Abstract
The serotonin 2C (5-HT2C) receptor has been identified as a potential drug target for the treatment of a variety of central nervous system (CNS) disorders, such as obesity, substance abuse, and schizophrenia. In this Viewpoint article, recent progress in developing selective 5-HT2C agonists for use in treating these disorders is summarized, including the work of our group. Challenges in this field and the possible future directions are described. Homology modeling as a method to predict the binding modes of 5-HT2C ligands to the receptor is also discussed. Compared to known ligands, the improved pharmacological profiles of the 2-phenylcyclopropylmethylamine-based 5-HT2C agonists make them preferred candidates for further studies.
Keywords: drug discovery, medicinal chemistry, receptors, schizophrenia, serotonin
Introduction
We have worked for a number of years to identify the best possible 5-HT2C receptor agonists for use in the treatment of a host of central nervous system (CNS) disorders. Herein, we provide a short overview of our efforts in this area of science together with our thoughts as to how these compounds might be used therapeutically.
The 5-HT2C Receptor as a Drug Target for CNS Disorders
The serotonin 2C (5-HT2C) receptor is a member of the super-family of G-protein-coupled receptors (GPCRs). First identified in 1984 from radioligand binding studies in the pig choroid plexus,[1] 5-HT2C belongs to the subfamily of serotonin receptors, of which 14 different members (5-HT1–7, some of these with further subclassifications) have been identified.[2] The 5-HT2C receptor was originally designated as 5-HT1C, but subsequent signal transduction pathway studies and amino sequence analysis revealed its close sequence homology with 5-HT2A and 5-HT2B; thus it was renamed as 5-HT2C.[3] It exhibits 46–50% overall sequence identity with 5-HT2A and 5-HT2B, and all of these subtypes couple preferentially to Gq/11 to increase the hydrolysis of inositol phosphates and elevate cytosolic calcium concentrations. The 5-HT2C receptor is the only known GPCR that has been found to undergo RNA editing, which affects its cell signaling, pharmacology, and brain function.[4]
Each subtype of serotonin receptors has a distinct distribution pattern. For the three 5-HT2 subtypes,[5] 5-HT2A is found in both central nervous system (CNS) and peripheral tissues such as gastrointestinal tissues and blood vessels. 5-HT2B receptors are localized mainly in vascular and cardiac tissues. The expression of 5-HT2C receptors, however, has been found to be restricted to the CNS, with negligible distribution in cardiac and vascular tissues. This feature of the 5-HT2C receptor makes it an ideal target for the treatment of CNS disorders, as drugs targeting it would have limited peripheral side effects. 5-HT2C has been found to play roles in a variety of CNS functions and pathological conditions, and 5-HT2C agonists have been proposed to be potential therapeutics for many CNS disorders, such as obesity, schizophrenia, and substance abuse.[6]
One significant challenge for developing 5-HT2C agonists as drug candidates is their selectivity against 5-HT2A and 5-HT2B, as the molecular determinants involved in ligand recognition by these receptors are highly conserved. More importantly, the activation of 5-HT2A and 5-HT2B receptors has been found to be related to hallucinogenic effects and cardiac valvulopathy, respectively.[7] Thus, the discovery of ligands possessing exquisite selectivity against 5-HT2A and 5-HT2B receptors is a key criterion for the advancement of 5-HT2C agonists.
Selective 5-HT2C receptor agonists
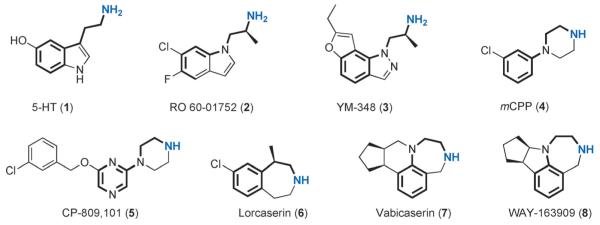
A number of selective 5-HT2C agonists have been discovered in the past two decades,[8] and representative structures are shown in Figure 1, along with the endogenous ligand serotonin (5-HT, 1). Pharmacological profiles of these compounds at the 5-HT2C, 5-HT2A, and 5-HT2B receptors are summarized in Table 1.
