Abstract
BACKGROUND
Patient decision aids (P‐DAs) inform medical decision making, but longer term effects are unknown. This article describes extended follow‐up from a thyroid cancer treatment P‐DA trial.
METHODS
In this single‐center, parallel‐design randomized controlled trial conducted at a Canadian tertiary/quaternary care center, early‐stage thyroid cancer patients from a P‐DA trial were contacted 15 to 23 months after randomization/radioactive iodine (RAI) decision making to evaluate longer term outcomes. It was previously reported that the use of the computerized P‐DA in thyroid cancer patients considering postsurgical RAI treatment significantly improved medical knowledge in comparison with usual care alone. The P‐DA and control groups were compared for the following outcomes: feeling informed about the RAI treatment choice, decision satisfaction, decision regret, cancer‐related worry, and physician trust. In a subgroup of 20 participants, in‐depth interviews were conducted for a qualitative analysis.
RESULTS
Ninety‐five percent (70 of 74) of the original population enrolled in follow‐up at a mean of 17.1 months after randomization. P‐DA users perceived themselves to be significantly more 1) informed about the treatment choice (P = .008), 2) aware of options (P = .009), 3) knowledgeable about treatment benefits (P = .020), and 4) knowledgeable about treatment risks/side effects (P = .001) in comparison with controls. There were no significant group differences in decision satisfaction (P = .142), decision regret (P = .199), cancer‐related worry (P = .645), mood (P = .211), or physician trust (P = .764). In the qualitative analysis, the P‐DA was perceived to have increased patient knowledge and confidence in decision making.
CONCLUSIONS
The P‐DA improved cancer survivors’ actual and long‐term perceived medical knowledge with no adverse effects. More research on the long‐term outcomes of P‐DA use is needed. Cancer 2015;121:3717–3726. © 2015 American Cancer Society.
Keywords: decision aids, decision making, decision support techniques, iodine radioisotopes, patient satisfaction, qualitative research, thyroid cancer
Short abstract
In this extended follow‐up study of a randomized controlled trial of a thyroid cancer treatment patient decision aid, patient decision aid users perceive themselves to be significantly more informed about the choice of radioactive iodine treatment, although their decision satisfaction and regret are not significantly different from those of controls not exposed to the patient decision aid. Patient decision aid utilization has no significant negative impact on cancer‐related worry, mood, or trust in the treating physician in long‐term follow‐up.
INTRODUCTION
According to the most recent statistics, the number of individuals diagnosed annually with thyroid cancer is approximately 289,000 worldwide,1 including 63,000 Americans,2 6000 Canadians,3 and 53,000 Europeans.4 Furthermore, the incidence of thyroid cancer is rising faster than that of any other malignancy in the United States and Canada.2, 3 Approximately two‐thirds of thyroid cancers are diagnosed at an early stage,2 and the 5‐year survival rate for early‐stage disease approaches 100%.2 Because of the excellent prognosis of early‐stage differentiated thyroid cancer, it is important for patients and their health care providers to carefully weigh the potential long‐term benefits and risks of associated treatments. However, there is a paucity of randomized controlled trials (RCTs) to guide evidence‐based radioactive iodine (RAI) treatment decision making.
We recently developed and tested a patient‐directed computerized decision aid for thyroid cancer (patient decision aid [P‐DA]), to be used as an adjunct to physician counseling, in explaining the risks, benefits, evidence uncertainties, and implications of the decision to accept or reject postsurgical RAI treatment (also known as RAI remnant ablation) for early‐stage papillary thyroid cancer.5, 6, 7, 8, 9, 10 RAI remnant ablation after total thyroidectomy may facilitate thyroid cancer disease surveillance, but its long‐term cancer outcome benefits are unclear because of a lack of long‐term RCTs and conflicting observational evidence. An important component of our P‐DA is that it provides an explicit explanation of evidence uncertainty related to the potential therapeutic benefit of RAI for long‐term cancer outcomes (ie, thyroid cancer–related mortality and recurrence).5, 6, 7, 8, 9, 10 In an RCT of patients with early‐stage thyroid cancer, compared with usual care, our P‐DA significantly increased patients’ medical knowledge and reduced decisional conflict at the time of RAI treatment decision making.9 However, the longer term effects of this and other decision P‐DAs are not well understood because P‐DA trials typically do not follow patients far beyond the period of decision making.
In this follow‐up study, our aim was to explore the impact of our thyroid cancer P‐DA on patients’ decision and psychosocial outcomes at longer term follow‐up (ie, after the completion of decision making) with complementary quantitative and qualitative approaches.
