Abstract
ABSTRACT. Caveolae are flask-shaped invaginations of the plasma membrane. The BAR domain proteins form crescent-shaped dimers, and their oligomeric filaments are considered to form spirals at the necks of invaginations, such as clathrin-coated pits and caveolae. PACSIN2/Syndapin II is one of the BAR domain-containing proteins, and is localized at the necks of caveolae. PACSIN2 is thought to function in the scission and stabilization of caveolae, through binding to dynamin-2 and EHD2, respectively. These two functions are considered to be switched by PACSIN2 phosphorylation by protein kinase C (PKC) upon hypotonic stress and sheer stress. The phosphorylation decreases the membrane binding affinity of PACSIN2, leading to its removal from caveolae. The removal of the putative oligomeric spiral of PACSIN2 from caveolar membrane invaginations could lead to the deformation of caveolae. Indeed, PACSIN2 removal from caveolae is accompanied by the recruitment of dynamin-2, suggesting that the removal provides space for the function of dynamin-2. Otherwise, the removal of PACSIN2 decreases the stability of caveolae, which could result in the flattening of caveolae. In contrast, an increase in the amount of EHD2 restored caveolar stability. Therefore, PACSIN2 at caveolae stabilizes caveolae, but its removal by phosphorylation could induce both caveolar endocytosis and flattening.
KEYWORDS: BAR domain, caveolae, mechanical stress, phosphorylation, protein kinase C
ABBREVIATIONS
- BAR
Bin/Amphiphysin/Rvs
- PKC
protein kinase C
- PRD
proline-rich domain
- TIRFM
total internal reflection fluorescence microscopy
INTRODUCTION
Caveolae are flask-shaped invaginations of the plasma membrane, with diameters from 50 to 100 nm. Caveolae are present in various cell types and have been implicated in many cellular processes, such as endocytosis and signal transduction.1 Initially, caveolae were thought to function as endocytic organelles that internalize extracellular materials or membrane. However, caveolae are immobile signaling platforms anchored to the actin cytoskeleton.1-3 Caveolin, the major structural protein in caveolae, is an evolutionarily conserved integral membrane protein.4 Caveolae contain many cellular components, such as sphingolipids, GM1 gangliosides, cholesterol, caveolin-1–3,1,4 cavin1–4,1,5,6 protein kinase C (PKC) α,7 EHD2,8,9 the F-actin cross-linking protein filamin,10 the promoter of actin-filament elongation mDia1,11 the Bin/Amphiphysin/Rvs (BAR) domain-containing proteins (BAR proteins, described below) 12,13 and others. Oligomerization of caveolin with cavins promotes the formation and maintenance of caveolae, by the generation of caveolar coats.14,15 Caveolar endocytosis is activated by PKCα,7,16 while scission from the plasma membrane is thought to be mediated by the ubiquitously expressed protein dynamin-2, a mechanochemical GTPase that oligomerizes at the necks of caveolae.17
In the resting state, caveolin-1 exhibits slow turnover in the plasma membrane, suggesting a tightly packed caveolar structure.18 However, under hypotonic conditions and during the activation of kinases such as PKC or the disruption of the actin cytoskeleton, caveolin-1 becomes relatively more mobile,2,19,20 indicating the dynamic regulation of caveolar molecules under such conditions. Therefore, it was suggested that caveolae function as mechanosensors by responding to membrane tension under regulation of protein kinases.
When cells are exposed to shear stress, caveolae formation is observed.21,22 Furthermore, caveolae act as membrane reservoirs in response to membrane tension under hypotonic conditions; i.e., they function as a buffer that unfolds upon membrane tension.19 The unfolding of the concave membrane of caveolae results in their flattening, which can increase the cellular surface area. During this process, the caveolar components, such as caveolin-1 and glycosphingolipids, are redistributed.19 These results indicate that caveolae play an important role in mechanotransduction,1,23 which might be related to the onset and progression of vascular proliferative disease.24 Furthermore, caveolae have been proposed to play crucial physiological roles in tumorigenesis, muscular disorders, cardiomyopathy, and other diseases,25,26 which might be dependent on the mechanical stress applied to cells. Hence, it is important to understand the mechanisms underlying the regulation of caveolae.