Figure 1.
Structures of 5-HT and representative 5-HT2C selective agonists.
Table 1.
Pharmacological profiles of representative 5-HT2C agonists.
| Compound | 5-HT2C | 5-HT2A | 5-HT2B | Ref. | |||
|---|---|---|---|---|---|---|---|
| EC50 [nm] | Emax [%] | EC50 [nm] | Emax [%] | EC50 [nm] | Emax [%] | ||
| RO 60-01752 | 52±3 | 88±20 | 400±20 | 91±5 | 2.4±1 | 130±30 | [10] |
| YM-348 | 1.0±0.2 | 76±1 | 93±10 | 97±2 | 3.2±3 | 110±10 | [10] |
| mCPP | 120±10 | 63±3 | 150±20 | 18±2 | 93±50 | 21±9 | [10] |
| CP-809101 | 0.11 | 93 | 153 | 67 | 65.3 | 57 | [13] |
| Lorcaserin | 9±0.5 | 100 | 168±11 | 75 | 943±90 | 100 | [15] |
| Vabicaserin | 8 | 100 | 1650 | –[a] | 1.5[b] >10000[c] |
80 – |
[16] |
| WAY-163909 | 8±3 | 90±6 | NE | NE | 185±105 | 40±3 | [17] |
Antagonist;
Receptor density at 5000 fmolmg−1;
Receptor density at 500 or 1500 fmolmg−1. Values with errors represent the mean±SD; NE: no effect; – not applicable.
As can be seen from Figure 1, all of these 5-HT2C agonists have an amino group as a mimic of the primary amine in 5-HT. This group can be protonated at physiological pH and then engage in a charge–charge interaction with the receptor. The linker between the aromatic ring and the ammonium “head” contains between two and three C or N atoms. Compound RO 60-01752 (2) shares a similar backbone with 5-HT, and the α-methyl group was incorporated to suppress metabolic side chain deamination and to increase the lipophilicity of the compound. Of its enantiomers, the (S)-isomer showed better potency and selectivity.[9] RO 60-01752 displayed a moderate potency at 5-HT2C receptors (EC50=52 nm) and an eightfold selectivity over 5-HT2A, but no selectivity against 5-HT2B (Table 1).[10] YM-348 (3) is an orally active 5-HT2C receptor agonist that was under investigation by Yamanouchi (now Astellas) for the potential treatment of obesity. It has a similar scaffold as RO 60-01752, into which a fused furan ring was incorporated. YM-348 showed excellent potency at 5-HT2C receptors and good selectivity over 5-HT2A, but little selectivity against 5-HT2B.[10]
meta-Chlorophenylpiperazine (mCPP, 4) was one of the earliest tool compounds used in pharmacological studies of the 5-HT2C receptor.[11] Although it is a nonspecific serotonergic agent, mCPP showed moderate potency at 5-HT2C and good “functional” selectivity over 5-HT2A and 5-HT2B, as the intrinsic efficacy of this compound is low at the latter two targets (Table 1). mCPP was one of the earliest compounds that was shown to induce satiety thereby reducing food intake in humans,[12] thus encouraging the development of 5-HT2C agonists for the treatment of obesity. Compound CP-809101 (5), which shares an arylpiperazine core structure with mCPP, is the most potent 5-HT2C agonist reported to date, showing an EC50 of 0.11 nm and good selectivity against both 5-HT2A and 5-HT2B receptors.[13] However, CP-809101 is relatively potent at 5-HT2B receptors (EC50=65.3 nm, Emax=57%), and its development has been discontinued due to the observation of genotoxicity in preclinical studies.[14]
