Summary
The aim of this paper is to critically review the literature documenting the imaging approach in adult Femoral Head Avascular Necrosis (FHAVN). For this purpose we described and evaluated different radiological techniques, such as X-ray, Computed Tomography (CT), Magnetic Resonance Imaging (MRI), and Nuclear Medicine.
Plain films are considered the first line imaging technique due to its ability to depict femoral head morphological changes, to its low costs and high availability. CT is not a routinely performed technique, but is useful to rule out the presence of a subchondral fracture when MRI is doubtful or contraindicated. MRI is unanimously considered the gold standard technique in the early stages, being capable to detect bone marrow changes such as edema and sclerosis. It may be useful also to guide treatment and, as CT, it is a validated technique in follow-up of patients with FHAVN. Nuclear medicine imaging is mostly applied in post-operative period to detect graft viability or infective complications. More advanced techniques may be useful in particular conditions but still need to be validated; thus new research trials are desirable.
In conclusion, X-ray examination is the first line approach, but lacks of sensitivity in early stage whereas MRI is indicated. CT easily depicts late stage deformation and may decrease MRI false positive results in detecting the subchondral fracture. However, the role of both Nuclear Medicine Imaging and advanced MR techniques in FHAVN still need to be investigated.
Keywords: osteonecrosis, femur head necrosis, diagnostic imaging, disease management
Background
Femoral head avascular necrosis is a common disease of the hip joint with an incidence of 10,000–20,000 new cases in the United States (1). Though most of patients are asymptomatic the prevalence is supposed to be markedly higher. Impaired blood perfusion and increased intraosseous pressure are identified as predominant responsible of the necrotic process. If not identified in the early stages, this condition leads to osteoarthritis and thus eventually to joint replacement. Radiologic staging of the disease is of pivotal importance allowing the identification and risk stratification in pre-collapse stages, prognosis, adequate treatment planning and post-operative follow-up (2). For this purpose, several techniques are nowadays available and new advanced ones are undergoing validation. While X-ray remains the mainstay of FHAVN imaging, CT, MRI and Nuclear Medicine hybrid techniques are gaining field of application in early stages of the disease. Moreover, the increasing number of conservative or mini-invasive treatments has prompted the need of more advanced imaging techniques to work as guidance, to evaluate bone reparative processes or graft viability. Therefore, the purpose of this paper is to review the available literature about the diagnostic imaging approach to FHAVN.
FHAVN classification overview
The first staging classification of the femoral head avascular necrosis was proposed in 1964 by Ficat and Arlet and was subsequently modified several times (3). Their purpose was to provide prognostic information to compare different treatment options dividing the disease course in 4 stages. Few years later a similar attempt was done by Marcus and Enneking dividing the disease in 6 stages (4). Since then several classification system were developed to provide insights in the prognosis, treatment planning and outcome evaluation (5).
According to Mont et al. (6), who reviewed 16 different classification schemes, just 4 of them were considered in more than the 85% of the evaluated studies; in particular Ficat classification was the most adopted accounting for the 63% of the considered studies and was followed by the Steinberg’s system which accounted for the 20%, by the Association Research Circulation Osseous (ARCO) system which accounted for the 12% and by the Japanese Orthopaedic Association (JOA) system which accounted for the 5%. However, which is the universally accepted classification is controversial and is still a matter of debate, leaving the choice to the different adopting centers. From a radiological point of view the X-ray evaluation represents the basis of all the proposed schemes. Only the latest adopted classifications, such as Steinberg’s, ARCO and JOA systems, were designed to include CT and MRI findings along with the increasing interest in early staging of the FHAVN. Another staging classification, developed by Mitchell et al. (7), relied only on MRI features to stage lesion severity and consists of 4 classes of FHAVN.
Conventional radiography
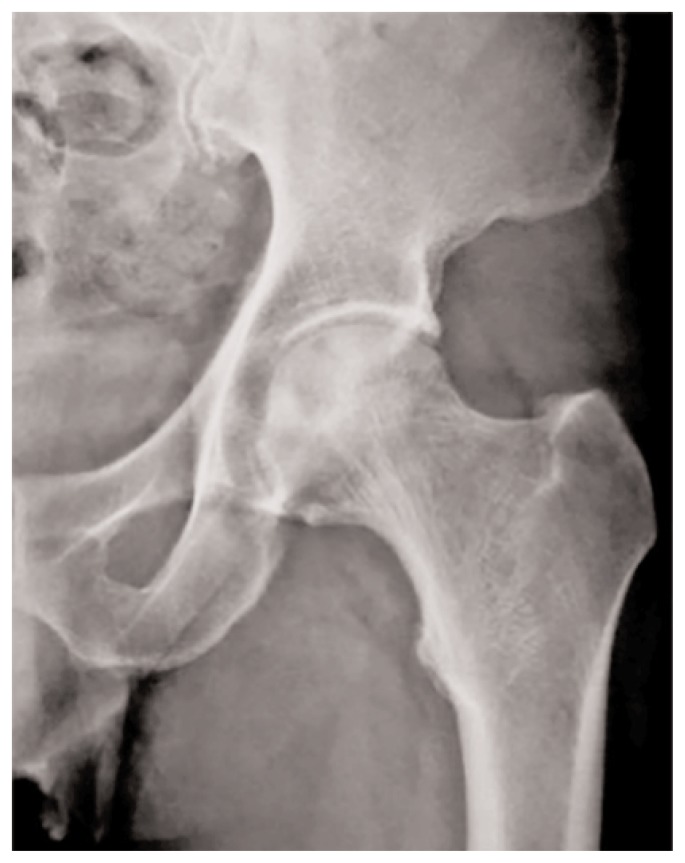
X-ray examination is still considered the mainstay and first line imaging technique in the FHAVN diagnosis. Radiographic assessment of the femoral head is also convenient and inexpensive. While it is insensitive in the early changes of the disease, imaging features of more advanced stages are often characteristic and may obviate the need for any additional radiologic evaluation. Plain films in both frontal and lateral “frog leg” projections are mandatory to increase the diagnostic accuracy. Femoral head appearance could be normal until 3 months since disease onset. Typical features, in order of appearance from early to late stage disease, are areas of sclerosis within the femoral head, presence of radiolucent lines “crescent sign” in keeping with subchondral fracture and collapse of the cortex with flattening of the femoral head, and eventual lateral subluxation (Figure 1). Sometimes cystic lesions may be