,929 (51.1) | 4,451 (53.4) |
| Occupation (housewife) | 4,995 (65.7) | 5,988 (69.1) |
Means and percentages correspond to average results for means and percentages of health centers.
Recruitment and attrition
Women were recruited between 2008 and 2010, and followed up for 2 years postrandomization. 34,987 women in the intervention clinics and 32,981 women in the control clinics were assessed for eligibility (Fig. 1); 7,436 (22.5%) and 8,419 (25.5%) women in intervention and control clinics respectively were enrolled in the study. Rates of prior mammographic screening, risk factors for breast cancer, and indicators of socioeconomic status were similar between arms (Table 1). Seven thousand four‐hundred and nineteen (99.8%) of women recruited into the intervention arm were screened by either CBE (N = 740; 10.0%) or mammography [N = 70 (0.9%)] or both [N = 6,609 (88.9%)], compared to 1,108 (13.1%) of women in the control arm [N = 160 (1.9%) CBE; 827 (9.8%) mammography; N = 121 (1.4%) both]. Attrition rates were similar in the two arms.
Results
Baseline characteristics of women enrolled in the study are shown in Table 1. On average, women were 58 years of age, and over 50% had had a previous mammogram.
Screening diagnoses and stage
More breast cancers were diagnosed in the intervention arm than in the control (23 and 18, respectively; p = 0.05, Table 2). Breast cancer was diagnosed in 34 women in the first year of the trial (21 intervention arm; 13 control arm) and in seven women in the second year (two intervention arm; five control). Early breast cancer (in situ‐IIB) was diagnosed in 15 (75%) of 21 cancer cases in the intervention arm during the first year, compared to in six (46%) of 13 cancer cases in the control. Twelve (57.1%) of the 21 cases in the intervention arm during the first year were in situ or Stage I, compared to only 1 (7.7%) of 13 cases in the control arm. In the second year, one in situ and one Stage 1 case was diagnosed in the intervention arm, whereas two Stage I and three Stage II cancers were found in the control arm.
Table 2.
TNM stage at diagnosis of breast cancer in the intervention and control groups
| Intervention | Control | |||||
|---|---|---|---|---|---|---|
| Category | Stage | Year 1 | Year 2 | Year 1 | Year 2 | Total |
| Early | In situ | 3 (14.3%) | 1 (50.0%) | 0 | 0 | 4 |
| I | 9 (42.9%) | 1 (50.0%) | 1 (7.7%) | 2 (40%) | 13 | |
| IIA | 3 (14.3%) | 0 | 5 (38.5%) | 1 (20%) | 9 | |
| Advanced | IIB | 3 (14.3%) | 0 | 5 (38.5%) | 2 (40%) | 10 |
| IIIA | 1 (4.8%) | 0 | 0 | 0 | 1 | |
| IIIB | 2 (9.5%) | 0 | 2 (15.4%) | 0 | 4 | |
| Total | 21 | 2 | 13 | 5 | 41 | |
Pearson χ 2 = 10.54 Pr = 0.05.
In the control arm, the basis for breast cancer diagnosis was mammography in all cases (N = 18). In the intervention arm, three cancers were diagnosed based on both tests; 14 diagnosed based only on mammography; six were diagnosed based only on CBE.
Distribution of cancer cases over time
At the end of year one, the overall incidence of breast cancer was greater in the intervention arm than in the control arm (Incidence Ratio (IR) = 1.9; 95%CI 0.9–4.1; Table 3). Overall cumulative rates tended to converge by the end of Year 2 (Fig. 2a). By the end of Year 1, the rates of advanced breast cancer did not differ between arms (IR = 1.0; 95%CI 0.3–3.5). During Year 2, there was an increase in advanced stage disease in the control but not in the intervention arm (Fig. 2 b). The rate of early breast cancer was significantly higher in the intervention arm (IR = 2.9; 95%CI 1.1–9.2) versus the control arm in Year 1, but not by the end of Year 2 (Fig. 2c). Hazard ratios (Fig. 2) changed very little when we used various combinations of adjustment variables from Table 1 in the model (data not shown).