Another series of 5-HT2C agonists bear the benz[d]azepine or benzodiazepine scaffold, exemplified by the compounds lorcaserin (6), vabicaserin (7), and WAY-163909 (8). Lorcaserin was developed by Arena Pharmaceuticals,[18] and it showed a good pharmacological profile, with an EC50 of 9 nm at 5-HT2C and good selectivity against the other two receptors.[15] It was approved by the US Food and Drug Administration (FDA) in 2012 for the treatment of obesity under the tradename Belviq. The homologues vabicaserin and WAY-163909 were developed by Wyeth (now Pfizer), both of which displayed good potency at the 5-HT2C receptor. Vabicaserin is an antagonist of the 5-HT2A receptor, while its functional efficacy at 5-HT2B depends on the receptor density: it showed no activity at low receptor density but greater potency when tested at higher receptor density.[16] Vabicaserin has been studied in clinical trials for the treatment of acute schizophrenia.[19] It was well tolerated with no significant safety issues emerging, and it caused no weight gain. A proof of concept was achieved as 200 mgkg−1 vabicaserin demonstrated therapeutic effects on both the positive and negative symptoms of the patients. However, vabicaserin failed to meet its primary endpoints in this trial (ClinicalTrials.gov Identifier: NCT00563706.). WAY-163909 was studied in various preclinical animal models of psychosis, but no clinical trial has been initiated.[17,20]
Developing 2-phenylcyclopropylmethylamines as selective serotonin 2C (5-HT2C) receptor agonists
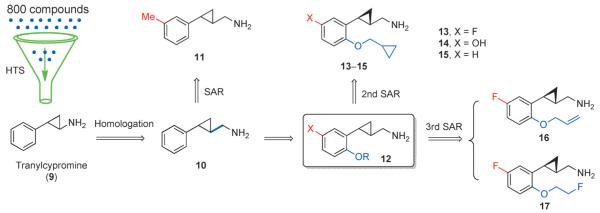
We initiated our research in this field by the high-throughput screening (HTS) of a chemical library of 800 compounds (Figure 2). Using this approach, the reversible monoamine oxidase (MAO) inhibitor tranylcypromine (9) was identified as a lead compound.[21] The first round of structure–activity relationship (SAR) studies identified its homolog 10 as a potent 5-HT2C agonist (EC50=13 nm), with good selectivity over 5-HT2A and moderate selectivity over 5-HT2B, respectively (Table 2). The introduction of a methyl group at position 5 gave compound 11, with enhanced 5-HT2C potency (EC50=4.8 nm) as well as good selectivity against 5-HT2B. Compounds with a trans configuration of the cyclopropane ring were found to show better potency than the cis isomers, and the (1S, 2S) absolute configuration is favored for compound 10.[21]
Figure 2.
Development of 2-phenylcyclopropylmethylamines as selective 5-HT2C receptor agonists.
Table 2.
Pharmacological profiles of 2-phenylcyclopropylmethylamine-based compounds.
| Compound | 5-HT2C | 5-HT2A | 5-HT2B | Ref. | |||
|---|---|---|---|---|---|---|---|
| EC50 [nm] | Emax [%] | EC50 [nm] | Emax [%] | EC50 [nm] | Emax [%] | ||
| 9 | 2697 | 109 | NA | – | >5000 | 29 | [21] |
| (±)-10 | 13 | 96 | 1399 | 74 | 85 | 93 | [21] |
| (+)-10 (1S, 2S) | 5.2 | 108 | 1396 | 79 | 37 | 111 | [21] |
| 11 | 4.8 | 95 | 585 | 86 | 65 | 93 | [21] |
| 13 (X=F) | 21 | 71 | 894 | 28 | 289 | 21 | [22] |
| 14 (X=OH) | 9.3 | 70 | 372 | 18 | NA | – | [22] |
| 15 (X=H) | 55 | 61 | NA | – | NA | – | [23] |
| 16 | 4.2 | 87 | 374 | 56 | NA | – | [24] |
| 17 | 3.4 | 89 | 359 | 76 | NA | – | [24] |
NA: no activity at 10 μm; – not applicable.
