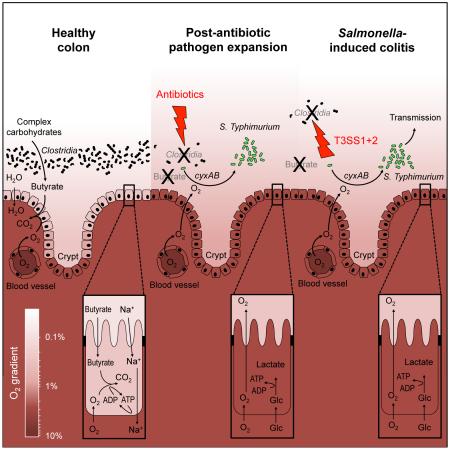
Abstract
The mammalian intestine is host to a microbial community that prevents pathogen expansion through unknown mechanisms, while antibiotic treatment can increase susceptibility to enteric pathogens. Here we show that streptomycin treatment depleted commensal, butyrate-producing Clostridia from the mouse intestinal lumen, leading to decreased butyrate levels, increased epithelial oxygenation and aerobic expansion of Salmonella enterica serovar Typhimurium. Epithelial hypoxia and Salmonella restriction could be restored by tributyrin treatment. Clostridia depletion and aerobic Salmonella expansion were also observed in the absence of streptomycin treatment in genetically resistant mice, but proceeded with slower kinetics and required the presence of functional Salmonella type III secretion systems. The Salmonella cytochrome bd-II oxidase synergized with nitrate reductases to drive luminal expansion, and both were required for fecal-oral transmission. We conclude that Salmonella virulence factors and antibiotic treatment promote pathogen expansion through the same mechanism: depletion of butyrate-producing Clostridia to elevate epithelial oxygenation, allowing aerobic Salmonella growth.
Graphical abstract
INTRODUCTION
Non-typhoidal Salmonella enterica serovars, such as S. enterica serovar Typhimurium (S. Typhimurium), are a common cause of human gastroenteritis (Majowicz et al., 2010). Studies using animal models of infection show that upon ingestion, S. Typhimurium uses the invasion-associated type III secretion system (T3SS-1) to enter intestinal epithelial cells (Galan and Curtiss, 1989). After crossing the epithelial barrier, the pathogen deploys a second type III secretion system (T3SS-2) to enhance its survival in the underlying tissue (Hensel et al., 1995). T3SS-1-mediated epithelial invasion and T3SS-2-mediated survival in tissue trigger acute intestinal inflammation and diarrhea (Tsolis et al., 1999), the hallmarks of gastroenteritis. Interestingly, intestinal inflammation confers a benefit to the pathogen because it drives its expansion in the lumen of the large bowel (Barman et al., 2008; Stecher et al., 2007), which is required for fecal oral transmission of S. Typhimurium to the next susceptible host (Lawley et al., 2008).
One mechanism that powers a luminal expansion of S. Typhimurium during colitis is the generation of respiratory electron acceptors as a by-product of the inflammatory host response (Ali et al., 2014; Lopez et al., 2015; Lopez et al., 2012; Rivera-Chavez et al., 2013; Thiennimitr et al., 2011; Winter et al., 2010). For example, reactive nitrogen species generated by the inflammatory host response can react to form nitrate, the preferred respiratory electron acceptor of S. Typhimurium under anaerobiosis (Lopez et al., 2015; Lopez et al., 2012).
A history of antibiotic usage is a risk factor for developing S. enterica-induced gastroenteritis (Pavia et al., 1990) and antibiotic treatment during convalescence may on occasion produce a bacteriologic and symptomatic relapse (Aserkoff and Bennett, 1969; Nelson et al., 1980). These effects of antibiotics can be modeled in mice, as treatment with streptomycin leads to a marked expansion of S. Typhimurium in the lumen of the murine large intestine (Que and Hentges, 1985). The mechanisms contributing to this post-antibiotic pathogen expansion remain poorly understood.
Antibiotic treatment increase epithelial oxygenation in the large intestine (Kelly et al., 2015), which is predicted to elevate diffusion of oxygen into the gut lumen (Espey, 2013). Oxygen is the only respiratory electron acceptor with a higher redox potential than nitrate. Work on oxygen respiration in Escherichia coli suggests that under conditions of high aeration, S. Typhimurium predominantly uses the low-affinity cytochrome bo3 oxidase encoded by cyoAB (Alexeeva et al., 2003; Cotter et al., 1990; Cotter et al., 1992; Cotter and Gunsalus, 1992; Fu et al., 1991). When the pathogen enters host tissue, it encounters an oxygen partial pressure (pO2) of 23–70 mmHg (3%-10% oxygen), which is considerably lower than the atmospheric pO2 of 160 mmHg (21% O2) (Carreau et al., 2011). S. Typhimurium relies on the high-affinity cytochrome bd oxidase encoded by the cydAB genes to support its growth in tissue during infection of mice (Craig et al., 2013).
Importantly, the S. Typhimurium chromosome encodes another enzyme with homology to cytochrome bd oxidase. This enzyme is termed cytochrome bd-II oxidase and is encoded by the S. Typhimurium cyxAB operon (Atlung and Brondsted, 1994; Dassa et al., 1991; McClelland et al., 2001). Cytochrome bd-II oxidase remains poorly characterized and its physiological role is not known. Regulation of the cyxAB operon in E. coli suggests that cytochrome bd-II oxidase might have a function under even more-oxygen-limiting conditions than cytochrome bd oxidase (Brondsted and Atlung, 1996), but this hypothesis has not been tested. Here we investigated whether cytochrome bd-II oxidase contributes to expansion of S. Typhimurium in the large intestine.
RESULTS
Cytochrome bd and bd-II oxidases are required for growth in different host niches
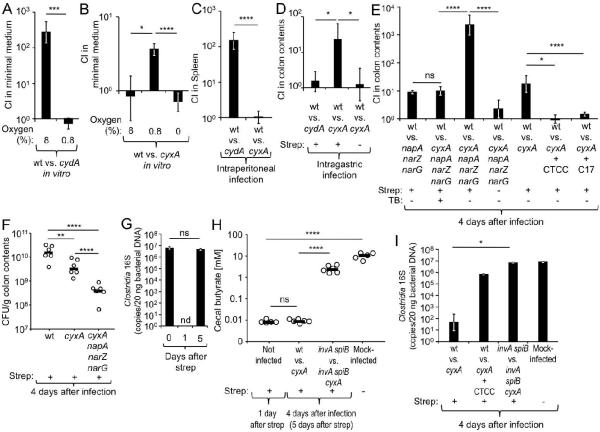
We generated S. Typhimurium mutants lacking either a functional cytochrome bd oxidase (cydA mutant) or a functional cytochrome bd-II oxidase (cyxA mutant) to investigate how aerobic respiration affects growth of the pathogen. The S. Typhimurium wild type exhibited a fitness advantage over the cydA mutant during growth with 8% oxygen, which corresponds to tissue oxygenation (3%-10% oxygen). However, the cydA gene did not confer a fitness advantage during growth with 0.8% oxygen (Fig. 1A), which was consistent with repression of the E. coli cydAB operon in low-oxygen environments (Tseng et al., 1996). The cyxA gene was not required for growth with 8% oxygen or with 0% oxygen, but provided a fitness advantage during growth with 0.8% oxygen (Fig. 1B).