demonstrated in association with sclerotic areas and are meant to be a negative prognostic factor predictive of future collapse. Osteoarthritis and ankylosis represent the latest evolution throughout the disease spectrum (8). Atypical radiographic features are identified in 18% of patients and in those undergoing steroid therapy (9). Joint space narrowing may be present and usually precedes the appearance of the crescent sign in this population. Furthermore, signs of bone repair, such as sclerosis, may be absent due to a decreased bone formation in the presence of normal bone resorption. While typical radiographic findings lead to a confident differential diagnosis between FHAVN and osteoarthritis or rapid onset osteoarthritis in which joint space narrowing and osteophytes are predominant, in atypical presentations the diagnosis could be insidious and is usually triggered by the clinician index of suspicion and confirmed by second line investigations (10). Plain films have also been used to evaluate lesion extension which is considered in the ARCO classification as mild, moderate and severe if involving less than the 15%, between 15 and 30% and more than the 30% of the femoral head, respectively. Several measurement methodics have been applied to anteroposterior and lateral radiography. In particular, Niimi et al. (11) considered the percentage of the weight-bearing area involved in the disease identifying 4 lesion types at AP radiographs. Kerboul et al. (12) measured the arc of the femoral surface involved considering two different angles, obtained drawing two lines from the center to the periphery of the affected femoral head on both AP and lateral radiographs.
Figure 1.
Plain radiograph showing an area of lucency surrounded by a rim of sclerosis compatible with FHAVN.
According to a recent diagnostic algorithm (13), when morphological changes of the femoral head are demonstrated at radiography and in particular when there is a depression of the articular surface between 2 and 4 mm associated or not with acetabular changes, no further imaging is required. An additional CT examination could be performed when the areas of sclerosis are associated with lucency lines of uncertain significance to rule out the presence of subchondral fractures.
However, conventional radiography may be not so accurate and sensitive as more advanced techniques, which can identify early stages in asymptomatic patients, but remains of pivotal importance in the late stages.
Computed tomography
Few studies in literature are focused on the role of multidetector CT in FHAVN and this is not routinely applied for diagnosis. CT technique has an intrinsic high spatial resolution and is more sensitive than X-ray in detecting bone typical osteonecrosis abnormalities, but it is still focused on the late stage of the disease. There are several proposed acquisition protocols, all characterized by a low slice thickness (1–2 mm) and pitch (1–1.5 mm). Commonly used image matrix is 512 × 512 and is usually associated to a reduced field of view (FOV). Multiplanar reformats associated with bone filtering are mandatory and possibly increase the accuracy in evaluating the articular surface (14). Typical FHAVN CT findings, in order of appearance from early to late stage disease, are the “asterix sign”, due to femoral head central sclerosis and trabeculae condensation, the calcifications, the subchondral fracture, and the bone collapse leading to final articular deformity. Several comparisons were made in literature between the staging performance of CT and MRI in FHAVN patients. Firstly, Mitchell et al. (15) in 1986 found the superiority of CT in displaying bone architecture while MR images better depicted the bone marrow alterations. More recently Yeh et al. (16) and Stevens et al. (17) compared the capacity of CT and MRI in the detection of the subchondral fractures concluding for the superiority of tomography examination. According to the former Authors, false positive diagnosis were more common at MRI and led to the erroneous attribution of patients in Steinberg’s stage III rather than II with consequent implications in treatment planning. Instead, Stevens et al. (17) evaluated sensitivity and specificity of CT and MR in the same task which accounted respectively 100% for the former and 38 and 100% for the latter, thus confirming the role and accuracy of CT examination in stages II-IV, when detection of the subchondral fracture is pivotal and in which MRI is unsatisfactory. However, the application of 3D isotropic sequences at MRI could smooth at least to a certain point this technique bias (17). Regarding early stages, a recent retrospective designed report by Barille et al. (18) documented a missed diagnosis in 89% of the hips with FHAVN at CT if compared to MRI; thus accounting for the low sensitivity of the former at these stages and also of the unawareness of the radiologists who did not included hips in their visual checklist. Multidetector CT is a useful technique also in post-operative ankle evaluation documenting mineralization state, the position of orthopaedic hardware and the presence or absence of bone bridging and fragmentation. Acquisition technique adjustments may be needed in this case to increase sensitivity in presence of beam hardening artifact due to metallic prosthesis (19, 20). CT examination is not recommended routinely for diagnosis, but it may be useful to exclude the presence of a subchondral fracture in FHAVN when MRI is doubtful or contraindicated, while it is a validated technique in the post-operative imaging follow up.