In the second round of SAR studies, we discovered that the introduction of an alkoxy group into the ortho-position relative to the cyclopropylmethylamine moiety on the benzene ring led to significant improvements in terms of ligand efficacy and selectivity. Cyclopropylmethyl ethers provided the best selectivity profiles, exemplified by compounds 13-15 (Table 2). Compound 13 showed an EC50 of 21 nm at 5-HT2C, while it is functionally selective against 5-HT2A and 5-HT2B, at which it showed Emax values below 30%. Compound 14 displayed a better profile, with an EC50<10 nm and no activity at 5-HT2B. Both compounds 13 and 14 were shown to normalize the phencyclidine (PCP)-disrupted prepulse inhibition (PPI) of startle in mice.[22] Removal of the 5-substituent led to compound 15, which showed no activity at 5-HT2B or 5-HT2A and acted as a moderately potent 5-HT2C partial agonist (EC50=55 nm, Emax =61%).[23]
In the third round of SAR work, a multiparameter optimization process involving ligand efficacy and selectivity, pharmacokinetic (PK) properties, brain penetration profile, as well as toxicity potentials led to the discovery of compounds 16 and 17 as the best two agonists from this series of compounds.[24] Both compounds showed EC50 values below 5 nm at 5-HT2C receptors, no activity at 5-HT2B, and around 100-fold selectivity versus 5-HT2A (Table 2). Their excellent pharmacological profiles position these two compounds as the best 5-HT2C agonists reported to date. Both compounds showed efficacy in the amphetamine-induced hyperactivity model in mice,[24] and results from other schizophrenia-like animal behavioral tests (data not published) strongly support their advancement as therapeutic candidates for treating schizophrenia.
Homology modeling and putative binding preference of 2-phenylcyclopropylmethyl-amine-based 5-HT2C agonists
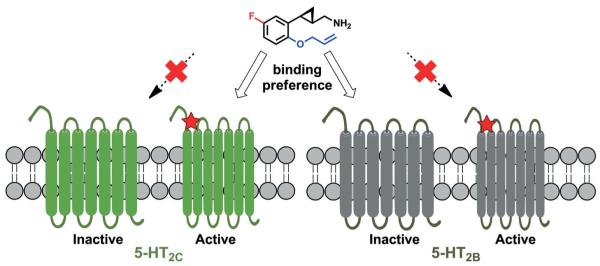
Around 30 different GPCR crystal structures have been solved from among the >800 GPCRs present in the human genome (http://gpcr.usc.edu/index.html). However, the 3D structure of the 5-HT2C receptor has yet to be disclosed. GPCR homology modeling is a commonly used approach for structure-based drug discovery (SBDD) and optimization when the structure of the target is unknown.[25] A β2-adrenergic receptor based homology model has been generated and used to predict the possible binding modes of putative 5-HT2C ligands to this receptor.[26] In our study, we used the β2-AR structure in its inactive mode for generating a homology model of 5-HT2C in its inactive mode, while the active mode model was generated by combining the resolved structure of the 5-HT2B and the β2-AR in its fully active state.[24] The binding modes of one of our best 5-HT2c ligands, namely compound 16, to the receptors were predicted by docking simulations, and the ligand was found to fit nicely into the active conformation of the 5-HT2C homology model. The binding is stabilized by the ion pair between the ligand’s ammonium group and Asp134 on TM3 along with various π–π and hydrophobic interactions.[24]
In the subfamily of serotonin receptors, the crystal structures of both the 5-HT1B and 5-HT2B receptors have been solved recently, both in their inactive conformations.[27] The homology model of the 5-HT2C receptor described above displays considerable similarity to the reported crystal structure of the 5-HT2B receptor. Interestingly, compound 16 shows nearly equivalent binding to both the 5-HT2B and 5-HT2C receptors (Ki=46 nm and 37 nm, respectively).[24] However, the intrinsic activity of this compound is different at these two receptors, as it is a full agonist for 5-HT2C while showing no agonism at 5-HT2B. Thus, we anticipate that compound 16 shows a preference for the active conformation of the 5-HT2C receptor and for the inactive conformation of 5-HT2B (Figure 3). A more precise delineation of the structural basis for these results will require the determination of the X-ray structures of the 5-HT2C receptor in both its active and inactive conformations, as well as of the 5-HT2B receptor in its active conformation. Such information would greatly facilitate the rational design of selective 5-HT2C agonists devoid of 5-HT2B agonism in the future.