Figure 1. Cytochrome bd-II oxidase contributes to post-antibiotic pathogen expansion.
(A and B) Minimal medium was inoculated with the indicated strains and the competitive index (CI) determined after 24 hours incubation in the presence of 8%, 0.8% or 0% oxygen. (C) Groups of C57BL/6 mice (N = 4) were infected intraperitoneally with the indicated strain mixtures and the CI determined four days after infection. (D–I) Streptomycin (Strep)-treated or mock-treated C57BL/6 mice were mock-infected or infected intragastrically with the indicated S. Typhimurium strains or strain mixtures (N is indicated in panels F and H or in Fig. S1A). (D, E) The CI in colon contents was determined four days after infection. (E) One day after infection, some mice were inoculated intragastrically with a culture of 17 human Clostridia isolates (C17) or received tributyrin (TB) supplementation daily. (E and I) One day after infection, some mice received chloroform-treated cecal contents (CTCC) of treatment naïve mice intragastrically. (F) CFU recovered from colon contents four days after infection with individual S. Typhimurium strains. (F and H) Each circle represents data from an individual animal. (G and I) Clostridia 16S rRNA gene copy numbers present in 20 ng of total bacterial DNA were determined at the indicated time points. (H) The concentration of butyrate was measured in cecal contents at the indicated time points after streptomycin treatment (see also Fig. S1B and S1C). Bars represent geometric means ± standard error. *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.001; ns, not statistically significantly different; nd, none detected; wt, S. Typhimurium wild type.
To assess the contribution of cydA and cyxA to growth in host tissue, mice (C57BL/6) were infected intraperitoneally with a 1:1 mixture of the wild type and the cydA mutant or a 1:1 mixture of the wild type and the cyxA mutant, respectively (competitive infection). Four days after infection, the wild type was recovered in significantly higher numbers from the spleen than the cydA mutant (Fig. 1C), confirming that cytochrome bd oxidase contributes to growth in tissue (Craig et al., 2013). In contrast, cyxA was dispensable for growth in tissue.
We next investigated the contribution of cydA and cyxA to post-antibiotic pathogen expansion in the colon contents of mice. To this end, mice (C57BL/6) received a single dose of streptomycin intragastrically and were infected one day later with a 1:1 mixture of the wild type and the cydA mutant or a 1:1 mixture of the wild type and the cyxA mutant. Four days after infection, the cydA gene was dispensable for post-antibiotic pathogen expansion while the cyxA gene conferred a significant (P < 0.05) fitness advantage (Fig. 1D). Importantly, the cyxA mutant did not confer a fitness advantage in the colon of mice that had not received antibiotics, suggesting that cytochrome bd-II oxidase contributed to post-antibiotic pathogen expansion in the gut (Fig. 1D).
Collectively, our data indicated that cydA was exclusively required during growth in host tissue (Fig. 1C), while cyxA contributed exclusively to growth under the more oxygen-limited conditions encountered in the antibiotic-treated gut (Fig. 1D).
Cytochrome bd-II oxidase and nitrate reductases synergistically drive post-antibiotic pathogen expansion
Consistent with previous studies suggesting that nitrate respiration contributes to a post-antibiotic pathogen expansion (Lopez et al., 2015; Lopez et al., 2012), the S. Typhimurium wild type was recovered in approximately 8-fold higher numbers than a nitrate respiration-deficient mutant (narG napA narZ mutant) four days after competitive infection of streptomycin-treated mice (C57BL/6) (Fig. 1E). To investigate whether nitrate respiration and aerobic respiration cooperated during post-antibiotic pathogen expansion, we constructed a cyxA narG napA narZ mutant and compared its fitness with that of wild-type S. Typhimurium. Recovery of bacteria four days after infection revealed a remarkable synergy between nitrate respiration and aerobic respiration, as the wild type was recovered in approximately 2,000-fold higher numbers than the respiration-deficient cyxA narG napA narZ mutant (Fig. 1E and S1A). Importantly, the S. Typhimurium wild type and cyxA narG napA narZ mutant were recovered in similar numbers from mice that had not received streptomycin, suggesting that nitrate respiration and aerobic respiration cooperated during post-antibiotic pathogen expansion. Similar results were observed when streptomycin-treated mice were infected with individual S. Typhimurium strains (Fig. 1F).
Cytochrome bd-II oxidase-dependent aerobic growth is driven by an antibiotic-mediated depletion of Clostridia
The finding that cyxA only conferred a growth advantage upon S. Typhimurium in streptomycin-treated mice suggested that aerobic pathogen expansion required depletion of a component of the gut-associated microbial community (gut microbiota). Analysis of DNA isolated from feces by quantitative real-time PCR with class-specific primers suggested that streptomycin treatment caused a marked depletion of members of the class Clostridia from the gut microbiota within a day (Fig. 1G) (Sekirov et al., 2008). By 5 days after streptomycin treatment, the Clostridia population had recovered to levels observed prior to antibiotic treatment. Clostridia are credited for producing the lion's share of the short-chain fatty acid butyrate, an important fermentation product produced by the gut microbiota (Louis and Flint, 2009; Vital et al., 2014). Cecal butyrate concentrations were diminished by four orders of magnitude one day after treatment with streptomycin (Fig 1H). In contrast, streptomycin treatment lowered the concentrations of acetate and propionate in the cecal contents by only one or two orders of magnitude, respectively (Fig. S1B and S1C).
We next tested the hypothesis that antibiotic-mediated depletion of Clostridia was responsible for an aerobic post-antibiotic pathogen expansion. To this end, streptomycin-treated mice (C57BL/6) were infected with the wild type and a cyxA mutant and inoculated one day later with chloroform-treated cecal contents of treatment naïve mice. Since chloroform kills vegetative bacterial cells but not spores, this treatment enriches for Clostridia, the dominant group of spore-forming bacteria present in cecal contents (Itoh and Freter, 1989). Inoculation of streptomycin-treated mice with chloroform-treated cecal contents increased the abundance of Clostridia (Fig. 1I) and annulled the fitness advantage conferred by cytochrome bd-II oxidase (Fig. 1E).
To directly test whether Clostridia depletion was responsible for driving an aerobic S. Typhimurium expansion, streptomycin-treated mice were infected with the wild type and a cyxA mutant and inoculated one day later with a community of 17 human Clostridia isolates (Atarashi et al., 2013; Atarashi et al., 2011; Narushima et al., 2014). Remarkably, inoculation with the 17 human Clostridia isolates abrogated the fitness advantage conferred by the cyxA gene (Fig. 1E and S1A).
