Summary
M ycobacterium tuberculosis (Mtb) infection can be cleared by the innate immune system before the initiation of an adaptive immune response. This innate protection requires a variety of robust cell autonomous responses from many different host immune cell types. However, Mtb has evolved strategies to circumvent some of these defences. In this mini‐review, we discuss these host–pathogen interactions with a focus on studies performed in human cells and/or supported by human genetics studies (such as genome‐wide association studies).
Introduction
Mycobacterium tuberculosis (Mtb) is particularly effective at subverting many of the host immune defences, and this is one reason why it is such a successful human pathogen that has been particularly hard to eradicate. The outcome of infection by Mtb and therefore the clinical manifestation of tuberculosis (TB) depend on many combined factors, such as host genetics, bacterial genetics (virulence factors), the health and nutritional status of the host and whether there has been any prior exposure/immunity and vaccination history. Around half of individuals exposed to Mtb do not exhibit a positive tuberculin skin test (Morrison et al., 2008), indicating that infection did not occur after exposure or that there has been no Th1‐type adaptive immune response (forming characteristic granulomas), indicating that there may have been an ‘early clearance’ of Mtb by the innate immune system (Verrall et al., 2014). All the information discussed in this review has been gained from studies using human cells or patients, because it has been shown that there are often differences with animal models that can affect the outcome of TB infection (Fortin et al., 2007). Although human primary cells are relevant models for studying human TB, there are difficulties associated with their use such as donor variability and genetic manipulation. Therefore, human macrophage‐like cell lines are also an important tool, as long as the data obtained using them are discussed with their aberrant nature in mind. Moreover, in both cell lines and primary cells, different aspects need to be considered such as multiple differentiation protocols that impairs useful comparisons across different laboratories and suboptimal culture conditions of the physiological environment (Vogt and Nathan, 2011).
First, we discuss the different cell types important for innate immunity against Mtb. Then, we discuss the mechanisms these cells use to clear the infection and the Mtb effectors that subvert the host defences.
Cells involved in the innate immune response to TB in humans
Innate defences to Mtb in the airways: the respiratory mucosa
Mtb is inhaled through the nose and mouth and passes along the trachea, bronchus, bronchioles and eventually to the alveoli in the lung (Fig. 1). Along the airway is the respiratory mucosa (Fig. 1A) that forms the first line of defence against Mtb (Middleton et al., 2002). It consists of (i) the epithelium, a layer of airway epithelial cells (AECs) forming a barrier that prevents invasion; (ii) the lamina propria, a layer of connective tissue and immune cells, including lymphocytes and macrophages; and (iii) a coating of a highly complex substance known as airway surface liquid (ASL), which contains mucus, immunoglobulin A and an array of other innate immune factors on the luminal surface. Also located in prime positions along the airways to encounter Mtb are bronchial‐ or nasal‐associated lymphoid tissues that are crucial for Mtb antigen sampling (Lugton, 1999).
Figure 1.
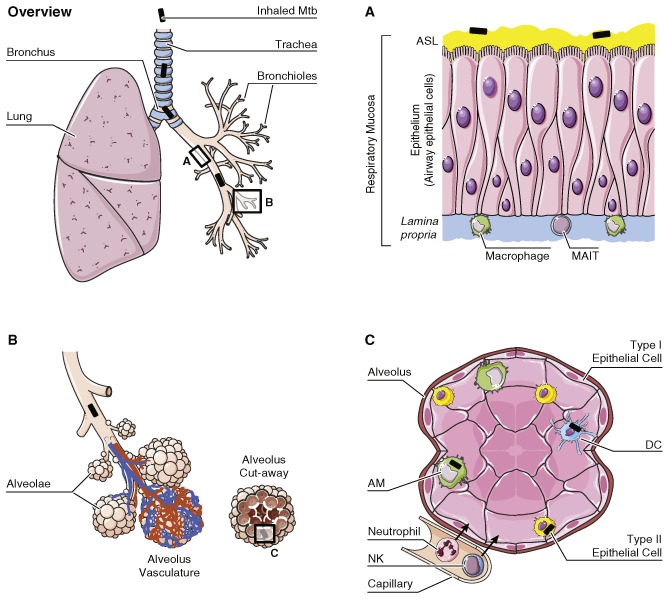
Cells involved in the human innate immune response to tuberculosis. Upon inhalation into the lung, Mtb (black rod) travels along the trachea, bronchus and bronchioles to the alveoli. Lining the airway is the respiratory mucosa (A). This consists of a layer of AECs that provide a tight barrier to prevent Mtb from invading the tissue and they have many receptors to detect Mtb. AECs control the composition of ASL, a substance containing mucus, anti‐microbial peptides, antibodies and cytokines/chemokines. The lamina propria supports the epithelium and also contains immune cells such as macrophages and MAIT that respond to infection. Mtb eventually reach the alveolae (B), which are surrounded by a network of capillaries to facilitate gas exchange. The alveolus (C) is structurally formed from type I epithelial cells, and type II epithelial cells are often found at the cell junctions. Type II cells secrete a variety of anti‐microbial substances including pulmonary surfactant. AMs and DCs are the primary resident defenders of the alveolus. They are effective phagocytes and have a range of intrinsic anti‐microbial capacities. In addition, neutrophils and NKs are recruited from the surrounding capillaries to bolster the host defence. Cells are not drawn to scale.