Surgical treatment and outcomes
Of the 23 cases diagnosed with early stage disease during 2 years of follow‐up, 18 (78.3%) had BCS compared to six (42.8%) of 14 women with late stage disease (p = 0.02; Table 4). Over the 2 year period, more women in the intervention arm had BCS (N = 15; 68.2%) than in the control arm (N = 9; 50%), although this difference was not statistically significant (Pearson χ 2= 2.15 Pr = 0.34 data not shown).
Table 4.
Clinical stage by type of surgery received
| Type of surgery received | |||||
|---|---|---|---|---|---|
| Stage Category | Stage | None | Breast conservation surgery (BCS) | Mastectomy | Totala |
| Early | In situ | 0 | 4 (16.7%) | 0 | 4 |
| I | 0 | 10 (41.7%) | 3 (23.1%) | 13 | |
| IIA | 0 | 4 (16.7%) | 5 (38.5%) | 9 | |
| Advanced | IIB | 1 (33.3%) | 6 (25.0%) | 2 (15.4%) | 9 |
| IIIA | 0 | 0 | 1 (7.7%) | 1 | |
| IIIB | 2 (66.7%) | 0 | 2 (15.4%) | 4 | |
| Total | 3 | 24 | 13 | 40 | |
Pearson χ 2 = 22.13 Pr = 0.02.
One patient omitted where details of surgery were unknown.
Nine (23%) of 23 cases in the intervention arm compared to seven (39%) of 18 cases in the control arm received neoadjuvant chemotherapy (data not shown). Thirty‐eight women in the intervention and 43 in the control arm died during the study; one woman in each arm died of breast cancer. One patient in the intervention arm developed a breast seroma requiring drainage. No other serious adverse events were reported.
Discussion
Here, we report that a CRT of an organized opportunistic breast‐screening program in asymptomatic women in Bogotá, Colombia, resulted in a significant increase in the detection of early stage cancers and greater utilization of BCS. Overall breast‐cancer incidence was higher in the intervention arm than in the control, due to an increase in detection of early‐stage disease during the first year of the trial when screening was being conducted. Rates of advanced breast cancer did not differ between groups during the first year. After two years of follow‐up, no further cases of advanced disease were observed in the intervention group, and the difference in the overall cumulative incidence rates of breast cancer in the two arms of the study converged. The increase in diagnosed in situ disease is in line with what occurs after a screening program has been implemented. Given the small numbers of in situ cases (four in the screened arm and none in the control arm) and the short follow‐up period, it is not possible to ascertain whether these cases are a result of overdiagnosis, or represent downstaging i.e. where these in situ cancers would eventually have been diagnosed at a later stage in the absence of screening.
Opportunistic screening occurs either as a result of a request from an individual or from contact with a health professional who offers the screening test. Our study used a modified opportunistic approach by offering screening to all women attending clinics for purposes other than breast screening or diagnosis. There is contradictory information about the effectiveness of opportunistic approaches in screening: some reports suggest that opportunistic screening may be less cost‐effective than organized screening particularly among women of low socioeconomic status.17, 18 On the other hand, the WHO recommends early diagnosis or down‐staging programs as appropriate in low‐resource settings to find prevalent clinically detectable cancers, stating that a cancer screening program is a more costly and complex undertaking than a down‐staging program based on clinical evaluation.19 Accordingly, opportunistic screening programs may represent an important option for LMICs where breast cancer is diagnosed in late stages and resources are limited: opportunistic screening might be implemented initially to downstage disease at diagnosis by identifying prevalent breast cancer cases within the target group, and at the same time providing the groundwork and infrastructure that later could allow LMICs to expand screening opportunities as more resources become available.
