Summary
The prevention of femoral head collapse and the maintenance of hip function would represent a substantial achievement in the treatment of osteonecrosis of the femoral head; however it is difficult to identify appropriate treatment protocols to manage patients with pre-collapse avascular necrosis in order to obtain a successful outcome in joint preserving procedures.
Conservative treatments, including pharmacological management and biophysical modalities, are not supported by any evidence and require further investigation. The appropriate therapeutic approach has not been identified. The choice of surgical procedures is based on patient clinical conditions and anatomopathological features; preservation of the femoral head by core decompression may be attempted in younger patients without head collapse. Biological factors, such as bone morphogenetic proteins and bone marrow stem cells, would improve the outcome of core decompression.
Another surgical procedure proposed for the treatment of avascular necrosis consists of large vascularized cortical bone grafts, but its use is not yet common due to surgical technical issues. Use of other surgical technique, such as osteotomies, is controversial, since arthroplasty is considered as the first option in case of severe femoral head collapse without previous intervention.
Keywords: femur head necrosis, osteonecrosis, orthopedic procedures, bone transplantation
Introduction
Avascular Necrosis (AVN) or Osteonecrosis of the Femoral Head (ONFH) is an increasing cause of musculoskeletal disability. It is an insidious and progressive disease that may lead to the complete deterioration of the hip joint which mostly occurs in individuals aged between the third and fifth decades of life. It was estimated that approximately 10% of the Total Hip Arthroplasties (THA) was performed for osteonecrosis (1).
Although several hypotheses have been proposed, the pathogenic mechanisms that lead to impaired blood supply to the bone resulting in death of osteocytes, progressive collapse of the subchondral bone and secondary Osteoarthritis (OA), are not well understood. However, both traumatic and nontraumatic causes have been described for AVN, including trauma, alcoholism, blood cell disorders, corticosteroid administration, pregnancy, collagen diseases, adipogenesis of bone marrow, immune deficiency conditions, but it can be idiopathic as well (2, 3).
Over the last 40 years different classification systems to define severity and progression of ONFH have been proposed. Kerboul et al. (4) estimated the extent of AVN at early stages measuring the combined necrotic angle of the femoral head involved, defined as ‘Kerboul angle’, on anteroposterior and lateral radiographs. This parameter can also be obtained from MRI scans and a necrotic angle <200° predicts lower risk of collapse in patients with femoral head necrosis (5). The Ficat and Arlet staging system (6), based on radiological findings, is still one of the most used classification method, even if it does not consider the extent of the lesion. Indeed, this parameter is helpful in predicting the collapse of the articular surface (7). Steinberg et al. (8) added quantification of femoral head involvement to the AVN classification, but this staging method is not widely used due to application issues. The Association Research Circulation Osseous (ARCO) classification system provided a more detailed quantification of the involvement of the femoral head, as well as MRI findings (9).
At an early-stage, osteonecrosis of the femoral head may be asymptomatic, whereas in a later stage it becomes painful and might cause a limitation of hip Range Of Motion (ROM). In most cases natural evolution of AVN of femoral head might lead to subsequent collapse of femoral surface and secondary osteoarthritis with typical clinical signs.
Management of patients with AVN consists of conservative and surgical approaches. The first one is not considered completely effective in most of cases (10), especially when used as single treatment, and is mostly prescribed in the precollapsed or early collapsed stage (<2 mm). Several hip preservation surgical treatments have been described to avoid the most invasive Total Hip Arthroplasty (THA) which is commonly indicated when there is a severe joint involvement.
Femoral head sparing procedures
The aim of surgical management of AVN is to preserve the joint and to avoid or delay the hip replacement, in particular in young patient. The following techniques have been proposed.
Core decompression
Core Decompression (CD) is the most commonly used procedure for the treatment of the pre-collapse stage of ONFH. The purpose of this treatment is to reduce the intraosseous pressure in the femoral head and increase the blood flow to the affected area in order to stimulate new bone formation. It is considered a simple and cost-effective procedure (11), but the success of the treatment is largely dependent on the causes and the stage of the lesion (12–14), as suggested by many Authors (15–20).
The standard CD technique is performed using 8–10 mm trephine or cannula introduced in the subtrochanteric femoral cortex. The necrotic lesion of the femoral head is then reached passing through the femoral neck. A potential risk of this technique is an excessive weakening of lateral femoral cortex which can cause a subtrochanteric fracture (19).
However, technical issues of CD improved over time (18), as confirmed by modifications of standard technique presented in the annual ARCO meeting in 2004. Kim et al. (16) described this modified technique with multiple small drillings with a 3 mm Steinman pin to perform the CD which theoretically reduced proximal femur weakening compared to the standard technique. The Authors compared 54 consecutive patients in which Multiple Drillings (MD) and conventional CD were performed; they observed both a longer time before collapse and a lower rate of collapse in the group receiving MD technique. Similar results were described by other Authors (18–20) so that this approach is believed to be an easy and simple procedure that can be safely performed percutaneously, under image intensifier guidance and with minimal risk of subtrochanteric fracture.
Mesenchymal stem cells or growth factors and core decompression
Since 2002, biological agents, including both osteogenic (Mesenchymal Stem Cells, MSCs) and/or osteoinductive factors (Bone Morphogenetic Proteins, BMPs), have been developed in order to improve surgical outcomes of CD technique (21–27). Hernigou et al. (21) hypothesized that there is an insufficient supply of progenitor cells enhancing bone remodeling in areas of AVN in patients with ONFH. MSCs are widely represented in different tissues, such as bone marrow, adipose tissue, muscle and tendon, synovial membrane, and blood vessels (28–30). Once separated and extracted from those tissues, MSCs maintained their capacity for multipotential differentiation and proliferation (31). They can differentiate into various cell types of several tissues under different conditions (32), and therefore MSCs have been widely applied to treat bone and cartilage defects, ONFH, osteoarthritis, and other diseases (33, 34). The combination of MSCs transplantation and CD surgery can enhance femoral head repair promoting reconstruction and creeping substitution of new bone (35, 36) (Figure 1). Hernigou et al. (22) performed for the first time a clinical study using core decompression and autologous bone marrow transplantation for the treatment of 189 hips in 116 ONFH cases. Only 9 cases of 145 hips, operated during Ficat stages I-II, required hip replacement after a mean 7-year follow-up, whereas 25 of 44 hips treated during Ficat stages III-IV, needed joint arthroplasty. Many studies confirmed the safety and efficacy of MSCs in ONFH (37–40). Gangji et al. (41) in a prospective, randomized, double blind trial, compared the surgical outcome of 2 groups of patients treated with isolated CD or CD and implantation of autologous bone marrow cells. In 24 cases at ARCO Stage I and II, they observed a significant improvement of pain and lower rate of radiographic progression in the group treated with the combined approach. However, they did not report a significant difference in both the groups in terms of subsequent THA. Cuervas-Mons et al. (42) demonstrated that infusion of MSCs during CD procedure improved hip function and avoided Total Hip Replacement (THR) in 75.3% of patients with ONFH during the first 2 years of treatment.