Clostridia depletion increases oxygenation of colonocytes to drive a cytochrome bd-II oxidase-dependent aerobic pathogen expansion
We next wanted to investigate how the prevalence of Clostridia could alter oxygen-availability in the gut. Clostridia are the main producers of butyrate (Louis and Flint, 2009; Vital et al., 2014), which serves as the preferred energy source for colonocytes (enterocytes of the colon). Colonocytes oxidize butyrate to carbon dioxide (CO2) (Donohoe et al., 2012), thereby rendering the epithelium hypoxic (< 7.6 mmHg or < 1% O2) (Kelly et al., 2015). However, in germ-free mice, where butyrate is absent, colonocytes obtain energy by fermenting glucose to lactate (Donohoe et al., 2012), which is accompanied by an increased oxygenation of the epithelium (Kelly et al., 2015). Since increased oxygenation of the epithelium is predicted to elevate oxygen diffusion into the gut lumen (Espey, 2013), we hypothesized that a depletion of Clostridia-derived butyrate would increase the oxygenation of colonocytes and increase diffusion of oxygen into the gut lumen to fuel a cyxA-dependent S. Typhimurium expansion.
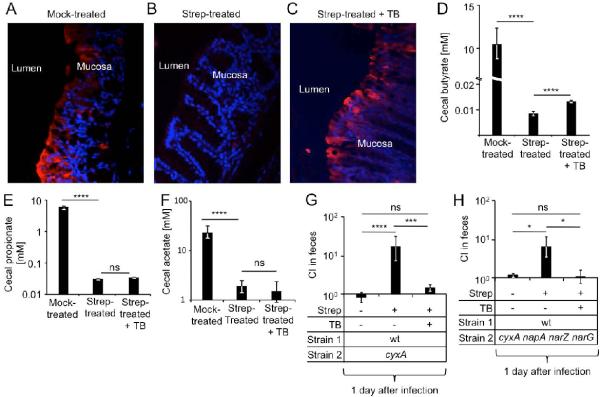
To test this hypothesis, we first investigated whether an antibiotic-mediated depletion of butyrate-producing Clostridia would elevate the oxygenation of colonocytes by using the exogenous hypoxia marker pimonidazole. Under hypoxic conditions, nitroreductase enzymes reduce pimonidazole to hydroxylamine intermediates, which bind irreversibly to nucleophilic groups in proteins or DNA (Kizaka-Kondoh and Konse-Nagasawa, 2009). Colonocytes of conventional mice (C57BL/6) exhibited hypoxia as indicated by pimonidazole staining (Fig. 2A) (Colgan and Taylor, 2010; Kelly et al., 2015). Streptomycin treatment eliminated staining of colonozytes with pimonidazole (Fig. 2B), indicative of a marked increase in colonocyte oxygenation. Next, we supplemented streptomycin-treated mice with 1,2,3-tributyrylglycerol (tributyrin), a food additive (butter flavoring) that exhibits delayed absorption in the proximal gut compared to butyrate, which renders it more effective for increasing butyrate concentrations in the large intestine (Kelly et al., 2015). Remarkably, tributyrin supplementation restored epithelial hypoxia in streptomycin-treated mice (Fig. 2C) and significantly (P 0.001) increased the concentration of cecal butyrate (Fig. 2D), while the concentrations of propionate and acetate remained unchanged (Fig. 2E and 2F). Thus, a streptomycin-mediated depletion of Clostridia-derived butyrate increased colonocyte oxygenation.
Figure 2. Tributyrin treatment restores physiologic hypoxia of colonocytes and prevents cytochrome bd-II oxidase-dependent post-antibiotic pathogen expansion.
(A–C) Groups of C57BL/6 mice (N = 4) were mock-treated (A), treated with streptomycin (Strep) (B) or streptomycin and tributyrin (TB) (C) and the colon collected one day later. Binding of pimonidazole (red fluorescence) was detected in sections of the colon counter stained with DAPI nuclear stain (blue fluorescence). Representative images are shown. (D–F) Groups of C57BL/6 mice (N = 5) were mock-treated, treated with streptomycin or streptomycin and TB. Concentrations of butyrate (D), propionate (E) and acetate (F) in cecal contents were determined 8 hours after TB supplementation. (G–H) Groups of C57BL/6 mice (N is shown in Fig. S1A except for TB supplementation where N = 4) were mock-treated or treated with streptomycin and infected one day later with the indicated strain mixtures. Some mice received mock supplementation or were supplemented with tributyrin 8 hours prior to pimonidazole injection. One day after infection, the competitive index (CI) in colon contents was determined. Bars represent geometric means ± standard error. *, P < 0.05; ***, P < 0.005; ****, P < 0.001; ns, not statistically significantly different; wt, S. Typhimurium wild type.
Since streptomycin treatment resulted in depletion of Clostridia and increased oxygenation of colonocytes within one day, we hypothesized that a cyxA-dependent fitness advantage might already be apparent at early time points after antibiotic treatment. A significant (P < 0.05) fitness advantage conferred by the cyxA gene was apparent just one day after infection of streptomycin-treated mice, however, no fitness advantage was observed in the absence of streptomycin treatment (Fig. 2G). Remarkably, tributyrin supplementation abrogated the fitness advantage conferred by the cyxA gene in streptomycin-treated mice (Fig. 2G). Similar results were obtained with a cyxA narG napA narZ mutant (Fig. 2H). Since a benefit of nitrate respiration is not observed one day after S. Typhimurium infection of streptomycin-treated mice (Lopez et al., 2015), these results point to cyxA as the main factor driving an early post-antibiotic expansion of S. Typhimurium in the lumen (i.e. one day after infection).
At four days after infection of streptomycin-treated mice, tributyrin supplementation reduced the fitness advantage conferred by the cyxA napA narZ narG genes to that conferred by the napA narZ narG genes in the absence of tributyrin supplementation (Fig. 1E). This outcome was consistent with the idea that tributyrin supplementation only reduced the fitness advantage conferred by cyxA.
T3SS-1 and T3SS-2 drive a cytochrome bd-II oxidase-mediated aerobic growth at later stages of infection
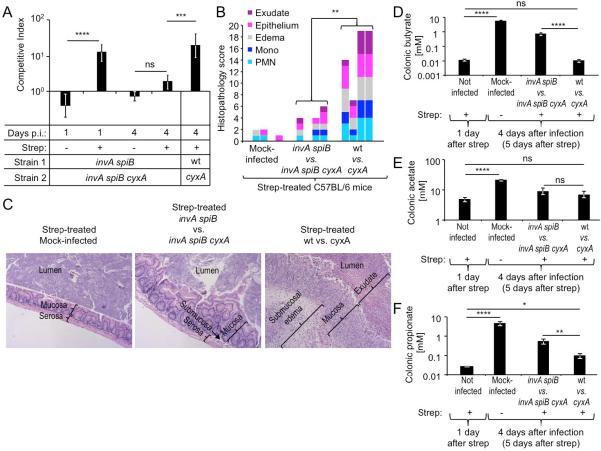
To investigate whether virulence factors were required to support luminal growth by aerobic respiration, T3SS-1 and T3SS-2 were inactivated using mutations in the invA and spiB genes, respectively. Similar to what was observed in the wild-type background, a fitness advantage of the avirulent invA spiB mutant over the invA spiB cyxA mutant was observed after one day of infection in the fecal contents of streptomycin-treated mice (C57BL/6), but not in mice that had not received antibiotics (Fig. 3A and S1A). Thus, virulence factors were not required for cytochrome bd-II oxidase-mediated growth one day after infection (corresponding to two days after streptomycin treatment). By four days after infection with virulent S. Typhimurium strains (i.e. a mixture of wild type and cyxA mutant), mice developed acute inflammation in the cecal mucosa, while no marked inflammatory changes were observed in mice infected with avirulent S. Typhimurium strains (i.e. a mixture of invA spiB mutant and invA spiB cyxA mutant) (Fig. 3B, C and S2). Interestingly, while the virulent S. Typhimurium wild type exhibited a fitness advantage over a cyxA mutant in colon contents four days after infection (corresponding to five days after streptomycin treatment), no fitness advantage was observed at this time point when streptomycin-treated mice were inoculated with a 1:1 mixture of the avirulent invA spiB mutant and a invA spiB cyxA mutant (Fig. 3A). Thus, virulence factors were required for cytochrome bd-II oxidase-mediated growth at later times after antibiotic treatment.