AECs can recognize pathogen‐associated molecular patterns (PAMPs) present on Mtb surfaces as they constitutively express pattern recognition receptors such as Toll‐like receptors, Dectin‐1, C‐type lectin receptors (CLRs), nucleotide‐binding oligomerization domain‐containing protein 2 (NOD2), dendritic cell (DC)‐specific intercellular adhesion molecule‐3‐grabbing non‐integrin and mannose receptor (reviewed in Li et al., 2012). These receptors have been implicated in Mtb infection in human AECs and mediate the production of cytokines and effector molecules to mount an effective immune response. AECs play a role as immune sentinels after exposure to Mtb by presenting antigen to mucosal‐associated invariant T cells (MAITs) (Harriff et al., 2014) and stimulating them to produce interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α and granzyme, factors that may contribute to Mtb clearance. MAIT cells rapidly respond to infection, providing an early IFN‐γ boost to activate macrophages (Gold et al., 2010). Crucially, AECs control the composition of ASL. ASL contains anti‐microbial peptides that have been implicated in Mtb resistance, such as β‐defensin 2 (Rivas‐Santiago et al., 2005), cathelicidin (LL‐37) (Rivas‐Santiago et al., 2008) and hepcidin (Sow et al., 2011), as well as a variety of cytokines and chemokines that are secreted by AECs to recruit and activate phagocytes (reviewed in Li et al., 2012).
Innate defences to Mtb in the airways: the alveoli
Mtb that successfully passes through the upper airways will be delivered to the alveoli (Fig. 1B andC). The alveoli consists of a thin lining of type I and II epithelial cells as well as other immune cells such as alveolar macrophages (AMs), DCs and neutrophils. Type I epithelial cells form the walls of the alveolus, and these cells are primarily involved with gas exchange. Whether they can be infected by Mtb remains to be seen. In contrast, infection of type II epithelial cells by Mtb has been extensively studied in vitro, and Mtb DNA has been detected within these cells in post‐mortem studies (Hernández‐Pando et al., 2000). Similar to AECs, these cells produce anti‐microbial molecules (Rivas‐Santiago et al., 2005). Additionally, type II cells produce and secrete pulmonary surfactant, hydrolytic enzymes and hydrolases in the extracellular lining of the lung. Surfactant proteins (members of the collectin family) bind to Mtb, causing agglutination (Ferguson et al., 1999) and enhanced phagocytosis by macrophages (Gaynor et al., 1995). Secreted hydrolases can alter the cell wall of Mtb and affect interactions with macrophages and host immune responses (Arcos et al., 2011).
Innate defences to Mtb in the airways: resident defenders
There are relatively few AMs per alveolus (around 10), but they live for around 3 months in humans (Thomas et al., 1976). AMs have a whole range of cell autonomous anti‐microbial mechanisms at their disposal (see below). On the other hand, evolution has equipped Mtb with the capability to evade and/or tolerate some of these anti‐microbial mechanisms (Table 1), and the outcome of the initial battle depends on the intrinsic microbicidal capacity of the host cell and the virulence factors of the ingested Mtb. If the host cell kills Mtb, then the infection is controlled, but if this response is ineffective, Mtb will replicate in this niche and the infection will spread.
Table 1.
Mtb virulence factors counteracting the innate immune response
| Mtb effectors | Action | Mechanism | Cell type | References |
|---|---|---|---|---|
| Intracellular trafficking and localization | ||||
| ESAT‐6 | Translocation into the cytosol | ESAT‐6 has a pore forming activity | THP‐1, DCs | van der Wel et al., 2007; Houben et al., 2012; Simeone et al., 2012 |
| LAM | Inhibits phagolysosome fusion | Unknown | THP‐1/MDMs | Hmama et al., 2004; Kang et al., 2005; Welin et al., 2008 |
| PtpA | Inhibits phagosome acidification | vATPase exclusion | THP‐1 | Bach et al., 2008; Wong et al., 2011; Wong and Jacobs, 2011 |
| Autophagy | ||||
| ESAT‐6 | Inhibits production of IFN‐γ | Affects TCR signalling | T cells | Wang et al., 2009 |
| ESX‐1 secretion system | Inhibition of autophagosomes/lysosome fusion | Unknown | DCs | Romagnoli et al., 2012 |
| LAM | Blocks transcriptional activation of IFN‐γ | Unknown | U937/THP‐1 | Chan et al., 1991 |
| miR‐30A | Inhibition of autophagy | Unknown | THP‐1 | Chen et al., 2015 |
| Sulfatide | Blocks IFN‐γ or lipopolysaccharide priming | Unknown | Monocytes | Pabst et al., 1988 |
| Host cell death | ||||
| ESAT‐6 | Induces necrotic death | Caspase‐1‐ and cathepsin B‐independent necrosis | MDMs | Welin et al., 2011 |
| ESX‐1 secretion system | Extracellular traps | Unknown | MDMs | Wong and Jacobs, 2013 |
| CpnT | Induces necrotic death | Unknown | Jurkat T | Danilchanka et al., 2014 |
| SecA2 and NuoG | Suppress apoptosis | Unknown | THP‐1 | Hinchey et al., 2007; Velmurugan et al., 2007; Miller et al., 2010 |
| PknE | Inhibits apoptosis | Unknown | THP‐1 | Jayakumar et al., 2008 |
| Rv3364c | Suppresses caspase‐1 and pyroptosis | Inhibition of cathepsin G activity | U937 | Danelishvili et al., 2011 |
| Reactive species and toxic metals | ||||
| Eis | Modulates ROS production | Targets JNK pathway | THP‐1 | Shin et al., 2010 |
| ctpC | Zinc detoxification | Zinc efflux | MDMs | Botella et al., 2011 |
| NuoG | Neutralizes ROS and TNF‐α‐mediated host cell apoptosis | Unknown | AMs | Miller et al., 2010 |
Mtb effector abbreviations: CpnT, C‐terminal domain of the channel protein with necrosis‐inducing toxin; Eis, enhanced intracellular survival protein; LAM, lipoarabinomannan; PknE, protein kinase E; PtpA, tyrosine phosphatase. Cell type abbreviations: AMs, alveolar macrophages; DCs, dendritic cells; MDMs, monocyte‐derived macrophages.