MATERIALS AND METHODS
Trial Design
As previously reported, we conducted a parallel‐design, single‐center RCT of consenting adult patients with early‐stage papillary thyroid cancer, who were randomly assigned to either a 1‐time viewing of a computerized P‐DA (with usual care) or no P‐DA exposure (usual care alone).8, 9 This trial was registered at ClinicalTrials.gov (NCT01083550). The trial was conducted at the University Health Network, a tertiary/quaternary care center for thyroid cancer, and it was approved by the research ethics board of the University Health Network. Informed consent was obtained for participation. Trial results were reported in accordance with Consolidated Standards of Reporting Trials guidelines.11
Participant Eligibility Criteria
Potentially eligible patient participants in the extended follow‐up study included those who had participated in the original RCT.8, 9 Specifically, they were adults who were 18 years old or older, had undergone total thyroidectomy (completed in 1 or 2 stages) on or after September 1, 2009, and had been diagnosed with early‐stage (low‐risk) papillary thyroid cancer according to a review of their original surgical pathology report (a primary tumor measuring 1 to 4 cm, no known positive lymph nodes or distant metastases, no extrathyroidal tumor extension, no vascular or lymphatic invasion, and no tall cell variant; ie, American Joint Committee on Cancer stage 1 or 2 in the absence of distant metastases12 and American Thyroid Association “low risk of recurrence” classification13). Participants had not received RAI treatment before randomization, and all were taking thyroid hormone. Participants were fluent in spoken and written English and were able to use a computer. The original trial recruitment was between March 2010 and June 2011.9 Contact for the extended follow‐up study was initiated by telephone approximately 15 to 23 months after randomization. This time point was selected to allow sufficient time to establish initial thyroid cancer outcomes and for acute side effects of RAI treatment side (if received) to resolve and yet be sufficiently close to the time of decision making to allow meaningful feedback and recollection about the experience. We excluded individuals diagnosed with thyroid cancer other than papillary thyroid cancer and those for whom a pathologic diagnosis could not be confirmed.8, 9
Intervention
Participants received their usual care and counseling from their respective treating physicians.8, 9 Participants who were randomized to the intervention arm self‐navigated the P‐DA Web site on a desktop personal computer once (up to 60 minutes) in a research office.8, 9 P‐DA access was password‐protected and was prohibited outside the study. There was no harm reported during the original trial9 or during the extended follow‐up.
Outcomes
In the extended follow‐up study, quantitative questionnaires were administered by a research assistant to patient participants who gave verbal consent for their participation (over the telephone). The questionnaires included an update of the thyroid cancer status and treatments, a questionnaire on feeling informed about the RAI treatment choice,14 a decision satisfaction questionnaire,14 a decision regret questionnaire,15, 16 a questionnaire measuring cancer‐related worry (Assessment of Survivor Concerns),17, 18 a screening mood questionnaire (Patient Health Questionnaire for Depression and Anxiety 4),19, 20 and a physician trust questionnaire.21 Verbal responses were recorded on a paper data form by the research assistant and were tabulated with Microsoft Excel. The thyroid cancer outcomes and associated treatments were verified by medical chart review for consenting participants.
The qualitative study comprised individual in‐person, in‐depth interviews exploring patients’ experiences with RAI treatment decision making and the overall thyroid cancer treatment. We performed stratified purposive sampling of participants for participation in the qualitative study22 to ensure sufficient representation of relevant demographic and clinical characteristics, including study group allocation. Criteria used in the purposive sampling included sex, group allocation (P‐DA or control), and RAI treatment status (receiving RAI or not) with the intention of achieving a subgroup with proportions representative of the original randomized trial sample. A researcher experienced in qualitative methods conducted the interviews, which were audio‐recorded and transcribed verbatim. Interview questions were designed to uncover patients’ perceptions in the following overarching areas: the process of RAI treatment decision making and the overall treatment experience.
Randomization, Allocation Concealment, Implementation, and Blinding
We performed central, computerized randomization with a 1:1 ratio at the patient level with variable block sizes of 2 and 4 (the block sizes were designed by a statistician).9 Randomization, revelation of allocation to participants, and intervention administration were performed during the same visit (with no revealing of allocation before the randomization) under the direction of a research assistant. There was no blinding in the follow‐up phase of this study.
Statistical Methods and Sample Size Considerations
Quantitative questionnaire analyses
For descriptive analyses, numbers and percentages were used to express categorical outcome data, and means and standard deviations (SDs) or ranges were used to describe continuous data. Quantitative questionnaires were scored according to the developers’ methods, and missing data for any questionnaire subscales were imputed with the mean of the remaining quantitative responses within that subscale (for questionnaires with predefined subscales). Individuals who did not consent to the extended follow‐up study were not included in the analyses. Independent (Welch) 2‐sample t tests were performed to compare continuous outcome questionnaire scores or Likert scale outcome data between the P‐DA and control groups. An α value of .05 was used as the cutoff for statistical significance for all statistical analyses. Quantitative statistical analyses were performed with PASW Statistics 18.0 (IBM, Chicago, Ill). As previously reported, this study was designed to be powered for the comparison of the short‐term outcome of medical knowledge at the time of medical decision making,9 so all outcomes evaluated in the extended follow‐up study should be considered secondary and hypothesis‐generating. By nature of the design of the extended follow‐up after the initiation of the original trial, only a convenience sample of participants from the original trial (maximum, 74) could be enrolled.