Figure 3. Virulence factors drive a cytochrome bd-II oxidase-dependent expansion of S. Typhimurium at later time points after infection.
(A–C) Groups of C57BL/6 mice (N is shown in Fig. S1A) were mock-treated or treated with streptomycin (Strep) and infected one day later with the indicated strain mixtures. (A) At one and four days after infection (Days p.i.) the competitive index (CI) in colon contents was determined. (B) Cecal histopathology was scored using tissue collected four days after infection from four mice per group using criteria listed in Table S1 (see also Fig. S2). Each bar represents data from one individual animal. (C) Representative images of H/E-stained cecal sections scored in panel B. All images were taken at the same magnification. (D–F) Groups of C57BL/6 mice were treated with streptomycin or mock-treated. One day after streptomycin treatment organs were collected for analysis or mice were mock infected or infected with the indicated S. Typhimurium strain mixtures. Concentrations of butyrate (D), acetate (E) and propionate (F) in colon contents were determined at the indicated time points. (A–C and D–F) Bars represent geometric means ± standard error. *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.001; ns, not statistically significantly different; wt, S. Typhimurium wild type.
We next investigated whether the degree of Clostridia depletion differed at later time points after infection between mice inoculated with virulent or avirulent S. Typhimurium strains. Strikingly, by four days after infection of streptomycin-treated mice with avirulent S. Typhimurium strains, the abundance of Clostridia had recovered to levels similar to those observed in mock-treated mice (Fig. 1I) and butyrate levels in the colon were significantly (P < 0.001) elevated compared to concentrations measured one day after streptomycin treatment (Fig. 3D). In stark contrast, a marked depletion of Clostridia DNA was still observed four days after infection with virulent S. Typhimurium strains (Fig. 1I) and colonic butyrate concentrations remained at levels observed one day after treatment with streptomycin (Fig. 3D). While virulence factors reduced butyrate levels by two orders of magnitude (Fig. 3D), acetate levels remained unchanged (Fig. 3E) and a 5.5-fold change in propionate levels was seen (Fig. 3F).
Collectively, these data suggested that virulence factors were unessential for aerobic S. Typhimurium growth during early stages of infection when antibiotics maintained a depletion of Clostridia. However, once the effect of antibiotics wore off, Clostridia levels began to rebound and butyrate concentrations started to rise again. During this recovery from antibiotic treatment, virulence factors contributed to maintaining Clostridia depletion and thus became essential for cyxA-dependent aerobic pathogen expansion.
Infection with virulent S. Typhimuirum triggers dysbiosis characterized by a depletion of Clostridia
Post-antibiotic pathogen expansion is not likely to represent the true physiological role of cytochrome bd-II oxidase, since the cyxAB operon is conserved among serovars of S. enterica, a species that formed long before the advent of antibiotic therapy. While streptomycin-treatment was solely responsible for cyxA-dependent growth of S. Typhimurium one day after infection, a contribution of virulence factors was apparent at four days after infection (Fig. 3A). We thus reasoned that at later time points after S. Typhimurium infection, the physiological role of cyxA might be apparent even in the absence of antibiotic treatment. Since genetically susceptible C57BL/6 mice become moribund at five days after infection, we switched to a genetically resistant mouse lineage (CBA mice) to test this idea.
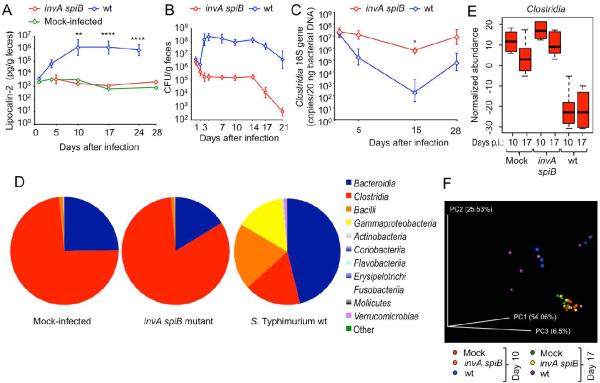
To investigate whether S. Typhimurium virulence factors could deplete Clostridia in the absence of antibiotic treatment, CBA mice were mock infected or infected with either the S. Typhimurium wild type or an invA spiB mutant. Measurement of fecal lipocalin 2, an inflammatory marker, suggested that intestinal inflammation peaked between days 10 and 17 after infection and required intact virulence factors (Fig. 4A). Transcript levels of Kc (encoding the neutrophil chemoattractant CXCL1), Mip2 (encoding the neutrophil chemoattractant CXCL2) and Il17a (encoding the pro-inflammatory cytokine interleukin [IL]-17A) were significantly elevated in the cecal mucosa at days 10 and 17 after infection with the S. Typhimurium wild type compared to mock-infected and invA spiB mutant-infected mice (Fig. S3A to S3F). Mice infected with the S. Typhimurium wild type shed approximately three orders of magnitude higher numbers of the pathogen in their feces than mice infected with the invA spiB mutant (Fig. 4B). Importantly, intestinal inflammation induced by the S. Typhimurium wild type was accompanied by a significant (P < 0.05) depletion of Clostridia (Fig. 4C).
Figure 4. S. Typhimurium virulence factors induce intestinal inflammation and are required for Clostridia depletion in the absence of antibiotic treatment.
Groups of CBA mice (N = 6) were mock-infected or infected intragastrically with 1 × 109 CFU/animal of the virulent S. Typhimurium wild type (wt) or the avirulent invA spiB mutant (invA spiB). (A) Fecal lipocalin-2 levels were determined by ELISA (see also Fig. S3). (B) S. Typhimurium CFU were determined in the feces over time. (C) Clostridia 16S rRNA gene copy numbers were determined by quantitative real-time PCR. (A–C) Data points represent geometric means ± standard error. (D) Average relative abundances of phylogenetic groupings at the class level determined by 16S profiling of the microbial community present in colon contents on day 10 after infection. (E) Normalized abundances of members of the class Clostridia in colon contents at the indicated days after infection (days p.i.). Boxes in whisker plots represent the second and third quartiles, while lines indicate the first and fourth quartiles. (F) Weighted principal coordinate analysis of 16S profiling data in which all reads identified as Enterobacteriaceae were excluded from analysis. For additional analysis of 16S profiling data see Fig. S4–S7. Each dot represents data from one animal. *, P < 0.05; **, P < 0.01; ****, P < 0.001.