DCs are also one of the first types of cell to encounter Mtb. They have a multitude of receptors to detect Mtb PAMPs and are highly efficient phagocytes (Henderson et al., 1997). After uptake of Mtb, DCs in the alveoli mature and present antigens via Major Histocompatibility Complex (MHC) class I and II to T cells in the local draining lymph node (Marino et al., 2004); thus, DCs are a link between the innate and adaptive immune response. However, the outcome of Mtb and DC interaction is complex and not well understood, likely to be due to variation in host genetics and bacterial virulence factors. Mtb is capable of replicating within DCs (Förtsch et al., 2000), and some reports show that Mtb actually manipulates DC function and impairs their ability to control infection (Hanekom et al., 2003). However, other studies find that DCs are beneficial to bolster the cellular immune response (Tailleux et al., 2003).
Innate defences to Mtb in the airways: recruited defenders
Neutrophils are the predominant cell type infected in the airways of individuals with active TB (Eum et al., 2010). These professional phagocytes play a very complex and conflicting role in the pathology of TB that likely depends upon the host genetics, Mtb virulence factors and also the stage of TB disease. Reflecting this, some studies have shown that human neutrophils can either restrict (Brown et al., 1987) or favour (Denis, 1991) Mtb growth. Highlighting the importance of virulence factors for the outcome of infection, a study showed that in primary human neutrophils, only virulent Mtb could survive the host‐generated respiratory burst by inducing necrotic cell death (Corleis et al., 2012). After stimulation with Mtb, neutrophils secrete chemokines and pro‐inflammatory cytokines leading to recruitment and activation of other immune cells (Riedel and Kaufmann, 1997). Human apoptotic neutrophils infected with Mtb can be phagocytosed by Mtb‐infected macrophages; in this case, the anti‐microbial contents of neutrophil granules can directly fuse with Mtb‐containing phagosomes in macrophages, leading to improved killing (Tan et al., 2006). Whether human neutrophils can control intracellular Mtb or act via neutrophil extracellular traps (NETs) is still debated (see below).
Natural killer cells (NKs) are also involved in Mtb infection. These innate immune cells are recruited to the site of infection early on and play a role in amplifying the anti‐microbial defence to TB. This is via recognition of infected macrophages through receptor molecules such as NKp44, NKp46 and NKG2D (Vankayalapati et al., 2005). NKs can lyse infected macrophages (Vankayalapati et al., 2002), produce IFN‐γ to further activate macrophages and can also secrete cytokines that expand CD8+ T cells and NK T cell (NKTs) populations (Vankayalapati and Barnes, 2009). NKTs recognize lipid antigens presented by CD1a molecules and NKT deficiency is associated with the development of active TB (Sutherland et al., 2009). There are other T‐cell subsets such as γδ T cells present in the alveoli; they have been shown to recognize Mtb phosphoantigens (Ismaili et al., 2002) and participate in the killing of infected macrophages through cytotoxic granules.
Cell autonomous defence mechanisms in TB
Trafficking and localization of Mtb in human cells
The phagosome is a central mediator of both the homeostatic and microbicidal functions of macrophages. After phagocytosis, Mtb blocks phagosome acidification as well acquisition of hydrolytic enzymes and anti‐microbial peptides. Two major Mtb virulence factors are involved in the blockage of the phagosomal maturation in human cell lines: the glycolipid lipoarabinomannan (LAM) (Hmama et al., 2004; Kang et al., 2005; Welin et al., 2008) and the secreted tyrosine phosphatase (PtpA) (Bach et al., 2008; Wong et al., 2011; Wong and Jacobs, 2011). Mtb lacking the surface lipid trehalose dimycolate (TDM) failed to block phagosome maturation in mouse macrophages (Katti et al., 2008) but to date, this has not been shown in human cells. However, in humans, there is a polymorphism in the CLR for TDM CLECSF8 (MCL) that is associated with susceptibility to pulmonary TB (Wilson et al., 2015) and implicates TDM as an important virulence factor in human infection.
In vitro studies using electron microscopy (EM) and a fluorescence resonance energy transfer‐based method showed that Mtb also localizes in the cytosol in THP‐1 macrophages, primary human macrophages and primary human DCs (van der Wel et al., 2007; Houben et al., 2012; Simeone et al., 2012). The ESX‐1 type VII secretion system (T7SS) that is lacking from most of the non‐pathogenic mycobacterial strains (Abdallah et al., 2007) is required for Mtb localization in the cytosol. As part of the T7SS, ESAT‐6 protein is believed to make pores on cellular membranes (Hsu et al., 2003; Jonge et al., 2007; Wong and Jacobs, 2011). However, mechanistically, how ESAT‐6 lyses the phagosomal membrane in host cells is still unknown.
Although cytosolic localization of Mtb has been reported in vitro, less is known regarding the subcellular localization of Mtb in cells from patients with TB. EM studies in infected AMs isolated by bronchoalveolar lavage from infected individuals revealed that Mtb localizes primarily in membrane‐bound compartments (Russell et al., 2002; Mwandumba et al., 2004). The subcellular localization of Mtb in other cells in vivo and the physiological relevance of the cytosolic localization is far from clear (Harriff et al., 2012), but it is becoming increasingly apparent that Mtb‐infected cells are likely to have a mixed population of bacteria that are free in the cytosol or found in membrane‐bound compartments; this should be considered in future interpretation of experimental data and also in drug development.
Autophagy in the immune response to TB
Autophagy plays a crucial role in resistance to pathogens and has been implicated as an important innate defence mechanism in controlling and eliminating Mtb (Gutierrez et al., 2004). Whereas many studies highlighted the role of autophagy during Mtb infection in the mouse model, less is known about the autophagic response in human cells or in patients with active TB. Mtb is able to evade autophagy by inhibiting fusion of autophagosomes with lysosomes through the ESX‐1 secretion system in primary human DCs (Romagnoli et al., 2012) and by expressing miR‐30A in THP‐1 macrophages (Chen et al., 2015). Several studies have linked vitamin D deficiency with an increased risk for susceptibility to active TB (Martineau et al., 2007; Wejse et al., 2007; Nnoaham and Clarke, 2008). The active form of vitamin D3 induces autophagy in primary human monocytes and THP‐1 macrophages via the expression of the anti‐microbial peptide cathelicidin, which activates transcription of the autophagy‐related genes encoding Beclin‐1 and ATG5 (Yuk et al., 2009). In primary human macrophages and THP‐1 macrophages, active vitamin D3 also induces the localization of Mtb in autophagosomes in a cathelicidin‐dependent manner (Yuk et al., 2009).