Qualitative analyses
A content analysis of the qualitative data collected during the individual interviews was completed manually by a researcher experienced in qualitative methods. The major themes were identified in transcripts with a grounded theory approach.23, 24, 25 Data analysis techniques to ensure the analytic rigor of qualitative data included checking, questioning, and theorizing.26 Once detailed coding was completed, patterns that were judged to be meaningful were categorized and reported.27, 28 The qualitative researcher reviewed extracted themes and related quotes with a clinical content expert (A.M.S.) for further clarification of concepts. Purposive participant sampling was continued until saturation of themes was achieved. An estimated sample size of approximately 20 to 25 participants was considered sufficient for in‐depth interview qualitative inquiry.29
RESULTS
Participant Characteristics
The acceptance rate for participation in the extended follow‐up study was 95% (70 of 74), and the participants included 34 from the P‐DA group and 36 from the control group (Fig. 1). Furthermore, 98.6% (69 of 70) of the participants in the extended follow‐up study provided permission for medical record review to confirm their thyroid cancer status and treatments. Fifty‐eight of these 70 participants were women (82.9%), 34 were in the P‐DA group (48.6%), and 17 had received RAI treatment (24.3%; 10 in the P‐DA group and 8 in the control group). The mean age was 47.1 years (SD, 12.3 years). The mean timing of participation in the extended follow‐up was 17.1 months after randomization (SD, 2.2 months) and 19.9 months (SD, 3.0 months) after first thyroid cancer surgery. The characteristics of the participants according to allocation are shown in Table 1. The subgroup of participants in the qualitative study included 20 individuals: 50% (10 of 20) had been exposed to the P‐DA, 85.0% (17 of 20) were female, the mean age was 48.4 years (SD, 13.5 years), and they were interviewed a mean of 17.8 months after randomization (SD, 2.7 months) and a mean of 20.7 months after their first thyroid cancer surgery (SD, 3.8 months). Approximately half of the patients in the respective P‐DA and control groups had been treated with RAI (ie, 5 of 10 participants in the P‐DA group and 4 of 10 in the control group).
Figure 1.
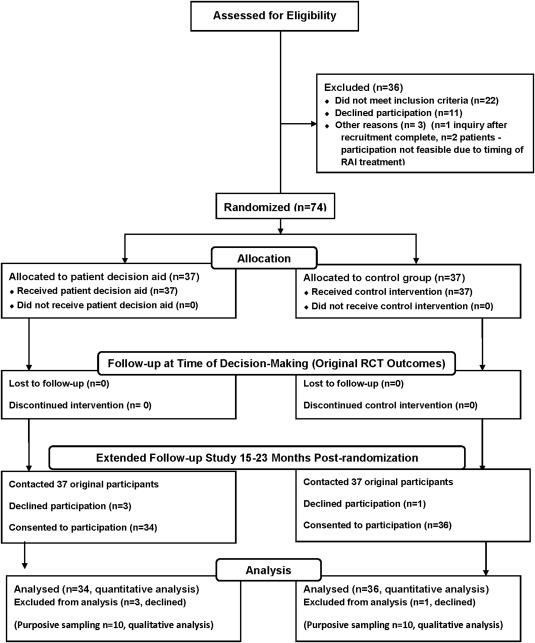
Participant flow in the study. RAI indicates radioactive iodine; RCT, randomized controlled study.
Table 1.
Characteristics of the Participants in the Follow‐Up Study
| Characteristic | Patient Decision Aid Group (n = 34) | Control Group (n = 36) | |
|---|---|---|---|
| Female sex, No. (%) | 28 (82.4) | 30 (83.3) | |
| Age, mean (SD), y | 48.8 (12.7) | 45.5 (11.8) | |
| Current marital status, No. (%) | Single | 10 (29.4) | 4 (11.1) |
| Married or common‐law | 22 (64.7) | 30 (83.3) | |
| Divorced or separated | 2 (5.9) | 2 (5.6) | |
| Highest level of education currently completed, No. (%) | High school or lower | 3 (8.8) | 3 (8.3) |
| College or university | 24 (70.6) | 18 (50.0) | |
| Postgraduate | 7 (20.6) | 15 (41.7) | |
| AJCC pathologic stage, No. (%)a | Stage 1 | 22 (64.7) | 30 (83.3) |
| Stage 2 | 12 (35.3) | 6 (16.7) | |
| Time since first thyroid cancer surgery (thyroidectomy), mean (SD), mo | 19.7 (2.7) | 20.0 (3.2) | |
| Time since randomization, mean (SD), mo | 16.9 (2.1) | 17.3 (2.4) | |
| Postsurgical radioactive iodine taken, No. (%) | 10 (29.4) | 8 (22.2) | |
| Treated disease recurrence, No. (%) | 0 (0) | 1 (2.8)b | |
Abbreviations: AJCC, American Joint Committee on Cancer; SD, standard deviation.
The AJCC staging system for thyroid cancer was used (AJCC Cancer Staging Manual, 7th ed).12 None of the patients had distant metastatic disease at their primary diagnosis. All patients were low‐risk according to the American Thyroid Association's recurrence risk classification system.13
One individual underwent reoperation of the neck for disease recurrence in central neck lymph nodes, and he was awaiting surgery at the time of the follow‐up; surgical pathology confirmed nodal recurrence in the central neck. Another individual underwent reoperation of the neck for what was found to be a benign thyroglossal cyst (no recurrent cancer was found, and this surgery had been completed before the time of the interview). Both of these individuals had no exposure to the patient decision aid and had received postsurgical radioactive iodine (ie, before the reoperation). The individual with neck nodal recurrence received another dose of radioactive iodine after his recurrence had been surgically treated.