The BAR proteins involved in caveolae
The BAR domains are evolutionarily conserved protein domains. The BAR domains form crescent-shaped homo-dimers, which sense and/or generate membrane curvature through binding to the membrane.27-29 BAR domains have positively charged surfaces, which are considered to function as templates for membrane curvature. The BAR domains polymerize into helical coats or oligomeric spirals, through lateral and tip-to-tip interactions, to deform the membrane into tubules, and these properties are thought to be important for the determination of membrane shape (Fig. 1).27,28 The BAR domain superfamily consists of 3 subfamilies: the BAR domain,30 the F-BAR/EFC domain,31 and the I-BAR/IMD domain.32 The BAR and F-BAR domain proteins primarily function in membrane invagination, such as endocytosis, whereas the IMD/I-BAR domain proteins are involved in the formation of membrane protrusions, such as filopodia.
Figure 1.
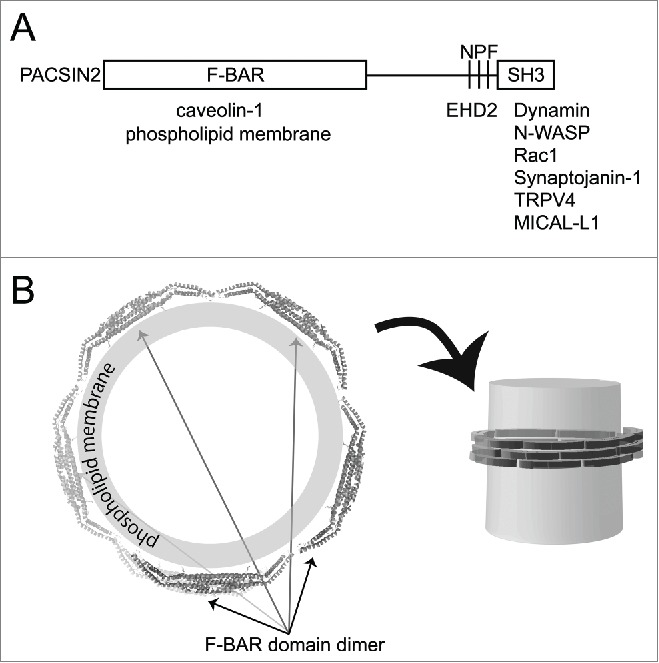
Domain structure of PACSIN2/Syndapin II and its binding proteins. (A) The domains of PACSIN2 and their binding proteins are illustrated. (B) The putative oligomeric spiral of PACSIN2 F-BAR domain around the membrane tubules. The F-BAR domain is supposed to form filamentous spiral, which assembles on the surface of membrane tubules such as those observed in the plasma membrane invaginations such as caveolae.
Caveolin is associated with the F-BAR domain proteins PACSIN2/Syndapin II and Nostrin.13,33 The F-BAR domain protein PACSIN2 regulates the morphogenesis and endocytosis of caveolae,12,13,34 through the positively charged concave surface that binds to membranes.35 The role of Nostrin in caveolar biogenesis has not been clarified yet.36
The PACSIN2 binding proteins connect to actin filaments
Caveolin-1 is tethered to the cortical actin cytoskeleton via filamin.10,37 However, PACSIN2 and the binding proteins also provide the connection to actin filaments. The BAR domain-containing proteins typically have SH3 domains.38 PACSIN2 has the F-BAR domain and the SH3 domain (Fig. 1). The C-terminal SH3 domain of PACSIN2 associates with the proline-rich domain (PRD) of dynamin-2 and N-WASP,39 that promotes membrane scission and activates the Arp2/3 complex-mediated actin polymerization, respectively.27 Nostrin binds to N-WASP through its SH3 domain.33 Although PACSIN and Nostrin bind to N-WASP, the roles of N-WASP and the Arp2/3 complex in caveolar functions remain unclear. Instead, mDia1 is reported to regulate actin polymerization in caveolae.11 However, the binding of mDia1 to PACSIN2 has not been examined.