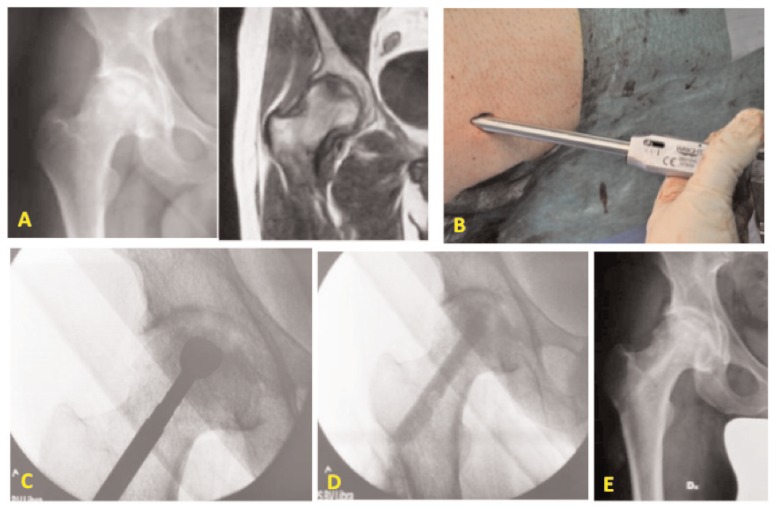
Figure 1.
Male patient of 44 years old with osteonecrosis of the right femoral head. A) Preoperative X-ray and MRI of the right hip; B) mini-invasive approach to the greater trochanter for the standard Core Decompression; C) burring the necrotic lesion in the femoral head through the femoral neck; D) fluoroscopic control at the end of the surgical procedure: conventional CD and application of bone substitute and autologous MSCs from iliac crest; E) X-rays at 3 months after surgical procedure.
Some of the growth factors produced by osteogenic cells, platelets, and inflammatory cells are implicated in bone healing (25) and BMPs are most commonly used to enhance this physiological process. The potential application of BMPs for the treatment of ONFH was investigated in experimental studies some years ago. Tang et al. (43) induced bilateral early-stage ONFH in adult goats ligating the lateral and medial circumflex arteries and delivering liquid nitrogen into the femoral head. CD was performed and porous Beta-Tricalcium Phosphate (β-TCP) was loaded with BMP-2 gene-modified or beta-galactosidase (beta-gal)-gene-transduced Bone-Marrow Mesenchymal Stem Cells (BMSCs) were implanted into the left and right femoral heads, respectively. The maximum compressive strength and Young’s modulus of femoral head treated with BMP-2 were similar to those of normal bone and significantly higher than those in the beta-gal group. Xiao et al. (44) induced early-stage ONFH in white rabbits delivering liquid nitrogen into the femoral head. The animals were divided in 3 groups according to the treatment received: a first control group received no treatment, a second group was treated with Bio-derived Bone Materials (BBM) combined with recombinant human Bone Morphogenetic Protein-2 (rhBMP-2) and a third group was treated with autologous BMSCs-seeded BBM combined with rhBMP-2. Authors observed histological improvements in femoral head in both groups treated with rhBMP-2. These positive findings derived from animal studies encouraged the use of BMPs in association with different scaffolds or bone substitutes also in humans. Mont et al. (45) used a demineralized bone matrix and a thermoplastic carrier plus the addition of BMP-7 for ONFH in 21 patients. In this study, a Harris Hip Score (HHS)>80 was observed in 86% of patients over a mean 48-month follow-up. Lieberman et al. (26) treated 15 patients at Ficat stage II or III with CD and autolyzed fibula allografts, combined with rhBMP-2 and Noncollagenous Proteins (NCPs). Radiographic progression of AVN was avoided in 14 of 17 hips at an average of 53 months. Only one patient, classified as Ficat stage IIA, developed collapse; among the patients reporting radiographic progression, two other hips already had collapse of the femoral head before the procedure. Seyler et al. (46) used autologous, nonvascularized bone-grafts combined with BMP-7 in 39 hips: 22 at Ficat stage II and 17 at Ficat stage III. At a mean time of 36 months, only 4 of 22 Ficat stage II hips and 11 of 17 Ficat stage III hips needed joint replacement.
Bone Grafting
Bone grafting has been used after removal of the necrotic lesion to provide structural support to the femoral head, or to act as a scaffold facilitating bone remodeling. The grafting can be performed through the CD tract, the most common and simple approach, but other techniques have been described. Merle d’Aubigne et al. (47) firstly described the “trap door technique”. After dislocating the femoral head, a flap was created on the chondral surface and the necrotic segment was removed with curettes and burrs to allow the filling of the lesion with the graft. Rosenwasser et al. (48) described a similar procedure, the “light bulb procedure”, to reach the subchondral bone of the femoral head through a window created at the head-neck junction.
Considering the use of bone grafts in the treatment of ONFH, many options have been described but nonvascularized graft has been more appealing because it is less technically demanding and reduce donor-site morbidity (49).