Quantitative Questionnaire Outcomes
Patients in the P‐DA group felt significantly more informed about the RAI cancer treatment choice than the usual‐care control group, as reflected by the following measures: 1) perception of having made an informed RAI treatment choice (P = .008), 2) awareness of the treatment choices for managing their cancer (P = .009), 3) knowledge of the benefits of RAI treatment for their cancer (P = .020), and 4) knowledge of the risks and side effects of RAI treatment for their cancer (P = .001; Table 2). Furthermore, when we summed the results of the feeling‐informed questionnaire, the overall mean score was significantly superior in the P‐DA group (mean, 18.3; SD, 1.8 [where 4 is worst and 20 is best]) versus the controls (mean, 15.6; SD, 4.8; P = .003). There were no significant differences between the P‐DA and no–P‐DA groups in the following outcomes: decision satisfaction (P = .142), decision regret (P = .199), cancer‐related worry (Assessment of Survivor Concerns; P = .645), mood (P = .211), and trust in the treating physician (P = .764; Table 2). Furthermore, there was no significant difference between groups for any of the respective subscale measures of cancer worry (P = .718) or health worry (0.191) of the Assessment of Survivor Concerns questionnaire. Also, there was no significant difference between groups for any of the respective anxiety (P = .107), worry (P = .251), interest (P = .912), or depression measures (P = .286) of the Patient Health Questionnaire for Depression and Anxiety 4 mood screening questionnaire.
Table 2.
Quantitative Questionnaire Comparison of the Patient Decision Aid Group and the Controls
| Characteristic | Patient Decision Aid Group (n = 34), Mean Score (SD) | Control Group (n = 36), Mean Score (SD) | P | |
|---|---|---|---|---|
| Feeling‐of‐being informed questionnaire (range, 1 [worst] to 5 [best]) | Made informed choice | 4.6 (0.5) | 4.0 (1.3) | .008 |
| Aware of treatment choices | 4.7 (0.5) | 4.0 (1.3) | .009 | |
| Knew treatment benefits | 4.5 (0.6) | 3.9 (1.2) | .020 | |
| Knew risks and side effects | 4.5 (0.7) | 3.6 (1.3) | .001 | |
| Decision satisfaction (range, 1 [worst] to 5 [best]) | 4.5 (0.7) | 4.2 (1.2) | .142 | |
| Decision regret (range, 0 [best] to 100 [worst]) | 12.7 (13.4) | 18.5 (23.1) | .199 | |
| Cancer‐related worry (Assessment of Survivor Concerns; range, 6 [best] to 24 [worst]) | 12.4 (4.3) | 12.9 (4.6) | .645 | |
| Mood (PHQ4; (range, 0 [best] to 12 [worst]) | 1.2 (1.6) | 1.8 (2.2) | .211 | |
| Trust in physician (range, 0 [worst] to 100 [best]) | 71.5 (16.2)a | 72.6 (14.3) | .764 | |
Abbreviations: PHQ‐4, Patient Health Questionnaire for Depression and Anxiety 4; SD, standard deviation.
Thirty‐three participants.
Qualitative Study Results
In the qualitative study, which included a subgroup of 20 representative participants, 4 main themes emerged: 1) the complexity of RAI treatment decision making along with the importance of physician counseling in affecting the ultimate treatment decision, 2) the value of study participation, 3) information needs and the necessity of human contact to meet psychosocial support needs, and 4) overall thyroid cancer treatment experience/satisfaction. A summary of these themes and representative quotations are shown in Table 3.
Table 3.
Main Themes From the Qualitative Study With Representative Quotations
| Theme | Representative Quotations |
|---|---|
| Complexity of RAI treatment decision making, importance of physician recommendations |
Patient decision aid group Information sources, importance of physician counseling “I would say it was a combination, but I weighted with what the doctors said probably most heavily.” “And based on what the doctor said to me, and what I actually read myself, and then I did some research myself, just generally on what it was all about. I was able to make my decision.” Patient involvement in decision making “I felt fairly involved ‘cause I had a lot of questions, and concerns, and they were all answered. So it was like once my mind was set at ease that it was the right decision to make. I felt okay making it.” “Basically went through the pro's and con's and from that I was fairly confident in saying to go ahead with it.” “I always feel you go away saying it has to be your decision, but you're not really the medical person, so it's always a negative kind of aspect” |
|
Control Group Information sources, importance of physician counseling “Pretty much it had to do with the doctor explaining to me what state my cancer was in and whether he thought I required more or less, and I asked him a few questions about it.” “The positive aspect was, you know, I felt that I was in good hands … only a doctor would have the right knowledge to make a decision. So still you have to rely and trust the doctors.” Patient involvement in decision making “I was totally involved, because I knew what was involved, and I knew from my own research, from what I've heard, that I was making the right decision.” “I didn't have a choice, it was just the doctor thought … I took his opinion and I figured he knew more than I did about it.” “Part of not having a choice is slightly liberating because you don't feel like you can make a wrong decision, because you're not making the decision.” “I kind of thought well, I'll just do whatever they say, because ‘they [physicians] know’. And I'll just do whatever they say and that's what I'll do. But then when I was faced with two doctors who said completely different things, for the first time I thought, well I don't know what to do because they're both saying different things … But I think it brought a human nature into healthcare for me, because I could see my doctors as people using information to make decisions. Once I could see myself as being part of the team, that is, giving valuable input and trying to make decisions, because it is at the end of the day, my body.” “There was an abundance of information [on the Internet] … So I tried to come out of from all different angles. I also tried to reach out to my own social network friends of friends or of relatives of family members who had thyroid cancer and knew somebody that had thyroid cancer.” | |
| Value of study participation |