To get a more detailed view of how S. Typhimurium infection alters the composition of the microbiota in the cecum, its composition was analyzed by 16S rRNA gene sequencing (16S profiling) at 10 and 17 days after infection. Compared to mock-infected mice, both the relative abundance (Fig. 4D, S4A, S5 and S6) and the normalized abundance (Fig. 4E) of Clostridia was significantly reduced in CBA mice at both 10 and 17 days after infection with the S. Typhimurium wild type strain (P < 0.0001), but not in invA spiB mutant-infected mice. Conversely, the abundance of members from the highly represented phylum Bacteroidetes remained unchanged during infection with the S. Typhimurium wild type strain compared to mock-treated mice (Fig. 4D, S4B, S5 and S7). Principle coordinate analysis revealed marked changes in the microbial community structure caused by infection with the S. Typhimurium wild type, while the microbiota composition of mice infected with the invA spiB mutant was similar to that in mock-infected mice (Fig. S3C), which was independent of changes in the abundance of Enterobacteriaceae (Fig. 4F). The strongest negative correlation in our data set was that Clostridia depletion was accompanied by an expansion of Gammaproteobacteria (Fig. S3D), the latter of which was due to an increased normalized abundance of Enterobacteriaceae (Fig. S3E). However, there was no correlation between abundances of Gammaproteobacteria and Bacteroidia (Fig. S3D).
A luminal aerobic S. Typhimurium expansion is observed in genetically resistant mice in the absence of antibiotic treatment
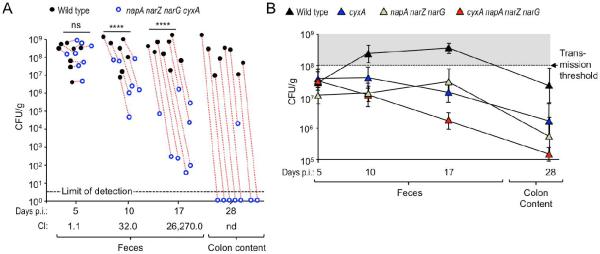
We next wanted to investigate whether a respiration-dependent expansion could also be observed in the absence of antibiotic treatment. The cyxA narG napA narZ genes did not provide a fitness advantage five days after competitive infection of CBA mice, however, at days 10 and 17 after infection, the wild type displayed a significant fitness advantage over the cyxA narG napA narZ mutant and by day 28 after infection the cyxA narG napA narZ mutant had been cleared from colon contents of most mice (Fig. 5A).
Figure 5. Cytochrome bd-II oxidase and nitrate reductases contribute to a luminal S. Typhimurium expansion in the absence of antibiotic treatment.
(A) Groups of CBA mice were infected intragastrically with a 1:1 mixture of the S. Typhimurium wild type (black circles) and the respiration-deficient napA narZ narG cyxA mutant (blue circles) and samples collected at the indicated days after infection (days p.i.). Dotted red lines connect strains recovered from the same animal. CI, competitive index; ****, P < 0.001; ns, not statistically significantly different; nd, not determined. (B) Groups of CBA mice (N = 4) were infected intragastrically with 1 × 108 CFU/animal of one of the indicated S. Typhimurium strains and samples collected at the indicated days after infection.
Infection of CBA mice with individual S. Typhimurium strains revealed that the wild type was shed in significantly higher numbers in the feces on days 10 and 17 after infection than a cyxA mutant (P < 0.05 and P < 0.01, respectively), a narG napA narZ mutant (P < 0.01 and P < 0.05, respectively) or a cyxA narG napA narZ mutant (P < 0.01 and P < 0.0001, respectively) (Fig. 5B). At the peak of fecal shedding (day 17 after infection), the cyxA narG napA narZ mutant was shed in significantly lower numbers with the feces than either the cyxA mutant (P < 0.05) or the narG napA narZ mutant (P < 0.05).
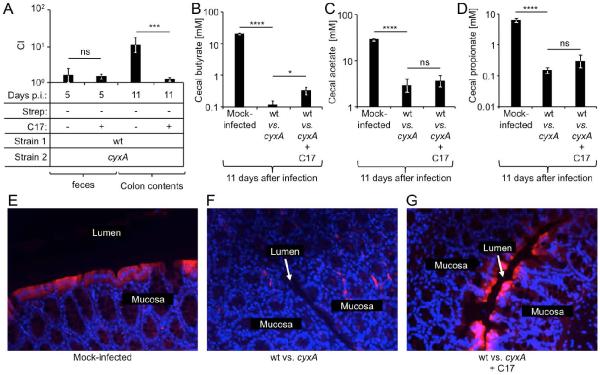
To test the hypothesis that a depletion of Clostridia was responsible for a cyxA-dependent pathogen expansion, CBA mice were infected intragastrically with a 1:1 mixture of the S. Typhimurium wild type and a cyxA mutant. At days 5, 7 and 10 after infection, mice were mock-treated or inoculated intragastrically with a community of 17 human Clostridia isolates. The cyxA gene conferred a significant (P < 0.005) fitness advantage at 11 days after infection of mock-treated mice (Fig. 6A), which was abrogated in mice inoculated with a community of 17 human Clostridia isolates. Furthermore, S. Typhimurium infection significantly (P < 0.001) diminished cecal butyrate concentrations. While inoculation with a community of 17 human Clostridia isolates resulted in a significant (P < 0.05) increase in butyrate levels (Fig. 6B), cecal levels of acetate and propionate remained unchanged (Fig. 6C and 6D). Importantly, colonocytes of mock-infected mice exhibited hypoxia (Fig. 6E), which was eliminated during S. Typhimurium infection (Fig. 6F), but could be restored by inoculation with a community of 17 human Clostridia isolates (Fig. 6G).
Figure 6. Depletion of Clostridia increases colonocyte oxygenation and drives a cytochrome bd-II oxidase-dependent expansion of S. Typhimurium.
Groups of CBA mice were mock-infected or infected intragastrically with a 1:1 mixture of the S. Typhimurium wild type (wt) and a cyxA mutant. At 5, 7 and 10 days after infection, mice were mock-inoculated or inoculated with 17 human Clostridia isolates (C17). (A) The competitive index (CI) in feces or colon contents was determined at the indicated time points. (B–D) Concentrations of butyrate (B), acetate (C) and propionate (D) in cecal contents are shown. (A–D) Bars represent geometric means ± standard error. (E–G) Binding of pimonidazole (red fluorescence) was detected in colonic sections counter stained with DAPI nuclear stain (blue fluorescence). Representative images are shown. *, P < 0.05; ***, P < 0.005; ****, P < 0.001; ns, not statistically significantly different.