Figure 3.
Putative binding preference of 2-phenylcyclopropylmethylamine-based 5-HT2C agonists.
Summary and Outlook
The discovery of selective 5-HT2C agonists has enabled the development of drugs or drug candidates that take advantage of the many roles this receptor plays in the CNS. To date, obesity is the only clinical indication for which a 5-HT2C agonist, the marketed drug lorcaserin, has been approved by the FDA. However, certain restrictions apply to the use of lorcaserin for obesity, including its use in patients with a body mass index (BMI) of over 30, or a BMI over 27 who have at least one weight-related health condition, such as high blood pressure, type 2 diabetes, or high cholesterol. Furthermore, the moderate 5-HT2B activity of lorcaserin has also been a concern as it might induce cardiac valvulopathy.[28] Due to the lack of availa bility of effective and safe anti-obesity drugs with other mechanisms of action in the marketplace,[29] the development of 5-HT2C agonists with better selectivity profiles for this worldwide disease would be of great value.
It is also well known that behavioral and neurobiological commonalities coexist between obesity and drug addiction,[30] and that the use of 5-HT2C agonists can be extended to the treatment of substance abuse.[6d] Lorcaserin attenuates self-stimulation and blocks the reward-enhancing effects of nicotine.[31] A clinical study of lorcaserin to evaluate its effect on smoking cessation has been completed; however, the results are yet to be disclosed (ClinicalTrials.gov Identifier: NCT02044874.). Considering the large number of tobacco smokers worldwide and the detrimental effects of this behavior on human health, as well as the limitations of existing therapies (e.g. varenicline and bupropion), including concerns about their safety,[32] the development of 5-HT2C agonists for smoking cessation or drug abuse is a new field that warrants further exploration.
As mentioned above, 5-HT2C agonists have been shown to function as potential therapeutics for the treatment of schizophrenia.[6b] The 5-HT2C receptor is an advantageous target for treating schizophrenia as the activation of it specifically decreases mesolimbic dopamine release without affecting nigrostriatal dopamine.[20] Thus it is predicted to have antipsychotic efficacy while causing few extrapyramidal side effects (EPS). Also, the fact that 5-HT2C agonists can induce weight loss means that such drugs would likely be devoid of the undesired side effect of weight gain and related metabolic disorders, which have been associated with most currently used antipsychotic drugs.[33] Although vabicaserin failed to meet its primary clinical endpoints in human trials, a proof of concept was achieved as some reduction in the positive symptoms of schizophrenia were observed. It should also be kept in mind that the high drop-out rates that were observed in this study may have contributed to the poor results.[19] We have evaluated our compounds in various schizophrenia-like animal behavioral models, such as amphetamine-induced hyperactivity and amphetamine/PCP-disrupted PPI.[22,24] Results of our reported studies together with unpublished data strongly support these agents for further advancement as therapeutic candidates for the treatment of schizophrenia.
In summary, the development of selective 5-HT2C agonists for the treatment of related CNS disorders such as obesity, substance abuse, and schizophrenia is a new and active direction. As stated above, selectivity is one of the most important criteria for the development of 5-HT2C agonists. Compared with other reported compounds, the improved pharmacological profiles of 2-phenylcyclopropylmethylamine derivatives make them preferred candidates for further studies.