Currently, bone grafting modalities are rarely used as isolated procedures; indeed many researchers performed these techniques in combination with growth factors and various bone-graft substitutes to enhance bone remodeling and to avoid the collapse of the femoral head (50). In a long-term study, Smith et al. (51) observed that 40 of 56 hips treated with nonvascularized grafts had poor clinical results after a mean 14-year follow-up. Another study, conducted by Keizer et al. (52) described the long-term results of CD and placement of a nonvascularized bone graft, from tibia or fibula, for the management of AVN of the femoral head in 80 hips. They observed a survival rate of 59% five years after surgery. They also found a significant difference in survivorship in favor of the tibial autograft. Seyler et al. (46) reported 83% survivorship in stage I and II ONFH and 78% survivorship at a minimum 2-year follow-up in 39 hips using the light bulb procedure. Many Authors (26, 45, 53) described the effects of autologous nonvascularized graft in combination with BMP-7 reporting good results and a lower rate of femoral head collapse.
Vascularized bone-grafting is recommended for the treatment of early ONFH (Ficat stage I to III) when the lesion involves <50% of the femoral head and its collapse is <2 mm. On the one hand, graft (usually vascularized iliac crest graft or vascularized fibula graft) provides a viable structural support for the subchondral bone and articular cartilage; on the other, as the vascularity is preserved and the graft has osteogenic potential, this approach may facilitate bone healing of the necrotic area. However, patients with a history of smoking, alcoholism, peripheral vascular disease or other risk factors for AVN should not be considered for this intervention (54).
Baksi et al. (55, 56) investigated the effects of Muscle-Pedicle Bone Graft (MPBG) alone, using Quadratus Femoris (QF) or sartorius or gluteus medius or Tensor Fascia Lata (TFL), in different stages of ONFH. These studies suggested that, since the necrotic area is located mainly in the anterosuperior aspect of the femoral head, intralesional curettage combined with the use of adjacent MPBG was the treatment of choice. Meyers et al. (57) demonstrated that application of MPBG for treatment of ONFH was effective in all patients with stage I and II but only in 33% of patients in stage III and IV. Lee and Rehmatullah reported a 70% success rate with the same approach in idiopathic ONFH (58). In our opinion, of the several available MPBG, the TFL for the anterior approach, and the QF for the posterior one, should be preferred.
The use of vascularized iliac crest graft is recommended for treatment of Ficat stage II and early-stage III. The success rate of this surgical technique is approximately of 74%. Iwato et al. (59) suggested that patients with no femoral head collapse preoperatively, progressed to radiographic collapse after a mean 3-year follow-up in >50% of cases. Similar findings have been reported by Eisenschenk et al. (60) that noted stable disease after 5-year follow-up only in 56% of patients treated with iliac crest graft perfused by the deep circumflex iliac artery. Better results are reported by Matsusaki et al. (61) who combined the use of vascularized pedicle iliac bone-graft with trans-trochanteric anterior rotational osteotomy; Authors demonstrated that there was no disease progression in 12 of 17 hips (71%) after a 50.7-month follow-up, and suggested that this surgical approach might be promising for joint preservation in patients with ONFH.
When compared with other methods, such as CD, nonvascularized bone graft, and osteotomies, free vascularized fibula grafting provides the most consistently successful results. In 1997 Sotereanos et al. (62) investigated the effects of this surgical approach for the treatment of ONFH, classified according to Steinberg staging system, demonstrating that the probability for conversion to THA within 5.5 years after interventions was 28% for stage II hips and 38% for stages III and IV hips. A previous study performed by Malizos et al. (63) demonstrated radiographic improvement, recovery of hip function, and a significant pain relief in all patients treated with vascularized fibula grafting for ONFH at precollapse stage. This surgical technique does not violate the capsula articularis as the other bone grafting procedures (pedicle iliac crest graft and QF pedicle graft) do (64). However, the success of this procedure is related to multiple factors: 1) decompression of the femoral head; 2) excision of the necrotic bone underneath the weight-bearing region that might inhibit revascularization of the femoral head; 3) buttress of the articular surface with the vascularized fibular graft by primary callus formation augmented with additional cancellous bone graft; and 4) a period of non-weight bearing to preserve bone healing.
Porous tantalum implants
The porous tantalum rod in combination with CD has been proposed to avoid the risk of autograft harvest or the infection of bone allograft (65). This method has the advantage of providing a mechanical support to the weakened subchondral bone of the femoral head and to fill the decompressed site (Figure 2). The standard technique provides the identification of core tract, the center of the necrotic lesion and a point immediately superior to the lesser trochanter. Subsequently at the insertion of a guide pin, using cannulated reamers, the core should be progressively ream from 8 mm to 10 mm under fluoroscopy, approximately 5 mm from the endosteal surface of the femoral head. Porous tantalum has osteoconductive capacity and provides a structural scaffold for bone ingrowth. It has been shaped in order to produce a high-porosity biomaterial screw with fully interconnected pores. This material is modeled in such way to reproduce the trabecular structure of cancellous bone (66).
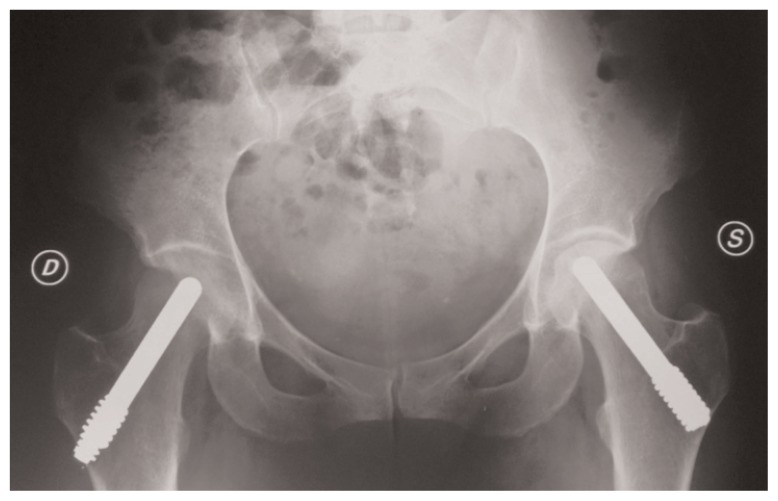
Figure 2.