Besides the SH3 domain, the Asn-Pro-Phe (NPF) sequence, in the linker between the F-BAR and SH3-domains of PACSIN2, binds to the Eps15 homology domain of the dynamin-like EHD2 ATPase.9,12 EHD2 binds to cavin1 and is localized to the necks of caveolae, where it stabilizes and constrains caveolae at the plasma membrane.8,9,40 Nucleotide hydrolysis by EHD2 is slower than that by dynamin 41; thus, EHD2 may control the slow dynamics of caveolae, which are considered to be important for the stabilization of caveolae.9 EHD2 exists at stationary caveolae and dissociates from caveolae after caveolar endocytosis.40 EHD2 constrains the lateral movement of caveolae, by linking the caveolae to actin filaments.40 Consistently, the depletion of EHD2 results in the mobilization of caveolae.9,40 Therefore, PACSIN2 and the PACSIN2-EHD2 complex play key roles in stabilizing caveolae, by associating with actin filaments.
There are several other binding proteins of PACSIN2, which include endosomal protein MICAL-L1,42 cation channel TRPV4,43 phosphoinositide phosphatase Synaptojanin-1,44 the Ras/Rac guanine nucleotide exchange factor Sos,45 and small GTPase Rac1.46 However, the roles of these proteins in caveolae have been not well understood.
Interestingly, the F-BAR domain of PACSIN2 was shown to directly bind to actin filaments on its concave surface, by stabilizing actin filaments in vitro.47 However, the concave surface also binds to the membrane for the caveolar localization of PACSIN2. Therefore, the role of the actin filament binding of PACSIN2 for caveolar dynamics is an issue that remains to be solved.
Phosphorylation of F-BAR domain protein PACSIN2 and caveolar dynamics
We identified PKC as the kinase that phosphorylates specific sites of PACSIN2.20 Surprisingly, the phosphorylation of PACSIN2 by PKC is induced by changes in hypotonic stress and shear stress, which accompany increases in the membrane tension, as well as by chemicals that directly activate PKC. The phosphorylation of PACSIN2 decreased its membrane-binding and tubulation abilities, which could be attributed to the repulsion between the negatively charged, phosphorylated serine 313 by PKCα and the relatively abundant negatively charged lipids, such as phosphatidylserine PI(4,5)P2. PACSIN2 phosphorylation did not affect its dimerization, auto-inhibition, or dynamin-2 interaction.20
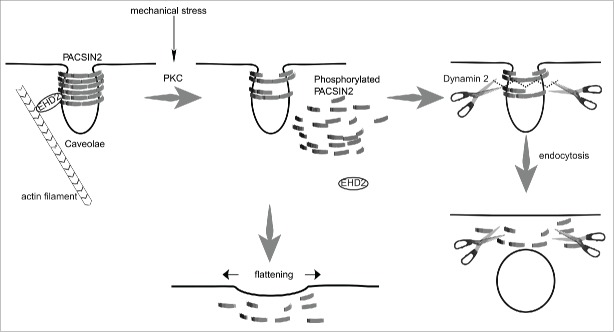
PACSIN2 phosphorylation at serine 313 decreased the lifetime of caveolae,20 which resulted in decreases in the number and the stability of caveolae and an increase in the mobility of the caveolae at the plasma membrane. These phenomena could be explained by the removal of PACSIN2 from caveolae, due to its decreased membrane binding.20 The removal of PACSIN2 could lead to 2 scenarios, endocytosis and flattening of caveolae, as discussed below (Fig. 2). However, we currently cannot distinguish between these 2 events by TIRFM, because these 2 scenarios are both observed by the disappearance of caveolae.
Figure 2.
A model of caveolar endocytosis mediated by PACSIN2 phosphorylation through PKC activation by mechanical stress. PACSIN2 oligomerizes and binds to the necks of caveolae. PACSIN2 is phosphorylated by PKC upon mechanical stimuli, such as hypotonic stress and shear stress, and phosphorylated PACSIN2 dissociates from the necks of caveolae. This enables dynamin-2 to occupy the space after the removal of PACSIN2, which induces caveolar scission and endocytosis. Otherwise, the loss of PACSIN2 at the caveolar neck leads to caveolar flattening, to buffer membrane tension.
If caveolae are flattened, then the disappearance of caveolae observed by TIRFM could be explained by the dilution of caveolin-1 from caveolae to the plasma membrane. PACSIN2 regulates the morphology of the necks of caveolae,13 and thus the dissociation of PACSIN2 by phosphorylation could make the caveolae deformable into flat membrane.