IFN‐γ induces autophagy in response to Mtb antigens in patients with active TB (Rovetta et al., 2014). In human primary macrophages, the protective effect of IFN‐γ depends on the timing of addition, concentration and magnitude of the ensuing microbial challenge (Vogt and Nathan, 2011). Mtb factors able to interfere with IFN‐γ response include ESAT‐6, which inhibits production of IFN‐γ by Mtb‐responsive primary human‐stimulated CD3+ T cells (Wang et al., 2009) and sulfatides present on the outer surface of Mtb that blocks IFN‐γ or lipopolysaccharide priming in primary human monocytes (Pabst et al., 1988). LAM is also able to block the transcriptional activation of IFN‐γ‐inducible genes in human macrophage‐like cell lines (Chan et al., 1991).
IFN‐γ‐inducible GTPases
IFN‐γ‐induced autophagy is also required to control intracellular pathogens via members of the immunity‐related GTPase family (IRG proteins, formerly known as p47 GTPases) and by the 65 kDa guanylate binding protein family. Compared with the mouse genome (containing 23 IRG genes), the human genome contains only three IRG genes, encoding IRGC, IRGQ and IRGM, but these are not inducible by IFN‐γ. Polymorphisms in the IRGM gene, which is functional in humans, are associated with susceptibility to TB among African‐Americans (King et al., 2011), Ghanese (Intemann et al., 2009) and Chinese (Che et al., 2010) populations, providing evidence that IRG proteins contribute to the control of Mtb in humans. However, functional polymorphisms in both IRGM and the autophagy gene ATG16L1 did not have a major impact on Mtb‐induced cytokine production in healthy volunteers, although a moderate effect was observed on IFN‐γ production by the ATG16L1 T300A polymorphism (Kleinnijenhuis et al., 2011). IRGM and other autophagic markers such as LC3 and ATG16L1 are recruited to Mtb‐containing compartments by the activation of the innate immune receptor NOD2 in Mtb‐infected human AMs (Juárez et al., 2012). However, the precise mechanism by which this family of proteins control the cell autonomous response to Mtb is not known.
Host cell death in immunity
The mode of host cell death after Mtb infection is crucial for the outcome of the disease. Mtb induces necrosis, a death modality defined by cell lysis, and inhibits apoptosis, a form of death that maintains an intact plasma membrane and that enables control of bacterial replication. Several Mtb proteins inhibit apoptosis in human cells such as the serine/threonine kinase PknE (Jayakumar et al., 2008) and the Rv3364c protein (Danelishvili et al., 2011). Moreover, the Mtb proteins SecA2 and NuoG suppress THP‐1 macrophage apoptosis (Hinchey et al., 2007; Velmurugan et al., 2007; Miller et al., 2010).
Once in the cytosol, Mtb induces necrosis as a strategy used by virulent bacteria to avoid innate host defence. In primary human macrophages, Mtb induces necrosis by causing mitochondrial inner membrane disruption (Chen et al., 2006) and inhibiting the lysosomal and Golgi‐mediated plasma membrane repair (Divangahi et al., 2009). In T cells, the C‐terminal domain of the channel protein with necrosis‐inducing toxin induces necrotic death (Danilchanka et al., 2014). Induction of necrosis is also dependent on bacterial load and a functional ESX‐1 system. Indeed, primary human macrophages infected with a high burden of ESAT‐6‐expressing Mtb undergo Caspase‐1‐ and Cathepsin B‐independent necrosis (Welin et al., 2011).
In neutrophils, Mtb induces NETs, which contain DNA and several biologically active cytosolic and granular proteins (Braian et al., 2013). The formation of NETs plays an essential function in the innate immune defence against Mtb infection by trapping mycobacteria and thereby preventing spread to other organs (Braian et al., 2013). In vitro, this mechanism has been observed in human but not in mouse macrophages infected by Mtb (Wong and Jacobs, 2013). The formation of extracellular traps by primary human macrophages during Mtb infection is inducible by IFN‐γ and requires the ESX‐1 secretion system (Wong and Jacobs, 2013).
Reactive species and toxic metals
Another cell autonomous mechanism that controls intracellular Mtb consists of directly exposing mycobacteria to a toxic intracellular environment containing, e.g. reactive oxygen and nitrogen species (ROS and RNS) as well as toxic metals. In the murine model of Mtb infection, the importance of nitric oxide (NO) and RNS for the control of intracellular mycobacterial replication and disease is well established (Chan et al., 1992). In human macrophages, however, the role of NO is less clear. Phagocytes induce oxidative killing by production of ROS including superoxide and hydrogen peroxide. The generation of ROS requires assembly of the superoxide‐generating NADPH oxidase 2 (NOX2) complex at phagolysosomal membranes (Bylund et al., 2010). The role of ROS in anti‐mycobacterial immunity has been highlighted by the discovery of a mutation in the gene encoding the catalytic subunit gp91phox of NOX2 linked to TB susceptibility in patients (Bustamante et al., 2011). Several Mtb factors counteract the production of ROS. The ‘enhanced intracellular survival’ (eis) gene modulates host cell ROS generation (Shin et al., 2010). Mtb can also neutralize NOX2‐derived ROS via a NuoG‐dependent mechanism in order to inhibit TNF‐α‐mediated host cell apoptosis in primary human AMs (Miller et al., 2010).