X-ray examination of a bilateral ONFH treated with porous tantalum rod in combination with core decompression: 7 years postoperative left hip and 3 month postoperative right hip.
Vitreous Tantalum (98% tantalum, 2% vitreous carbon) screw has a threaded lateral extremity that can be inserted into the femoral neck to support the articular cartilage, stimulating the repair process, and interrupting the interface between necrotic and healthy tissue, thus improving local vascularization (67, 68). The use of this material, together with biological agents, such as growth factors, or drugs, such as bisphosphonates (BPs), can improve bone formation around and within trabecular metal implants (69–73).
This procedure might be a useful alternative to conservative treatment for young individuals with AVN at first or second stage in order to reduce the period of non-weight-bearing. This method was first proposed by Pedersen et al. (74) that suggested porous tanta lum rods as reasonable mechanical substi tute of a fibular graft. Since 2005, this material has been used in a lot of clinical studies, following CD, for ONFH treatment (75–81).
However, the role of porous tantalum implant and the clinical outcomes related to its use, including postoperative weight-bearing time, are still controversial and only few studies reported the long-term survivorship of the implants (13–18). Veillette et al. (82) investigating 54 patients with ONFH treated with CD and the insertion of a porous tantalum rod, reported overall survival rates of 91.8% at 24 months, and 68.1% at 48 months. Aldegheri et al. (67) performed 15 implants of tantalum screw, and assessed the clinical outcome and AVN staging using the HHS and the Steinberg classification, respectively. They reported in all cases extension of osteonecrosis not exceed 30% of the joint surface and no collapse of femoral head. After a mean 15.43 ± 5.41 month follow-up, 10 implants (in 7 patients) were assessed and all but one patient showed a marked improvement in HHS (+127.9%), with no further progression of the disease. These findings appeared promising, but there are some concerns about the release of metal debris in the joint as well as technical issues during surgery. Moreover, histological studies demonstrated poor bone ingrowth and insufficient mechanical support of the subchondral bone, which are relevant limitations when THR is required (83–85). Recently, Papapietro et al. (86) suggested that the trabecular metal implants are not always clinically satisfactory because of pain and functional limitations that could require conversion to a THA in some cases.
Proximal femoral osteotomies
Proximal femoral osteotomies, for the treatment of ONFH, consist of rotating the necrotic area of femoral head away from the load-bearing area and then replacing it with the uninvolved healthy portion of the head. This approach also reduces the intraosseous venous pressure improving local vascularity.
Two main types of osteotomies are used: transtrochanteric rotational and intertrochanteric varus or valgus osteotomy (combined with flexion or extension). The success rates of these surgical techniques for the treatment of ONFH have been reported between 70% and 93% (87–90). Jacobs et al. (91) had 73% success rate at 5.3-year follow-up after intertrochanteric osteotomy. Maistrelli et al. (92) reported satisfactory surgical outcome in 71% of AVN after 2 years of intertrochanteric varus or valgus osteotomy and it dropped to 58% at 8.2 years.
The main reason for these results is the technical complexity of this approach. Proximal femur osteotomies have never been compared to any other surgical technique and it is difficult to define the superiority of this approach to other methods described. This method of treatment is suitable for patients not being treated with long-term steroids, with minimal osteoarthritic changes, without acetabular involvement, and with a Kerboul angle <200° (93, 94).
Conclusions
Symptomatic AVN of the hip is a disabling condition for which has not yet been identified the exact pathophysiological mechanisms; several treatment options have been described including nonoperative modalities, joint preservation procedures, and THR. Joint preservation treatment may improve the clinical outcomes for small lesions at the pre-collapse stage. The finding of crescent sign, femoral head flattening, and acetabular involvement suggest an advanced stage of AVN in which joint preservation options are less effective than THA (Table 1). Pharmacological treatments and biological agents, including growth and differentiation factors, may influence the surgical management of patients with ONFH, but further studies with long-term follow-up are needed in order to support the available data. However, the successful outcome of joint saving procedures depends on accurate diagnosis and prompt intervention before the collapse of the femoral head.
Table 1.
Data about conservative surgery for ONFH. NA= not available, CD= Core Decompression, MD= Multiple Drilling, NVBG= Non-Vascularized Bone Grafts, MPBG= Muscle Pedicle Bone Graft, ARCO= Association Research Circulation Osseous.
| Clinical Study | No. | Surgical technique | Disease Stage | Results |
|---|---|---|---|---|
| Kim et al. (16) | 65 hips (54 patients) | Conventional CD vs MD | NA | Lower rate of collapse 55% vs 85.7% (3-year follow-up) |
| Hernigou et al. (22) | 189 hips (116 patients) | CD + bone marrow from iliac crest |
Ficat-Arlet stage I and II = 145 stage III and IV = 44 |
Survival rate 13.05% (7-year follow-up) |
| Keizer et al. (52) | 80 hips (78 evaluated) | CD + NVBGs |