If caveolae are internalized by endocytosis, then the disappearance of caveolin-1 can be explained by its uptake into the interior of the cell, beyond the illumination of TIRFM. The disappearance of PACSIN2 was followed by the recruitment of dynamin-2,20 suggesting that the removal of PACSIN2 provides some spaces for the association of dynamin for membrane scission upon endocytosis.20 Independently, the removal of PACSIN2 alone could be predicted to be a trigger for the scission from theoretical approach,48 which have shown that BAR proteins have a scaffolding function, involved in stabilizing the neck of the invagination and preventing membrane scission.48 Thus, the removal of BAR domain protein from the neck of the invagination could induce membrane destabilization and scission.48
Concluding remarks
Other caveolar proteins, such as cavin1–4, might be co-regulated with PACSIN2 phosphorylation, as cavins bind to and/or recruit PKCα.49,50 Cavin-1 dissociated from caveolin-1 upon caveolae flattening.19 Therefore, the mechanical stress-induced removal of PACSIN2 by PKC-mediated phosphorylation would cooperatively function with these caveolar components to promote caveolar endocytosis and flattening. Biomechanical and theoretical analyses of the mechanics of caveolar deformation will facilitate the clarification of the behaviors of caveolae upon various stimuli.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the Funding Program for Next Generation World-Leading Researchers (NEXT program); Japan Society for the Promotion of Science (JSPS) KAKENHI [grant numbers 26291037, 15H01641, 15H05902].
REFERENCES
- 1.Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol 2013; 14:98-112; PMID:23340574; http://dx.doi.org/ 10.1038/nrm3512 [DOI] [PubMed] [Google Scholar]
- 2.Tagawa A, Mezzacasa A, Hayer A, Longatti A, Pelkmans L, Helenius A. Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J Cell Biol 2005; 170:769-79; PMID:16129785; http://dx.doi.org/ 10.1083/jcb.200506103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomsen P, Roepstorff K, Stahlhut M, van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol Biol Cell 2002; 13:238-50; PMID: 11809836; http://dx.doi.org/ 10.1091/mbc.01-06-0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell 1992; 68:673-82; PMID:1739974; http://dx.doi.org/ 10.1016/0092-8674(92)90143-Z [DOI] [PubMed] [Google Scholar]
- 5.Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM, Martin S, et al.. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell 2008; 132:113-24; PMID:18191225; http://dx.doi.org/ 10.1016/j.cell.2007.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen CG, Nichols BJ. Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol 2010; 20:177-86; PMID:20153650; http://dx.doi.org/ 10.1016/j.tcb.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 7.Oka N, Yamamoto M, Schwencke C, Kawabe J, Ebina T, Ohno S, Couet J, Lisanti MP, Ishikawa Y. Caveolin interaction with protein kinase C. Isoenzyme-dependent regulation of kinase activity by the caveolin scaffolding domain peptide. J Biol Chem 1997; 272:33416-21; PMID:9407137; http://dx.doi.org/ 10.1074/jbc.272.52.33416 [DOI] [PubMed] [Google Scholar]