Due to its decentralized nature and lack of systematic reporting, the quality of opportunistic screening is difficult to evaluate in countries lacking centralized cancer registries and fragmented healthcare systems such as those in low‐resource settings. Thus, few data exist on the results of opportunistic breast screening programs and, to our knowledge; none have been evaluated in a randomized controlled trial (RCT). Also, no RCT of mammography screening has been conducted in LMICs. A Japanese study reported on opportunistic screening in 12,823 women and found that opportunistic screening had a greater net benefit for women in their 40s compared to women aged >50.20 European studies compared screen‐detected cancers vs. those detected by opportunistic screening, and despite a higher percentage diagnosed at a lower grade by population‐based screening, overall prognostic factors were comparable between cancers diagnosed by either method.21, 22 Given the specific barriers to population based screening in low‐resource settings, opportunistic screening may represent a good preliminary method for downstaging breast cancer. A recent study based in Malaysia comparing opportunistic with diagnostic and high‐risk screened population found that early stage breast cancer was diagnosed in 84.6% in the screening group vs. in 61.1% of the diagnostic group.23
While mortality is the end‐point by which screening programs should ultimately be judged, the rate of later‐stage tumors can be used as a reliable surrogate indicator of the effect of a screening program before mortality results are available.24, 25 The lack of long‐term follow‐up in our study meant we were unable to ascertain whether this will translate to reductions in mortality; we also are not able to address the frequency of interval cancers (cancers diagnosed during the interval between screening episodes) using this opportunistic approach. However, detection of breast‐cancer in women at earlier stages was associated with greater rates of BCS rather than mastectomy, less need for more complex and toxic systemic therapies and therefore, less morbidity, resulting in cost savings for health systems.
The value of CBE as a screening modality to reduce mortality has not been established, and as a result neither IARC nor the U.S. Preventative Services Task Force recommends CBE as part of a screening modality.26, 27 IARC has proposed that RCTs of CBE versus no screening should be conducted in a country where resources are unavailable to implement mammographic screening; and a CBE versus mammography trial be performed in countries where resources permit only limited mammography to evaluate their utility in LMICs.27 The effects of CBE with and without mammography on downstaging have been the subject of a few RCT where downstaging of disease has been observed, but insufficient follow‐up had elapsed to observe differences in mortality between arms.28, 29 Furthermore, the 25‐year follow‐up of the Canadian National Breast Screening Study found that annual mammography in women aged 40–59 had no additional benefit beyond that of CBE in terms of mortality.30 The inclusion of CBE in the Colombian guidelines was intended to increase detection rates and reduce the chance of interval cancers. Our results show that CBE detected nine out of 21 cancer cases, while mammography detected 17 cancers, suggesting better overall sensitivity for mammography. Nonetheless, these results indicate that CBE can increase overall detection rates beyond mammographic screening alone. More specific analysis regarding the precise effect on screening performance of the two modalities is beyond the objectives of this study.
Screening programs can induce harms including overdiagnosis and false‐positive work‐ups. The magnitude of overdiagnosis is controversial with estimates ranging from 0 to 54%.31, 32, 33, 34, 35 A recent paper based on European breast cancer service screening outcomes suggests that the chance of a breast cancer death being avoided by population‐based screening is greater than that of overdiagnosis.5 In LMICs, breast cancer incidence is lower than in high income countries, and is more likely to be diagnosed at a younger age; in younger populations, more women will have to be screened in LMICs to detect one cancer, increasing the rate of false‐positive work‐ups and placing increased burdens on resource‐poor health systems.36 Thus, the relative benefits and risks of screening should be individually evaluated for the population being screened and the implementation resource requirements for the program being planned.
One of the major challenges faced during the implementation of the study is the high turnover rate of GPs in primary care centers (35% during the study period), which required ongoing efforts for training and education in order to maintain high quality standards for CBE as it is highly dependent on the provider.
Strengths of our study include its randomized nature, a high response rate among those who were invited to participate, and the fact that both radiologists and pathologists were blinded to study arm. Limitations include duration of follow‐up, the need to use advanced disease as a surrogate endpoint for mortality, and a relatively low numbers of cases. However, results are all in the expected direction, and rates of early breast‐cancer diagnosis during the first year were statistically significant between arms. Another limitation is screening in women randomized to the control arm. As shown in Figure 1, 11.2% of the women in the control arm had a mammogram with or without a CBE, and another 1.9% had CBE alone, for an overall screening estimate of 13.1%. This, however, is probably an overestimate of the actual amount of screening because some of the mammograms were likely to have been diagnostic in nature. The screening in the control group would tend to result in an underestimation the benefit of screening, and would not lead to a spuriously observed beneficial effect.