Ficat-Arlet stage 0 = 6 stage I = 3 stage IIA = 31 stage IIB = 16 stage III = 13 stage IV = 9 |
Survival rate 59% (5-year follow-up) |
| Rehmatullah et al. (58) | 10 patients | MPBGs | NA | Survival rate 70% (2-year follow-up) |
| Eisenschenk et al. (60) | 8 patients | Iliac crest vascularized graft |
ARCO stage I, II and III |
Survival rate 50% (5-year follow-up) |
| Sotereanos et al. (62) | 88 hips (65 patients) | Free vascularized fibula grafting |
Steinberg stage II, III and IV |
Survival rate 100% stages IC and IIA, 94% for stage IIB, 50% for stage IIC, 80% for stage IIIB, 58% for stage IIIC, 72% for stage IVA, 58% for stage IVB (5.5-year follow-up) |
| Maistrelli et al.(92) | 106 hips | Intertrochanteric osteotomy (varus or valgus) | NA | Survival rate 71% (2-year follow-up) Survival rate 58% (8.2-year follow-up) |
References
- 1.Mont MA, Hungerford DS. Non traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:45974. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis) N Engl J Med. 1992;326:14739. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- 3.Seamon J, Keller T, Saleh J, Cui Q. The pathogenesis of nontraumatic osteonecrosis. Arthritis. 2012;2012:601763. doi: 10.1155/2012/601763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerboul M, Thomine J, Postel M, Merle d’Aubigné R. The conservative surgical treatment of idiopathic aseptic necrosis of the femoral head. J Bone Joint Surg Br. 1974;56:291–6. [PubMed] [Google Scholar]
- 5.Ha YC, Jung WH, Kim JR, Seong NH, Kim SY, Koo KH. Prediction of collapse in femoral head osteonecrosis: A modified Kerboul method with use of magnetic resonance images. J Bone Joint Surg Am. 2006;88(Suppl 3):35–40. doi: 10.2106/JBJS.F.00535. [DOI] [PubMed] [Google Scholar]
- 6.Ficat RP. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br. 1985;67:3–9. doi: 10.1302/0301-620X.67B1.3155745. [DOI] [PubMed] [Google Scholar]
- 7.Tripathy SK, Goyal T, Sen RK. Management of femoral head osteonecrosis: Current concepts. Indian J Orthop. 2015;49(1):28–45. doi: 10.4103/0019-5413.143911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77:34–41. [PubMed] [Google Scholar]
- 9.Sugano N, Atsumi T, Ohzono K, Kubo T, Hotokebuchi T, Takaoka K. The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci. 2002;7:601–5. doi: 10.1007/s007760200108. [DOI] [PubMed] [Google Scholar]
- 10.Mont MA, Carbone JJ, Fairbank AC. Core decompression versus nonoperative management for osteonecrosis of the hip. Clin Orthop Relat Res. 1996;324:169–78. doi: 10.1097/00003086-199603000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Soohoo NF, Vyas S, Manunga J, Sharifi H, Kominski G, Lieberman JR. Cost-effectiveness analysis of core decompression. J Arthroplasty. 2006;21:670–81. doi: 10.1016/j.arth.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Ficat RP, Arlet J. In: Ischemia and Necroses of Bone. Hungerford DS, editor. Baltimore: Williams and Wilkins; 1980. [Google Scholar]
- 13.Hungerford DS. Treatment of ischemic necrosis of the femoral head. In: Evarts CD, editor. Surgery of the Musculoskeletal System. Vol. 3. New York: Churchill Livingstone; 1983. pp. 5029–43. [Google Scholar]
- 14.Bozic KJ, Zurakowski D, Thornhill TS. Survivorship analysis of hips treated with core decompression for nontraumatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 1999;81:200–9. doi: 10.2106/00004623-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Wang GJ, Dughman SS, Reger SI, Stamp WG. The effect of core decompression on femoral head blood flow in steroid-induced avascular necrosis of the femoral head. J Bone Joint Surg Am. 1985;67:121–4. [PubMed] [Google Scholar]
- 16.Kim SY, Kim DH, Park IH. Multiple drilling compared with core decompression for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Br. 2004;86:149. [Google Scholar]
- 17.Mont MA, Ragland PS, Etienne G. Core decompression of the femoral head for osteonecrosis using percutaneous multiple small-diameter drilling. Clin Orthop Relat Res. 2004;429:131–8. doi: 10.1097/01.blo.0000150128.57777.8e. [DOI] [PubMed] [Google Scholar]
- 18.Marker DR, Seyler TM, Ulrich SD, Srivastava S, Mont MA. Do modern techniques improve core decompression outcomes for hip osteonecrosis? Clin Orthop Relat Res. 2008;466:1093–103. doi: 10.1007/s11999-008-0184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al Omran A. Multiple drilling compared with standard core decompression for avascular necrosis of the femoral head in sickle cell disease patients. Arch Orthop Trauma Surg. 2013;133:609–13. doi: 10.1007/s00402-013-1714-9. [DOI] [PubMed] [Google Scholar]
- 20.Song WS, Yoo JJ, Kim YM, Kim HJ. Results of multiple drilling compared with those of conventional methods of core decompression. Clin Orthop Relat Res. 2007;454:139–46. doi: 10.1097/01.blo.0000229342.96103.73. [DOI] [PubMed] [Google Scholar]
- 21.Hernigou P, Poignard A, Zilber S, Rouard H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2009;43:40–5. doi: 10.4103/0019-5413.45322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;(405):14–23. doi: 10.1097/00003086-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Gangji V, Hauzeur JP, Matos C, De Maertelaer V, Toungouz M, Lambermont M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am. 2004;86-A:1153–160. doi: 10.2106/00004623-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Hernigou P, Poignard A, Manicom O, Mathieu G, Rouard H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg Br. 2005;87:896–902. doi: 10.1302/0301-620X.87B7.16289. [DOI] [PubMed] [Google Scholar]