- 8.Shah C, Hegde BG, Moren B, Behrmann E, Mielke T, Moenke G, Spahn CM, Lundmark R, Daumke O, Langen R. Structural insights into membrane interaction and caveolar targeting of dynamin-like EHD2. Structure 2014; 22:409-20; PMID:24508342; http://dx.doi.org/ 10.1016/j.str.2013.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moren B, Shah C, Howes MT, Schieber NL, McMahon HT, Parton RG, Daumke O, Lundmark R. EHD2 regulates caveolar dynamics via ATP-driven targeting and oligomerization. Mol Biol Cell 2012; 23:1316-29; PMID:22323287; http://dx.doi.org/ 10.1091/mbc.E11-09-0787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stahlhut M, van Deurs B. Identification of filamin as a novel ligand for caveolin-1: evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol Biol Cell 2000; 11:325-37; PMID:10637311; http://dx.doi.org/ 10.1091/mbc.11.1.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Echarri A, Muriel O, Pavon DM, Azegrouz H, Escolar F, Terron MC, Sanchez-Cabo F, Martínez F, Montoya MC, Llorca O, et al.. Caveolar domain organization and trafficking is regulated by Abl kinases and mDia1. J Cell Sci 2012; 125:3097-113; PMID:22454521; http://dx.doi.org/ 10.1242/jcs.090134 [DOI] [PubMed] [Google Scholar]
- 12.Hansen CG, Howard G, Nichols BJ. Pacsin 2 is recruited to caveolae and functions in caveolar biogenesis. J Cell Sci 2011; 124:2777-85; PMID:21807942; http://dx.doi.org/ 10.1242/jcs.084319 [DOI] [PubMed] [Google Scholar]
- 13.Senju Y, Itoh Y, Takano K, Hamada S, Suetsugu S. Essential role of PACSIN2/syndapin-II in caveolae membrane sculpting. J Cell Sci 2011; 124:2032-40; PMID:21610094; http://dx.doi.org/ 10.1242/jcs.086264 [DOI] [PubMed] [Google Scholar]
- 14.Kovtun O, Tillu VA, Jung WR, Leneva N, Ariotti N, Chaudhary N, Mandyam RA, Ferguson C, Morgan GP, Johnston WA, et al.. Structural Insights into the Organization of the Cavin Membrane Coat Complex. Dev Cell 2014; 31:405-19; PMID:25453557; http://dx.doi.org/ 10.1016/j.devcel.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 15.Ludwig A, Howard G, Mendoza-Topaz C, Deerinck T, Mackey M, Sandin S, Ellisman MH, Nichols BJ. Molecular composition and ultrastructure of the caveolar coat complex. PLoS Biol 2013; 11:e1001640; PMID:24013648; http://dx.doi.org/ 10.1371/journal.pbio.1001640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mineo C, Ying YS, Chapline C, Jaken S, Anderson RG. Targeting of protein kinase Calpha to caveolae. J Cell Biol 1998; 141:601-10; PMID:9566962; http://dx.doi.org/ 10.1083/jcb.141.3.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelkmans L, Puntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 2002; 296:535-9; http://dx.doi.org/ 10.1126/science.1069784 [DOI] [PubMed] [Google Scholar]
- 18.Pelkmans L, Burli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 2004; 118:767-80; PMID:15369675; http://dx.doi.org/ 10.1016/j.cell.2004.09.003 [DOI] [PubMed] [Google Scholar]
- 19.Sinha B, Koster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, et al.. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 2011; 144:402-13; PMID:21295700; http://dx.doi.org/ 10.1016/j.cell.2010.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senju Y, Rosenbaum E, Shah C, Hamada-Nakahara S, Itoh Y, Yamamoto K, Hanawa-Suetsugu K, Daumke O, Suetsugu S. Phosphorylation of PACSIN2 by protein kinase C triggers the removal of caveolae from the plasma membrane. J Cell Sci 2015; 128:2766-80; PMID:26092940; http://dx.doi.org/ 10.1242/jcs.167775 [DOI] [PubMed] [Google Scholar]
- 21.Boyd NL, Park H, Yi H, Boo YC, Sorescu GP, Sykes M, Jo H. Chronic shear induces caveolae formation and alters ERK and Akt responses in endothelial cells. Am J Physiol Heart Circulatory Physiol 2003; 285:H1113-22; http://dx.doi.org/ 10.1152/ajpheart.00302.2003 [DOI] [PubMed] [Google Scholar]
- 22.Rizzo V, Morton C, DePaola N, Schnitzer JE, Davies PF. Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. Am J physiol Heart Circulatory Physiol 2003; 285:H1720-9; http://dx.doi.org/ 10.1152/ajpheart.00344.2002 [DOI] [PubMed] [Google Scholar]
- 23.Gervasio OL, Phillips WD, Cole L, Allen DG. Caveolae respond to cell stretch and contribute to stretch-induced signaling. J Cell Sci 2011; 124:3581-90; PMID:22045729; http://dx.doi.org/ 10.1242/jcs.084376 [DOI] [PubMed] [Google Scholar]