Conventional magnetic resonance imaging
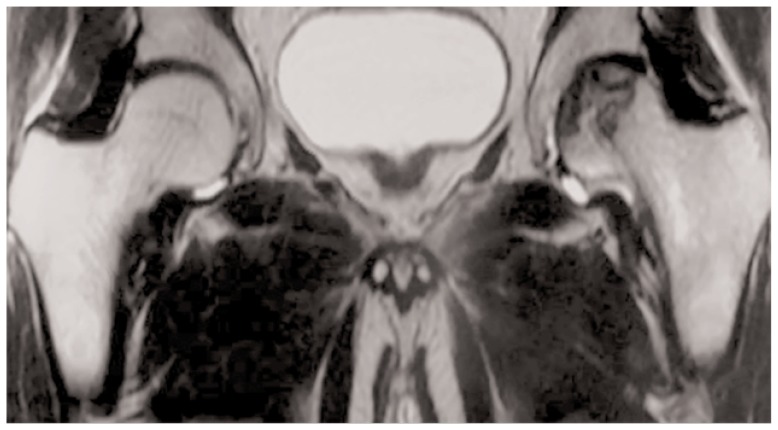
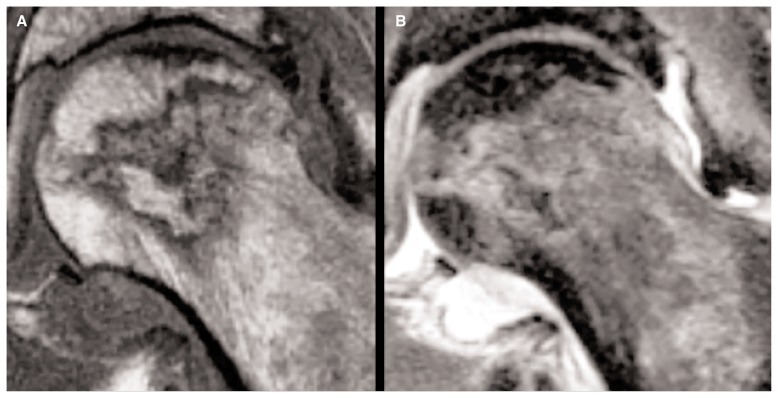
MRI is considered the gold standard technique in the early stages of FHAVN with a sensitivity of more than 99%; thus this technique is considered by both Steinberg and ARCO classification in the early disease stage (21–23). Signal changes in bone marrow have been demonstrated within 1 week of vascular injury in animals. Diagnostic MRI protocols include sagittal T1 fast spin echo (FSE) and Short Tau Inversion Recovery (STIR) sequences, coronal and axial PD and axial T2 FSE. Post-operative scans may include T2 Spectral Adiabatic Inversion Recovery (SPAIR) sequences according to different performing centers which is less degraded by metallic artifacts. Diagnostic post-contrast imaging usually is not performed, although it could demonstrate the lack of the enhancement of the necrotic area or a ring enhancement due to the granulation tissue at the border of the lesion. Osteonecrosis area appears circumscribed by a “band like” hypointensity in T1 weighted images which correspond to the rim of reacting sclerosis surrounding the involved bone marrow (Figure 2) (24–28). Crescentic, serpentine, ring like or wedge lesion are also common depending on the extension of the FHAVN. According to Mitchell et al. (29), the area of necrosis may have different signal intensities other than bone marrow adipose tissue in a minority of cases, due to the eventual haemorrhagic, cystic or fibrous evolution. Another pathognomonic alteration, evident in 65–85% of patients, is the “double line” sign on T2 weighted images which is considered a chemical shift artifact in its origin and is characterized by an outer low signal of intensity rim with an inner zone of high intensity referred to the granulation tissue (Figure 3) (30). Subchondral fractures are evident as smooth low signal intensity lines on T1 weighted images concave to the articular surface; thus they differ from insufficiency fractures which are irregular in shape and convex to the surface. Fracture gap may have several signal intensities on T2 weighted images due to the eventual presence of fluid collections or gas. Bone Marrow Edema (BME) is usually well documented at T2 fat suppressed weighted images and may involve only the epiphysis or could extend to the metaphysis. It has been the focus of several papers regarding its possible role as first marker of FHAVN and its association with subchondral fracture, symptomatology and prognosis (31, 32). BME is also seen in conjunction with joint effusion in at least 50% of FHAVN patients. Morphologic changes of the femoral head, such as contour alteration and collapse, are also identified at conventional MRI examination. Quantification of the extent of the osteonecrosis is also possible and is believed to carry pivotal prognosis information. Several methods have been proposed for this purpose, but the overall volume of the femoral head is considered the most validated predictor of future collapse. When more than 25–50% of the femoral head is affected articular collapse is seen in 43–87% of patient, while only 5% of them with an involvement of less than 25% will develop future deformity (33–35). While there is no role in lesion follow-up after head collapse, some Authors recently pointed out the possible utilization of MRI in follow-up small pre-collapse lesion which might heal in time with conservative therapy (36). Intra-operative use of MRI has recently been proposed by Kerimaa et al. (37) who performed femoral head core decompression under its guidance. The technical success rate of the procedure was 100%, resembling the benefit obtained of acquiring images in arbitrary planes and the ability to depict the most affected areas of the femoral head. MRI could be used also to assess post-operative outcome, particularly in patients undergoing Vascularized Fibular Grafting (VFG). Although gadolinium administration is not routinely performed in post-operative period unless there is the suspicion of infection, in case of VFG, it may add important prognosis informations. The presence of enhancement surrounding the graft and in the necrotic area means revascularization and is often associated with reduction of BME which stands for a favorable outcome (38, 39).
Figure 2.
Crescent sign at coronal T2 weighted images with associated initial articular deformity in the left femoral head in a 41-year-old patient.
Figure 3.
T1 weighted (A) and T2 weighted (B) images show the “double line” sign associated with neck edema in the left femoral head of a 52-year-old male patient.
In summary, conventional MRI is considered the gold standard technique in the early stages when plain films are negative and can be used as guidance and follow-up of FHAVN conservative and surgical treatments.