- 25.Rackwitz L, Eden L, Reppenhagen S, Reichert JC, Jakob F, Walles H, et al. Stem cell- and growth factor-based regenerative therapies for avascular necrosis of the femoral head. Stem Cell Res Ther. 2012;3:7. doi: 10.1186/scrt98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieberman JR, Conduah A, Urist MR. Treatment of osteonecrosis of the femoral head with core decompression and human bone morphogenetic protein. Clin Orthop Relat Res. 2004;429:139–45. doi: 10.1097/01.blo.0000150312.53937.6f. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Liu S, Su X. Core decompression and implantation of bone marrow mononuclear cells with porous hydroxylapatite composite filler for the treatment of osteonecrosis of the femoral head. Arch Orthop Trauma Surg. 2013;133:125–33. doi: 10.1007/s00402-012-1623-3. [DOI] [PubMed] [Google Scholar]
- 28.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 29.Tintut Y, Alfonso Z, Saini T, Radcliff K, Watson K, Bostrom K, Demer LL. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–10. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- 30.Kim SH, Kim YS, Lee SY, Kim KH, Lee YM, Kim WK, Lee YK. Gene expression profile in mesenchymal stem cells derived from dental tissues and bone marrow. J Periodontal Implant Sci. 2011;41:192–200. doi: 10.5051/jpis.2011.41.4.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ratajczak MZ, Jadczyk T, Pedziwiatr D, Wojakowski W. New advances in stem cell research: practical implications for regenerative medicine. Pol Arch Med Wewn. 2014;124(7–8):417–26. doi: 10.20452/pamw.2355. [DOI] [PubMed] [Google Scholar]
- 32.Abudusaimi A, Aihemaitijiang Y, Wang YH, Cui L, Maimaitiming S, Abulikemu M. Adipose-derived stem cells enhance bone regeneration in vascular necrosis of the femoral head in the rabbit. J Int Med Res. 2011;39:1852–60. doi: 10.1177/147323001103900528. [DOI] [PubMed] [Google Scholar]
- 33.Lee HS, Huang GT, Chiang H, Chiou LL, Chen MH, Hsieh CH, Jiang CC. Multipotential mesenchymal stem cells from femoral bone marrow near the site of osteonecrosis. Stem Cells. 2003;21:190–9. doi: 10.1634/stemcells.21-2-190. [DOI] [PubMed] [Google Scholar]
- 34.Otto WR, Rao J. Tomorrow’s skeleton staff: mesenchymal stem cells and the repair of bone and cartilage. Cell Prolif. 2004;37:97–110. doi: 10.1111/j.1365-2184.2004.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, Liu B, Yu X. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50:325–30. doi: 10.1016/j.bone.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Sen RK, Tripathy SK, Aggarwal S, Marwaha N, Sharma RR, Khandelwal N. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: a randomized control study. J Arthroplasty. 2012;27:679–86. doi: 10.1016/j.arth.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Gangji V, Toungouz M, Hauzeur JP. Stem cell therapy for osteonecrosis of the femoral head. Expert Opin Biol Ther. 2005;5:437–42. doi: 10.1517/14712598.5.4.437. [DOI] [PubMed] [Google Scholar]
- 38.Gangji V, Hauzeur JP. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. Surgical technique. J Bone Joint Surg Am. 2005;87(Suppl 1):106–12. doi: 10.2106/JBJS.D.02662. [DOI] [PubMed] [Google Scholar]
- 39.Yan ZQ, Chen YS, Li WJ, Yang Y, Huo JZ, Chen ZR, et al. Treatment of osteonecrosis of the femoral head by percutaneous decompression and autologous bone marrow mononuclear cell infusion. Chin J Traumatol. 2006;9:3–7. [PubMed] [Google Scholar]
- 40.Daltro GC, Fortuna VA, de Araújo SA, Ferraz Lessa PI, Sobrinho UA, Borojevic R. Femoral head necrosis treatment with autologous stem cells in sickle cell disease. Acta Orthop Bras. 2008;16:44–8. [Google Scholar]
- 41.Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: Five year followup of a prospective controlled study. Bone. 2011;49:1005–9. doi: 10.1016/j.bone.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 42.Cuervas-Mons M, Narbona J, Laguna R, Vaquero J. (Autologous concentrated bone marrow graft in the treatment of femoral head avascular necrosis: clinical outcome after two years of follow up in a non-controlled prospective study) Rev Esp Cir Ortop Traumatol. 2013;57:106–110. doi: 10.1016/j.recot.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Tang TT, Lu B, Yue B, Xie XH, Xie YZ, Dai KR, Lu JX, Lou JR. Treatment of osteonecrosis of the femoral head with hBMP-2-gene-modified tissue-engineered bone in goats. J Bone Joint Surg Br. 2007;89(1):127–9. doi: 10.1302/0301-620X.89B1.18350. [DOI] [PubMed] [Google Scholar]
- 44.Xiao ZM, Jiang H, Zhan XL, Wu ZG, Zhang XL. Treatment of osteonecrosis of femoral head with BMSCs-seeded bio-derived bone materials combined with rhBMP-2 in rabbits. Chin J Traumatol. 2008;11(3):165–70. doi: 10.1016/s1008-1275(08)60035-8. [DOI] [PubMed] [Google Scholar]
- 45.Mont MA, Etienne G, Ragland PS. Outcome of nonvascularized bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2003;417:84–92. doi: 10.1097/01.blo.0000096826.67494.38. [DOI] [PubMed] [Google Scholar]
- 46.Seyler TM, Marker DR, Ulrich SD, Fatscher T, Mont MA. Nonvascularized bone grafting defers joint arthroplasty in hip osteonecrosis. Clin Orthop Relat Res. 2008;466:1125–32. doi: 10.1007/s11999-008-0211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merle d’Aubigne R, Postel M, Mazabraud A, Massias P, Gueguen J. Idiopathic necrosis of the femoral head in adults. J Bone Joint Surg Br. 1965;47:612–33. [PubMed] [Google Scholar]
- 48.Rosenwasser MP, Garino JP, Kiernan HA, Michelsen CB. Long term follow-up of thorough debridement and cancellous bone grafting of the femoral head for avascular necrosis. Clin Orthop Relat Res. 1994;306:17–7. [PubMed] [Google Scholar]
- 49.Keizer SB, Kock NB, Dijkstra PD, Taminiau AH, Nelissen RG. Treatment of avascular necrosis of the hip by a non-vascularized cortical graft. J Bone Joint Surg Br. 2006 Apr;88(4):460–6. doi: 10.1302/0301-620X.88B4.16950. [DOI] [PubMed] [Google Scholar]