Heavy metal poisoning is emerging as a very effective cell autonomous mechanism of bacterial elimination. Transcriptional studies show that during infection of primary human macrophages, Mtb faces a burst of free zinc, which accumulates within the mycobacterial phagosome (Botella et al., 2011). To counteract this mechanism, Mtb up‐regulates expression of the P‐type ATPase‐encoding gene ctpC, which regulates the intra‐bacterial levels of Zn2+ through efflux of the metal ion (Botella et al., 2011).
Conclusion
The host innate immune response to TB requires a variety of different host cell types to successfully protect the host from infection. Physical barriers and anti‐microbial substances are just as important as immune cells for protection. There are many different factors that influence the outcome of the initial battle between host and pathogen, including a variety of mechanisms that Mtb has evolved to subvert host defences. If Mtb is not killed by the innate immune response, it will replicate and disseminate and the host adaptive immune response will become critical for control. This review focuses solely on studies performed in humans or in human cells. The majority of the known innate mechanisms involved in Mtb infection have been studied in murine cells. Considering that there are key differences in host cell responses between humans and other animal models, one of the major challenges for the future will be to confirm the relevance of these findings in humans. Better understanding of the mechanisms involved in innate immunity in humans will enable us to develop improved treatments for TB.
Acknowledgements
This work was supported by the Francis Crick Institute, which receives its core funding principally from Cancer Research UK, the UK Medical Research Council (MC_UP_1202/11) and the Wellcome Trust.
Lerner, T. R. , Borel, S. , and Gutierrez, M. G. (2015), The innate immune response in human tuberculosis. Cell Microbiol, 17, 1277–1285. doi: 10.1111/cmi.12480.
References
- Abdallah, A.M. , Gey van Pittius, N.C. , Champion, P.A. , Cox, J. , Luirink, J. , Vandenbroucke‐Grauls, C.M. , et al (2007) Type VII secretion – mycobacteria show the way. Nat Rev Microbiol 5: 883–891. [DOI] [PubMed] [Google Scholar]
- Arcos, J. , Sasindran, S.J. , Fujiwara, N. , Turner, J. , Schlesinger, L.S. , and Torrelles, J.B. (2011) Human lung hydrolases delineate Mycobacterium tuberculosis‐macrophage interactions and the capacity to control infection. J Immunol 187: 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach, H. , Papavinasasundaram, K.G. , Wong, D. , Hmama, Z. , and Av‐Gay, Y. (2008) Mycobacterium tuberculosis virulence is mediated by PtpA dephosphorylation of human vacuolar protein sorting 33B. Cell Host Microbe 3: 316–322. [DOI] [PubMed] [Google Scholar]
- Botella, H. , Peyron, P. , Levillain, F. , Poincloux, R. , Poquet, Y. , Brandli, I. , et al (2011) Mycobacterial p(1)‐type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10: 248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braian, C. , Hogea, V. , and Stendahl, O. (2013) Mycobacterium tuberculosis‐ induced neutrophil extracellular traps activate human macrophages. J Innate Immun 5: 591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, A.E. , Holzer, T.J. , and Andersen, B.R. (1987) Capacity of human neutrophils to kill Mycobacterium tuberculosis . J Infect Dis 156: 985–989. [DOI] [PubMed] [Google Scholar]
- Bustamante, J. , Arias, A.A. , Vogt, G. , Picard, C. , Galicia, L.B. , Prando, C. , et al (2011) Germline CYBB mutations that selectively affect macrophages in kindreds with X‐linked predisposition to tuberculous mycobacterial disease. Nat Immunol 12: 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund, J. , Brown, K.L. , Movitz, C. , Dahlgren, C. , and Karlsson, A. (2010) Intracellular generation of superoxide by the phagocyte NADPH oxidase: how, where, and what for? Free Radic Biol Med 49: 1834–1845. [DOI] [PubMed] [Google Scholar]
- Chan, J. , Fan, X.D. , Hunter, S.W. , Brennan, P.J. , and Bloom, B.R. (1991) Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun 59: 1755–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, J. , Xing, Y. , Magliozzo, R.S. , and Bloom, B.R. (1992) Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med 175: 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che, N. , Li, S. , Gao, T. , Zhang, Z. , Han, Y. , Zhang, X. , et al (2010) Identification of a novel IRGM promoter single nucleotide polymorphism associated with tuberculosis. Clin Chim Acta 411: 1645–1649. [DOI] [PubMed] [Google Scholar]
- Chen, M. , Gan, H. , and Remold, H.G. (2006) A mechanism of virulence: virulent Mycobacterium tuberculosis strain H37Rv, but not attenuated H37Ra, causes significant mitochondrial inner membrane disruption in macrophages leading to necrosis. J Immunol 176: 3707–3716. [DOI] [PubMed] [Google Scholar]
- Chen, Z. , Wang, T. , Liu, Z. , Zhang, G. , Wang, J. , Feng, S. , and Liang, J. (2015) Mycobacteria tuberculosis induced miR‐30A inhibit autophagy process as a possible mechanism of immune escape in human macrophage. Jpn J Infect Dis. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Corleis, B. , Korbel, D. , Wilson, R. , Bylund, J. , Chee, R. , and Schaible, U.E. (2012) Escape of Mycobacterium tuberculosis from oxidative killing by neutrophils. Cell Microbiol 14: 1109–1121. [DOI] [PubMed] [Google Scholar]