Cytochrome bd-II oxidase and nitrate reductases are required for transmission by the fecal oral route
Maximal bacterial shedding is relevant, because S. Typhimurium numbers in the feces need to exceed a threshold of 108 bacteria per gram feces for transmission (Lawley et al., 2008). Interestingly, only the S. Typhimurium wild type always exceeded this critical threshold during infection of CBA mice (Fig. 5B). To test the prediction that respiration is required for transmission, we studied transmission of S. Typhimurium from infected CBA mice to naïve CBA mice. All naïve mice cohoused with S. Typhimurium wild type-infected mice started to shed the pathogen within 7 days of cohousing (Fig. 7). In contrast, the pathogen could not be isolated from any of the naïve mice cohoused with either cyxA mutant-infected mice or with cyxA narG napA narZ mutant-infected mice after 7 days of cohousing. After 18 days of cohousing, 33% of naïve mice cohoused with cyxA mutant-infected mice became colonized with the pathogen. However, none of the naïve mice cohoused for 18 days with cyxA narG napA narZ mutant-infected mice became colonized with the pathogen. Thus, aerobic respiration contributed to transmission, but an inability to respire both oxygen and nitrate resulted in complete loss of S. Typhimurium transmissibility by the fecal oral route.
Figure 7. Respiration is required for fecal-oral transmission.
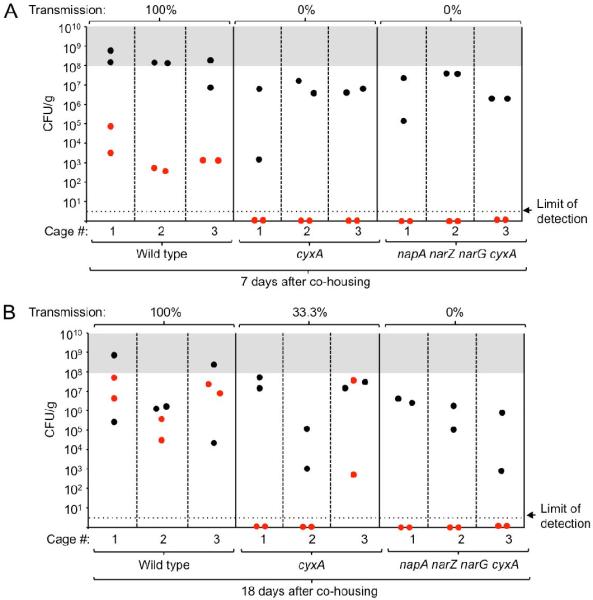
Groups of CBA mice (N = 6) were infected intragastrically with 1 × 108 CFU/animal of either the S. Typhimurium wild type, a cyxA mutant or a napA narZ narG cyxA mutant. 10 days after infection, two infected mice (donors, black circles) were co-housed with two naïve mice (recipients, red circles) per cage and feces (A) or colon contents (B) were collected after 7 (A) or 18 days of co-housing (B).
DISCUSSION
The intestine is home to a large microbial community that confers benefit by preventing pathogen expansion (Bohnhoff et al., 1954). Streptomycin treatment of mice depletes populations of spore-forming bacteria belonging to the class Clostridia (Sekirov et al., 2008), which are the members within the community that are most effective in preventing expansion of commensal E. coli in the mouse intestine (Itoh and Freter, 1989). Clostridia become depleted at later stages of an S. Typhimurium infection in streptomycin-treated mice through a neutrophil-dependent mechanism (Gill et al., 2012). Here we show that inflammation induced by S. Typhimurium virulence factors leads to a depletion of Clostridia in genetically resistant mice even in the absence of antibiotic treatment.
Changes in the microbiota composition induced either by streptomycin treatment or by S. Typhimurium infection were accompanied by a depletion of the short-chain fatty acid butyrate. Butyrate production by the gut microbiota proceeds through the acetyl-CoA pathway, the glutarate pathway, the 4-aminobutyrate pathway or the lysine pathway and the majority of bacteria encoding these pathways are members of the class Clostridia (Louis and Flint, 2009; Vital et al., 2014). An antibiotic-induced depletion of short-chain fatty acids has been previously implicated in the loss of colonization resistance against S. Typhimurium (Garner et al., 2009; Meynell, 1963). However, the mechanism by which short-chain fatty acids limit pathogen expansion is not fully resolved. Butyrate has a marked influence on host cell physiology because it serves as the main energy source for colonocytes (Donohoe et al., 2012). The main function of the colon is to absorb water by generating an osmotic gradient through the absorption of sodium (Na+). Na+ diffuses along an electrochemical gradient through channels located in the apical membrane of surface colonocytes and is then actively extruded by a Na+ pump (Na+ K+ ATPase) located in their basolateral membrane (Sandle, 1998). The ATP required by surface colonocytes to energize Na+ transport comes from the oxidation of microbiota-derived butyrate to carbon dioxide (CO2) (Velazquez et al., 1997). The oxidation of butyrate to CO2 consumes considerable quantities of oxygen within the host cell, thereby rendering the epithelium hypoxic (< 7.6 mmHg or < 1% O2) (reviewed in (Colgan and Taylor, 2010)). Importantly, a streptomycin-mediated depletion of Clostridia-derived butyrate changes the energy metabolism of colonocytes towards fermentation of glucose, thereby conserving oxygen within the host cell and increasing oxygenation of the epithelium (Kelly et al., 2015). Since oxygen diffuses freely across biological membranes, increased oxygenation of the epithelium is predicted to elevate oxygen availability in the intestinal lumen (Espey, 2013). Consistent with this idea, we found that streptomycin treatment fueled a cyxA-dependent aerobic expansion of S. Typhimurium, which could be blunted by increasing the abundance of Clostridia or by restoring the physiologic hypoxia of colonocytes through tributyrin supplementation.
Streptomycin treatment depleted Clostridia within a day, while S. Typhimurium infection alone reduced the prevalence of Clostridia within the community more slowly. It has been reported previously that streptomycin-treatment can rescue the ability of a S. Typhimurium mutant lacking both T3SS-1 and T3SS-2 to colonize the lumen of the large bowel at early stages of infection (Barthel et al., 2003). Our data indicate that this effect is at least in part due to increased oxygenation of the intestinal epithelium driving a cyxA-dependent aerobic expansion of S. Typhimurium. Interestingly, our data suggest that virulence factors and antibiotic treatment drive pathogen expansion through the same mechanism, namely a depletion of Clostridia. The biological significance of this pathogen expansion is that shedding of the pathogen in high numbers with the feces is required for its successful transmission by the fecal oral route (Lawley et al., 2008). Consistent with this idea, we found that a respiration-dependent expansion of S. Typhimurium was essential for transmission of the pathogen in the mouse model.
The picture emerging from this study is that S. Typhimurium uses its virulence factors to deplete butyrate-producing Clostridia from the gut-associated microbial community. The resulting increase in epithelial oxygenation drives a cytochrome bd-II oxidase-dependent aerobic expansion of S. Typhimurium within the gut lumen, which synergizes with a nitrate respiration-driven expansion to enhance transmission. This pathogenic strategy is exacerbated by oral antibiotic therapy since it enhances and accelerates Clostridia depletion, which might explain why treatment with oral antibiotics often precedes infection with antibiotic-sensitive S. enterica serovars causing human gastroenteritis (Pavia et al., 1990).
