Summary
Complex Regional Pain Syndrome (CRPS) is a multifactorial and disabling disorder with complex etiology and pathogenesis. Goals of therapy in CRPS should be pain relief, functional restoration, and psychological stabilization, but early interventions are needed in order to achieve these objectives. Several drugs have been used to reduce pain and to improve functional status in CRPS, despite the lack of scientific evidence supporting their use in this scenario. They include anti-inflammatory drugs, analgesics, anesthetics, anticonvulsants, antidepressants, oral muscle relaxants, corticosteroids, calcitonin, bisphosphonates, calcium channel blockers and topical agents. NSAIDs showed no value in treating CRPS. Glucocorticoids are the only anti-inflammatory drugs for which there is direct clinical trial evidence in early stage of CRPS. Opioids are a reasonable second or third-line treatment option, but tolerance and long term toxicity are unresolved issues. The use of anticonvulsants and tricyclic antidepressants has not been well investigated for pain management in CRPS. During the last years, bisphosphonates have been the mostly studied pharmacologic agents in CRPS treatment and there are good evidence to support their use in this condition. Recently, the efficacy of intravenous (IV) administration of neridronate has been reported in a randomized controlled trial. Significant improvements in VAS score and other indices of pain and quality of life in patients who received four 100 mg IV doses of neridronate versus placebo were reported. These findings were confirmed in the open-extension phase of the study, when patients formerly enrolled in the placebo group received neridronate at the same dosage, and these results were maintained at 1 year follow-up. The current literature concerning sympathetic blocks and sympathectomy techniques lacks evidence of efficacy. Low evidence was recorded for a free radical scavenger, dimethylsulphoxide (DMSO) cream (50%). The same level of efficacy was noted for vitamin C (500 mg per day for 50 days) in prevention of CRPS in patients affected by wrist fracture. In conclusion, the best available therapeutic approach to CRPS is multimodal and is based on the use of several classes of drugs, associated to early physiotherapy. Neridronate at appropriate doses is associated with clinically relevant and persistent benefits in CRPS patients.
Keywords: complex regional pain syndrome, therapy, pain management, neridronate
Background
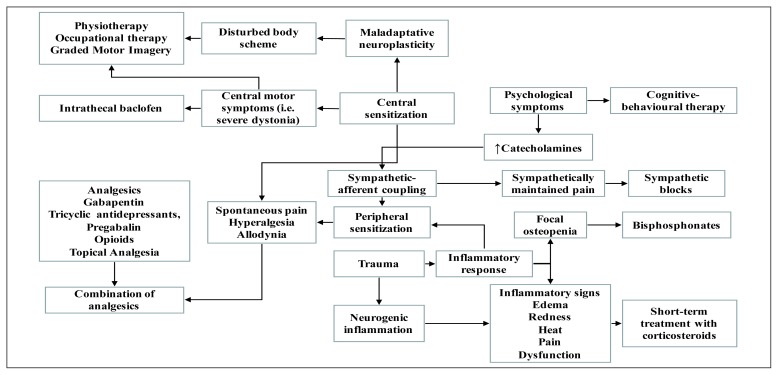
Complex Regional Pain Syndrome (CRPS) is a multifactorial and disabling disorder with complex etiology and pathogenesis. This condition affects vascular, nervous, skeletal, integumentary and immune systems (1). Usually, CRPS develops in a peripheral limb after a trauma or surgery and is characterized by pain, as typical burning pain, accompanied by allodynia and hyperalgesia, disproportionate to the inciting event and by a variety of autonomic disturbances and trophic abnormalities. Management of CRPS is challenging, partly because of a lack of clinical data regarding the efficacy of the available therapies and partly because successful treatment of CRPS requires a multidisciplinary approach (2) (Figure 1). The interdisciplinary approach for treating patients with CRPS is the most pragmatic, helpful, and cost-effective one available today (3). Therefore, this approach should be multimodal, including the following interventions: occupational, physical, vocational, and recreational therapy; psychological/behavioral therapy; drugs; invasive procedures as nerve blocks, spinal cord stimulation and sympathectomy. Goals of therapies in CRPS should be pain relief, functional restoration, and psychological stabilization and to achieve these objectives, treatments should be performed as early as possible (4). Physical and occupational therapy is the cornerstone and first line treatment for CRPS (5, 6). Early mobilization is essential and should be encouraged, avoiding the painful symptoms of the patient. The objectives of physical and occupational therapy are to reduce edema, to normalize sensation of the painful limb through desensitizing therapies, and to decrease functional impairment avoiding maladaptive positioning of the limb affected by CRPS. In particular, the rehabilitative approach should be tailored to specific body functions and consists of progressive loading exercise programs including regular passive and active exercises to mobilize joints in order to improve or maintain the range of motion (1). These interventions are generally used in combination with pain control medications (7).