Patient decision aid group “I think the most important part [of the patient decision aid] was that it was an objective way for me to see information … And I really felt in control.” “If anything, it's empowered me … Knowledge is power, for sure.” “I'm not calling them [treating physicians] and going crazy with questions, and then going hysterical because I can't get a response. (…) Hell is actually the fear of the unknown. So the more knowledge that someone has, I think the more they're at peace. It's not like you're groping in the dark.” “It's really made me feel more confident about my situation. The more information you have I think, the more secure you feel. Because you know exactly what you're dealing with, and what the expectation is.” “In my own research, I wasn't completely aware of how ambiguous it was, until I did this study. Then when I did this study, I was like, ‘Wow,’ there is a lot of ambiguity over whether I should do this or not.” “What I found really valuable about the tool was that it didn't pressure you—it educated you and you made your choice.” “It [study participation] makes you feel good that hey, I'm not the only one going through this alone.” “Kind of taking from my experience and being able to help somebody else … that's the reason why I'm here [participating in the study].” |
|
Control group “It made me think, ‘you better do your own research’ because making decisions is a critical part of your care.” [study participation] “I was feeling jealous of the people who used it [the patient decision aid] ‘cause I was thinking, ‘That would've been so helpful.’” “Maybe knowing in the sense that I'm not the only one getting it [thyroid cancer].” [study participation benefit] | |
| Unmet information and psychosocial support needs |
Patient decision aid group General information needs “So I feel there's an information void on life after in terms of … what could happen and what one should look for—post‐operation or post‐treatment.” “I'd say the biggest concern is when mostly trying to get my hormones balanced, so I feel normal again.” Psychosocial support needs “I think, you know, you just have basic concerns that you just want to talk to a human being I think sometimes? I mean, it's fine to talk to a computer, but it's awfully helpful to get someone's personal experience (from another survivor) … So I think the combination of computer access to lots of information plus being able to talk to a person beforehand, is really, really helpful.” |
|
Control group Unmet information needs related to RAI treatment decision making “I didn't feel like I had a comprehensive, ‘these are the risks [of RAI treatment], these are …” “Boy, I don't know what's going to happen, And that's a yucky feeling, you know?” [experience at RAI treatment] “I don't think the whole experience [of RAI treatment] was driven by anxiety but there was a certain level of mystery surrounding the experience and not knowing what to expect.” General information needs “I was frustrated because you get online and it's such basic information [about thyroid cancer], and I just … You know, I wanted a real opinion based on exactly what my … . You know, what my condition was, and you know, based on what they found from me. But it's hard to get that information just from viewing general websites.” “The Internet is sort of difficult, like I'm not a medical person, so I don't fully understand all the research papers and everything.” Psychosocial support needs “I relied very heavily on the forum support group online … I felt that that was a huge support.” | |
| Overall treatment experience |
Patient decision aid group “The after effects of the treatment were certainly not anything more than what the literature provided by the medical personal prepared me for.” “They hadn't found anything else [ie, recurrence]. So I'm very satisfied.” “Well, I guess everyone has the same worry right? That it'll come back! But everything I've been told is that my prognosis is very good … so knowing that, I'm not particularly concerned.” “All the staff I felt were very approachable. And the fact that it felt like it was my decision.” “There is a worry about having any kind of cancer coming back in your lifetime.” |
|
Control group “I think overall it's been a positive experience. The way it was dealt with as well … it's been you know having the opportunity to ask questions to Dr. X [thyroid cancer specialist] as well; it was fairly open.” “I think I'm very satisfied. I didn't have any long‐term side effects.” “I don't worry about it at all. I really don't. … I mean they're giving me adequate follow up care, I'm being seen by Drs. X [thyroid cancer specialists], and I'm feeling fine.” “There's always a little bit of worry of recurrences. You know, if it's going to come back or if it's going to manifest itself into another cancer. So it's certainly in the back of my mind. But I have faith in you know, the doctors that are caring for me and … I just gotta do what I have to do.” |
Abbreviation: RAI, radioactive iodine.
The first theme was the complexity of RAI treatment decision making from a patient perspective, and this included consideration of information from a variety of sources (eg, physicians, personal research on the Internet, other thyroid cancer survivors, and family/friends). Counseling by physicians was consistently reported as the most important information source affecting the ultimate treatment choice in both the P‐DA and control groups, and this mirrored findings at earlier time point evaluations.10 The degree to which individual participants in each of the groups was involved in RAI treatment decision making was variable. Individuals in the P‐DA group reported that the P‐DA was helpful in providing them with objective information and making them feel more comfortable with the decision‐making process, with some individuals feeling empowered. The concept of evidence uncertainty, explained in the P‐DA, was considered an important revelation in acquiring knowledge relevant to the treatment choice. In contrast, control group participants expressed negative feelings about not having the opportunity to use the P‐DA (eg, disappointment, frustration, and jealousy), and they indicated that they felt they may have missed out on helpful information.