- 24.Sedding DG, Braun-Dullaeus RC. Caveolin-1: dual role for proliferation of vascular smooth muscle cells. Trends Cardiovascular Med 2006; 16:50-5; http://dx.doi.org/ 10.1016/j.tcm.2005.11.007 [DOI] [PubMed] [Google Scholar]
- 25.Bonilla E, Fischbeck K, Schotland DL. Freeze-fracture studies of muscle caveolae in human muscular dystrophy. Am J Pathol 1981; 104:167-73; PMID:7258302 [PMC free article] [PubMed] [Google Scholar]
- 26.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, et al.. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 2001; 293:2449-52; http://dx.doi.org/ 10.1126/science.1062688 [DOI] [PubMed] [Google Scholar]
- 27.Suetsugu S, Kurisu S, Takenawa T. Dynamic shaping of cellular membranes by phospholipids and membrane-deforming proteins. Physiol Rev 2014; 94:1219-48; PMID:25287863; http://dx.doi.org/ 10.1152/physrev.00040.2013 [DOI] [PubMed] [Google Scholar]
- 28.Daumke O, Roux A, Haucke V. BAR domain scaffolds in dynamin-mediated membrane fission. Cell 2014; 156:882-92; PMID:24581490; http://dx.doi.org/ 10.1016/j.cell.2014.02.017 [DOI] [PubMed] [Google Scholar]
- 29.Frost A, Unger VM, De Camilli P. The BAR domain superfamily: membrane-molding macromolecules. Cell 2009; 137:191-6; PMID:19379681; http://dx.doi.org/ 10.1016/j.cell.2009.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science (New York, NY) 2004; 303:495-9; http://dx.doi.org/ 10.1126/science.1092586 [DOI] [PubMed] [Google Scholar]
- 31.Shimada A, Niwa H, Tsujita K, Suetsugu S, Nitta K, Hanawa-Suetsugu K, Akasaka R, Nishino Y, Toyama M, Chen L, et al.. Curved EFC/F-BAR-domain dimers are joined end to end into a filament for membrane invagination in endocytosis. Cell 2007; 129:761-72; PMID:17512409; http://dx.doi.org/ 10.1016/j.cell.2007.03.040 [DOI] [PubMed] [Google Scholar]
- 32.Suetsugu S, Murayama K, Sakamoto A, Hanawa-Suetsugu K, Seto A, Oikawa T, Mishima C, Shirouzu M, Takenawa T, Yokoyama S. The RAC binding domain/IRSp53-MIM homology domain of IRSp53 induces RAC-dependent membrane deformation. J Biol Chem 2006; 281:35347-58; PMID:17003044; http://dx.doi.org/ 10.1074/jbc.M606814200 [DOI] [PubMed] [Google Scholar]
- 33.Schilling K, Opitz N, Wiesenthal A, Oess S, Tikkanen R, Muller-Esterl W, Icking A. Translocation of endothelial nitric-oxide synthase involves a ternary complex with caveolin-1 and NOSTRIN. Mol Biol Cell 2006; 17:3870-80; PMID:16807357; http://dx.doi.org/ 10.1091/mbc.E05-08-0709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koch D, Westermann M, Kessels MM, Qualmann B. Ultrastructural freeze-fracture immunolabeling identifies plasma membrane-localized syndapin II as a crucial factor in shaping caveolae. Histochem Cell Biol 2012; 138:215-30; PMID:22718246; http://dx.doi.org/ 10.1007/s00418-012-0945-0 [DOI] [PubMed] [Google Scholar]
- 35.Shimada A, Takano K, Shirouzu M, Hanawa-Suetsugu K, Terada T, Toyooka K, Umehara T, Yamamoto M, Yokoyama S, Suetsugu S. Mapping of the basic amino-acid residues responsible for tubulation and cellular protrusion by the EFC/F-BAR domain of pacsin2/Syndapin II. FEBS Letters 2010; 584:1111-8; PMID:20188097; http://dx.doi.org/ 10.1016/j.febslet.2010.02.058 [DOI] [PubMed] [Google Scholar]
- 36.Kovacevic I, Hu J, Siehoff-Icking A, Opitz N, Griffin A, Perkins AC, Munn AL, Müller-Esterl W, Popp R, Fleming I, et al.. The F-BAR protein NOSTRIN participates in FGF signal transduction and vascular development. EMBO J 2012; 31:3309-22; PMID:22751148; http://dx.doi.org/ 10.1038/emboj.2012.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muriel O, Echarri A, Hellriegel C, Pavon DM, Beccari L, Del Pozo MA. Phosphorylated filamin A regulates actin-linked caveolae dynamics. J Cell Sci 2011; 124:2763-76; PMID:21807941; http://dx.doi.org/ 10.1242/jcs.080804 [DOI] [PubMed] [Google Scholar]