Figure 1.
Possible treatment on the basis of the pathogenic mechanisms (2).
Pharmacological management of CRPS
Currently there are no guidelines that clearly define what medication should be administered to patients affected by CRPS. Several drugs have been used to reduce pain and pain sensitization and to improve functional status in CRPS, despite the lack of scientific evidence supporting their use in this scenario. The most common medications used to manage this condition are anti-inflammatory, analgesics, anesthetics, anticonvulsants, antidepressants, oral muscle relaxants, corticosteroids, calcitonin, bisphosphonates, calcium channel blockers and topical agents (7). Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), Cyclooxygenase (COX)-2 inhibitors, corticosteroids, and free-radical scavengers are used in order to treat pain and inflammation in CRPS. NSAIDs inhibit COX and prevent the synthesis of prostaglandins, which mediate inflammation and hyperalgesia, and thus may block spinal nociceptive processing (8). Some NSAIDs seem to be more useful than others. For example, ketoprofen may have substantial anti-bradykinin and anti-prostacyclin effects in addition to the typical anti-prosta glandin effect. However, NSAIDs, including selective COX-2 inhibitors, have not supporting evidence in treating CRPS (9). Oral Glucocorticoids (GCs), such as prednisolone at dosage of 40mg/day for 14 days followed by a gradual tapering of 10mg/week (10) are the only anti-inflammatory drug for which there is direct clinical trial evidence in CRPS. These drugs have proved to be effective in the treatment of CRPS, suggesting that a short course of steroids may be indicated in early CRPS with prominent inflammation (11). However longer courses of treatment with steroids are unproven, and there are several serious contraindications to their chronic use (3).
In a recent systematic review and meta-analysis published in Lancet Neurology (12), Authors emphasize that the use of opioids for neuropathic pain is a reasonable second or third-line treatment option to try. However, tolerance and long-term toxicity of these agents remain unresolved issues (13), and long-term high-dose opioid use might even worsen allodynia and/or hyperpathia.
Anticonvulsants, such as gabapentin and pregabalin, interact with neuronal L-type calcium channels, reducing neurotransmitter release. Both drugs demonstrated to be effective in the treatment of trigeminal neuralgia pain, although there is little evidence to recommend their use in other neuropathic pain conditions (14, 15). As both mood disorders and chronic pain share multiple neurochemical pathways, it has been hypothesized that some pharmacological agents used to treat depression might be useful to treat neuropathic pain, including CRPS. Furthermore, both the European Federation of Neurological Societies (EFNS) and the American Academy of Neurology (AAN) recommend the use of Tricyclic Antidepressants (TCA) and selective Serotonin-Norepinephrine Reuptake Inhibitors (SNRI) for the treatment of neuropathic pain (16). However, these drugs are generally used at lower doses for pain relief than that used for the management of depression, but no clinical trial aimed to treat CRPS has been yet performed (3).