Acknowledgements
The authors are grateful for financial support from the US National Institutes of Health (NIH) (R01MH99993) and also thank Dr. Werner Tueckmantel for proofreading the manuscript.
References
- [1].Pazos A, Hoyer D, Palacios JM. Eur. J. Pharmacol. 1984;106:539–546. doi: 10.1016/0014-2999(84)90057-8. [DOI] [PubMed] [Google Scholar]
- [2] a).Berger M, Gray JA, Roth BL. Annu. Rev. Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nichols DE, Nichols CD. Chem. Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- [3].Humphrey PPA, Hartig P, Hoyer D. Trends Pharmacol. Sci. 1993;14:233–236. doi: 10.1016/0165-6147(93)90016-d. [DOI] [PubMed] [Google Scholar]
- [4] a).Werry TD, Loiacono R, Sexton PM, Christopoulos A. Pharmacol. Ther. 2008;119:7–23. doi: 10.1016/j.pharmthera.2008.03.012. [DOI] [PubMed] [Google Scholar]; b) Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, sBush E. Sander, Emeson RB. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- [5].Meltzer HY, Roth BL. J. Clin. Invest. 2013;123:4986–4991. doi: 10.1172/JCI70678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6] a).Nilsson BM. J. Med. Chem. 2006;49:4023–4034. doi: 10.1021/jm058240i. [DOI] [PubMed] [Google Scholar]; b) Rosenzweig-Lipson S, Comery TA, Marquis KL, Gross J, Dunlop J. Handb. Exp. Pharmacol. 2012;213:147–165. doi: 10.1007/978-3-642-25758-2_6. [DOI] [PubMed] [Google Scholar]; c) Filip M, Spampinato U, McCreary AC, Przegalinski E. Brain Res. 2012;1476:132–153. doi: 10.1016/j.brainres.2012.03.035. [DOI] [PubMed] [Google Scholar]; d) Higgins GA, Sellers EM, Fletcher PJ. Trends Pharmacol. Sci. 2013;34:560–570. doi: 10.1016/j.tips.2013.08.001. [DOI] [PubMed] [Google Scholar]
- [7] a).Nichols DE. Pharmacol. Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]; b) Roth BL. N. Engl. J. Med. 2007;356:6–9. doi: 10.1056/NEJMp068265. [DOI] [PubMed] [Google Scholar]; c) Huang XP, Setola V, Yadav PN, Allen JA, Rogan SC, Hanson BJ, Revankar C, Robers M, Doucette C, Roth BL. Mol. Pharmacol. 2009;76:710–722. doi: 10.1124/mol.109.058057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee J, Jung ME, Lee J. Expert Opin. Ther. Pat. 2010;20:1429–1455. doi: 10.1517/13543776.2010.518956. [DOI] [PubMed] [Google Scholar]
- [9].Bös M, Jenck F, Martin JR, Moreau JL, Sleight AJ, Wichmann J, Widmer U. J. Med. Chem. 1997;40:2762–2769. doi: 10.1021/jm970030l. [DOI] [PubMed] [Google Scholar]
- [10].Kimura Y, Hatanaka K, Naitou Y, Maeno K, Shimada I, Koakutsu A, Wanibuchi F, Yamaguchi T. Eur. J. Pharmacol. 2004;486:353–353. doi: 10.1016/j.ejphar.2003.10.004. [DOI] [PubMed] [Google Scholar]
- [11] a).Kennett GA, Curzon G. Psychopharmacology. 1988;96:93–100. doi: 10.1007/BF02431539. [DOI] [PubMed] [Google Scholar]; b) Sargent PA, Sharpley AL, Williams C, Goodall EM, Cowen PJ. Psychopharmacology. 1997;133:309–312. doi: 10.1007/s002130050407. [DOI] [PubMed] [Google Scholar]