- 38.Suetsugu S, Toyooka K, Senju Y. Subcellular membrane curvature mediated by the BAR domain superfamily proteins. Seminars Cell Dev Biol 2010; 21:340-9; http://dx.doi.org/ 10.1016/j.semcdb.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 39.Kessels MM, Qualmann B. The syndapin protein family: linking membrane trafficking with the cytoskeleton. J Cell Sci 2004; 117:3077-86; PMID:15226389; http://dx.doi.org/ 10.1242/jcs.01290 [DOI] [PubMed] [Google Scholar]
- 40.Stoeber M, Stoeck IK, Hanni C, Bleck CK, Balistreri G, Helenius A. Oligomers of the ATPase EHD2 confine caveolae to the plasma membrane through association with actin. EMBO J 2012; 31:2350-64; PMID: 22505029; http://dx.doi.org/ 10.1038/emboj.2012.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daumke O, Lundmark R, Vallis Y, Martens S, Butler PJ, McMahon HT. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature 2007; 449:923-7; PMID: 17914359; http://dx.doi.org/ 10.1038/nature06173 [DOI] [PubMed] [Google Scholar]
- 42.Giridharan SS, Cai B, Vitale N, Naslavsky N, Caplan S. Cooperation of MICAL-L1, syndapin2, and phosphatidic acid in tubular recycling endosome biogenesis. Mol Biol Cell 2013; 24:1776-90, S1–15; http://dx.doi.org/ 10.1091/mbc.E13-01-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuajungco MP, Grimm C, Oshima K, D'Hoedt D, Nilius B, Mensenkamp AR, Bindels RJ, Plomann M, Heller S. PACSINs bind to the TRPV4 cation channel. PACSIN 3 modulates the subcellular localization of TRPV4. J Biol Chem 2006; 281:18753-62; PMID:16627472; http://dx.doi.org/ 10.1074/jbc.M602452200 [DOI] [PubMed] [Google Scholar]
- 44.Modregger J, Ritter B, Witter B, Paulsson M, Plomann M. All three PACSIN isoforms bind to endocytic proteins and inhibit endocytosis. J Cell Sci 2000; 113 Pt 24:4511-21; PMID:11082044 [DOI] [PubMed] [Google Scholar]
- 45.Wasiak S, Quinn CC, Ritter B, de Heuvel E, Baranes D, Plomann M, McPherson PS. The Ras/Rac guanine nucleotide exchange factor mammalian Son-of-sevenless interacts with PACSIN 1/syndapin I, a regulator of endocytosis and the actin cytoskeleton. J Biol Chem 2001; 276:26622-8; PMID:11352907; http://dx.doi.org/ 10.1074/jbc.M100591200 [DOI] [PubMed] [Google Scholar]
- 46.de Kreuk B J, Nethe M, Fernandez-Borja M, Anthony EC, Hensbergen P J, Deelder A M, Plomann M, Hordijk P L. The F-BAR domain protein PACSIN2 associates with Rac1 and regulates cell spreading and migration. J Cell Sci 2011; 124:2375-88; PMID:21693584; http://dx.doi.org/ 10.1242/jcs.080630 [DOI] [PubMed] [Google Scholar]
- 47.Kostan J, Salzer U, Orlova A, Toro I, Hodnik V, Senju Y, Zou J, Schreiner C, Steiner J, Meriläinen J, et al.. Direct interaction of actin filaments with F-BAR protein pacsin2. EMBO Reports 2014; 15:1154-62; PMID:25216944; http://dx.doi.org/ 10.15252/embr.201439267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dmitrieff S, Nedelec F. Membrane Mechanics of Endocytosis in Cells with Turgor. PLoS Computational Biol 2015; 11:e1004538; http://dx.doi.org/ 10.1371/journal.pcbi.1004538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill MM, Daud NH, Aung CS, Loo D, Martin S, Murphy S, Black DM, Barry R, Simpson F, Liu L, et al.. Co-regulation of cell polarization and migration by caveolar proteins PTRF/Cavin-1 and caveolin-1. PloS One 2012; 7:e43041; PMID:22912783; http://dx.doi.org/ 10.1371/journal.pone.0043041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Izumi Y, Hirai S, Tamai Y, Fujise-Matsuoka A, Nishimura Y, Ohno S. A protein kinase Cdelta-binding protein SRBC whose expression is induced by serum starvation. J Biol Chem 1997; 272:7381-9; PMID:9054438; http://dx.doi.org/ 10.1074/jbc.272.11.7381 [DOI] [PubMed] [Google Scholar]