EXPERIMENTAL PROCEDURES
Bacterial strains and plasmids
The 17 human Clostridia isolates kindly provided by K. Honda (Atarashi et al., 2011; Narushima et al., 2014) and were cultured individually as described previously (Atarashi et al., 2013). S. Typhimurium and E. coli strains used in this study are listed in Table S1 and culture conditions are described in the supplemental experimental procedures. The plasmids and primers used in this study are listed in Tables S2 and S3, respectively. Construction of S. Typhimurium mutants and plasmids is described in the supplemental experimental procedures.
Animal Experiments
All experiments in this study were approved by the Institutional Animal Care and Use Committee at the University of California at Davis.
Female C57BL/6 mice, aged 8–12 weeks, and female CBA mice, aged 6–8 weeks, were obtained from The Jackson Laboratory, Bar Harbor (C57BL/6J mice; CBA/J mice). C57BL/6 mice were treated with 20 mg/animal streptomycin or mock-treated and orally inoculated 24 hours later with S. Typhimurium strains as described in the supplementary experimental procedures. For tributyrin supplementation, mice were mock-treated or received tributyrin (5g/kg) by oral gavage three hours after infection. CBA mice were inoculated with S. Typhimurium strains as described in the supplementary experimental procedures. For microbiota analysis, mice were euthanized at 10 and 17 days after infection and DNA from the cecal contents was extracted using the PowerSoil DNA Isolation kit (Mo-Bio, Carlsbad, CA) according to the manufacturer's protocol. Generation and analysis of sequencing data is described in detail in the supplemental experimental procedures. Transmission was studied as described previously (Lawley et al., 2008), and is described in detail in the supplemental experimental procedures. Inoculation of mice with a community of 17 Clostridia strains or with spore preparations is described in the supplemental experimental procedures. In some experiments Pimonidazole hydrochloride (PMDZ) (Hypoxyprobe, Inc., Burlington, MA) was administered by intraperitoneal injection at a dosage of approximately 60mg/kg body weight 60 minutes prior to euthanasia.
Analysis of animal samples
Hypoxia staining was performed as described previously (Kelly et al., 2015) and is described in detail in the supplemental experimental procedures. Blinded evaluation of histopathological changes was performed as described previously (Spees et al., 2013) using the criteria listed in Table S4. Short-chain fatty acid concentrations in cecal and colon contents were determined by mass spectrometer as described in the supplemental experimental procedures. RNA was isolated from tissue using standard methods (see supplemental experimental procedures for details) and was reverse transcribed using random hexamers and Moloney murine leukemia virus reverse transcriptase (Applied Biosystems). Quantitative real-time PCR was performed using SYBR green (Applied Biosystems) PCR mix and the appropriate primer sets (Table S2) at a final concentration of 0.25 mM. Absolute values were calculated using a plasmid carrying the cloned gene to generate a standard curve using concentrations ranging from 108 to 101 copies/μl diluted in a 0.02 mg/ml yeast RNA (Sigma) solution.
Statistical analysis
Fold-changes of ratios (bacterial numbers or mRNA levels) were transformed logarithmically prior to statistical analysis. An unpaired Student's t-test was used to determine whether differences in fold-changes between groups were statistically significant (P < 0.05). Significance of differences in histopathology scores was determined by a one-tailed non-parametric test (Mann-Whitney).
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge K. Honda for kindly providing 17 human Clostridia isolates and the Host-Microbe Systems Biology Core at UC for expert technical assistance with microbiota sequence analysis.
This work was supported by Public Health Service Grants AI096528 (A.J.B.), AI112949 (A.J.B.), AI103248 (S.E.W.), AI112241 (C.A.L.), OD010931 (E.M.V.) and AI060555 (E.M.V. and F.R.-C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS F.R.-C., L.F.Z., F.F., C.A.L., M.X.B. and E.E.O. performed and analyzed the experiments. G.X., C.B.L. and S.E.W. performed experiments. F.R.-C. and A.J.B. were responsible for the overall study design and for writing the manuscript.
SUPPLEMENTARY DATA Supplementary data include Figures S1–S7 and Tables S1–S4.
REFERENCES
- Alexeeva S, Hellingwerf KJ, Teixeira de Mattos MJ. Requirement of ArcA for redox regulation in Escherichia coli under microaerobic but not anaerobic or aerobic conditions. Journal of bacteriology. 2003;185:204–209. doi: 10.1128/JB.185.1.204-209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MM, Newsom DL, Gonzalez JF, Sabag-Daigle A, Stahl C, Steidley B, Dubena J, Dyszel JL, Smith JN, Dieye Y, et al. Fructose-asparagine is a primary nutrient during growth of Salmonella in the inflamed intestine. PLoS pathogens. 2014;10:e1004209. doi: 10.1371/journal.ppat.1004209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aserkoff B, Bennett JV. Effect of antibiotic therapy in acute salmonellosis on the fecal excretion of salmonellae. The New England journal of medicine. 1969;281:636–640. doi: 10.1056/NEJM196909182811202. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlung T, Brondsted L. Role of the transcriptional activator AppY in regulation of the cyx appA operon of Escherichia coli by anaerobiosis, phosphate starvation, and growth phase. Journal of bacteriology. 1994;176:5414–5422. doi: 10.1128/jb.176.17.5414-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman M, Unold D, Shifley K, Amir E, Hung K, Bos N, Salzman N. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infection and immunity. 2008;76:907–915. doi: 10.1128/IAI.01432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Russmann H, Hardt WD. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infection and immunity. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnhoff M, Drake BL, Miller CP. Effect of streptomycin on susceptibility of intestinal tract to experimental Salmonella infection. Proc Soc Exp Biol Med. 1954;86:132–137. doi: 10.3181/00379727-86-21030. [DOI] [PubMed] [Google Scholar]
- Brondsted L, Atlung T. Effect of growth conditions on expression of the acid phosphatase (cyx-appA) operon and the appY gene, which encodes a transcriptional activator of Escherichia coli. Journal of bacteriology. 1996;178:1556–1564. doi: 10.1128/jb.178.6.1556-1564.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. Journal of cellular and molecular medicine. 2011;15:1239–1253. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nature reviews Gastroenterology & hepatology. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter PA, Chepuri V, Gennis RB, Gunsalus RP. Cytochrome o (cyoABCDE) and d (cydAB) oxidase gene expression in Escherichia coli is regulated by oxygen, pH, and the fnr gene product. Journal of bacteriology. 1990;172:6333–6338. doi: 10.1128/jb.172.11.6333-6338.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter PA, Darie S, Gunsalus RP. The effect of iron limitation on expression of the aerobic and anaerobic electron transport pathway genes in Escherichia coli. FEMS Microbiol Lett. 1992;100:227–232. doi: 10.1111/j.1574-6968.1992.tb14045.x. [DOI] [PubMed] [Google Scholar]