- Danelishvili, L. , Everman, J.L. , McNamara, M.J. , and Bermudez, L.E. (2011). Inhibition of the plasma‐membrane‐associated serine protease cathepsin G by Mycobacterium tuberculosis Rv3364c suppresses caspase‐1 and pyroptosis in macrophages. Front Microbiol 2: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilchanka, O. , Sun, J. , Pavlenok, M. , Maueröder, C. , Speer, A. , Siroy, A. , et al (2014) An outer membrane channel protein of Mycobacterium tuberculosis with exotoxin activity. Proc Natl Acad Sci USA 111: 6750–6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis, M. (1991) Human neutrophils, activated with cytokines or not, do not kill virulent Mycobacterium tuberculosis . J Infect Dis 163: 919–920. [DOI] [PubMed] [Google Scholar]
- Divangahi, M. , Chen, M. , Gan, H. , Desjardins, D. , Hickman, T.T. , Lee, D.M. , et al (2009) Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat Immunol 10: 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum, S.Y. , Kong, J.H. , Hong, M.S. , Lee, Y.J. , Kim, J.H. , Hwang, S.H. , et al (2010) Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 137: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, J.S. , Voelker, D.R. , McCormack, F.X. , and Schlesinger, L.S. (1999) Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate‐lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J Immunol 163: 312–321. [PubMed] [Google Scholar]
- Fortin, A. , Abel, L. , Casanova, J.L. , and Gros, P. (2007) Host genetics of mycobacterial diseases in mice and men: forward genetic studies of BCG‐osis and tuberculosis. Annu Rev Genomics Hum Genet 8: 163–192. [DOI] [PubMed] [Google Scholar]
- Förtsch, D. , Röllinghoff, M. , and Stenger, S. (2000) IL‐10 converts human dendritic cells into macrophage‐like cells with increased antibacterial activity against virulent Mycobacterium tuberculosis . J Immunol 165: 978–987. [DOI] [PubMed] [Google Scholar]
- Gaynor, C.D. , McCormack, F.X. , Voelker, D.R. , McGowan, S.E. , and Schlesinger, L.S. (1995) Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J Immunol 155: 5343–5351. [PubMed] [Google Scholar]
- Gold, M.C. , Cerri, S. , Smyk‐Pearson, S. , Cansler, M.E. , Vogt, T.M. , Delepine, J. , et al (2010) Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol 8: e1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez, M.G. , Master, S.S. , Singh, S.B. , Taylor, G.A. , Colombo, M.I. , and Deretic, V. (2004) Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119: 753–766. [DOI] [PubMed] [Google Scholar]
- Hanekom, W.A. , Mendillo, M. , Manca, C. , Haslett, P.A. , Siddiqui, M.R. , Barry, C. , and Kaplan, G. (2003) Mycobacterium tuberculosis inhibits maturation of human monocyte‐derived dendritic cells in vitro. J Infect Dis 188: 257–266. [DOI] [PubMed] [Google Scholar]
- Harriff, M.J. , Purdy, G.E. , and Lewinsohn, D.M. (2012). Escape from the phagosome: the explanation for mhc‐i processing of mycobacterial antigens? Front Immunol 3: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harriff, M.J. , Cansler, M.E. , Toren, K.G. , Canfield, E.T. , Kwak, S. , Gold, M.C. , and Lewinsohn, D.M. (2014) Human lung epithelial cells contain Mycobacterium tuberculosis in a late endosomal vacuole and are efficiently recognized by CD8+ T cells. PLoS ONE 9: e97515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, R.A. , Watkins, S.C. , and Flynn, J.L. (1997) Activation of human dendritic cells following infection with Mycobacterium tuberculosis . J Immunol 159: 635–643. [PubMed] [Google Scholar]
- Hernández‐Pando, R. , Jeyanathan, M. , Mengistu, G. , Aguilar, D. , Orozco, H. , Harboe, M. , et al (2000) Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet 356: 2133–2138. [DOI] [PubMed] [Google Scholar]
- Hinchey, J. , Lee, S. , Jeon, B.Y. , Basaraba, R.J. , Venkataswamy, M.M. , Chen, B. , et al (2007) Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis . J Clin Invest 117: 2279–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hmama, Z. , Sendide, K. , Talal, A. , Garcia, R. , Dobos, K. , and Reiner, N.E. (2004) Quantitative analysis of phagolysosome fusion in intact cells: inhibition by mycobacterial lipoarabinomannan and rescue by an 1alpha,25‐dihydroxyvitamin D3‐phosphoinositide 3‐kinase pathway. J Cell Sci 117: 2131–2140. [DOI] [PubMed] [Google Scholar]
- Houben, D. , Demangel, C. , van Ingen, J. , Perez, J. , Baldeón, L. , Abdallah, A.M. , et al (2012) ESX‐1‐mediated translocation to the cytosol controls virulence of mycobacteria. Cell Microbiol 14: 1287–1298. [DOI] [PubMed] [Google Scholar]
- Hsu, T. , Hingley‐Wilson, S.M. , Chen, B. , Chen, M. , Dai, A.Z. , Morin, P.M. , et al (2003) The primary mechanism of attenuation of bacillus Calmette‐Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci USA 100: 12420–12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intemann, C.D. , Thye, T. , Niemann, S. , Browne, E.N.L. , Amanua Chinbuah, M. , Enimil, A. , et al (2009) Autophagy gene variant IRGM‐261T contributes to protection from tuberculosis caused by Mycobacterium tuberculosis but not by M. africanum strains. PLoS Pathog 5: e1000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismaili, J. , Olislagers, V. , Poupot, R. , Fournié, J.J. , and Goldman, M. (2002) Human gamma delta T cells induce dendritic cell maturation. Clin Immunol 103: 296–302. [DOI] [PubMed] [Google Scholar]
- Jayakumar, D. , Jacobs, W.R. , and Narayanan, S. (2008) Protein kinase E of Mycobacterium tuberculosis has a role in the nitric oxide stress response and apoptosis in a human macrophage model of infection. Cell Microbiol 10: 365–374. [DOI] [PubMed] [Google Scholar]