Another theme that emerged from the qualitative analysis of both study groups was the general benefits of study participation. Participants in both the P‐DA and control groups indicated that participating in this study strengthened their realization that there was a treatment choice and encouraged their engagement in learning about their cancer and its medical management. Furthermore, patients in both groups indicated that they felt some comfort in knowing that they were part of a greater community of individuals afflicted by the same condition, and they hoped that their participation would help future patients.
Information and psychosocial support needs were raised as issues by participants in both groups, but unmet RAI treatment information needs were reported only by controls. Unmet knowledge translation needs relating to long‐term survivorship care, including thyroid hormone treatment, were reported, regardless of P‐DA use. The importance of psychosocial support by human contact with survivors was reported by both groups.
Overall thyroid cancer treatment satisfaction in both groups was related to positive outcomes (eg, a lack of treatment side effects and no evidence of disease recurrence) or outcomes meeting expectations and the availability of health care providers for ongoing care and support. However, some lingering worries about future disease recurrence or development of another cancer were reported by participants in both groups.
DISCUSSION
We found in this single‐site RCT that a computerized P‐DA, used as an adjunct to usual care, was associated with greater patient medical knowledge around the time of RAI treatment decision making9 and at extended follow‐up approximately 17 months later in comparison with usual care. Importantly, although our P‐DA explicitly explained the uncertainties in medical evidence related to postsurgical RAI treatment decision making (including a lack of RCTs and conflicting observational data), P‐DA participants’ decisional conflict was reduced at the time of decision making.9 Furthermore, longer term decision satisfaction, decision regret, mood, trust in physicians, and cancer‐related worry did not appear to be negatively affected by the disclosure of evidence uncertainty to patients.
Our qualitative study results highlighted how positive clinical outcomes and comfort during ongoing medical follow‐up were important components of patients’ overall satisfaction with their cancer care experience. With respect to decision making, we learned in the qualitative component of the study that the P‐DA was perceived as an objective source of medical information and empowered some patients in the decision‐making process (in part, this depended on the degree to which they desired to participate in that process). However, patients reported that physicians’ counseling/recommendations were the dominant factor influencing the ultimate treatment decision. Patients’ preferences for their degree of involvement in medical decision making was variable in both groups. These findings may explain some conflicting results of trials, as reported in recent systematic reviews specifically examining the impact of P‐DAs on patient involvement in decision making.30, 31
In a recent systematic review of decision aids for cancer screening and treatment, Trikalinos et al31 reported that patients’ satisfaction with the decision‐making process was significantly increased among P‐DA users versus controls in approximately half of the trials examining this outcome (2 of 4); furthermore, in 8 cancer decision‐aid trials examining the outcome of decision regret, among P‐DA users, decision regret was lower in 4 trials and higher in 3 trials in comparison with individuals not using the P‐DAs. Trikalinos et al also reported that no large differences in emotional distress or worry were evident between cancer P‐DA users and those not exposed to P‐DAs in 7 trials. These findings as well as our results suggest inconsistency in the impact of oncology P‐DAs on patients’ decision satisfaction and decision regret and the lack of a significant impact on cancer‐related emotional distress (including cancer‐related worry and physician trust). The complexity of the overall cancer care experience, disease and treatment‐related outcomes, individual patient characteristics, and the life situation of the individual may be important contributing factors and should be explored in future cancer decision‐making research.
Increased confidence in decision making and decision self‐efficacy has been reported as a positive effect of P‐DAs in some RCTs.30 However, in a recent RCT of a diabetes management P‐DA, patient empowerment was not significantly increased when it was measured on a disease‐specific quantitative self‐efficacy questionnaire.32 In the field of oncology P‐DAs, Alden33 recently described a complex theoretical model of cancer patient empowerment in treatment decision making. This model includes specific antecedents of an individual's desire for medical information and overall life satisfaction with effects mediated by 1) the patient's confidence in cancer‐related comprehension and participation in decision making and 2) the patient's attitude to the malignancy.33 Furthermore, Alden reported that utilization of a cancer treatment P‐DA appeared to increase patient empowerment by directly interacting with a patient's desire for medical information and indirectly interacting with the individual's attitude to cancer. Yet, Joseph‐Williams et al34 suggested that a power imbalance between health care providers and patients may be an important barrier to patient involvement in medical decision making, and they suggested that attitudinal changes are needed in the health care provider community to enable patients to be more involved in their medical decisions. The complex relation between P‐DA utilization, patient–health care provider interactions, and patient empowerment deserves further in‐depth study in other longer term follow‐up P‐DA trials.
Another important finding of our study was that thyroid cancer patients in the control group expressed some disappointment in not being able to view the P‐DA, and this has some relevance to the disclosure of risks to participants in future decision‐aid trials. Control group participants also reported limitations in applying existing information from the Internet to their situation, in understanding available medical literature, and in obtaining information on potential RAI side effects and treatment expectations. These findings echo the results of prior studies and indicate that thyroid cancer patients do not feel fully informed about important aspects of treatment decision making, including the disease prognosis,35, 36, 37 possible treatment side effects,35, 36, 37 and evidence uncertainty associated with RAI use.35 In fact, the awareness of such knowledge gaps was part of the rationale for the development of our P‐DA.35
The strengths of this RCT follow‐up study include the measurement of longer term follow‐up P‐DA RCT data (well beyond the period of treatment decision making), a relatively high rate of patient participation (95% or 70 of 74), the use of complementary quantitative and qualitative methodology in evaluating patient outcomes, the strict avoidance of external contamination of the control group (P‐DA access was not available outside the study), and the completeness of the data collection. Furthermore, the results of the quantitative questionnaire analyses were enhanced by the qualitative analyses. Some of our study limitations include a relatively small sample size, a limited population scope (ie, English‐speaking, computer‐literate, Canadian thyroid cancer survivors), a lack of quantitative measurements of self‐efficacy or empowerment, and the secondary nature of follow‐up outcome analyses (ie, hypothesis‐generating). Furthermore, the clinical importance of the statistically significant quantitative difference in the perception of feeling informed between the study arms is not known. Our findings need to be confirmed and built upon in other long‐term follow‐up P‐DA RCTs.