Perez et al. (17) found second-line evidence of efficacy for vitamin C (500 mg per day for 50 days) in prevention of CRPS in patients affected by wrist fracture. Two recent meta-analyses reported conflicting conclusions about the role of vitamin C in preventing CRPS in patients with distal radius fractures (18, 19). Considering the good safety profile and low costs, vitamin C might represent a cost-effective approach in preventing CRPS after an orthopedic trauma or surgery.
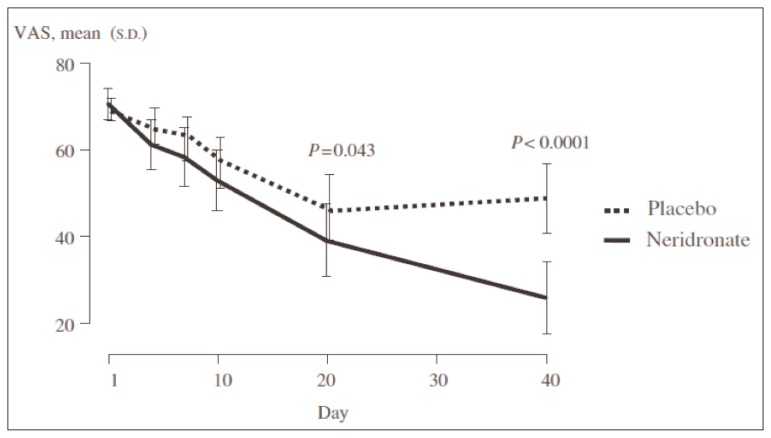
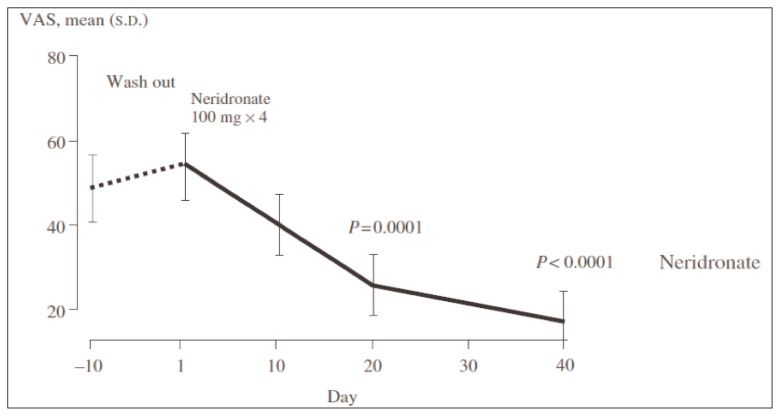
During the last years, bisphosphonates (BPs) have been the most studied pharmacological agents for treatment of CRPS (20). BPs are potent inhibitors of osteoclast activity widely used for the management of osteoporosis and other metabolic bone diseases. However, potential beneficial effects of BPs in CRPS might be not related to their well known antiresorptive activity, but to a more complex interaction between these drugs and the pathophysiological events triggering and maintaining CRPS. Several studies have demonstrated the efficacy of these medications compared to placebo for the treatment of CRPS. Oral or intravenous (IV) alendronate, IV pamidronate, clodronate and ibandronate reduced pain and improved physical function in patients with CRPS with a good profile of safety and tolerability (21–23). In CRPS, patchy osteoporosis due to osteoclast hyperactivity is a common finding, but the role of osteoclast activation in this condition is not well understood. The main mechanism of action of BPs is the inhibition of osteoclast activity and consequently bone resorption. A secondary action of these molecules could be the interference with the inflammatory and nociceptive pathways in CRPS (24). In fact, it seems that acidification of the extracellular milieu caused by osteoclast activation may activate nociceptive acid-sensing receptors and the release of pro-inflammatory cytokines (25, 26). Moreover, BPs could interfere with macrophages activation, implicated with overexpression on Nerve Growth Factor (NGF) responsible for neurogenic inflammation (24). On the basis of these findings and of available data from clinical trials, there is sufficient evidence to support the use of BPs as first line drug in the management of CRPS in clinical practice. On the other hand, the main limitations of these studies were the small number of patients enrolled and the supporting sponsorship for most of the them. Recently the efficacy of IV administration of neridronate has been reported by Varenna et al. (24) in a randomized controlled trial on a population of 82 patients with CRPS type I. The Authors reported a significant decrease in Visual Analogue Scale (VAS) score and other indices of pain and quality of life in patients who received four 100 mg IV doses of neridronate versus placebo (Figure 2). The same trend was noted in the open-extension phase of the study, when patients formerly enrolled in the placebo group received neridronate at the same dosage (Figure 3). The positive results were confirmed at 1-year follow-up. The Authors concluded that neridronate at appropriate doses is associated with clinically relevant and persistent benefits in CRPS patients. Moreover, a randomized, double-blind, placebo controlled study to assess the safety and the efficacy of intramuscular administration of neridronate at doses of 25 mg for consecutive 16 days in patients with CRPS type I is ongoing (27). The expected results would be similar to the findings of trial performed by Varenna et al.