- 50.Banerjee S, Issa K, Pivec R, Kapadia BH, Khanuja HS, Mont MA. Osteonecrosis of the hip: Treatment options and outcomes. Orthop Clin North Am. 2013;44:463–76. doi: 10.1016/j.ocl.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Smith KR, Bonfiglio M, Montgomery WJ. Non-traumatic necrosis of the femoral head treated with tibial bone-grafting: A follow-up note. J Bone Joint Surg Am. 1980;62:845–7. [PubMed] [Google Scholar]
- 52.Keizer SB, Kock NB, Dijkstra PD, Taminiau AH, Nelissen RG. Treatment of avascular necrosis of the hip by a non-vascularized cortical graft. J Bone Joint Surg Br. 2006;88:460–6. doi: 10.1302/0301-620X.88B4.16950. [DOI] [PubMed] [Google Scholar]
- 53.Papanagiotou M, Malizos KN, Vlychou M, Dailiana ZH. Autologous (non-vascularised) fibular grafting with recombinant bone morphogenetic protein-7 for the treatment of femoral head osteonecrosis: Preliminary report. Bone Joint J. 2014;96-B:31–5. doi: 10.1302/0301-620X.96B1.32773. [DOI] [PubMed] [Google Scholar]
- 54.Tripathy SK, Goyal T, Sen RK. Management of femoral head osteonecrosis: Current concepts. Indian J Orthop. 2015;49(1):28–45. doi: 10.4103/0019-5413.143911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baksi DP. Treatment of post-traumatic avascular necrosis of the femoral head by multiple drilling and muscle-pedicle bone grafting. Preliminary report. J Bone Joint Surg Br. 1983;65:268–73. doi: 10.1302/0301-620X.65B3.6341373. [DOI] [PubMed] [Google Scholar]
- 56.Baksi DP. Treatment of osteonecrosis of the femoral head by drilling and muscle-pedicle bone grafting. J Bone Joint Surg Br. 1991;73:241–5. doi: 10.1302/0301-620X.73B2.2005147. [DOI] [PubMed] [Google Scholar]
- 57.Meyers MH. The treatment of osteonecrosis of the hip with fresh osteochondral allografts and with the muscle pedicle graft technique. Clin Orthop Relat Res. 1978;130:202–9. [PubMed] [Google Scholar]
- 58.Lee CK, Rehmatullah N. Muscle-pedicle bone graft and cancellous bone graft for the “silent hip” of idiopathic ischemic necrosis of the femoral head in adults. Clin Orthop Relat Res. 1981;158:185–94. [PubMed] [Google Scholar]
- 59.Iwato H, Torii S, Hasegawa Y, Itoh H, Mizuno M, Genda E, et al. Indications and results of vascularized pedicle iliac bone graft in avascular necrosis of the femoral head. Clin Orthop Relat Res. 1993;295:281–8. [PubMed] [Google Scholar]
- 60.Eisenschenk A, Lautenbach M, Schwetlick G, Weber U. Treatment of femoral head necrosis with vascularized iliac crest transplants. Clin Orthop Relat Res. 2001;386:100–5. doi: 10.1097/00003086-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 61.Matsusaki H, Noguchi M, Kawakami T, Tani T. Use of vascularized pedicle iliac bone graft combined with transtrochanteric rotational osteotomy in the treatment of avascular necrosis of the femoral head. Arch Orthop Trauma Surg. 2005;125:95–101. doi: 10.1007/s00402-004-0777-z. [DOI] [PubMed] [Google Scholar]
- 62.Sotereanos DG, Plakseychuk AY, Rubash HE. Free vascularized fibula grafting for the treatment of osteonecrosis of the femoral head. Clin Orthop Relat Res. 1997;344:243–56. [PubMed] [Google Scholar]
- 63.Malizos KN, Soucacos PN, Beris AE. Osteonecrosis of the femoral head: Hip salvaging with implantation of a vascularized fibular graft. Clin Orthop Relat Res. 1995;314:67–75. [PubMed] [Google Scholar]
- 64.Korompilias AV, Lykissas MG, Beris AE, Urbaniak JR, Soucacos PN. Vascularised fibular graft in the management of femoral head osteonecrosis: Twenty years later. J Bone Joint Surg Br. 2009;91:287–93. doi: 10.1302/0301-620X.91B3.21846. [DOI] [PubMed] [Google Scholar]
- 65.Shuler MS, Rooks MD, Roberson JR. Porous tantalum implant in early osteonecrosis of the hip: preliminary report on operative, survival, and outcomes results. J Arthroplasty. 2007;22:26–31. doi: 10.1016/j.arth.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 66.Osteonecrosis Intervention Implant. Zimmer; Allendale, New Jersey, USA: Trabecular Metal. [Google Scholar]
- 67.Aldegheri R, Taglialavoro G, Berizzi A. The tantalum screw for treating femoral head necrosis: rationale and results. Strategies Trauma Limb Reconstr. 2007;2(2–3):63–8. doi: 10.1007/s11751-007-0021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsao AK, Roberson JR, Christie MJ, Dore DD, Heck DA, Robertson DD, Poggie RA. Biomechanical and clinical evaluations of a porous tantalum implant for the treatment of early-stage osteonecrosis. J Bone Joint Surg Am. 2005;87(Suppl 2):22–7. doi: 10.2106/JBJS.E.00490. [DOI] [PubMed] [Google Scholar]
- 69.Plakseychuk AY, Kim SY, Park BC, Varitimidis SE, Rubash HE, Sotereanos DG. Vascularized compared with nonvascularized fibular grafting for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Am. 2003;85A(4):589–96. doi: 10.2106/00004623-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Vail TP, Urbaniak JR. Donor-site morbidity with use of vascularized autogenous fibular grafts. J Bone Joint Surg Am. 1996;78(2):204–11. doi: 10.2106/00004623-199602000-00006. [DOI] [PubMed] [Google Scholar]
- 71.Tanzer M, Karabasz D, Krygier JJ, Cohen R, Bobyn JD. The Otto Aufranc Award: bone augmentation around and within porous implants by local bisphosphonate elution. Clin Orthop Relat Res. 2005;441:30–9. doi: 10.1097/01.blo.0000194728.62996.2d. [DOI] [PubMed] [Google Scholar]
- 72.Cornell CN, Lane JM. Current understanding of osteoconduction in bone regeneration. Clin Orthop Relat Res. 1998;355(Suppl):S267–S73. doi: 10.1097/00003086-199810001-00027. [DOI] [PubMed] [Google Scholar]