Advanced magnetic resonance imaging
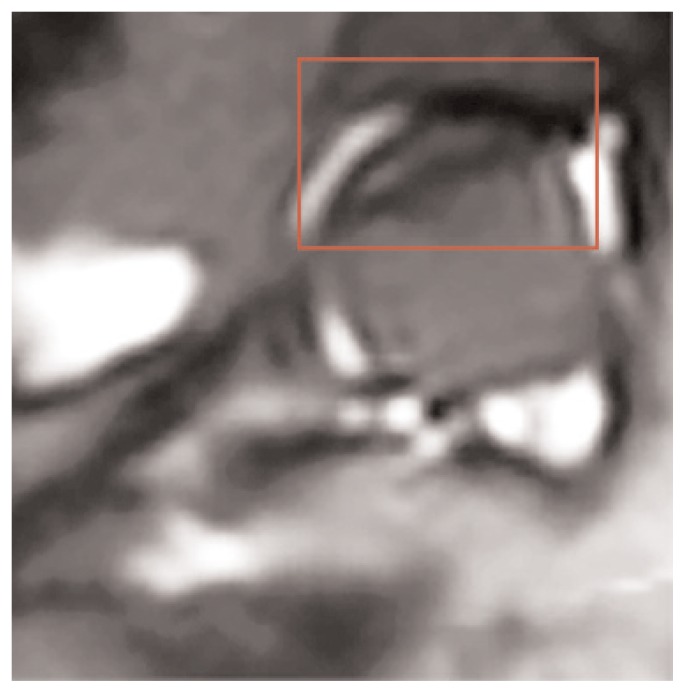
The search for improvements in MR sensitivity and specificity in FHAVN has prompted the attention focus on different MR technologies such as Diffusion Weighted Imaging (DWI), Perfusion Imaging (PWI), T2 Mapping and Magnetic Resonance Spectroscopy (MRS). Diffusion has recently been proposed as a promising tool to assess several skeletal diseases, given its ability to depict water shifting between different tissue compartments. Contrarily to other organs where diffusion is restricted in ischaemic condition, the majority of papers on FHAVN have shown its increase in the femoral epiphysis (Figure 4) (40). Similar results were obtained also considering the Legg-Calve-Perthes (LCP) disease (41, 42). This phenomenon is likely due to the different cell population which constitutes the bone marrow and to a late identification of the pathologic process, when cells death with membranes lysis and BME has already occurred. Previous FHAVN animal models, indeed, were in agreement with the theory of a transient restriction followed by an increase in diffusion of water molecules (43, 44). Although there is common agreement on diffusion increase in adult FHAVN, Oner et al. (45) analyzed diffusion in different disease stages, showing no significant difference. Mueller et al. (46), despite the evidence of diffusion increase in the epiphysis and metaphysis, failed to demonstrate any difference in Apparent Diffusion Coefficient (ADC) between Transient Bone Marrow Edema (TBME), FHAVN, and Subchondral Insufficiency Fractures (SIV). In our opinion previously mentioned results and the absence of a stage dependent ADC value, in contrast to what was recently demonstrated in LCP disease, limit the actual value of this technique.
Figure 4.
DWI image obtained from the same patient of Figure 3 showing increase diffusion in the left femoral head.
The role of PWI is to demonstrate perfusion changes in ischaemic bone marrow. It has been previously demonstrated that osteonecrosis is the result of decreased perfusion associated to a stasis in the venous outflow. As the necrosis progresses, an increase of the blood flow is also due to the proliferation of the granulation tissue. Dynamic contrast enhancement imaging has been used to demonstrate these changes and it was shown to increase MRI sensitivity in detecting osteonecrosis foci in animal models (47). Dynamic T1 weighted sequences are usually acquired in the coronal plane, but there is no agreement in technical parameters and dynamic time intervals which differ between Authors. Hayashi et al. (48) showed a decrease of the initial slope and in the enhancement ratio of the time-intensity curve, which was associated to VEGF increase and DNA oxidative damage in a rabbit model of steroid induced FHAVN. As previously demonstrated in animals, Chan et al. (49) showed a delayed time to peak enhancement also in humans, and found an associated increase in the peak enhancement absolute value that was suggested to reflect reduced venous outflow. Both changes were found to be time dependent. Recently, Mueller et al. (46) following the experience of Malizos et al. (50) and Aaron et al. (51) on TBME, confirmed previous results showing a decreased time to peak, enhancement slope and ratio in patients with FHAVN, whose values were statistically different from results obtained in patients with SIV or TBME; thus perfusion technique could be used to recognize these distinct entities.
MRS has been used to identify metabolic changes in the bone marrow whose lipid and water compartments are readily altered in an ischaemic environment. According to this and following the previous experience of Bluemke et al. (52) who measured the fat content of the femoral head, Hou et al. (53) used single voxel PRESS technique to analyze contralateral normal appearing femoral head in patients with FHAVN and comparing it to normal subject. The Authors concluded that the lipid and water line width (LW) were narrower than in control subjects and that the lipid/water ratio showed only borderline differences between groups. Considering that previously cited work is the only one present in literature, to our knowledge, on MRS in FHAVN patients, other insights on this topic are needed to support clinical implementation of this technique.
T2 mapping is one of the recently proposed techniques to evaluate articular cartilage. It has been proposed that in FHAVN patients the articular deformation is responsible of cartilage degeneration and that this event coincides with symptoms presentation. On this basis, this novel technique was applied by Yamamoto et al. (54) who found, instead, that T2 abnormalities were present also in asymptomatic patients with Systemic Lupus Erythematosus and non-collapsed osteonecrosis. However, further research is needed to discover the full potential of this technique which could be applied in pre-collapse stages.
Ultra-high field magnets
While new magnets are being developed, 7T magnets are already used worldwide for research in different radiological fields. Ultra-high field magnet advantages comprise a 2-fold or 4-fold signal To Noise Ratio (SNR) over 3T and 1.5T respectively, higher spatial resolution and image contrast. Although 7T MRI studies focused preferentially on brain and some musculoskeletal structures, recently, some Authors investigated the performance of 7T magnets in FHAVN patients. In two different papers, Theysohn et al. (55, 56) analyzed 7T advantages and compared it to 3T magnets. Images obtained using a home-made coil, were judged according to a 4 point scale. 7T performance could not yet surpass 3T experience, although full technical potential of the former was not exploited due to technological immature equipment. Depiction of critical structures, such as the articular cartilage or the labrum, was at least comparable to that obtained with 3T magnets. Further research and coil development is needed to understand the full potential of these novel machines.