Our results demonstrate that an opportunistic approach offering screening systematically to women, was successful at detecting early‐stage disease. The results should be interpreted with caution given the small sample size; longer follow‐up is also required to evaluate whether the initial increase in early stage disease promotes long‐term improvement in cancer morbidity and mortality with the use of similar or fewer healthcare resources. Nonetheless, in the absence of population‐based screening programs, a well‐designed opportunistic program embedded within a functional healthcare system may represent an attractive method for downstaging breast cancer as a first step toward improving breast cancer mortality in LMICs.
Clinicaltrials.gov registration number: NCT02337582
Conflict of interest: The authors have no conflict of interest to disclose.
References
- 1. DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. Cancer J Clin 2014;64:52–62. [DOI] [PubMed] [Google Scholar]
- 2. Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005;353:1784–92. [DOI] [PubMed] [Google Scholar]
- 3. Broeders M, Moss S, Nystrom L, et al. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen 2012;19:14–25. [DOI] [PubMed] [Google Scholar]
- 4. Njor S, Nystrom L, Moss S, et al. Breast cancer mortality in mammographic screening in Europe: a review of incidence‐based mortality studies. J Med Screen 2012;19:33–41. [DOI] [PubMed] [Google Scholar]
- 5. Paci E, Broeders M, Hofvind S, et al. European breast cancer service screening outcomes: a first balance sheet of the benefits and harms. Cancer Epidemiol Biomarkers Prev 2014;23:1159–63. [DOI] [PubMed] [Google Scholar]
- 6. Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893–907. [DOI] [PubMed] [Google Scholar]
- 7. Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population‐based study (CONCORD). Lancet Oncol 2008;9:730–56. [DOI] [PubMed] [Google Scholar]
- 8. Pineros M, Gamboa O, Hernandez‐Suarez G, et al. Patterns and trends in cancer mortality in Colombia 1984‐2008. Cancer Epidemiol 2013;37:233–9. [DOI] [PubMed] [Google Scholar]
- 9. Pedraza AM, Pollan M, Pastor‐Barriuso R, et al. Disparities in breast cancer mortality trends in a middle income country. Breast Cancer Res Treat 2012;134:1199–207. [DOI] [PubMed] [Google Scholar]
- 10. Anderson BO, Jakesz R. Breast cancer issues in developing countries: an overview of the Breast Health Global Initiative. World J Surg 2008;32:2578–85. [DOI] [PubMed] [Google Scholar]
- 11. Murillo R, Díaz S, Sánchez O, et al. Pilot Implementation of Breast Cancer Early Detection Programs in Colombia. Breast Care (Basel, Switzerland) 2008;3:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barton MB, Harris R, Fletcher SW. The rational clinical examination. Does This Patient Have Breast Cancer? the Screening Clinical Breast Examination: Should It Be Done? How? J Am Med Assoc 1999;282:1270–80. [DOI] [PubMed] [Google Scholar]
- 13. American College of Radiology (ACR) . Breast Imaging Reporting and Data System Atlas (BI‐RADS Atlas)ed. Reston, VA: American College of Radiology, 2003. [Google Scholar]
- 14. Cancer Incidence in Five Continentsed , Vol. 10. Lyon, France: IARC, 2013.
- 15. Pardo C, Cendales R. Incidencia estimada y mortalidad por cáncer en Colombia 2002‐2006. Bogotá; Instituto Nacional de Cancerología; 2010.
- 16. Hayes RJ, Bennett S. Simple sample size calculation for cluster‐randomized trials. Int J Epidemiol 1999;28:319–26. [DOI] [PubMed] [Google Scholar]
- 17. de Gelder R, Bulliard JL, de Wolf C, et al. Cost‐effectiveness of opportunistic versus organised mammography screening in Switzerland. Eur J Cancer (Oxford, England: 1990) 2009;45:127–38. [DOI] [PubMed] [Google Scholar]
- 18. Bulliard JL, Ducros C, Jemelin C, et al. Effectiveness of organised versus opportunistic mammography screening. Ann Oncol 2009;20:1199–202. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization . Early detection. In: Organization WH, ed. Cancer Control: WHO Guide for Effective Programmes, vol. 2015: World Health Organization, 2014. Available at: http://www.who.int/cancer/detection/breastcancer/en/index3.html. Accession on 15 June 2015.