- [12] a).Dourish CT, Thomas JM, Higgs S. Neuropsychopharmacology. 2013;38:S506–S507. [Google Scholar]; b) Thomas JM, Dourish CT, Tomlinson JW, Hassan-Smith Z, Higgs S. Psychopharmacology. 2014;231:2449–2459. doi: 10.1007/s00213-013-3409-x. [DOI] [PubMed] [Google Scholar]
- [13].Siuciak JA, Chapin DS, McCarthy SA, Guanowsky V, Brown J, Chiang P, Marala R, Patterson T, Seymour PA, Swick A, Iredale PA. Neuropharmacology. 2007;52:279–290. doi: 10.1016/j.neuropharm.2006.07.024. [DOI] [PubMed] [Google Scholar]
- [14].Kalgutkar AS, Dalvie DK, Aubrecht J, Smith EB, Coffing SL, Cheung JR, Vage C, Lame ME, Chiang P, McClure KF, Maurer TS, Coelho RV, Soliman VF, Schildknegt K. Drug Metab. Dispos. 2007;35:848–858. doi: 10.1124/dmd.106.013649. [DOI] [PubMed] [Google Scholar]
- [15].Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, Al-Shamma H, Smith B, Chalmers D, Behan D. J. Pharmacol. Exp. Ther. 2008;325:577–587. doi: 10.1124/jpet.107.133348. [DOI] [PubMed] [Google Scholar]
- [16].Dunlop J, Watts SW, Barrett JE, Coupet J, Harrison B, Mazandarani H, Nawoschik S, Pangalos MN, Ramamoorthy S, Schechter L, Smith D, Stack G, Zhang J, Zhang GM, Rosenzweig-Lipson S. J. Pharmacol. Exp. Ther. 2011;337:673–680. doi: 10.1124/jpet.111.179572. [DOI] [PubMed] [Google Scholar]
- [17].Dunlop J, Sabb AL, Mazandarani H, Zhang J, Kalgaonker S, Shukhina E, Sukoff S, Vogel RL, Stack G, Schechter L, Harrison BL, Rosenzweig-Lipson S. J. Pharmacol. Exp. Ther. 2005;313:862–869. doi: 10.1124/jpet.104.075382. [DOI] [PubMed] [Google Scholar]
- [18].Smith BM, Smith JM, Tsai JH, Schultz JA, Gilson CA, Estrada SA, Chen RR, Park DM, Prieto EB, Gallardo CS, Sengupta D, Dosa P, Covel A, Ren A, Webb RR, Beeley NRA, Martin M, Morgan M, Espitia S, SaIdana HR, Bjenning C, Whelan KT, Grottick AJ, Menzaghi F, Thomsen WJ. J. Med. Chem. 2008;51:305–313. doi: 10.1021/jm0709034. [DOI] [PubMed] [Google Scholar]
- [19].Shen JHQ, Zhao YG, Rosenzweig-Lipson S, Popp D, Williams JBW, Giller E, Detke MJ, Kane JM. J. Psychiatr. Res. 2014;53:14–22. doi: 10.1016/j.jpsychires.2014.02.012. [DOI] [PubMed] [Google Scholar]
- [20].Marquis KL, Sabb AL, Logue SF, Brennan JA, Piesla MJ, Comery TA, Grauer SM, Ashby CR, Nguyen HQ, Dawson LA, Barrett JE, Stack G, Meltzer HY, Harrison BL, Rosenzweig-Lipson S. J. Pharmacol. Exp. Ther. 2007;320:486–496. doi: 10.1124/jpet.106.106989. [DOI] [PubMed] [Google Scholar]
- [21].Cho SJ, Jensen NH, Kurome T, Kadari S, Manzano ML, Malberg JE, Caldarone B, Roth BL, Kozikowski AP. J. Med. Chem. 2009;52:1885–1902. doi: 10.1021/jm801354e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kozikowski AP, Cho SJ, Jensen NH, Allen JA, Svennebring AM, Roth BL. ChemMedChem. 