Nuclear medicine
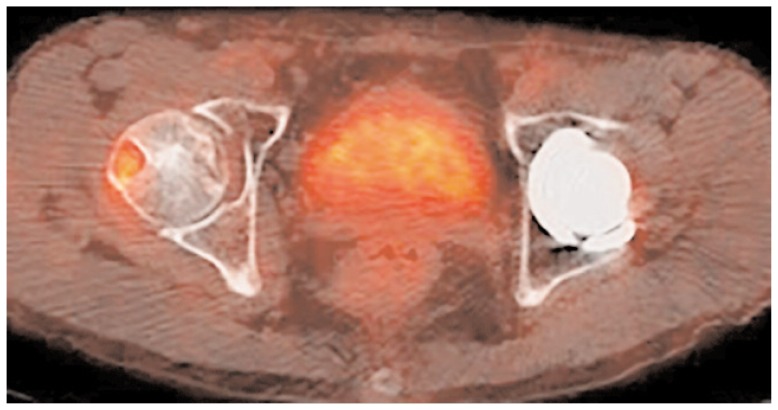
Nuclear medicine techniques, such as bone scan, SPECT, SPECT/CT and PET or PET/CT are not routinely used in FHAVN patients and their role is still under investigation. Osteonecrosis areas appear as photopenic lesions circumscribed by a reactive (hot) interface (bull’s eye sign) at 99 technetium bone scan (57). Although this technique has been demonstrated to be more sensitive than plain films and particularly suitable for non-collaborative patients in early stages, its performance against MRI was unsatisfactory, with only 56% of sensitivity compared to 100% sensitivity of the latter (58). SPECT was developed to overcome bone scan limits, but it still suffered for the lack of anatomical details; thus hybrid SPECT/CT was introduced and its superiority against previous techniques was documented in a recent study performed by Agarwal et al. (59). On the other hand, Motomura et al. (60), following Miki et al. (61) experience with planar scintigraphy, analyzed the femoral osteonecrosis reparative process showing that the increased tracer uptake is stage-dependent and may predict imminent collapse. Recently, SPECT/CT was also used in treatment follow-up to determine the viability of the graft after VFG intervention which is characterized by a time dependent increase of tracer uptake suggesting revascularization (62). Few papers in literature deal with PET in FHAVN. Dasa et al. compared the performance of SPECT, conventional MRI and PET in their series of patients concluding for an advantage of PET on the other techniques in early stages where also MRI was negative (63). Finally, just case reports are available in literature, to our knowledge, regarding PET/CT and FHAVN suggesting it as an effective alternative to MRI in patients with absolute contraindications (Figure 5) (64, 65). While Nuclear Medicine techniques are of limited value and not recommended in the diagnosis of FHAVN with some exceptions, is our opinion that they can play an important role in surgical therapy follow-up not only identifying bone graft viability, but also excluding infections and other inflammatory processes.
Figure 5.
PET/CT showing intensive uptake in an area of osteonecrosis within the right femoral head in a 45-year-old asymptomatic patient.
References
- 1.Lieberman JR, Berry DJ, Mont MA, Aaron RK, Callaghan JJ, Rajadhyaksha AD. Osteonecrosis of the hip: management in the 21st century. Instr Course Lect. 2003;52:337–55. [PubMed] [Google Scholar]
- 2.Murphey MD, Foreman KL, Klassen-Fischer MK, Fox MG, Chung EM, Kransdorf MJ. From the radiologic pathology archives imaging of osteonecrosis: radiologic-pathologic correlation. Radiographics. 2014;34( 4):1003–28. doi: 10.1148/rg.344140019. [DOI] [PubMed] [Google Scholar]
- 3.Arlet J, Ficat RP. Forage-biopsie de la tete femorale dans I’osteonecrose primitive. Observations histopathologiques portant sur huit forances. Rev Rheum. 1964;31(31):257–64. [Google Scholar]
- 4.Marcus ND, Enneking WF, Massam RA. The silent hip in idiopathic aseptic necrosis. Treatment by bone-grafting. J Bone Joint Surg Am. 1973;55(7):1351–66. [PubMed] [Google Scholar]
- 5.Choi HR, Steinberg ME, Y Cheng E. Osteonecrosis of the femoral head: diagnosis and classification systems. Curr Rev Musculoskelet Med. 2015;8(3):210–20. doi: 10.1007/s12178-015-9278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mont MA, Marulanda GA, Jones LC, Saleh KJ, Gordon N, Hungerford DS, et al. Systematic analysis of classification systems for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88( Suppl 3):16–26. doi: 10.2106/JBJS.F.00457. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell DG, Rao VM, Dalinka MK, et al. Femoral head avascular necrosis: correlation of MR imaging, radiographic staging, radionuclide imaging, and clinical findings. Radiology. 1987;162(3):709–15. doi: 10.1148/radiology.162.3.3809484. [DOI] [PubMed] [Google Scholar]
- 8.Gross TP, Liu F. Is there added risk in resurfacing a femoral head with cysts? J Orthop Surg Res. 2011;6:55. doi: 10.1186/1749-799X-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aiello MR, et al. Imaging in Avascular Necrosis of the Femoral Head: Overview. emedicine.medscape.com/article/386808-overview.