Figure 2.
Efficacy of intravenous administration of neridronate vs placebo (24).
Figure 3.
Efficacy of intravenous administration of neridronate vs placebo. Results from open extension phase of the study, when patients formerly enrolled in the placebo group received neridronate at the same dosing regimen (24).
Interventional approach to CRPS
When conservative therapies fail, invasive treatment modalities are attempted. Sympathetic nerve blocks (cervicothoracic block for the upper extremity and lumbar block for the lower extremity) under fluoroscopic control are commonly indicated for CRPS treatment especially for patients with prevalent sympathetic symptoms (3). Reduction of pain and functional impairment after a single block are considered a positive response and may suggest potential benefits of an extended series of blocks, especially if associated to physical and occupational therapy. Therefore, the pharmacological block must be selective, sparing sensory and motor function of the limb. Skin temperature rise, along with superficial vein engorgement and Horner’s syndrome in the cervicothoracic block, are other signs of successful sympathetic block (3). In patients responsive to sympathetic nerve blocks, surgical or chemical sympathectomy have also been proposed. Chemical sympathectomy consists in phenol or alcohol injection performed with the same techniques and targets of sympathetic blocks. Surgical sympathectomy may be performed with an open approach or more frequently with a minimally invasive technique using thermoablation, which results might be more lasting and reproducible according to some Authors. Technical demand and potential complications are similar to sympathetic blocks (1).
The current literature concerning sympathetic blocks and sympathectomy techniques showed a lack of evidence to support efficacy and also practical guidelines about drugs selection are currently missing. Clinicians should therefore carefully consider the balance between potential risks and benefits of these techniques before proceeding to the treatment (3).
Spinal Cord Stimulation (SCS) consists in stimulating the dorsal columns of the spinal cord via electrodes placed in the epidural space. The mechanism of action is not clear, probably involving an altered local neurochemistry in dorsal horn, suppressing the hyperexcitability of the neurons (28).
When applied in the early stages of CRPS, SCS can significantly increase limb functionality and pain relief, but its use remains controversial and should be reserved to carefully selected patients (28). Clinically detectable goals of SCS in CRPS include reduction in pain perception and muscle dysfunction, and improvement in blood flow (1). Patients should undergo a test stimulation with a temporary device to assess responsiveness and confirm the potential benefit of a permanent implant. Electrodes are placed in the lower cervical region for upper extremity involvement and in the lower thoracic region for the lower extremity impairment, adjusting final placement on the basis of patients referral of paresthesias in the affected region. Complications of this technique range from infection and bleeding to spinal cord or nerve injury, and implant migration, malposition or breakage, and pulse-generator failure may also occur (29).