In conclusion, not only does a computerized thyroid cancer P‐DA improve medical knowledge significantly at the time of thyroid cancer treatment decision making,9 but at longer term follow‐up, individuals exposed to the P‐DA perceive themselves to be better informed about the decision in comparison with usual‐care controls. The value placed by patients on physician counseling in the cancer treatment decision‐making process was a central theme observed regardless of whether individuals were exposed to the P‐DA or not. A significant finding of our study is that for malignancies associated with treatment evidence uncertainty, disclosing this uncertainty to patients (via a computerized P‐DA) does not appear to result in adverse psychosocial outcomes or mistrust in physicians. This critical finding is relevant to many other malignancies for which evidence uncertainty exists either because there is a lack of high‐quality RCTs or because existing trials have conflicting results. Moreover, in such circumstances, cancer treatment decisions need to be made with the explicit disclosure to patients of related uncertainties as part of a fully informed shared decision‐making process. Although great advancements have been made in the scientific critical appraisal of medical literature, more research is needed on how to effectively to translate uncertainty in medical knowledge to oncology patients facing complex treatment decisions.
FUNDING SUPPORT
The extended follow‐up study of this trial was funded by an operating grant from the Institute of Cancer Research of the Canadian Institutes of Health Research (FRN 111416). The original trial was supported by an operating grant from the Ontario Ministry of Health and Long‐Term Care (Alternate Funding Plan: Innovation Fund). Anna M. Sawka currently holds a Chair in Health Services Research from Cancer Care Ontario, which is funded by the Ontario Ministry of Health and Long‐Term Care. Sharon Straus holds a Tier 1 Canada Research Chair. The funding agencies had no role in the study design, execution, data analysis, or results reporting.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
Supporting information
Supplementary Information
This study was presented in part at the following scientific meetings: 95th Annual Meeting of the Endocrine Society (June 15‐18, 2013; San Francisco, CA) and the 84th Annual Meeting of the American Thyroid Association (October 29 to November 2, 2014; Coronado, CA).
Anna M. Sawka, David P. Goldstein, Gary Rodin, Shereen Ezzat, Sharon Straus, and Amiram Gafni contributed to the conception or design of this work. Anna M. Sawka, David P. Goldstein, Shereen Ezzat, and Gary Rodin contributed to securing operating grant funding for this work. Anna M. Sawka, James D. Brierley, Richard W. Tsang, Lorne Rotstein, Shereen Ezzat, Phillip Segal, Lineke Heus, and David P. Goldstein contributed to the data acquisition. Anna M. Sawka, Lineke Heus, and Kevin E. Thorpe contributed to the data analysis. Kevin E. Thorpe designed the central randomization strategy. All authors contributed to the interpretation of data for this work. Anna M. Sawka drafted the work, and all coauthors contributed to revising it critically for important intellectual content and gave final approval of the final article.
REFERENCES
- 1.Cancer Research UK. Thyroid cancer incidence statistics. http://www.cancerresearchuk.org/cancer-info/cancerstats/types/thyroid/incidence/uk-thyroid-cancer-incidence-statistics. Accessed March 9, 2015.
- 2. American Cancer Society . Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- 3.Canadian Cancer Society's Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2014. Toronto, Canada: Canadian Cancer Society; 2014.
- 4.EUCAN. Thyroid cancer. http://eco.iarc.fr/eucan/Cancer.aspx?Cancer=35. Accessed March 9, 2015.
- 5. Sawka AM, Straus S, Gafni A, et al. A usability study of a computerized decision aid to help patients with, early stage papillary thyroid carcinoma in, decision‐making on adjuvant radioactive iodine treatment. Patient Educ Couns. 2011;84:e24‐e27. [DOI] [PubMed] [Google Scholar]
- 6. Sawka AM, Meiyappan S, David D, et al. A mixed methods evaluation of a computerized decision aid for patients considering radioactive iodine remnant ablation: developing person‐centered medicine for thyroid cancer. Int J Pers Cent Med. 2011;1:559‐563. [Google Scholar]
- 7. Sawka AM, Straus S, Gafni A, et al. How can we meet the information needs of patients with early stage papillary thyroid cancer considering radioactive iodine remnant ablation? Clin Endocrinol (Oxf). 2011;74:419‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sawka AM, Straus S, Brierley JD, et al. Decision aid on radioactive iodine treatment for early stage papillary thyroid cancer—a randomized controlled trial. Trials. 2010;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sawka AM, Straus S, Rotstein L, et al. Randomized controlled trial of a computerized decision aid on adjuvant radioactive iodine treatment for patients with early‐stage papillary thyroid carcinoma. J Clin Oncol. 2012;10:2906‐2911. [DOI] [PubMed] [Google Scholar]
- 10. Sawka AM, Rilkoff H, Tsang RW, et al. The rationale of patients with early stage papillary thyroid cancer for accepting or rejecting radioactive iodine remnant ablation. Thyroid. 2013;23:246‐247. [DOI] [PubMed] [Google Scholar]
- 11. Schulz KF, Altman DG, Moher D; CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edge SB, Byrd DR, Carducci MA, et al. AJCC Cancer Staging Manual. 7th ed New York, NY: Springer‐Verlag; 2009. [Google Scholar]
- 13. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer , Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167‐1214. [DOI] [PubMed] [Google Scholar]
- 14. Martinez LS, Schwartz JS, Freres D, et al. Patient‐clinician information engagement increases treatment decision satisfaction among cancer patients through feeling of being informed. Patient Educ Couns. 2009;77:384‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Connor AM. User Manual—Decision Regret Scale. https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Regret_Scale.pdf. Updated 2003. Accessed January 12, 2015.