- 10.Pivec R, Johnson AJ, Harwin SF, Mont MA. Differentiation, diagnosis, and treatment of osteoarthritis, osteonecrosis, and rapidly progressive osteoarthritis. Orthopedics. 2013;36(2):118–25. doi: 10.3928/01477447-20130122-04. [DOI] [PubMed] [Google Scholar]
- 11.Niimi R, Sudo A, Hasegawa M, Uchida A. Course of avascular necrosis of femoral head without collapse of femoral head at first examination: minimum 8-year follow-up. Orthopedics. 2008;31(8):755. [PubMed] [Google Scholar]
- 12.Kerboul M, Thomine J, Postel M, Merle d’Aubigne R. The conservative surgical treatment of idiopathic aseptic necrosis of the femoral head. J Bone Joint Surg Br. 1974;56(2):291–6. [PubMed] [Google Scholar]
- 13.Pierce TP, Jauregui JJ, Cherian JJ, Elmallah RK, Mont MA. Imaging evaluation of patients with osteonecrosis of the femoral head. Curr Rev Musculoskelet Med. 2015;8(3):221–7. doi: 10.1007/s12178-015-9279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu LB, Huang ZG, Wei HY, Wang W, Ren A, Xu YY. Osteonecrosis of the femoral head: using CT, MRI and gross specimen to characterize the location, shape and size of the lesion. Br J Radiol. 2015;88(1046):20140508. doi: 10.1259/bjr.20140508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell DG, Kressel HY, Arger PH, Dalinka M, Spritzer CE, Steinberg ME. Avascular necrosis of the femoral head: morphologic assessment by MR imaging, with CT correlation. Radiology. 1986;161(3):739–42. doi: 10.1148/radiology.161.3.3786725. [DOI] [PubMed] [Google Scholar]
- 16.Yeh LR, Chen CK, Huang YL, Pan HB, Yang CF. Diagnostic performance of MR imaging in the assessment of subchondral fractures in avascular necrosis of the femoral head. Skeletal Radiol. 2009;38(6):559–64. doi: 10.1007/s00256-009-0659-0. [DOI] [PubMed] [Google Scholar]
- 17.Stevens K, Tao C, Lee SU, Salem N, Vandevenne J, Cheng C, Neumann G, Valentin-Opran A, Lang P. Subchondral fractures in osteonecrosis of the femoral head: comparison of radiography, CT, and MR imaging. AJR Am J Roentgenol. 2003;180(2):363–8. doi: 10.2214/ajr.180.2.1800363. [DOI] [PubMed] [Google Scholar]
- 18.Barille MF, Wu JS, McMahon CJ. Femoral head avascular necrosis: a frequently missed incidental finding on multidetector CT. Clin Radiol. 2014;69(3):280–5. doi: 10.1016/j.crad.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Douglas-Akinwande AC, Buckwalter KA, Rydberg J, Rankin JL, Choplin RH. Multichannel CT: evaluating the spine in postoperative patients with orthopedic hardware. Radiographics. 2006;26( Suppl 1):S97–110. doi: 10.1148/rg.26si065512. [DOI] [PubMed] [Google Scholar]
- 20.Lee MJ, Kim S, Lee SA, Song HT, Huh YM, Kim DH, Han SH, Suh JS. Overcoming artifacts from metallic orthopedic implants at high-field-strength MR imaging and multi-detector CT. Radiographics. 2007;27(3):791–803. doi: 10.1148/rg.273065087. [DOI] [PubMed] [Google Scholar]
- 21.Glickstein MF, Burk DL, Jr, Schiebler ML, et al. Avascular necrosis versus other diseases of the hip: sensitivity of MR imaging. Radiology. 1988;169(1):213–5. doi: 10.1148/radiology.169.1.3420260. [DOI] [PubMed] [Google Scholar]
- 22.Piyakunmala K, Sangkomkamhang T, Chareonchonvanitch K. Is magnetic resonance imaging necessary for normal plain radiography evaluation of contralateral non-traumatic asymptomatic femoral head in high osteonecrosis risk patient. J Med Assoc Thai. 2009;92( suppl 6):S147–S51. [PubMed] [Google Scholar]
- 23.Kamata N, Oshitani N, Sogawa M, et al. Usefulness of magnetic resonance imaging for detection of asymptomatic osteonecrosis of the femoral head in patients with inflammatory bowel disease on long-term corticosteroid treatment. Scand J Gastroenterol. 2008;43(3):308–13. doi: 10.1080/00365520701676773. [DOI] [PubMed] [Google Scholar]
- 24.Zhao DW, Hu YC. Chinese experts’ consensus on the diagnosis and treatment of osteonecrosis of the femoral head in adults. Orthop Surg. 2012;4(3):125. doi: 10.1111/j.1757-7861.2012.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malizos KN, Karantanas AH, Varitimidis SE, Dailiana ZH, Bargiotas K, Maris T. Osteonecrosis of the femoral head: etiology, imaging and treatment. Eur J Radiol. 2007;63(1):16. doi: 10.1016/j.ejrad.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Fordyce MJ, Solomon L. Early detection of avascular necrosis of the femoral head by MRI. J Bone Joint Surg Br. 1993;75(3):365. doi: 10.1302/0301-620X.75B3.8496201. [DOI] [PubMed] [Google Scholar]
- 27.Coleman BG, Kressel HY, Dalinka MK, Scheibler ML, Burk DL, Cohen EK. Radiographically negative avascular necrosis: detection with MR imaging. Radiology. 1988;168(2):525. doi: 10.1148/radiology.168.2.3393676. [DOI] [PubMed] [Google Scholar]
- 28.Lang P, Genant HK, Jergesen HE, Murray WR. Imaging of the hip joint. Computed tomography versus magnetic resonance imaging. Clin Orthop Relat Res. 1992;274:135. [PubMed] [Google Scholar]
- 29.Mitchell DG, Joseph PM, Fallon M, et al. Chemical-shift MR imaging of the femoral head: an in vitro study of normal hips and hips with avascular necrosis. AJR Am J Roentgenol. 1987;148(6):1159–64. doi: 10.2214/ajr.148.6.1159. [DOI] [PubMed] [Google Scholar]