Intravenous Regional Anesthesia (IVRA) has been tried with some efficacy reported for bretylium and ketanserin use (1). Given the multifactorial pathogenesis and the polymorphous clinical picture of CRPS, the appropriate therapy will be tailored for each specific patient. The therapeutic approach should be multimodal, in particular in chronic pain (>6 months). At an early stage, an intensive pharmacological approach, including neridronate and/or other drugs (corticosteroids, analgesics, vitamin C) combined with a rehabilitative approach focused on maximizing functional independence and pain relief will provide the best result. In a chronic phase it is mandatory a multimodal approach, including as key treatments sensory modulation modalities for pain control through conservative and interventional techniques. In any case these treatments should be associated to BPs, such as neridronate as first line drugs.
References
- 1.Freedman M, Greis AC, Marino L, et al. Complex Regional Pain Syndrome. Diagnosis and Treatment. Phys Med Rehabil Clin N Am. 2014;25:291–303. doi: 10.1016/j.pmr.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Gierthmuhlen J, Binder A, Baron R. Mechanism-based treatment in complex regional pain syndromes. Nat Rev Neurol. 2014;10(9):518–28. doi: 10.1038/nrneurol.2014.140. [DOI] [PubMed] [Google Scholar]
- 3.Harden RN, Oaklander AL, Burton AW, et al. Complex Regional Pain Syndrome: practical diagnostic and treatment guidelines, 4th edition. Pain Medicine. 2013;14:180–229. doi: 10.1111/pme.12033. [DOI] [PubMed] [Google Scholar]
- 4.Rockett M. Diagnosis, mechanisms and treatment of complex regional pain syndrome. Curr Opin Anaesthesiol. 2014;27:494–500. doi: 10.1097/ACO.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 5.Rho RH, Brewer RP, Lamer TJ, et al. Complex regional pain syndrome. Mayo Clin Proc. 2002;77:174–80. doi: 10.4065/77.2.174. [DOI] [PubMed] [Google Scholar]
- 6.Barnhoorn KJ, Staal JB, van Dongen RT, et al. Are pain-related fears mediators for reducing disability and pain in patients with complex regional pain syndrome type 1? An explorative analysis on pain exposure physical therapy. PLoS One. 2015;10(4):e0123008. doi: 10.1371/journal.pone.0123008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackey S, Feinberg S. Pharmacologic therapies for complex regional pain syndrome. Curr Pain Headache Rep. 2007;11(1):38–43. doi: 10.1007/s11916-007-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geisslinger G, Yaksh T. Spinal actions of cyclooxygenase isoenzyme inhibitors. In: Devor M, Rowbotham M, Wiesenfeld-Halin Z, editors. Proceedings of the 9th World Congress on Pain. Seattle, WA: IASP Press; 2000. pp. 833–55. [Google Scholar]
- 9.Breuer AJ, Mainka T, Hansel N, et al. Short-term treatment with parecoxib for complex regional pain syndrome: a randomized, placebo-controlled double-blind trial. Pain Physician. 2014;17(2):127–37. [PubMed] [Google Scholar]
- 10.Kalita J, Vajpayee A, Misra UK. Comparison of prednisolone with piroxicam in complex regional pain syndrome following stroke: a randomized controlled trial. QJM. 2006;99:89–95. doi: 10.1093/qjmed/hcl004. [DOI] [PubMed] [Google Scholar]
- 11.Wesseldijk F, Huygen FJ, Heijmans-Antonissen C, et al. Six years follow-up of the levels of TNF-alpha and IL-6 in patients with complex regional pain syndrome type 1. Mediators Inflamm. 2008;2008:469439. doi: 10.1155/2008/469439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–73. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendlik MT, Uritsky TJ. Treatment of Neuropathic Pain. Curr Treat Options Neurol. 2015;17(12):50. doi: 10.1007/s11940-015-0381-2. [DOI] [PubMed] [Google Scholar]