- Jonge, M.I.D. , Pehau‐Arnaudet, G. , Fretz, M.M. , Romain, F. , Bottai, D. , Brodin, P. , et al (2007) ESAT‐6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP‐10 under acidic conditions and exhibits membrane‐lysing activity. J Bacteriol 189: 6028–6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez, E. , Carranza, C. , Hernández‐Sánchez, F. , León‐Contreras, J.C. , Hernández‐Pando, R. , Escobedo, D. , et al (2012) NOD2 enhances the innate response of alveolar macrophages to Mycobacterium tuberculosis in humans. Eur J Immunol 42: 880–889. [DOI] [PubMed] [Google Scholar]
- Kang, P.B. , Azad, A.K. , Torrelles, J.B. , Kaufman, T.M. , Beharka, A. , Tibesar, E. , et al (2005) The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan‐mediated phagosome biogenesis. J Exp Med 202: 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katti, M.K. , Dai, G. , Armitige, L.Y. , Rivera Marrero, C. , Daniel, S. , Singh, C.R. , et al (2008) The Delta fbpA mutant derived from Mycobacterium tuberculosis H37Rv has an enhanced susceptibility to intracellular antimicrobial oxidative mechanisms, undergoes limited phagosome maturation and activates macrophages and dendritic cells. Cell Microbiol 10: 1286–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, K.Y. , Lew, J.D. , Ha, N.P. , Lin, J.S. , Ma, X. , Graviss, E.A. , and Goodell, M.A. (2011) Polymorphic allele of human IRGM1 is associated with susceptibility to tuberculosis in African Americans. PLoS ONE 6: e16317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinnijenhuis, J. , Oosting, M. , Plantinga, T.S. , van der Meer, J.W.M. , Joosten, L.A.B. , Crevel, R.V. , and Netea, M.G. (2011) Autophagy modulates the Mycobacterium tuberculosis‐induced cytokine response. Immunology 134: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Wang, Y. , and Liu, X. (2012) The role of airway epithelial cells in response to mycobacteria infection. Clin Dev Immunol 2012: 791392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugton, I. (1999) Mucosa‐associated lymphoid tissues as sites for uptake, carriage and excretion of tubercle bacilli and other pathogenic mycobacteria. Immunol Cell Biol 77: 364–372. [DOI] [PubMed] [Google Scholar]
- Marino, S. , Pawar, S. , Fuller, C.L. , Reinhart, T.A. , Flynn, J.L. , and Kirschner, D.E. (2004) Dendritic cell trafficking and antigen presentation in the human immune response to Mycobacterium tuberculosis . J Immunol 173: 494–506. [DOI] [PubMed] [Google Scholar]
- Martineau, A.R. , Honecker, F.U. , Wilkinson, R.J. , and Griffiths, C.J. (2007) Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol 103: 793–798. [DOI] [PubMed] [Google Scholar]
- Middleton, A.M. , Chadwick, M.V. , Nicholson, A.G. , Dewar, A. , Groger, R.K. , Brown, E.J. , et al (2002) Interaction of Mycobacterium tuberculosis with human respiratory mucosa. Tuberculosis (Edinb) 82: 69–78. [DOI] [PubMed] [Google Scholar]
- Miller, J.L. , Velmurugan, K. , Cowan, M.J. , and Briken, V. (2010) The type I NADH dehydrogenase of Mycobacterium tuberculosis counters phagosomal NOX2 activity to inhibit TNF‐α‐mediated host cell apoptosis. PLoS Pathog 6: e1000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison, J. , Pai, M. , and Hopewell, P.C. (2008) Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low‐income and middle‐income countries: a systematic review and meta‐analysis. Lancet Infect Dis 8: 359–368. [DOI] [PubMed] [Google Scholar]
- Mwandumba, H.C. , Russell, D.G. , Nyirenda, M.H. , Anderson, J. , White, S.A. , Molyneux, M.E. , and Squire, S.B. (2004) Mycobacterium tuberculosis resides in nonacidified vacuoles in endocytically competent alveolar macrophages from patients with tuberculosis and HIV infection. J Immunol 172: 4592–4598. [DOI] [PubMed] [Google Scholar]
- Nnoaham, K.E. , and Clarke, A. (2008) Low serum vitamin D levels and tuberculosis: a systematic review and meta‐analysis. Int J Epidemiol 37: 113–119. [DOI] [PubMed] [Google Scholar]
- Pabst, M.J. , Gross, J.M. , Brozna, J.P. , and Goren, M.B. (1988) Inhibition of macrophage priming by sulfatide from Mycobacterium tuberculosis . J Immunol 140: 634–640. [PubMed] [Google Scholar]
- Riedel, D.D. , and Kaufmann, S.H. (1997) Chemokine secretion by human polymorphonuclear granulocytes after stimulation with Mycobacterium tuberculosis and lipoarabinomannan. Infect Immun 65: 4620–4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas‐Santiago, B. , Schwander, S.K. , Sarabia, C. , Diamond, G. , Klein‐Patel, M.E. , Hernandez‐Pando, R. , et al (2005) Human {beta}‐defensin 2 is expressed and associated with Mycobacterium tuberculosis during infection of human alveolar epithelial cells. Infect Immun 73: 4505–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas‐Santiago, B. , Hernandez‐Pando, R. , Carranza, C. , Juarez, E. , Contreras, J.L. , Aguilar‐Leon, D. , et al (2008) Expression of cathelicidin LL‐37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect Immun 76: 935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli, A. , Etna, M.P. , Giacomini, E. , Pardini, M. , Remoli, M.E. , Corazzari, M. , et al (2012) ESX‐1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy 8: 1357–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovetta, A.I. , Peña, D. , Hernández Del Pino, R.E. , Recalde, G.M. , Pellegrini, J. , Bigi, F. , et al (2014) IFNG‐mediated immune responses enhance autophagy against Mycobacterium tuberculosis antigens in patients with active tuberculosis. Autophagy 10: 2109–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, D.G. , Mwandumba, H.C. , and Rhoades, E.E. (2002) Mycobacterium and the coat of many lipids. J Cell Biol 158: 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, D.‐M. , Jeon, B.‐Y. , Lee, H.‐M. , Jin, H.S. , Yuk, J.‐M. , Song, C.‐H. , et al (2010) Mycobacterium tuberculosis eis regulates autophagy, inflammation, and cell death through redox‐dependent signaling. PLoS Pathog 6: e1001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone, R. , Bobard, A. , Lippmann, J. , Bitter, W. , Majlessi, L. , Brosch, R. , and Enninga, J. (2012) Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog 8: e1002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sow, F.B. , Nandakumar, S. , Velu, V. , Kellar, K.L. , Schlesinger, L.S. , Amara, R.R. , et al (2011) Mycobacterium tuberculosis components stimulate production of the antimicrobial peptide hepcidin. Tuberculosis (Edinb) 91: 314–321. [DOI] [PubMed] [Google Scholar]