- Cotter PA, Gunsalus RP. Contribution of the fnr and arcA gene products in coordinate regulation of cytochrome o and d oxidase (cyoABCDE and cydAB) genes in Escherichia coli. FEMS Microbiol Lett. 1992;70:31–36. doi: 10.1016/0378-1097(92)90558-6. [DOI] [PubMed] [Google Scholar]
- Craig M, Sadik AY, Golubeva YA, Tidhar A, Slauch JM. Twin-arginine translocation system (tat) mutants of Salmonella are attenuated due to envelope defects, not respiratory defects. Molecular microbiology. 2013;89:887–902. doi: 10.1111/mmi.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassa J, Fsihi H, Marck C, Dion M, Kieffer-Bontemps M, Boquet PL. A new oxygen-regulated operon in Escherichia coli comprises the genes for a putative third cytochrome oxidase and for pH 2.5 acid phosphatase (appA) Molecular & general genetics : MGG. 1991;229:341–352. doi: 10.1007/BF00267454. [DOI] [PubMed] [Google Scholar]
- Donohoe DR, Wali A, Brylawski BP, Bultman SJ. Microbial regulation of glucose metabolism and cell-cycle progression in mammalian colonocytes. PLoS One. 2012;7:e46589. doi: 10.1371/journal.pone.0046589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espey MG. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic Biol Med. 2013;55:130–140. doi: 10.1016/j.freeradbiomed.2012.10.554. [DOI] [PubMed] [Google Scholar]
- Fu HA, Iuchi S, Lin EC. The requirement of ArcA and Fnr for peak expression of the cyd operon in Escherichia coli under microaerobic conditions. Molecular & general genetics : MGG. 1991;226:209–213. doi: 10.1007/BF00273605. [DOI] [PubMed] [Google Scholar]
- Galan JE, Curtiss R., 3rd Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner CD, Antonopoulos DA, Wagner B, Duhamel GE, Keresztes I, Ross DA, Young VB, Altier C. Perturbation of the small intestine microbial ecology by streptomycin alters pathology in a Salmonella enterica serovar typhimurium murine model of infection. Infection and immunity. 2009;77:2691–2702. doi: 10.1128/IAI.01570-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill N, Ferreira RB, Antunes LC, Willing BP, Sekirov I, Al-Zahrani F, Hartmann M, Finlay BB. Neutrophil elastase alters the murine gut microbiota resulting in enhanced Salmonella colonization. PLoS One. 2012;7:e49646. doi: 10.1371/journal.pone.0049646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel M, Shea JE, Gleeson C, Jones MD, Dalton E, Holden DW. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- Itoh K, Freter R. Control of Escherichia coli populations by a combination of indigenous clostridia and lactobacilli in gnotobiotic mice and continuous-flow cultures. Infection and immunity. 1989;57:559–565. doi: 10.1128/iai.57.2.559-565.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizaka-Kondoh S, Konse-Nagasawa H. Significance of nitroimidazole compounds and hypoxia-inducible factor-1 for imaging tumor hypoxia. Cancer science. 2009;100:1366–1373. doi: 10.1111/j.1349-7006.2009.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley TD, Bouley DM, Hoy YE, Gerke C, Relman DA, Monack DM. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infection and immunity. 2008;76:403–416. doi: 10.1128/IAI.01189-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez CA, Rivera-Chavez F, Byndloss MX, Baumler A. The periplasmic nitrate reductase NapABC supports luminal growth of Salmonella enterica serovar Typhimurium during colitis. Infection and immunity. 2015 doi: 10.1128/IAI.00351-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez CA, Winter SE, Rivera-Chavez F, Xavier MN, Poon V, Nuccio SP, Tsolis RM, Baumler AJ. Phage-mediated acquisition of a type III secreted effector protein boosts growth of salmonella by nitrate respiration. mBio. 2012;3 doi: 10.1128/mBio.00143-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ, Jones TF, Fazil A, Hoekstra RM. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- McClelland M, Sanderson KE, Spieth J, Clifton SW, Latreille P, Courtney L, Porwollik S, Ali J, Dante M, Du F, et al. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature. 2001;413:852–856. doi: 10.1038/35101614. [DOI] [PubMed] [Google Scholar]
- Meynell GG. Antibacterial mechanisms of the mouse gut. II. The role of Eh and volatile fatty acids in the normal gut. British journal of experimental pathology. 1963;44:209–219. [PMC free article] [PubMed] [Google Scholar]
- Narushima S, Sugiura Y, Oshima K, Atarashi K, Hattori M, Suematsu M, Honda K. Characterization of the 17 strains of regulatory T cell-inducing human-derived Clostridia. Gut Microbes. 2014;5:333–339. doi: 10.4161/gmic.28572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JD, Kusmiesz H, Jackson LH, Woodman E. Treatment of Salmonella gastroenteritis with ampicillin, amoxicillin, or placebo. Pediatrics. 1980;65:1125–1130. [PubMed] [Google Scholar]
- Pavia AT, Shipman LD, Wells JG, Puhr ND, Smith JD, McKinley TW, Tauxe RV. Epidemiologic evidence that prior antimicrobial exposure decreases resistance to infection by antimicrobial-sensitive Salmonella. The Journal of infectious diseases. 1990;161:255–260. doi: 10.1093/infdis/161.2.255. [DOI] [PubMed] [Google Scholar]
- Que JU, Hentges DJ. Effect of streptomycin administration on colonization resistance to Salmonella typhimurium in mice. Infection and immunity. 1985;48:169–174. doi: 10.1128/iai.48.1.169-174.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Chavez F, Winter SE, Lopez CA, Xavier MN, Winter MG, Nuccio SP, Russell JM, Laughlin RC, Lawhon SD, Sterzenbach T, et al. Salmonella uses energy taxis to benefit from intestinal inflammation. PLoS pathogens. 2013;9:e1003267. doi: 10.1371/journal.ppat.1003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandle GI. Salt and water absorption in the human colon: a modern appraisal. Gut. 1998;43:294–299. doi: 10.1136/gut.43.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirov I, Tam NM, Jogova M, Robertson ML, Li Y, Lupp C, Finlay BB. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infection and immunity. 2008;76:4726–4736. doi: 10.1128/IAI.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spees AM, Wangdi T, Lopez CA, Kingsbury DD, Xavier MN, Winter SE, Tsolis RM, Baumler AJ. Streptomycin-Induced Inflammation Enhances Escherichia coli Gut Colonization Through Nitrate Respiration. mBio. 2013;4:e00430–00413. doi: 10.1128/mBio.00430-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, Sterzenbach T, Tsolis RM, Roth JR, Baumler AJ. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17480–17485. doi: 10.1073/pnas.1107857108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CP, Albrecht J, Gunsalus RP. Effect of microaerophilic cell growth conditions on expression of the aerobic (cyoABCDE and cydAB) and anaerobic (narGHJI, frdABCD, and dmsABC) respiratory pathway genes in Escherichia coli. Journal of bacteriology. 1996;178:1094–1098. doi: 10.1128/jb.178.4.1094-1098.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolis RM, Adams LG, Ficht TA, Baumler AJ. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infection and immunity. 1999;67:4879–4885. doi: 10.1128/iai.67.9.4879-4885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez OC, Lederer HM, Rombeau JL. Butyrate and the colonocyte. Production, absorption, metabolism, and therapeutic implications. Adv Exp Med Biol. 1997;427:123–134. [PubMed] [Google Scholar]
- Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio. 2014;5:e00889. doi: 10.1128/mBio.00889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.