- 16. Brehaut JC, O'Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23:281‐292. [DOI] [PubMed] [Google Scholar]
- 17. Gotay CC, Pagano IS. Assessment of Survivor Concerns (ASC): a newly proposed brief questionnaire. Health Qual Life Outcomes. 2007;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bresner L, Banach R, Rodin G, et al. Cancer‐related worry in Canadian thyroid cancer survivors. J Clin Endocrinol Metab. 2015;100:977‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kroenke K, Spitzer RL, Williams JB, et al. An ultra‐brief screening scale for anxiety and depression: the PHQ‐4. Psychosomatics. 2009;50:613‐621. [DOI] [PubMed] [Google Scholar]
- 20. Lowe B, Wahl I, Rose M, et al. A 4‐item measure of depression and anxiety: validation and standardization of the Patient Health Questionnaire‐4 (PHQ‐4) in the general population. J Affect Disord. 2010;122:86‐95. [DOI] [PubMed] [Google Scholar]
- 21. Anderson LA, Dedrick RF. Development of the Trust in Physician Scale: a measure to assess interpersonal trust in patient‐physician relationships. Psychol Rep. 1990;67(pt 2):1091‐1100. [DOI] [PubMed] [Google Scholar]
- 22. Sandelowski M. Combining qualitative and quantitative sampling, data collection, and analysis techniques in mixed‐method studies. Res Nurs Health. 2000;23:246‐255. [DOI] [PubMed] [Google Scholar]
- 23. Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine; 1967. [Google Scholar]
- 24. Finlay L. “Outing” the researcher: the provenance, process, and practice of reflexivity. Qual Health Res. 2002;12:531‐545. [DOI] [PubMed] [Google Scholar]
- 25. Denzin LL, Lincoln YS. The Sage Handbook of Qualitative Research. 3rd ed Newbury Park, CA: Sage Publications; 2005:230. [Google Scholar]
- 26. Kvale S. Interviews: An Introduction to Qualitative Research Interviewing. Thousand Oaks, CA: Sage Publications; 1996. [Google Scholar]
- 27. Reissman CK. Narrative Analysis. Thousand Oaks, CA: Sage Publications; 1993. [Google Scholar]
- 28. Czarniawska B. Narratives in Social Science Research. London, England: Sage Publications; 2004. [Google Scholar]
- 29. Crouch M, McKenzie H. The logic of small samples in interview‐based qualitative research. Soc Sci Inf. 2006;45:83‐99. [Google Scholar]
- 30. Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions . Cochrane Database Syst Rev. 2014:CD001431. [DOI] [PubMed] [Google Scholar]
- 31. Trikalinos TA, Wieland LS, Adam GP, Zgodic A, Ntzani EE. Decision Aids for Cancer Screening and Treatment. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Comparative Effectiveness Reviews 145. [PubMed] [Google Scholar]
- 32. Denig P, Schuling J, Haaijer‐Ruskamp F, Voorham J. Effects of a patient oriented decision aid for prioritising treatment goals in diabetes: pragmatic randomised controlled trial. BMJ. 2014;349:g5651. [DOI] [PubMed] [Google Scholar]
- 33. Alden DL. Decision aid influences on factors associated with patient empowerment prior to cancer treatment decision making. Med Decis Making. 2014;34:884‐898. [DOI] [PubMed] [Google Scholar]
- 34. Joseph‐Williams N, Edwards A, Elwyn G. Power imbalance prevents shared decision making. BMJ. 2014;348:g3178. [DOI] [PubMed] [Google Scholar]
- 35. Sawka AM, Goldstein DP, Brierley JD, et al. The impact of thyroid cancer and post‐surgical radioactive iodine treatment on the lives of thyroid cancer survivors: a qualitative study. PLoS One. 2009;4:e4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roberts KJ, Lepore SJ, Urken ML. Quality of life after thyroid cancer: an assessment of patient needs and preferences for information and support. J Cancer Educ. 2008;23:186‐191. [DOI] [PubMed] [Google Scholar]
- 37. Goldfarb M, Casillas J. Unmet information and support needs in newly diagnosed thyroid cancer: comparison of adolescents/young adults (AYA) and older patients. J Cancer Surviv. 2014;8:394‐401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