- Sutherland, J.S. , Jeffries, D.J. , Donkor, S. , Walther, B. , Hill, P.C. , Adetifa, I.M. , et al (2009) High granulocyte/lymphocyte ratio and paucity of NKT cells defines TB disease in a TB‐endemic setting. Tuberculosis (Edinb) 89: 398–404. [DOI] [PubMed] [Google Scholar]
- Tailleux, L. , Neyrolles, O. , Honoré‐Bouakline, S. , Perret, E. , Sanchez, F. , Abastado, J.P. , et al (2003) Constrained intracellular survival of Mycobacterium tuberculosis in human dendritic cells. J Immunol 170: 1939–1948. [DOI] [PubMed] [Google Scholar]
- Tan, B.H. , Meinken, C. , Bastian, M. , Bruns, H. , Legaspi, A. , Ochoa, M.T. , et al (2006) Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J Immunol 177: 1864–1871. [DOI] [PubMed] [Google Scholar]
- Thomas, E.D. , Ramberg, R.E. , Sale, G.E. , Sparkes, R.S. , and Golde, D.W. (1976) Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science 192: 1016–1018. [DOI] [PubMed] [Google Scholar]
- Vankayalapati, R. , and Barnes, P.F. (2009) Innate and adaptive immune responses to human Mycobacterium tuberculosis infection. Tuberculosis (Edinb) 89 (Suppl. 1): S77–S80. [DOI] [PubMed] [Google Scholar]
- Vankayalapati, R. , Wizel, B. , Weis, S.E. , Safi, H. , Lakey, D.L. , Mandelboim, O. , et al (2002) The NKp46 receptor contributes to NK cell lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol 168: 3451–3457. [DOI] [PubMed] [Google Scholar]
- Vankayalapati, R. , Garg, A. , Porgador, A. , Griffith, D.E. , Klucar, P. , Safi, H. , et al (2005) Role of NK cell‐activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol 175: 4611–4617. [DOI] [PubMed] [Google Scholar]
- Velmurugan, K. , Chen, B. , Miller, J.L. , Azogue, S. , Gurses, S. , Hsu, T. , et al (2007) Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog 3: e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrall, A.J. , Netea, M.G. , Alisjahbana, B. , Hill, P.C. , and van Crevel, R. (2014) Early clearance of Mycobacterium tuberculosis: a new frontier in prevention. Immunology 141: 506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt, G. , and Nathan, C. (2011) In vitro differentiation of human macrophages with enhanced antimycobacterial activity. J Clin Invest 121: 3889–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Barnes, P.F. , Dobos‐Elder, K.M. , Townsend, J.C. , Chung, Y.‐T. , Shams, H. , et al (2009) ESAT‐6 inhibits production of IFN‐γ by Mycobacterium tuberculosis‐responsive human T cells. J Immunol 182: 3668–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wejse, C. , Olesen, R. , Rabna, P. , Kaestel, P. , Gustafson, P. , Aaby, P. , et al (2007) Serum 25‐hydroxyvitamin D in a West African population of tuberculosis patients and unmatched healthy controls. Am J Clin Nutr 86: 1376–1383. [DOI] [PubMed] [Google Scholar]
- van der Wel, N. , Hava, D. , Houben, D. , Fluitsma, D. , van Zon, M. , Pierson, J. , et al (2007) M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 129: 1287–1298. [DOI] [PubMed] [Google Scholar]
- Welin, A. , Winberg, M.E. , Abdalla, H. , Särndahl, E. , Rasmusson, B. , Stendahl, O. , and Lerm, M. (2008) Incorporation of Mycobacterium tuberculosis lipoarabinomannan into macrophage membrane rafts is a prerequisite for the phagosomal maturation block. Infect Immun 76: 2882–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welin, A. , Eklund, D. , Stendahl, O. , and Lerm, M. (2011) Human macrophages infected with a high burden of ESAT‐6‐expressing M. tuberculosis undergo caspase‐1‐ and cathepsin B‐independent necrosis. PLoS ONE 6: e20302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, G.J. , Marakalala, M.J. , Hoving, J.C. , van Laarhoven, A. , Drummond, R.A. , Kerscher, B. , et al (2015) The C‐type lectin receptor CLECSF8/CLEC4D is a key component of anti‐mycobacterial immunity. Cell Host Microbe 17: 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, D. , Bach, H. , Sun, J. , Hmama, Z. , and Av‐Gay, Y. (2011) Mycobacterium tuberculosis protein tyrosine phosphatase (PtpA) excludes host vacuolar‐H+‐ATPase to inhibit phagosome acidification. Proc Natl Acad Sci USA 108: 19371–19376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, K.‐W. , and Jacobs, W.R., Jr (2011) Critical role for NLRP3 in necrotic death triggered by Mycobacterium tuberculosis . Cell Microbiol 13: 1371–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, K.W. , and Jacobs, W.R., Jr (2013) Mycobacterium tuberculosis exploits human interferon gamma to stimulate macrophage extracellular trap formation and necrosis. J Infect Dis 208: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk, J.‐M. , Shin, D.‐M. , Lee, H.‐M. , Yang, C.‐S. , Jin, H.S. , Kim, K.‐K. , et al (2009) Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 6: 231–243. [DOI] [PubMed] [Google Scholar]