- 30.Vande Berg BE, Malghem JJ, Labaisse MA, Noel HM, Maldague BE. MR imaging of avascular necrosis and transient marrow edema of the femoral head. RadioGraphics. 1993;13(3):501–20. doi: 10.1148/radiographics.13.3.8316660. [DOI] [PubMed] [Google Scholar]
- 31.Koo KH, Ahn IO, Kim R, Song HR, Jeong ST, Na JB, et al. Bone marrow edema and associated pain in early stage osteonecrosis of the femoral head: prospective study with serial MR images. Radiology. 1999;213(3):715. doi: 10.1148/radiology.213.3.r99dc06715. [DOI] [PubMed] [Google Scholar]
- 32.Huang GS, Chan WP, Chang YC, Chang CY, Chen CY, Yu JS. MR imaging of bone marrow edema and joint effusion in patients with osteonecrosis of the femoral head: relationship to pain. AJR Am J Roentgenol. 2003;181(2):545. doi: 10.2214/ajr.181.2.1810545. [DOI] [PubMed] [Google Scholar]
- 33.Nam KW, Kim YL, Yoo JJ, Koo KH, Yoon KS, Kim HJ. Fate of untreated asymptomatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 2008;90(3):477–84. doi: 10.2106/JBJS.F.01582. CrossRef, Medline. [DOI] [PubMed] [Google Scholar]
- 34.Beltran J, Knight CT, Zuelzer WA, et al. Core decompression for avascular necrosis of the femoral head: correlation between long-term results and preoperative MR staging. Radiology. 1990;175(2):533–6. doi: 10.1148/radiology.175.2.2326478. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu K, Moriya H, Akita T, Sakamoto M, Suguro T. Prediction of collapse with magnetic resonance imaging of avascular necrosis of the femoral head. J Bone Joint Surg Am. 1994;76(2):215–23. doi: 10.2106/00004623-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Zhao FC, Li ZR, Zhang NF, Wang BL, Sun W, Cheng LM, et al. Lesion size changes in osteonecrosis of the femoral head: a long-term prospective study using MRI. Int Orthop. 2010;34(6):799. doi: 10.1007/s00264-009-0829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerimaa P, Väänänen M, Ojala R, Hyvönen P, Lehenkari P, Tervonen O, Blanco Sequeiros R. MRI-guidance in percutaneous core decompression of osteonecrosis of the femoral head. Eur Radiol. 2015 doi: 10.1007/s00330-015-3905-y. [DOI] [PubMed] [Google Scholar]
- 38.Elias I, Zoga AC, Schweitzer ME, Ballehr L, Morrison WB, Raikin SM. A specific bone marrow edema around the foot and ankle following trauma and immobilization therapy: pattern description and potential clinical relevance. Foot Ankle Int. 2007;28(4):463–71. doi: 10.3113/FAI.2007.0463. [DOI] [PubMed] [Google Scholar]
- 39.Buchan CA, Pearce DH, Lau J, White LM. Imaging of postoperative avascular necrosis of the ankle and foot. Semin Musculoskelet Radiol. 2012;16(3):192–204. doi: 10.1055/s-0032-1320060. [DOI] [PubMed] [Google Scholar]
- 40.Hong N, Du X, Nie Z, Li S. Diffusion-weighted MR study of femoral head avascular necrosis in severe acute respiratory syndrome patients. J Magn Reson Imaging. 2005;22(5):661–4. doi: 10.1002/jmri.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mackenzie JD, Hernandez A, Pena A, Ruppert K, Khrichenko D, Gonzalez L, Jawad AF, Wells L, Smith-Whitley K, Jaramillo D. Magnetic resonance imaging in children with sickle cell disease—detecting alterations in the apparent diffusion coefficient in hips with avascular necrosis. Pediatr Radiol. 2012;42(6):706–13. doi: 10.1007/s00247-011-2327-5. [DOI] [PubMed] [Google Scholar]
- 42.Ozel BD, Ozel D, Ozkan F, Halefoglu AM. Diffusion-weighted magnetic resonance imaging of femoral head osteonecrosis in two groups of patients: Legg-Perthes-Calve and Avascular necrosis. Radiol Med. 2015 doi: 10.1007/s11547-015-0589-y. [DOI] [PubMed] [Google Scholar]
- 43.Menezes NM, Connolly SA, Shapiro F, Olear EA, Jimenez RM, Zurakowski D, Jaramillo D. Early ischemia in growing piglet skeleton: MR diffusion and perfusion imaging. Radiology. 2007 Jan;242(1):129–36. doi: 10.1148/radiol.2421050680. [DOI] [PubMed] [Google Scholar]
- 44.Jaramillo D, Connolly SA, Vajapeyam S, Robertson RL, Dunning PS, Mulkern RV, Hayward A, Maier SE, Shapiro F. Normal and ischemic epiphysis of the femur: diffusion MR imaging study in piglets. Radiology. 2003;227(3):825–32. doi: 10.1148/radiol.2273011673. [DOI] [PubMed] [Google Scholar]
- 45.Öner AY, Aggunlu L, Akpek S, Celik A, Le Roux P, Tali T, Gultekin S. Staging of hip avascular necrosis: is there a need for DWI? Acta Radiol. 2011;52(1):111–4. doi: 10.1258/ar.2010.100231. [DOI] [PubMed] [Google Scholar]
- 46.Mueller D, Schaeffeler C, Baum T, Walter F, Rechl H, Rummeny EJ, Woertler K. Magnetic resonance perfusion and diffusion imaging characteristics of transient bone marrow edema, avascular necrosis and subchondral insufficiency fractures of the proximal femur. Eur J Radiol. 2014;83(10):1862–9. doi: 10.1016/j.ejrad.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 47.Nadel SN, Debatin JF, Richardson WJ, et al. Detection of acute avascular necrosis of the femoral head in dogs: dynamic contrast-enhanced MR imaging vs spin-echo and STIR sequences. AJR Am J Roentgenol. 1992;159(6):1255–61. doi: 10.2214/ajr.159.6.1442396. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi S, Fujioka M, Ikoma K, Saito M, Ueshima K, Ishida M, Kuribayashi M, Ikegami A, Mazda O, Kubo T. Evaluation of femoral perfusion in a rabbit model of steroid-induced osteonecrosis by dynamic contrast-enhanced MRI with a high magnetic field MRI system. J Magn Reson Imaging. 2015;41(4):935–40. doi: 10.1002/jmri.24632. [DOI] [PubMed] [Google Scholar]