- 73.Urbaniak JR, Harvey EJ. Revascularization of the femoral head in osteonecrosis. J Am Acad Orthop Surg. 1998;6(1):44–54. doi: 10.5435/00124635-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 74.Pedersen DR, Brown TD, Poggie RA. Finite element characterization of a porous tantalum material for treatment of avascular necrosis. Trans Orthop Res Soc. 1997;22:598. [Google Scholar]
- 75.Shuler MS, Rooks MD, Roberson JR. Porous tantalum implant in early osteonecrosis of the hip: preliminary report on operative, survival, and outcomes results. J Arthroplasty. 2007;22:26–31. doi: 10.1016/j.arth.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 76.Tsao AK, Roberson JR, Christie MJ, Dore DD, Heck DA, Robertson DD, Poggie RA. Biomechanical and clinical evaluations of a porous tantalum implant for the treatment of early-stage osteonecrosis. J Bone Joint Surg Am. 2005;87( Suppl 2):22–7. doi: 10.2106/JBJS.E.00490. [DOI] [PubMed] [Google Scholar]
- 77.Nadeau M, Seguin C, Theodoropoulos JS, Harvey EJ. Short term clinical outcome of a porous tantalum implant for the treatment of advanced osteonecrosis of the femoral head. Mcgill J Med. 2007;10:4–10. [PMC free article] [PubMed] [Google Scholar]
- 78.Tanzer M, Bobyn JD, Krygier JJ, Karabasz D. Histopathologic retrieval analysis of clinically failed porous tantalum osteonecrosis implants. J Bone Joint Surg Am. 2008;90:1282–9. doi: 10.2106/JBJS.F.00847. [DOI] [PubMed] [Google Scholar]
- 79.Varitimidis SE, Dimitroulias AP, Karachalios TS, Dailiana ZH, Malizos KN. Outcome after tantalum rod implantation for treatment of femoral head osteonecrosis: 26 hips followed for an average of 3 years. Acta Orthop. 2009;80:20–5. doi: 10.1080/17453670902804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Floerkemeier T, Lutz A, Nackenhorst U, Thorey F, Waizy H, Windhagen H, von Lewinski G. Core decompression and osteonecrosis intervention rod in osteonecrosis of the femoral head: clinical outcome and finite element analysis. Int Orthop. 2011;35:1461–6. doi: 10.1007/s00264-010-1138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Floerkemeier T, Thorey F, Daentzer D, Lerch M, Klages P, Windhagen H, von Lewinski G. Clinical and radiological outcome of the treatment of osteonecrosis of the femoral head using the osteonecrosis intervention implant. Int Orthop. 2011;35:489–95. doi: 10.1007/s00264-009-0940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Veillette CJ, Mehdian H, Schemitsch EH, McKee MD. Survivorship analysis and radiographic outcome following tantalum rod insertion for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88( Suppl 3):48–55. doi: 10.2106/JBJS.F.00538. [DOI] [PubMed] [Google Scholar]
- 83.Aldegheri R, Taglialavoro G, Berizzi A. The tantalum screw for treating femoral head necrosis: rationale and results. Strategies Trauma Limb Reconstr. 2007;2(2–3):63–8. doi: 10.1007/s11751-007-0021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Y, Su X, Zhou S, Wang L, Wang C, Liu S. A modified porous tantalum implant technique for osteonecrosis of the femoral head: survivorship analysis and prognostic factors for radiographic progression and conversion to total hip arthroplasty. Int J Clin Exp Med. 2015;8(2):1918–30. [PMC free article] [PubMed] [Google Scholar]
- 85.Tanzer M, Bobyn JD, Krygier JJ, Karabasz D. Histopathologic retrieval analysis of clinically failed porous tantalum osteonecrosis implants. J Bone Joint Surg Am. 2008;90:1282–9. doi: 10.2106/JBJS.F.00847. [DOI] [PubMed] [Google Scholar]
- 86.Papapietro N, Di Martino A, Niccoli G, Palumbo A, Salvatore G, Forriol F, Denaro V. Trabecular metal screw implanted for avascular necrosis of the femoral head may complicate subsequent arthroplasty surgery. Eur J Orthop Surg Traumatol. 2014;24(6):931–8. doi: 10.1007/s00590-013-1275-1. [DOI] [PubMed] [Google Scholar]
- 87.Ito H, Tanino H, Yamanaka Y, Nakamura T, Takahashi D, Minami A, et al. Long term results of conventional varus half-wedge proximal femoral osteotomy for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Br. 2012;94:308–14. doi: 10.1302/0301-620X.94B3.27814. [DOI] [PubMed] [Google Scholar]
- 88.Sugioka Y, Katsuki I, Hotokebuchi T. Transtrochanteric rotational osteotomy of the femoral head for the treatment of osteonecrosis. Followup statistics. Clin Orthop Relat Res. 1982;169:115–26. [PubMed] [Google Scholar]
- 89.Sugioka Y. Transtrochanteric anterior rotational osteotomy of the femoral head in the treatment of osteonecrosis affecting the hip: A new osteotomy operation. Clin Orthop Relat Res. 1978;130:191–201. [PubMed] [Google Scholar]
- 90.Hasegawa Y, Sakano S, Iwase T, Iwasada S, Torii S, Iwata H. Pedicle bone grafting versus transtrochanteric rotational osteotomy for avascular necrosis of the femoral head. J Bone Joint Surg Br. 2003;85:191–8. doi: 10.1302/0301-620x.85b2.13190. [DOI] [PubMed] [Google Scholar]
- 91.Jacobs MA, Hungerford DS, Krackow KA. Intertrochanteric osteotomy for avascular necrosis of the femoral head. J Bone Joint Surg Br. 1989;71:200–4. doi: 10.1302/0301-620X.71B2.2925735. [DOI] [PubMed] [Google Scholar]
- 92.Maistrelli G, Fusco U, Avai A, Bombelli R. Osteonecrosis of the hip treated by intertrochanteric osteotomy. A four-to 15-year follow-up. J Bone Joint Surg Br. 1988;70:761–6. doi: 10.1302/0301-620X.70B5.3192575. [DOI] [PubMed] [Google Scholar]
- 93.Sen RK. Management of avascular necrosis of femoral head at pre-collapse stage. Indian J Orthop. 2009;43:6–16. doi: 10.4103/0019-5413.45318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Banerjee S, Issa K, Pivec R, Kapadia BH, Khanuja HS, Mont MA. Osteonecrosis of the hip: Treatment options and outcomes. Orthop Clin North Am. 2013;44:463–76. doi: 10.1016/j.ocl.2013.07.004. [DOI] [PubMed] [Google Scholar]