2010;5:1221–1225. doi: 10.1002/cmdc.201000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen G, Cho SJ, Huang XP, Jensen NH, Svennebring A, Sassano MF, Roth BL, Kozikowski AP. ACS Med. Chem. Lett. 2011;2:929–932. doi: 10.1021/ml200206z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cheng J, Giguere PM, Onajole OK, Lv W, Gaisin A, Gunosewoyo H, Schmerberg CM, Pogorelov VM, Rodriguiz RM, Vistoli G, Wetsel WC, Roth BL, Kozikowski AP. J. Med. Chem. 2015;58:1992–2002. doi: 10.1021/jm5019274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cavasotto CN, Palomba D. Chem. Commun. 2015;51:13576–13594. doi: 10.1039/c5cc05050b. [DOI] [PubMed] [Google Scholar]
- [26].Storer RI, Brennan PE, Brown AD, Bungay PJ, Conlon KM, Corbett MS, DePianta RP, Fish PV, Heifetz A, Ho DKH, Jessiman AS, McMurray G, de Oliveira CAF, Roberts LR, Root JA, Shanmugasundaram V, Shapiro MJ, Skerten M, Westbrook D, Wheeler S, Whitlock GA, Wright J. J. Med. Chem. 2014;57:5258–5269. doi: 10.1021/jm5003292. [DOI] [PubMed] [Google Scholar]
- [27] a).Liu W, Wacker D, Gati C, Han GW, James D, Wang DJ, Nelson G, Weierstall U, Katritch V, Barty A, Zatsepin NA, Li DF, Messer-schmidt M, Boutet S, Williams GJ, Koglin JE, Seibert MM, Wang C, Shah STA, Basu S, Fromme R, Kupitz C, Rendek KN, Grotjohann I, Fromme P, Kirian RA, Beyerlein KR, White TA, Chapman HN, Caffrey M, Spence JCH, Stevens RC, Cherezov V. Science. 2013;342:1521–1524. doi: 10.1126/science.1244142. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wacker D, Wang C, Katritch V, Han GW, Huang XP, Vardy E, McCorvy JD, Jiang Y, Chu MH, Siu FY, Liu W, Xu HE, Cherezov V, Roth BL, Stevens RC. Science. 2013;340:615–619. doi: 10.1126/science.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wang C, Jiang Y, Ma JM, Wu HX, Wacker D, Katritch V, Han GW, Liu W, Huang XP, Vardy E, McCorvy JD, Gao X, Zhou XE, Melcher K, Zhang CH, Bai F, Yang HY, Yang LL, Jiang HL, Roth BL, Cherezov V, Stevens RC, Xu HE. Science. 2013;340:610–614. doi: 10.1126/science.1232807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].DiNicolantonio JJ, Chatterjee S, O’Keefe JH, Meier P. Open Heart. 2014;1:e000173. doi: 10.1136/openhrt-2014-000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Derosa G, Maffioli P. Expert Opin. Drug Saf. 2012;11:459–471. doi: 10.1517/14740338.2012.675326. [DOI] [PubMed] [Google Scholar]
- [30].Kenny PJ. Nat. Rev. Neurosci. 2011;12:638–651. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- [31].Zeeb FD, Higgins GA, Fletcher PJ. ACS Chem. Neurosci. 2015;6:1231–1240. doi: 10.1021/acschemneuro.5b00017. [DOI] [PubMed] [Google Scholar]
- [32].Syed BA, Chaudhari K. Nat. Rev. Drug Discovery. 2013;12:97–98. doi: 10.1038/nrd3914. [DOI] [PubMed] [Google Scholar]
- [33] a).Musil R, Obermeier M, Russ P, Hamerle M. Expert Opin. Drug Saf. 2015;14:73–96. doi: 10.1517/14740338.2015.974549. [DOI] [PubMed] [Google Scholar]; b) Kroeze WK, Hufeisen SJ, Popadak BA, Renock S, Steinberg SA, Ernsberger P, Jayathilake K, Meltzer HY, Roth BL. Neuropsychopharmacology. 2003;28:519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]