- 49.Chan WP, Liu YJ, Huang GS, Lin MF, Huang S, Chang YC, Jiang CC. Relationship of idiopathic osteonecrosis of the femoral head to perfusion changes in the proximal femur by dynamic contrast-enhanced MRI. AJR Am J Roentgenol. 2011;196(3):637–43. doi: 10.2214/AJR.10.4322. [DOI] [PubMed] [Google Scholar]
- 50.Malizos KN, Zibis AH, Dailiana Z, Hantes M, Karachalios T, Karantanas AH. MR imaging findings in transient osteoporosis of the hip. Eur J Radiol. 2004;50(3):238–44. doi: 10.1016/j.ejrad.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 51.Aaron RK, Dyke JP, Ciombor DM, Ballon D, Lee J, Jung E, Tung GA. Perfusion abnormalities in subchondral bone associated with marrow edema, osteoarthritis, and avascular necrosis. Ann N Y Acad Sci. 2007;1117:124–37. doi: 10.1196/annals.1402.069. [DOI] [PubMed] [Google Scholar]
- 52.Bluemke DA, Petri M, Zerhouni EA. Femoral head perfusion and composition: MR imaging and spectroscopic evaluation of patients with systemic lupus erythematosus and at risk for avascular necrosis. Radiology. 1995;197(2):433–8. doi: 10.1148/radiology.197.2.7480688. [DOI] [PubMed] [Google Scholar]
- 53.Hou CH, Shih TT, Liu CY, Li YD, Enright T. Proton MR spectroscopy of the femoral head—evaluation of patients at risk for avascular necrosis. J Magn Reson Imaging. 2006;24(2):409–17. doi: 10.1002/jmri.20653. [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto S, Watanabe A, Nakamura J, Ohtori S, Harada Y, Kishida S, Wada Y, Takahashi K. Quantitative T2 mapping of femoral head cartilage in systemic lupus erythematosus patients with noncollapsed osteonecrosis of the femoral head associated with corticosteroid therapy. J Magn Reson Imaging. 2011;34(5):1151–8. doi: 10.1002/jmri.22685. [DOI] [PubMed] [Google Scholar]
- 55.Theysohn JM, Kraff O, Theysohn N, Orzada S, Landgraeber S, Ladd ME, Lauenstein TC. Hip imaging of avascular necrosis at 7 Tesla compared with 3 Tesla. Skeletal Radiol. 2014;43(5):623–32. doi: 10.1007/s00256-014-1818-5. [DOI] [PubMed] [Google Scholar]
- 56.Theysohn JM, Kraff O, Orzada S, Theysohn N, Classen T, Landgraeber S, Ladd ME, Lauenstein TC. Bilateral hip imaging at 7 Tesla using a multi-channel transmit technology: initial results presenting anatomical detail in healthy volunteers and pathological changes in patients with avascular necrosis of the femoral head. Skeletal Radiol. 2013;42(11):1555–63. doi: 10.1007/s00256-013-1698-0. [DOI] [PubMed] [Google Scholar]
- 57.Brenner AI, Koshy J, Morey J, Lin C, DiPoce J. The bone scan. Semin Nucl Med. 2012;42(1):11–26. doi: 10.1053/j.semnuclmed.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 58.Mont MA, Ulrich SD, Seyler TM, Smith JM, Marker DR, McGrath MS, Hungerford DS, Jones LC. Bone scanning of limited value for diagnosis of symptomatic oligofocal and multifocal osteonecrosis. J Rheumatol. 2008;35(8):1629–34. [PubMed] [Google Scholar]
- 59.Agarwal KK, Mukherjee A, Sharma P, Bal C, Kumar R. Incremental value of 99mTc-MDP hybrid SPECT/CT over planar scintigraphy and SPECT in avascular necrosis of the femoral head. Nucl Med Commun. 2015;36(10):1055–62. doi: 10.1097/MNM.0000000000000357. [DOI] [PubMed] [Google Scholar]
- 60.Motomura G, Yamamoto T, Abe K, Nakashima Y, Ohishi M, Hamai S, Doi T, Honda H, Iwamoto Y. Scintigraphic assessments of the reparative process in osteonecrosis of the femoral head using SPECT/CT with 99mTc hydroxymethylene diphosphonate. Nucl Med Commun. 2014;35(10):1047–51. doi: 10.1097/MNM.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 61.Miki T, Yamamuro T, Okumura H, Ueo T, Kasai R, Yamamoto I. Scintigraphy in nontraumatic femoral head necrosis. Acta Orthop Scand. 1987;58(4):375–8. doi: 10.3109/17453678709146359. [DOI] [PubMed] [Google Scholar]
- 62.Fontecha CG, Roca I, Barber I, Menendez ME, Collado D, Mascarenhas VV, Barrera-Ochoa S, Soldado F. Femoral head bone viability after free vascularized fibular grafting for osteonecrosis: SPECT/CT study. Microsurgery. 2015 doi: 10.1002/micr.22452. [DOI] [PubMed] [Google Scholar]
- 63.Dasa V, Adbel-Nabi H, Anders MJ, Mihalko WM. F-18 fluoride positron emission tomography of the hip for osteonecrosis. Clin Orthop Relat Res. 2008;466(5):1081–6. doi: 10.1007/s11999-008-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gayana S, Bhattacharya A, Kashyap R, Sen RK, Mittal BR. (18)F-fluoride PET/CT in avascular necrosis of the femoral head. Clin Nucl Med. 2013;38(6):e265–6. doi: 10.1097/RLU.0b013e318266d036. [DOI] [PubMed] [Google Scholar]
- 65.Choi KH, Oh JK, Kim SH, Yoo ID, Choi EK, Han EJ. Osteonecrosis Mimicking Bone Metastasis in Femoral Head on (18)F-FDG PET/CT: A Case Report. Nucl Med Mol Imaging. 2011;45(1):68–71. doi: 10.1007/s13139-010-0060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]