- 20. Kikuchi M, Tsunoda H, Koyama T, et al. Opportunistic breast cancer screening by mammography in Japan for women in their 40s at our preventive medical center: harm or benefit?. Breast Cancer (Tokyo, Japan) 2014;21:135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vanier A, Leux C, Allioux C, et al. Are prognostic factors more favorable for breast cancer detected by organized screening than by opportunistic screening or clinical diagnosis? A study in Loire‐Atlantique (France). Cancer Epidemiol 2013;37:683–7. [DOI] [PubMed] [Google Scholar]
- 22. Hoff SR, Klepp O, Hofvind S. Asymptomatic breast cancer in non‐participants of the national screening‐programme in Norway: a confounding factor in evaluation? J Med Screen 2012;19:177–83. [DOI] [PubMed] [Google Scholar]
- 23. Teh YC, Tan GH, Taib NA, et al. Opportunistic mammography screening provides effective detection rates in a limited resource healthcare system. BMC Cancer 2015;15:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swedish Organised Service Screening Evaluation Group . Effect of mammographic service screening on stage at presentation of breast cancers in Sweden. Cancer 2007;109:2205–12. [DOI] [PubMed] [Google Scholar]
- 25. Buiatti E, Barchielli A, Bartolacci S, et al. The impact of organised screening programmes on the stage‐specific incidence of breast cancer in some Italian areas. European Journal of Cancer (Oxford, England: 1990) 2003;39:1776–82. [DOI] [PubMed] [Google Scholar]
- 26. U.S. Preventive Services Task Force . Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009;151:716–26, W‐236. [DOI] [PubMed] [Google Scholar]
- 27. IARC , Breast Cancer Screening. Lyon, France: IARC, 2002.
- 28. Mittra I, Mishra GA, Singh S, et al. A cluster randomized, controlled trial of breast and cervix cancer screening in Mumbai, India: methodology and interim results after three rounds of screening. Int J Cancer 2010;126:976–84. [DOI] [PubMed] [Google Scholar]
- 29. Sankaranarayanan R, Ramadas K, Thara S, et al. Clinical breast examination: preliminary results from a cluster randomized controlled trial in India. J Natl Cancer Inst 2011;103:1476–80. [DOI] [PubMed] [Google Scholar]
- 30. Miller AB, Wall C, Baines CJ, et al. Twenty five year follow‐up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ 2014;348:g366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paci E, Duffy S. Overdiagnosis and overtreatment of breast cancer: overdiagnosis and overtreatment in service screening. Breast Cancer Res 2005;7:266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puliti D, Duffy SW, Miccinesi G, et al. Overdiagnosis in mammographic screening for breast cancer in Europe: a literature review. J Med Screen 2012;19 Suppl 1:42–56. [DOI] [PubMed] [Google Scholar]
- 33. White E, Lee CY, Kristal AR. Evaluation of the increase in breast cancer incidence in relation to mammography use. J Natl Cancer Inst 1990;82:1546–52. [DOI] [PubMed] [Google Scholar]
- 34. Zackrisson S, Andersson I, Janzon L, et al. Rate of over‐diagnosis of breast cancer 15 years after end of Malmo mammographic screening trial: follow‐up study. BMJ 2006;332:689–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gotzsche PC, Jorgensen KJ, Maehlen J, et al. Estimation of lead time and overdiagnosis in breast cancer screening. Br J Cancer 2009;100:219; author reply 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Corbex M, Burton R, Sancho‐Garnier H. Breast cancer early detection methods for low and middle income countries, a review of the evidence. Breast (Edinburgh, Scotland) 2012;21:428–34. [DOI] [PubMed] [Google Scholar]