- 15.van de Vusse AC, Stomp-van den Berg SG, Kessels AH, et al. Randomised controlled trial of gabapentin in complex regional pain syndrome type 1 [ISRCTN84121379] BMC Neurol. 2004;4:13. doi: 10.1186/1471-2377-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baltenberger EP, Buterbaugh WM, Martin BS, et al. Review of antidepressants in the treatment of neuropathic pain. Mental Health Clinician. 2015;5(3):123–33. [Google Scholar]
- 17.Perez RS, Zollinger PE, Dijkstra PU, et al. Evidence based guidelines for complex regional pain syndrome type 1. BMC Neurology. 2010;10:20. doi: 10.1186/1471-2377-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meena S, Sharma P, Gangary SK, et al. Role of vitamin C in prevention of complex regional pain syndrome after distal radius fractures: a meta-analysis. Eur J Orthop Surg Traumatol. 2015;25(4):637–41. doi: 10.1007/s00590-014-1573-2. [DOI] [PubMed] [Google Scholar]
- 19.Evaniew N, McCarthy C, Kleinlugtenbelt YV, et al. Vitamin C to Prevent Complex Regional Pain Syndrome in Patients With Distal Radius Fractures: A Meta-Analysis of Randomized Controlled Trials. J Orthop Trauma. 2015;29(8):e235–41. doi: 10.1097/BOT.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 20.Varenna M, Adami S, Sinigaglia L. Bisphosphonates in complex regional pain syndrome type I: how do they work ? Clin Exp Rheumatol. 2014;32:451–4. [PubMed] [Google Scholar]
- 21.Manicourt DH, Brasseur JP, Boutsen Y, et al. Role of alendronate in therapy for posttraumatic complex regional pain syndrome type I of the lower extremity. Arthritis Rheum. 2004;50(11):3690–7. doi: 10.1002/art.20591. [DOI] [PubMed] [Google Scholar]
- 22.Simm PJ, Briody J, McQuade M, et al. The successful use of pamidronate in an 11-year-old girl with complex regional pain syndrome: Response to treatment demonstrated by serial peripheral quantitative computerised tomographic scans. Bone. 2010;46(4):885–8. doi: 10.1016/j.bone.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 23.Breuer B, Pappagallo M, Ongseng F, et al. An Open-label Pilot Trial of Ibandronate for Complex Regional Pain Syndrome. Clin J Pain. 2008;24:685–9. doi: 10.1097/AJP.0b013e318175920f. [DOI] [PubMed] [Google Scholar]
- 24.Varenna M, Adami S, Rossini M, et al. Treatment of complex regional pain syndrome type I with neridronate: a randomized, double-blind, placebo-controlled study. Rheumatology (Oxford) 2013;52(3):534–42. doi: 10.1093/rheumatology/kes312. [DOI] [PubMed] [Google Scholar]
- 25.Evans JR, Robertson WG, Morgan DB, et al. Effect of pyrophosphate and diphosphonates on the dissolution of hydroxyapatites using a flow system. Calcif Tissue Int. 1980;31:1539. doi: 10.1007/BF02407176. [DOI] [PubMed] [Google Scholar]
- 26.Fast DK, Felix R, Dowse C, et al. The effects of diphosphonates on the growth and glycolysis of connective tissue cells in culture. Biochem J. 1978;477:83107. doi: 10.1042/bj1720097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. [Accessed 29 December 2015]. Available: https://www.clinicaltrialsregister.eu/ctr-search/trial/2014-001156-28/IT.
- 28.Oakley JC, Prager JP. Spinal cord stimulation: mechanisms of action. Spine (Phila Pa 1976) 2002;27(22):2574–83. doi: 10.1097/00007632-200211150-00034. [DOI] [PubMed] [Google Scholar]
- 29.Christo PJ, Jones CT. Complex Regional Pain Syndrome: Treatment Approaches. In: Smith HS, editor. Current therapy in Pain. Elsevier; 2009. [Google Scholar]